 Found 222 hits with Last Name = 'shimazaki' and Initial = 'm'
Found 222 hits with Last Name = 'shimazaki' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50165518
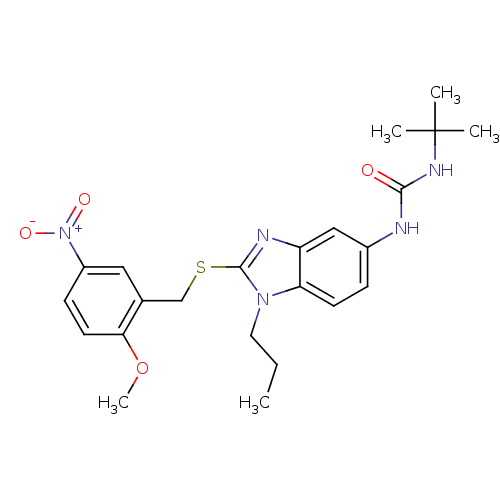
(1-tert-Butyl-3-[2-(2-methoxy-5-nitro-benzylsulfany...)Show SMILES CCCn1c(SCc2cc(ccc2OC)[N+]([O-])=O)nc2cc(NC(=O)NC(C)(C)C)ccc12 Show InChI InChI=1S/C23H29N5O4S/c1-6-11-27-19-9-7-16(24-21(29)26-23(2,3)4)13-18(19)25-22(27)33-14-15-12-17(28(30)31)8-10-20(15)32-5/h7-10,12-13H,6,11,14H2,1-5H3,(H2,24,26,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 2265-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.030
BindingDB Entry DOI: 10.7270/Q2GB23K1 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50165530

(6-(3-tert-Butyl-ureido)-2-(2-methoxy-5-nitro-benzy...)Show SMILES CCCn1c(SCc2cc(ccc2OC)[N+]([O-])=O)nc2cc(NC(=O)NC(C)(C)C)cc(C(=O)NCCCN(C)C)c12 Show InChI InChI=1S/C29H41N7O5S/c1-8-13-35-25-22(26(37)30-12-9-14-34(5)6)16-20(31-27(38)33-29(2,3)4)17-23(25)32-28(35)42-18-19-15-21(36(39)40)10-11-24(19)41-7/h10-11,15-17H,8-9,12-14,18H2,1-7H3,(H,30,37)(H2,31,33,38) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rat leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 2265-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.030
BindingDB Entry DOI: 10.7270/Q2GB23K1 |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50149812
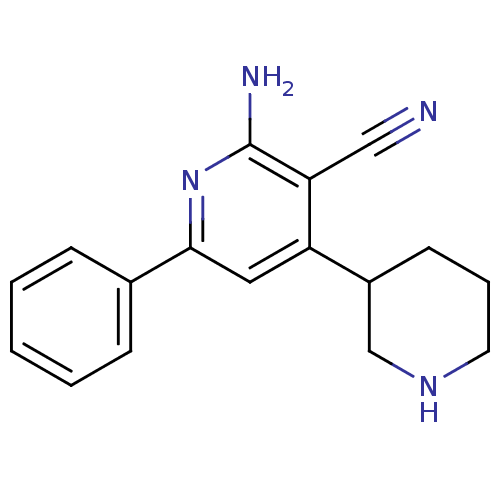
(2''-Amino-6''-phenyl-1,2,3,4,5,6-hexahydro-[3,4'']...)Show InChI InChI=1S/C17H18N4/c18-10-15-14(13-7-4-8-20-11-13)9-16(21-17(15)19)12-5-2-1-3-6-12/h1-3,5-6,9,13,20H,4,7-8,11H2,(H2,19,21) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >20 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human I-kappa-B-kinase beta |
Bioorg Med Chem Lett 14: 4013-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.040
BindingDB Entry DOI: 10.7270/Q2PC31VC |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50165553
(6-(3-tert-Butyl-ureido)-2-(2-methoxy-5-nitro-benzy...)Show SMILES CCCn1c(SCc2cc(ccc2OC)[N+]([O-])=O)nc2cc(NC(=O)NC(C)(C)C)cc(C(=O)NCCCN(C)Cc3ccccc3)c12 Show InChI InChI=1S/C35H45N7O5S/c1-7-17-41-31-28(32(43)36-16-11-18-40(5)22-24-12-9-8-10-13-24)20-26(37-33(44)39-35(2,3)4)21-29(31)38-34(41)48-23-25-19-27(42(45)46)14-15-30(25)47-6/h8-10,12-15,19-21H,7,11,16-18,22-23H2,1-6H3,(H,36,43)(H2,37,39,44) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rat leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 2265-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.030
BindingDB Entry DOI: 10.7270/Q2GB23K1 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50165538

(6-(3-tert-Butyl-ureido)-2-(2-methoxy-5-nitro-benzy...)Show SMILES CCCn1c(SCc2cc(ccc2OC)[N+]([O-])=O)nc2cc(NC(=O)NC(C)(C)C)cc(C(O)=O)c12 Show InChI InChI=1S/C24H29N5O6S/c1-6-9-28-20-17(21(30)31)11-15(25-22(32)27-24(2,3)4)12-18(20)26-23(28)36-13-14-10-16(29(33)34)7-8-19(14)35-5/h7-8,10-12H,6,9,13H2,1-5H3,(H,30,31)(H2,25,27,32) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 2265-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.030
BindingDB Entry DOI: 10.7270/Q2GB23K1 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50165528

(6-(3-tert-Butyl-ureido)-2-(2-methoxy-5-nitro-benzy...)Show SMILES CCCn1c(SCc2cc(ccc2OC)[N+]([O-])=O)nc2cc(NC(=O)NC(C)(C)C)cc(C(=O)NC)c12 Show InChI InChI=1S/C25H32N6O5S/c1-7-10-30-21-18(22(32)26-5)12-16(27-23(33)29-25(2,3)4)13-19(21)28-24(30)37-14-15-11-17(31(34)35)8-9-20(15)36-6/h8-9,11-13H,7,10,14H2,1-6H3,(H,26,32)(H2,27,29,33) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rat leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 2265-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.030
BindingDB Entry DOI: 10.7270/Q2GB23K1 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50165539
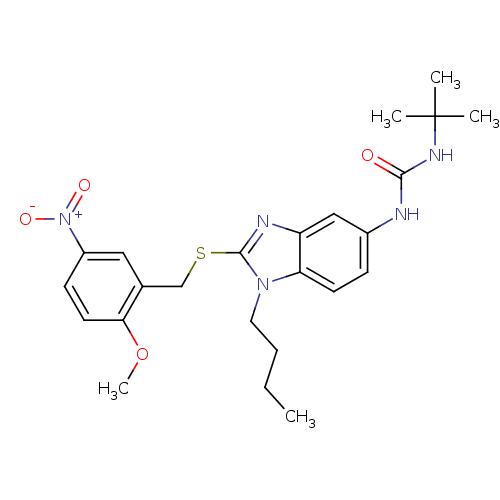
(1-tert-Butyl-3-[1-butyl-2-(2-methoxy-5-nitro-benzy...)Show SMILES CCCCn1c(SCc2cc(ccc2OC)[N+]([O-])=O)nc2cc(NC(=O)NC(C)(C)C)ccc12 Show InChI InChI=1S/C24H31N5O4S/c1-6-7-12-28-20-10-8-17(25-22(30)27-24(2,3)4)14-19(20)26-23(28)34-15-16-13-18(29(31)32)9-11-21(16)33-5/h8-11,13-14H,6-7,12,15H2,1-5H3,(H2,25,27,30) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 2265-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.030
BindingDB Entry DOI: 10.7270/Q2GB23K1 |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit alpha
(Homo sapiens (Human)) | BDBM50149806
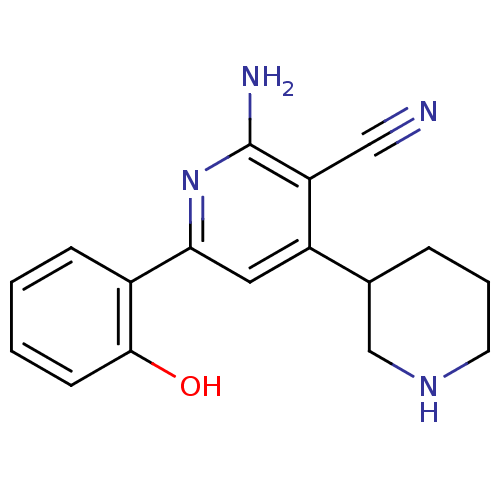
(2''-Amino-6''-(2-hydroxy-phenyl)-1,2,3,4,5,6-hexah...)Show InChI InChI=1S/C17H18N4O/c18-9-14-13(11-4-3-7-20-10-11)8-15(21-17(14)19)12-5-1-2-6-16(12)22/h1-2,5-6,8,11,20,22H,3-4,7,10H2,(H2,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibition of I-kappa-B-kinase alpha |
Bioorg Med Chem Lett 14: 4013-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.040
BindingDB Entry DOI: 10.7270/Q2PC31VC |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50149806

(2''-Amino-6''-(2-hydroxy-phenyl)-1,2,3,4,5,6-hexah...)Show InChI InChI=1S/C17H18N4O/c18-9-14-13(11-4-3-7-20-10-11)8-15(21-17(14)19)12-5-1-2-6-16(12)22/h1-2,5-6,8,11,20,22H,3-4,7,10H2,(H2,19,21) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human I-kappa-B-kinase beta |
Bioorg Med Chem Lett 14: 4013-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.040
BindingDB Entry DOI: 10.7270/Q2PC31VC |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50165533

(6-(3-tert-Butyl-ureido)-2-(2-methoxy-5-nitro-benzy...)Show SMILES CCCn1c(SCc2cc(ccc2OC)[N+]([O-])=O)nc2cc(NC(=O)NC(C)(C)C)cc(C(=O)NCCc3ccccn3)c12 Show InChI InChI=1S/C31H37N7O5S/c1-6-15-37-27-24(28(39)33-14-12-21-9-7-8-13-32-21)17-22(34-29(40)36-31(2,3)4)18-25(27)35-30(37)44-19-20-16-23(38(41)42)10-11-26(20)43-5/h7-11,13,16-18H,6,12,14-15,19H2,1-5H3,(H,33,39)(H2,34,36,40) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 2265-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.030
BindingDB Entry DOI: 10.7270/Q2GB23K1 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50165544
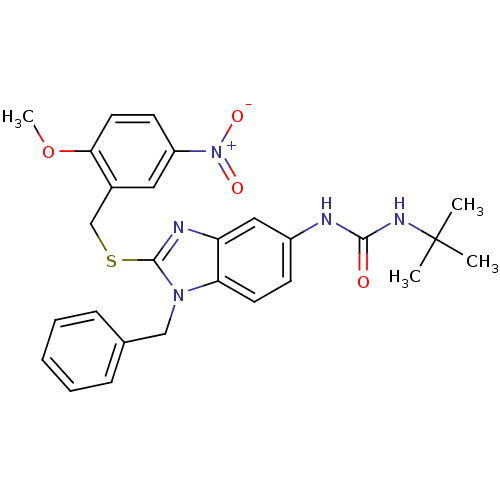
(1-[1-Benzyl-2-(2-methoxy-5-nitro-benzylsulfanyl)-1...)Show SMILES COc1ccc(cc1CSc1nc2cc(NC(=O)NC(C)(C)C)ccc2n1Cc1ccccc1)[N+]([O-])=O Show InChI InChI=1S/C27H29N5O4S/c1-27(2,3)30-25(33)28-20-10-12-23-22(15-20)29-26(31(23)16-18-8-6-5-7-9-18)37-17-19-14-21(32(34)35)11-13-24(19)36-4/h5-15H,16-17H2,1-4H3,(H2,28,30,33) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 2265-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.030
BindingDB Entry DOI: 10.7270/Q2GB23K1 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50165521
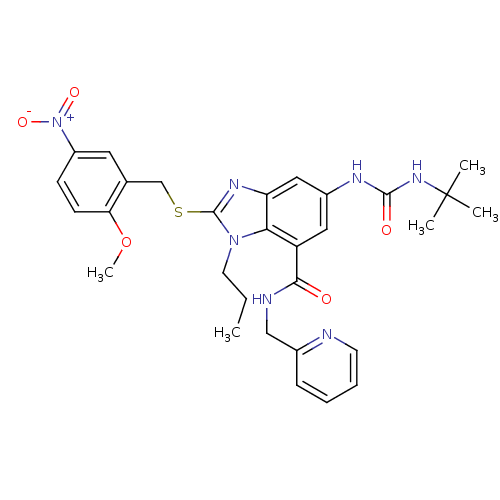
(6-(3-tert-Butyl-ureido)-2-(2-methoxy-5-nitro-benzy...)Show SMILES CCCn1c(SCc2cc(ccc2OC)[N+]([O-])=O)nc2cc(NC(=O)NC(C)(C)C)cc(C(=O)NCc3ccccn3)c12 Show InChI InChI=1S/C30H35N7O5S/c1-6-13-36-26-23(27(38)32-17-20-9-7-8-12-31-20)15-21(33-28(39)35-30(2,3)4)16-24(26)34-29(36)43-18-19-14-22(37(40)41)10-11-25(19)42-5/h7-12,14-16H,6,13,17-18H2,1-5H3,(H,32,38)(H2,33,35,39) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 2265-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.030
BindingDB Entry DOI: 10.7270/Q2GB23K1 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50165563
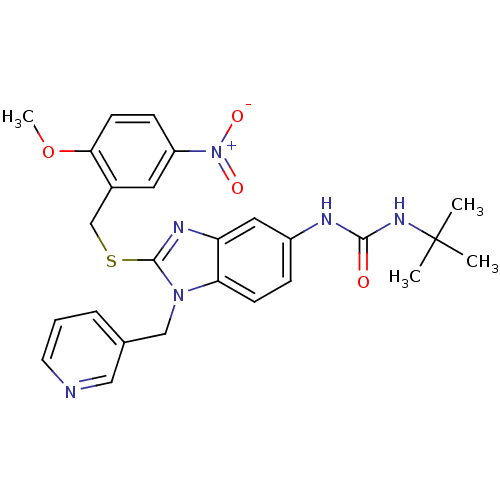
(1-tert-Butyl-3-[2-(2-methoxy-5-nitro-benzylsulfany...)Show SMILES COc1ccc(cc1CSc1nc2cc(NC(=O)NC(C)(C)C)ccc2n1Cc1cccnc1)[N+]([O-])=O Show InChI InChI=1S/C26H28N6O4S/c1-26(2,3)30-24(33)28-19-7-9-22-21(13-19)29-25(31(22)15-17-6-5-11-27-14-17)37-16-18-12-20(32(34)35)8-10-23(18)36-4/h5-14H,15-16H2,1-4H3,(H2,28,30,33) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 2265-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.030
BindingDB Entry DOI: 10.7270/Q2GB23K1 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50165557
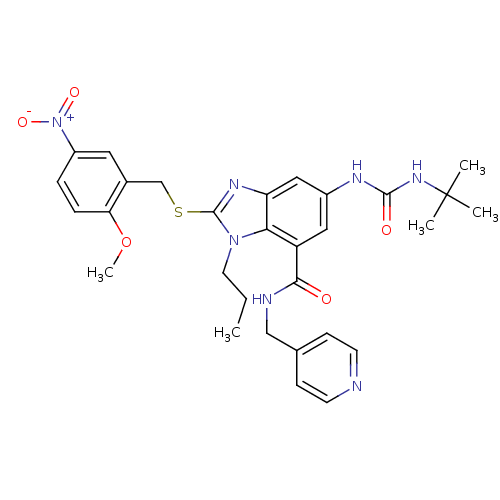
(6-(3-tert-Butyl-ureido)-2-(2-methoxy-5-nitro-benzy...)Show SMILES CCCn1c(SCc2cc(ccc2OC)[N+]([O-])=O)nc2cc(NC(=O)NC(C)(C)C)cc(C(=O)NCc3ccncc3)c12 Show InChI InChI=1S/C30H35N7O5S/c1-6-13-36-26-23(27(38)32-17-19-9-11-31-12-10-19)15-21(33-28(39)35-30(2,3)4)16-24(26)34-29(36)43-18-20-14-22(37(40)41)7-8-25(20)42-5/h7-12,14-16H,6,13,17-18H2,1-5H3,(H,32,38)(H2,33,35,39) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rat leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 2265-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.030
BindingDB Entry DOI: 10.7270/Q2GB23K1 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50165551

(6-(3-tert-Butyl-ureido)-2-(2-methoxy-5-nitro-benzy...)Show SMILES CCCn1c(SCc2cc(ccc2OC)[N+]([O-])=O)nc2cc(NC(=O)NC(C)(C)C)cc(C(=O)OC)c12 Show InChI InChI=1S/C25H31N5O6S/c1-7-10-29-21-18(22(31)36-6)12-16(26-23(32)28-25(2,3)4)13-19(21)27-24(29)37-14-15-11-17(30(33)34)8-9-20(15)35-5/h8-9,11-13H,7,10,14H2,1-6H3,(H2,26,28,32) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 2265-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.030
BindingDB Entry DOI: 10.7270/Q2GB23K1 |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50149822

(2-Amino-4-[1-amino-2-(4-fluoro-phenyl)-ethyl]-6-(2...)Show InChI InChI=1S/C20H17FN4O/c21-13-7-5-12(6-8-13)9-17(23)15-10-18(25-20(24)16(15)11-22)14-3-1-2-4-19(14)26/h1-8,10,17,26H,9,23H2,(H2,24,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human I-kappa-B-kinase beta |
Bioorg Med Chem Lett 14: 4013-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.040
BindingDB Entry DOI: 10.7270/Q2PC31VC |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50165528

(6-(3-tert-Butyl-ureido)-2-(2-methoxy-5-nitro-benzy...)Show SMILES CCCn1c(SCc2cc(ccc2OC)[N+]([O-])=O)nc2cc(NC(=O)NC(C)(C)C)cc(C(=O)NC)c12 Show InChI InChI=1S/C25H32N6O5S/c1-7-10-30-21-18(22(32)26-5)12-16(27-23(33)29-25(2,3)4)13-19(21)28-24(30)37-14-15-11-17(31(34)35)8-9-20(15)36-6/h8-9,11-13H,7,10,14H2,1-6H3,(H,26,32)(H2,27,29,33) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 2265-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.030
BindingDB Entry DOI: 10.7270/Q2GB23K1 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50165523

(1-tert-Butyl-3-[1-ethyl-2-(2-methoxy-5-nitro-benzy...)Show SMILES CCn1c(SCc2cc(ccc2OC)[N+]([O-])=O)nc2cc(NC(=O)NC(C)(C)C)ccc12 Show InChI InChI=1S/C22H27N5O4S/c1-6-26-18-9-7-15(23-20(28)25-22(2,3)4)12-17(18)24-21(26)32-13-14-11-16(27(29)30)8-10-19(14)31-5/h7-12H,6,13H2,1-5H3,(H2,23,25,28) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 2265-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.030
BindingDB Entry DOI: 10.7270/Q2GB23K1 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50165532
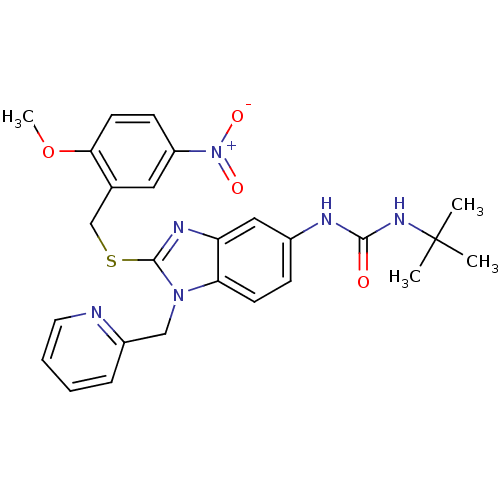
(1-tert-Butyl-3-[2-(2-methoxy-5-nitro-benzylsulfany...)Show SMILES COc1ccc(cc1CSc1nc2cc(NC(=O)NC(C)(C)C)ccc2n1Cc1ccccn1)[N+]([O-])=O Show InChI InChI=1S/C26H28N6O4S/c1-26(2,3)30-24(33)28-18-8-10-22-21(14-18)29-25(31(22)15-19-7-5-6-12-27-19)37-16-17-13-20(32(34)35)9-11-23(17)36-4/h5-14H,15-16H2,1-4H3,(H2,28,30,33) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 2265-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.030
BindingDB Entry DOI: 10.7270/Q2GB23K1 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50165562
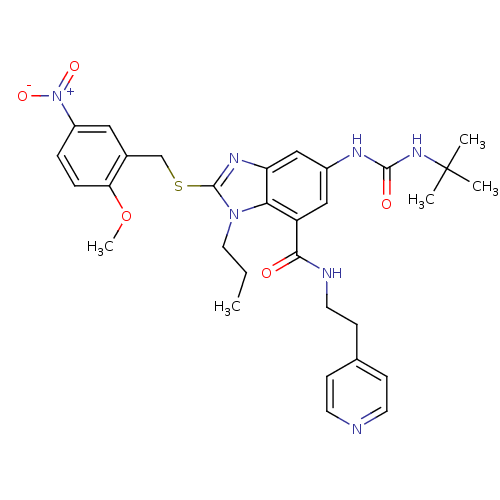
(6-(3-tert-Butyl-ureido)-2-(2-methoxy-5-nitro-benzy...)Show SMILES CCCn1c(SCc2cc(ccc2OC)[N+]([O-])=O)nc2cc(NC(=O)NC(C)(C)C)cc(C(=O)NCCc3ccncc3)c12 Show InChI InChI=1S/C31H37N7O5S/c1-6-15-37-27-24(28(39)33-14-11-20-9-12-32-13-10-20)17-22(34-29(40)36-31(2,3)4)18-25(27)35-30(37)44-19-21-16-23(38(41)42)7-8-26(21)43-5/h7-10,12-13,16-18H,6,11,14-15,19H2,1-5H3,(H,33,39)(H2,34,36,40) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rat leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 2265-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.030
BindingDB Entry DOI: 10.7270/Q2GB23K1 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50165525
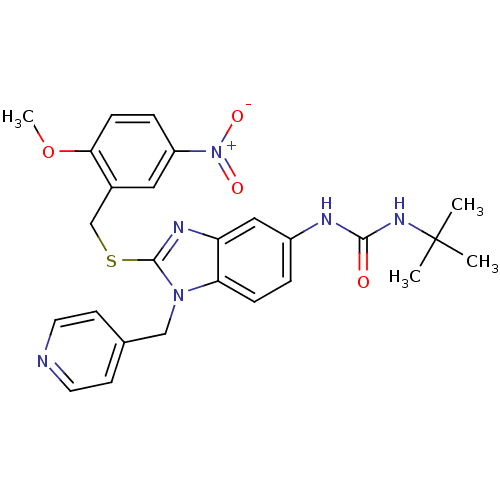
(1-tert-Butyl-3-[2-(2-methoxy-5-nitro-benzylsulfany...)Show SMILES COc1ccc(cc1CSc1nc2cc(NC(=O)NC(C)(C)C)ccc2n1Cc1ccncc1)[N+]([O-])=O Show InChI InChI=1S/C26H28N6O4S/c1-26(2,3)30-24(33)28-19-5-7-22-21(14-19)29-25(31(22)15-17-9-11-27-12-10-17)37-16-18-13-20(32(34)35)6-8-23(18)36-4/h5-14H,15-16H2,1-4H3,(H2,28,30,33) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 2265-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.030
BindingDB Entry DOI: 10.7270/Q2GB23K1 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50165521

(6-(3-tert-Butyl-ureido)-2-(2-methoxy-5-nitro-benzy...)Show SMILES CCCn1c(SCc2cc(ccc2OC)[N+]([O-])=O)nc2cc(NC(=O)NC(C)(C)C)cc(C(=O)NCc3ccccn3)c12 Show InChI InChI=1S/C30H35N7O5S/c1-6-13-36-26-23(27(38)32-17-20-9-7-8-12-31-20)15-21(33-28(39)35-30(2,3)4)16-24(26)34-29(36)43-18-19-14-22(37(40)41)10-11-25(19)42-5/h7-12,14-16H,6,13,17-18H2,1-5H3,(H,32,38)(H2,33,35,39) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rat leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 2265-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.030
BindingDB Entry DOI: 10.7270/Q2GB23K1 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50165540
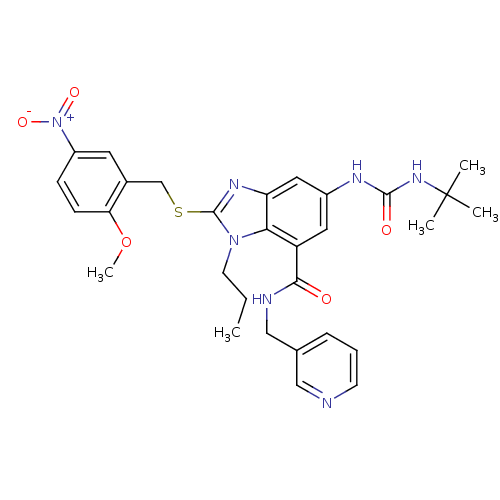
(6-(3-tert-Butyl-ureido)-2-(2-methoxy-5-nitro-benzy...)Show SMILES CCCn1c(SCc2cc(ccc2OC)[N+]([O-])=O)nc2cc(NC(=O)NC(C)(C)C)cc(C(=O)NCc3cccnc3)c12 Show InChI InChI=1S/C30H35N7O5S/c1-6-12-36-26-23(27(38)32-17-19-8-7-11-31-16-19)14-21(33-28(39)35-30(2,3)4)15-24(26)34-29(36)43-18-20-13-22(37(40)41)9-10-25(20)42-5/h7-11,13-16H,6,12,17-18H2,1-5H3,(H,32,38)(H2,33,35,39) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rat leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 2265-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.030
BindingDB Entry DOI: 10.7270/Q2GB23K1 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50165524
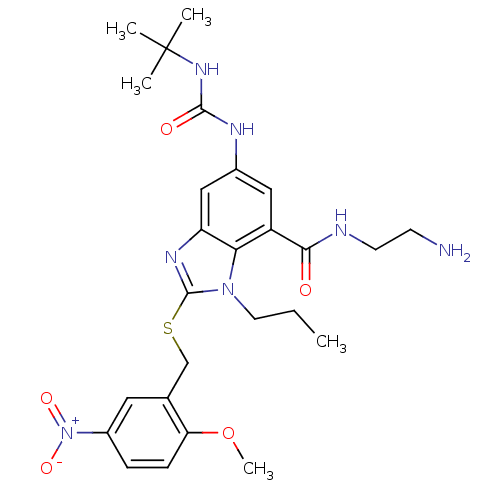
(6-(3-tert-Butyl-ureido)-2-(2-methoxy-5-nitro-benzy...)Show SMILES CCCn1c(SCc2cc(ccc2OC)[N+]([O-])=O)nc2cc(NC(=O)NC(C)(C)C)cc(C(=O)NCCN)c12 Show InChI InChI=1S/C26H35N7O5S/c1-6-11-32-22-19(23(34)28-10-9-27)13-17(29-24(35)31-26(2,3)4)14-20(22)30-25(32)39-15-16-12-18(33(36)37)7-8-21(16)38-5/h7-8,12-14H,6,9-11,15,27H2,1-5H3,(H,28,34)(H2,29,31,35) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rat leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 2265-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.030
BindingDB Entry DOI: 10.7270/Q2GB23K1 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50165533

(6-(3-tert-Butyl-ureido)-2-(2-methoxy-5-nitro-benzy...)Show SMILES CCCn1c(SCc2cc(ccc2OC)[N+]([O-])=O)nc2cc(NC(=O)NC(C)(C)C)cc(C(=O)NCCc3ccccn3)c12 Show InChI InChI=1S/C31H37N7O5S/c1-6-15-37-27-24(28(39)33-14-12-21-9-7-8-13-32-21)17-22(34-29(40)36-31(2,3)4)18-25(27)35-30(37)44-19-20-16-23(38(41)42)10-11-26(20)43-5/h7-11,13,16-18H,6,12,14-15,19H2,1-5H3,(H,33,39)(H2,34,36,40) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rat leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 2265-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.030
BindingDB Entry DOI: 10.7270/Q2GB23K1 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50165555
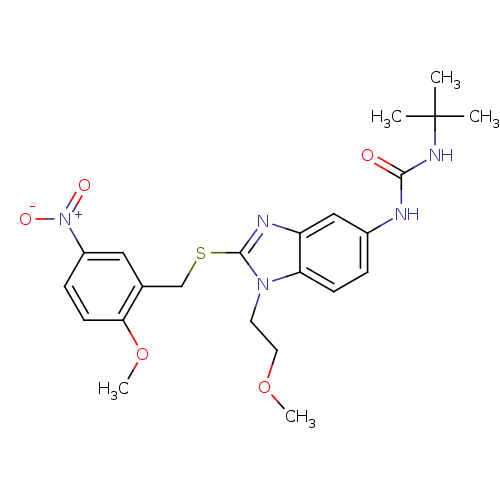
(1-tert-Butyl-3-[1-(2-methoxy-ethyl)-2-(2-methoxy-5...)Show SMILES COCCn1c(SCc2cc(ccc2OC)[N+]([O-])=O)nc2cc(NC(=O)NC(C)(C)C)ccc12 Show InChI InChI=1S/C23H29N5O5S/c1-23(2,3)26-21(29)24-16-6-8-19-18(13-16)25-22(27(19)10-11-32-4)34-14-15-12-17(28(30)31)7-9-20(15)33-5/h6-9,12-13H,10-11,14H2,1-5H3,(H2,24,26,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 2265-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.030
BindingDB Entry DOI: 10.7270/Q2GB23K1 |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50149815
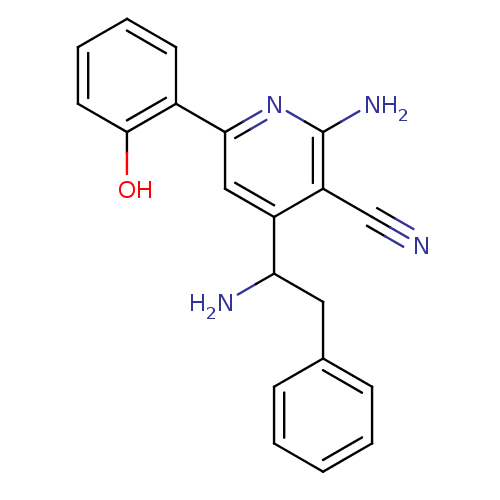
(2-Amino-4-(1-amino-2-phenyl-ethyl)-6-(2-hydroxy-ph...)Show InChI InChI=1S/C20H18N4O/c21-12-16-15(17(22)10-13-6-2-1-3-7-13)11-18(24-20(16)23)14-8-4-5-9-19(14)25/h1-9,11,17,25H,10,22H2,(H2,23,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human I-kappa-B-kinase beta |
Bioorg Med Chem Lett 14: 4013-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.040
BindingDB Entry DOI: 10.7270/Q2PC31VC |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50165557

(6-(3-tert-Butyl-ureido)-2-(2-methoxy-5-nitro-benzy...)Show SMILES CCCn1c(SCc2cc(ccc2OC)[N+]([O-])=O)nc2cc(NC(=O)NC(C)(C)C)cc(C(=O)NCc3ccncc3)c12 Show InChI InChI=1S/C30H35N7O5S/c1-6-13-36-26-23(27(38)32-17-19-9-11-31-12-10-19)15-21(33-28(39)35-30(2,3)4)16-24(26)34-29(36)43-18-20-14-22(37(40)41)7-8-25(20)42-5/h7-12,14-16H,6,13,17-18H2,1-5H3,(H,32,38)(H2,33,35,39) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 2265-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.030
BindingDB Entry DOI: 10.7270/Q2GB23K1 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50165520
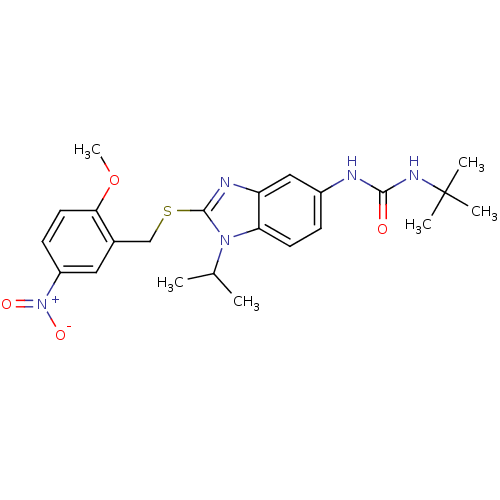
(1-tert-Butyl-3-[1-isopropyl-2-(2-methoxy-5-nitro-b...)Show SMILES COc1ccc(cc1CSc1nc2cc(NC(=O)NC(C)(C)C)ccc2n1C(C)C)[N+]([O-])=O Show InChI InChI=1S/C23H29N5O4S/c1-14(2)27-19-9-7-16(24-21(29)26-23(3,4)5)12-18(19)25-22(27)33-13-15-11-17(28(30)31)8-10-20(15)32-6/h7-12,14H,13H2,1-6H3,(H2,24,26,29) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rat leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 2265-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.030
BindingDB Entry DOI: 10.7270/Q2GB23K1 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50165520

(1-tert-Butyl-3-[1-isopropyl-2-(2-methoxy-5-nitro-b...)Show SMILES COc1ccc(cc1CSc1nc2cc(NC(=O)NC(C)(C)C)ccc2n1C(C)C)[N+]([O-])=O Show InChI InChI=1S/C23H29N5O4S/c1-14(2)27-19-9-7-16(24-21(29)26-23(3,4)5)12-18(19)25-22(27)33-13-15-11-17(28(30)31)8-10-20(15)32-6/h7-12,14H,13H2,1-6H3,(H2,24,26,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 2265-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.030
BindingDB Entry DOI: 10.7270/Q2GB23K1 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50165540

(6-(3-tert-Butyl-ureido)-2-(2-methoxy-5-nitro-benzy...)Show SMILES CCCn1c(SCc2cc(ccc2OC)[N+]([O-])=O)nc2cc(NC(=O)NC(C)(C)C)cc(C(=O)NCc3cccnc3)c12 Show InChI InChI=1S/C30H35N7O5S/c1-6-12-36-26-23(27(38)32-17-19-8-7-11-31-16-19)14-21(33-28(39)35-30(2,3)4)15-24(26)34-29(36)43-18-20-13-22(37(40)41)9-10-25(20)42-5/h7-11,13-16H,6,12,17-18H2,1-5H3,(H,32,38)(H2,33,35,39) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 2265-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.030
BindingDB Entry DOI: 10.7270/Q2GB23K1 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50590536
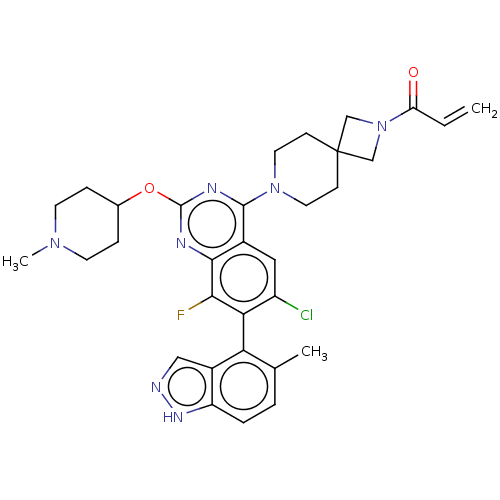
(CHEMBL5209329)Show SMILES CN1CCC(CC1)Oc1nc(N2CCC3(CN(C3)C(=O)C=C)CC2)c2cc(Cl)c(c(F)c2n1)-c1c(C)ccc2[nH]ncc12 |(8.57,-2.62,;7.23,-3.39,;5.9,-2.62,;4.56,-3.39,;4.56,-4.93,;5.9,-5.7,;7.23,-4.93,;3.23,-5.7,;1.9,-4.93,;1.89,-3.41,;.56,-2.64,;.56,-1.1,;-.77,-.33,;-.77,1.21,;.56,1.98,;-.55,3.09,;.54,4.17,;1.65,3.07,;.54,5.71,;-.79,6.48,;1.88,6.48,;1.88,8.02,;1.9,1.21,;1.9,-.33,;-.76,-3.41,;-2.09,-2.64,;-3.43,-3.4,;-4.76,-2.63,;-3.43,-4.94,;-2.09,-5.72,;-2.09,-7.26,;-.76,-4.94,;.58,-5.71,;-4.76,-5.71,;-4.76,-7.25,;-3.43,-8.02,;-6.08,-8.02,;-7.42,-7.26,;-7.42,-5.72,;-8.57,-4.68,;-7.94,-3.28,;-6.41,-3.44,;-6.09,-4.95,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116949
BindingDB Entry DOI: 10.7270/Q23T9N5B |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50149818

(2-Amino-4-(2-amino-ethyl)-6-(2-hydroxy-phenyl)-nic...)Show InChI InChI=1S/C14H14N4O/c15-6-5-9-7-12(18-14(17)11(9)8-16)10-3-1-2-4-13(10)19/h1-4,7,19H,5-6,15H2,(H2,17,18) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human I-kappa-B-kinase beta |
Bioorg Med Chem Lett 14: 4013-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.040
BindingDB Entry DOI: 10.7270/Q2PC31VC |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50590537
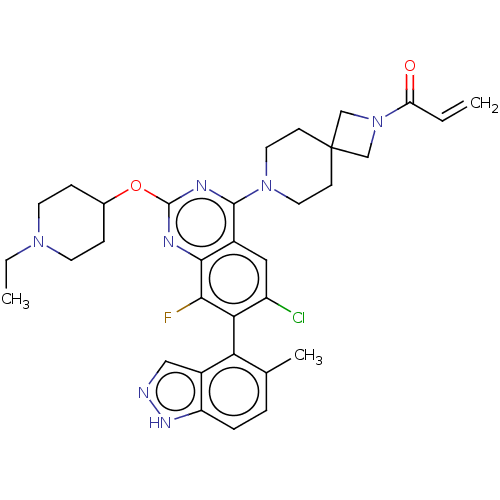
(CHEMBL5205152)Show SMILES CCN1CCC(CC1)Oc1nc(N2CCC3(CN(C3)C(=O)C=C)CC2)c2cc(Cl)c(c(F)c2n1)-c1c(C)ccc2[nH]ncc12 |(8.57,-1.08,;8.57,-2.62,;7.23,-3.39,;5.9,-2.62,;4.56,-3.39,;4.56,-4.93,;5.9,-5.7,;7.23,-4.93,;3.23,-5.7,;1.9,-4.93,;1.89,-3.41,;.56,-2.64,;.56,-1.1,;-.77,-.33,;-.77,1.21,;.56,1.98,;-.55,3.09,;.54,4.17,;1.65,3.07,;.54,5.71,;-.79,6.48,;1.88,6.48,;1.88,8.02,;1.9,1.21,;1.9,-.33,;-.76,-3.41,;-2.09,-2.64,;-3.43,-3.4,;-4.76,-2.63,;-3.43,-4.94,;-2.09,-5.72,;-2.09,-7.26,;-.76,-4.94,;.58,-5.71,;-4.76,-5.71,;-4.76,-7.25,;-3.43,-8.02,;-6.08,-8.02,;-7.42,-7.26,;-7.42,-5.72,;-8.57,-4.68,;-7.94,-3.28,;-6.41,-3.44,;-6.09,-4.95,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116949
BindingDB Entry DOI: 10.7270/Q23T9N5B |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50165537
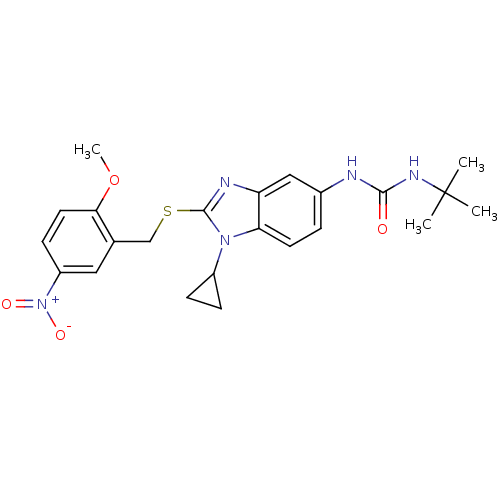
(1-tert-Butyl-3-[1-cyclopropyl-2-(2-methoxy-5-nitro...)Show SMILES COc1ccc(cc1CSc1nc2cc(NC(=O)NC(C)(C)C)ccc2n1C1CC1)[N+]([O-])=O Show InChI InChI=1S/C23H27N5O4S/c1-23(2,3)26-21(29)24-15-5-9-19-18(12-15)25-22(27(19)16-6-7-16)33-13-14-11-17(28(30)31)8-10-20(14)32-4/h5,8-12,16H,6-7,13H2,1-4H3,(H2,24,26,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 2265-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.030
BindingDB Entry DOI: 10.7270/Q2GB23K1 |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50149819
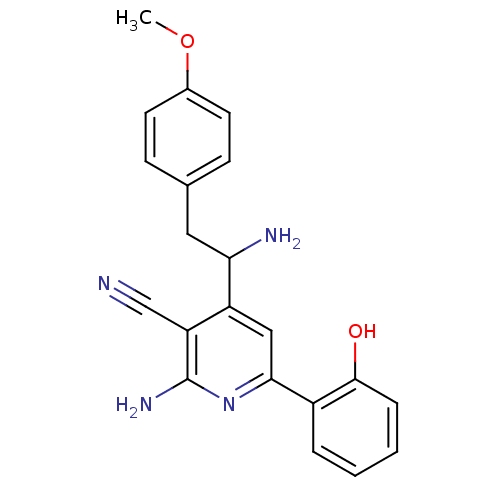
(2-Amino-4-[1-amino-2-(4-methoxy-phenyl)-ethyl]-6-(...)Show SMILES COc1ccc(CC(N)c2cc(nc(N)c2C#N)-c2ccccc2O)cc1 Show InChI InChI=1S/C21H20N4O2/c1-27-14-8-6-13(7-9-14)10-18(23)16-11-19(25-21(24)17(16)12-22)15-4-2-3-5-20(15)26/h2-9,11,18,26H,10,23H2,1H3,(H2,24,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human I-kappa-B-kinase beta |
Bioorg Med Chem Lett 14: 4013-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.040
BindingDB Entry DOI: 10.7270/Q2PC31VC |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50165536
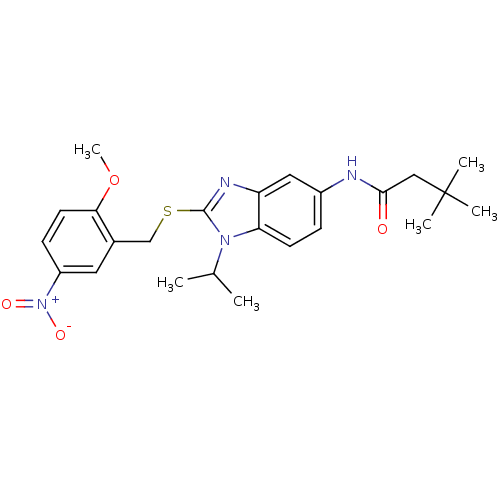
(CHEMBL196337 | N-[1-Isopropyl-2-(2-methoxy-5-nitro...)Show SMILES COc1ccc(cc1CSc1nc2cc(NC(=O)CC(C)(C)C)ccc2n1C(C)C)[N+]([O-])=O Show InChI InChI=1S/C24H30N4O4S/c1-15(2)27-20-9-7-17(25-22(29)13-24(3,4)5)12-19(20)26-23(27)33-14-16-11-18(28(30)31)8-10-21(16)32-6/h7-12,15H,13-14H2,1-6H3,(H,25,29) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rat leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 2265-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.030
BindingDB Entry DOI: 10.7270/Q2GB23K1 |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50149820
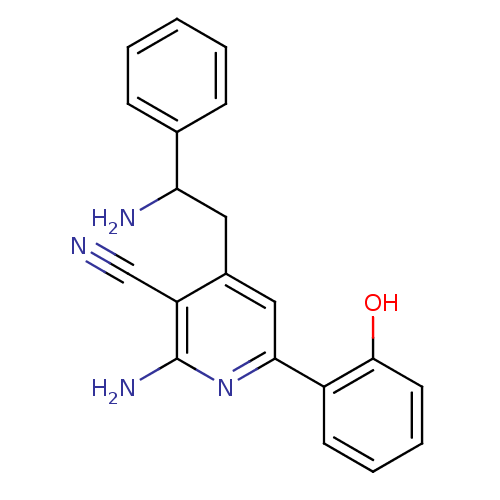
(2-Amino-4-(2-amino-2-phenyl-ethyl)-6-(2-hydroxy-ph...)Show InChI InChI=1S/C20H18N4O/c21-12-16-14(10-17(22)13-6-2-1-3-7-13)11-18(24-20(16)23)15-8-4-5-9-19(15)25/h1-9,11,17,25H,10,22H2,(H2,23,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human I-kappa-B-kinase beta |
Bioorg Med Chem Lett 14: 4013-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.040
BindingDB Entry DOI: 10.7270/Q2PC31VC |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50165552
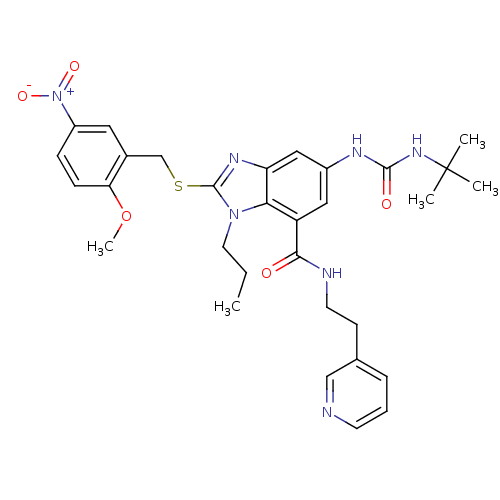
(6-(3-tert-Butyl-ureido)-2-(2-methoxy-5-nitro-benzy...)Show SMILES CCCn1c(SCc2cc(ccc2OC)[N+]([O-])=O)nc2cc(NC(=O)NC(C)(C)C)cc(C(=O)NCCc3cccnc3)c12 Show InChI InChI=1S/C31H37N7O5S/c1-6-14-37-27-24(28(39)33-13-11-20-8-7-12-32-18-20)16-22(34-29(40)36-31(2,3)4)17-25(27)35-30(37)44-19-21-15-23(38(41)42)9-10-26(21)43-5/h7-10,12,15-18H,6,11,13-14,19H2,1-5H3,(H,33,39)(H2,34,36,40) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rat leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 2265-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.030
BindingDB Entry DOI: 10.7270/Q2GB23K1 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50165518

(1-tert-Butyl-3-[2-(2-methoxy-5-nitro-benzylsulfany...)Show SMILES CCCn1c(SCc2cc(ccc2OC)[N+]([O-])=O)nc2cc(NC(=O)NC(C)(C)C)ccc12 Show InChI InChI=1S/C23H29N5O4S/c1-6-11-27-19-9-7-16(24-21(29)26-23(2,3)4)13-18(19)25-22(27)33-14-15-12-17(28(30)31)8-10-20(15)32-5/h7-10,12-13H,6,11,14H2,1-5H3,(H2,24,26,29) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rat leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 2265-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.030
BindingDB Entry DOI: 10.7270/Q2GB23K1 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50160488
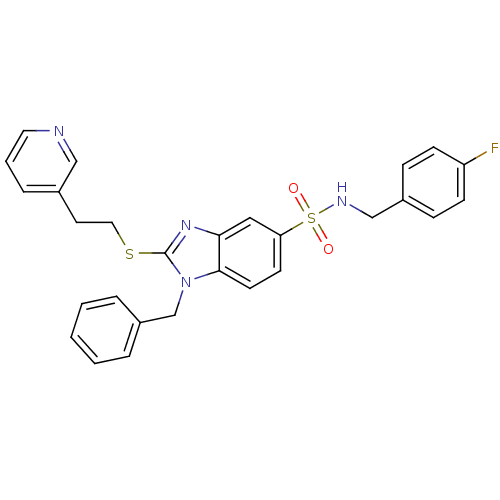
(1-Benzyl-2-(2-pyridin-3-yl-ethylsulfanyl)-1H-benzo...)Show SMILES Fc1ccc(CNS(=O)(=O)c2ccc3n(Cc4ccccc4)c(SCCc4cccnc4)nc3c2)cc1 Show InChI InChI=1S/C28H25FN4O2S2/c29-24-10-8-22(9-11-24)19-31-37(34,35)25-12-13-27-26(17-25)32-28(33(27)20-23-5-2-1-3-6-23)36-16-14-21-7-4-15-30-18-21/h1-13,15,17-18,31H,14,16,19-20H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration towards human leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 799-803 (2005)
Article DOI: 10.1016/j.bmcl.2004.10.089
BindingDB Entry DOI: 10.7270/Q2CC105F |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50165541
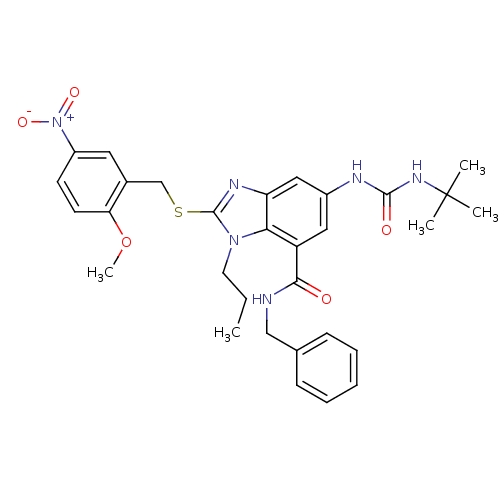
(6-(3-tert-Butyl-ureido)-2-(2-methoxy-5-nitro-benzy...)Show SMILES CCCn1c(SCc2cc(ccc2OC)[N+]([O-])=O)nc2cc(NC(=O)NC(C)(C)C)cc(C(=O)NCc3ccccc3)c12 Show InChI InChI=1S/C31H36N6O5S/c1-6-14-36-27-24(28(38)32-18-20-10-8-7-9-11-20)16-22(33-29(39)35-31(2,3)4)17-25(27)34-30(36)43-19-21-15-23(37(40)41)12-13-26(21)42-5/h7-13,15-17H,6,14,18-19H2,1-5H3,(H,32,38)(H2,33,35,39) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 2265-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.030
BindingDB Entry DOI: 10.7270/Q2GB23K1 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50165541

(6-(3-tert-Butyl-ureido)-2-(2-methoxy-5-nitro-benzy...)Show SMILES CCCn1c(SCc2cc(ccc2OC)[N+]([O-])=O)nc2cc(NC(=O)NC(C)(C)C)cc(C(=O)NCc3ccccc3)c12 Show InChI InChI=1S/C31H36N6O5S/c1-6-14-36-27-24(28(38)32-18-20-10-8-7-9-11-20)16-22(33-29(39)35-31(2,3)4)17-25(27)34-30(36)43-19-21-15-23(37(40)41)12-13-26(21)42-5/h7-13,15-17H,6,14,18-19H2,1-5H3,(H,32,38)(H2,33,35,39) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rat leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 2265-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.030
BindingDB Entry DOI: 10.7270/Q2GB23K1 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50590541
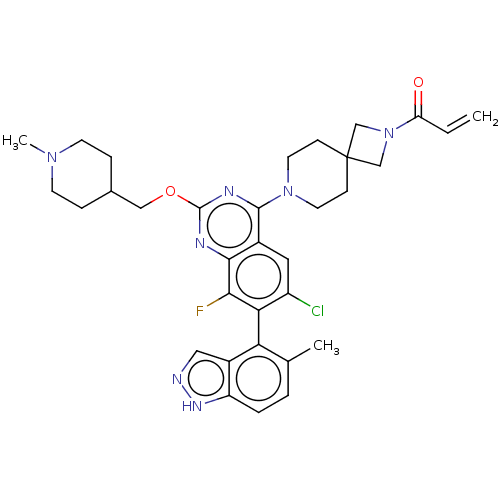
(CHEMBL5181467)Show SMILES CN1CCC(COc2nc(N3CCC4(CN(C4)C(=O)C=C)CC3)c3cc(Cl)c(c(F)c3n2)-c2c(C)ccc3[nH]ncc23)CC1 |(9.23,-8.01,;7.9,-7.24,;7.9,-5.7,;6.56,-4.93,;5.23,-5.7,;3.9,-4.93,;2.56,-5.7,;1.23,-4.93,;1.22,-3.41,;-.11,-2.64,;-.11,-1.1,;-1.44,-.33,;-1.44,1.21,;-.11,1.98,;-1.21,3.09,;-.12,4.17,;.98,3.07,;-.12,5.71,;-1.46,6.48,;1.21,6.48,;1.21,8.02,;1.23,1.21,;1.23,-.33,;-1.43,-3.41,;-2.76,-2.64,;-4.09,-3.4,;-5.43,-2.63,;-4.09,-4.94,;-2.75,-5.72,;-2.75,-7.26,;-1.42,-4.94,;-.09,-5.71,;-5.43,-5.71,;-5.43,-7.25,;-4.09,-8.02,;-6.75,-8.02,;-8.09,-7.26,;-8.09,-5.71,;-9.23,-4.68,;-8.61,-3.28,;-7.07,-3.44,;-6.75,-4.95,;5.23,-7.24,;6.56,-8.01,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116949
BindingDB Entry DOI: 10.7270/Q23T9N5B |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50165562

(6-(3-tert-Butyl-ureido)-2-(2-methoxy-5-nitro-benzy...)Show SMILES CCCn1c(SCc2cc(ccc2OC)[N+]([O-])=O)nc2cc(NC(=O)NC(C)(C)C)cc(C(=O)NCCc3ccncc3)c12 Show InChI InChI=1S/C31H37N7O5S/c1-6-15-37-27-24(28(39)33-14-11-20-9-12-32-13-10-20)17-22(34-29(40)36-31(2,3)4)18-25(27)35-30(37)44-19-21-16-23(38(41)42)7-8-26(21)43-5/h7-10,12-13,16-18H,6,11,14-15,19H2,1-5H3,(H,33,39)(H2,34,36,40) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 2265-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.030
BindingDB Entry DOI: 10.7270/Q2GB23K1 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50160488

(1-Benzyl-2-(2-pyridin-3-yl-ethylsulfanyl)-1H-benzo...)Show SMILES Fc1ccc(CNS(=O)(=O)c2ccc3n(Cc4ccccc4)c(SCCc4cccnc4)nc3c2)cc1 Show InChI InChI=1S/C28H25FN4O2S2/c29-24-10-8-22(9-11-24)19-31-37(34,35)25-12-13-27-26(17-25)32-28(33(27)20-23-5-2-1-3-6-23)36-16-14-21-7-4-15-30-18-21/h1-13,15,17-18,31H,14,16,19-20H2 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration towards rat leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 799-803 (2005)
Article DOI: 10.1016/j.bmcl.2004.10.089
BindingDB Entry DOI: 10.7270/Q2CC105F |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50165559
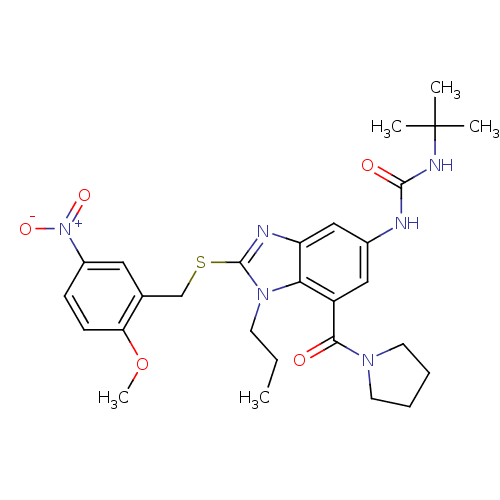
(1-tert-Butyl-3-[2-(2-methoxy-5-nitro-benzylsulfany...)Show SMILES CCCn1c(SCc2cc(ccc2OC)[N+]([O-])=O)nc2cc(NC(=O)NC(C)(C)C)cc(C(=O)N3CCCC3)c12 Show InChI InChI=1S/C28H36N6O5S/c1-6-11-33-24-21(25(35)32-12-7-8-13-32)15-19(29-26(36)31-28(2,3)4)16-22(24)30-27(33)40-17-18-14-20(34(37)38)9-10-23(18)39-5/h9-10,14-16H,6-8,11-13,17H2,1-5H3,(H2,29,31,36) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rat leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 2265-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.030
BindingDB Entry DOI: 10.7270/Q2GB23K1 |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50149809

(2-Amino-4-(1-amino-ethyl)-6-(2-hydroxy-phenyl)-nic...)Show InChI InChI=1S/C14H14N4O/c1-8(16)10-6-12(18-14(17)11(10)7-15)9-4-2-3-5-13(9)19/h2-6,8,19H,16H2,1H3,(H2,17,18) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human I-kappa-B-kinase beta |
Bioorg Med Chem Lett 14: 4013-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.040
BindingDB Entry DOI: 10.7270/Q2PC31VC |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50165563

(1-tert-Butyl-3-[2-(2-methoxy-5-nitro-benzylsulfany...)Show SMILES COc1ccc(cc1CSc1nc2cc(NC(=O)NC(C)(C)C)ccc2n1Cc1cccnc1)[N+]([O-])=O Show InChI InChI=1S/C26H28N6O4S/c1-26(2,3)30-24(33)28-19-7-9-22-21(13-19)29-25(31(22)15-17-6-5-11-27-14-17)37-16-18-12-20(32(34)35)8-10-23(18)36-4/h5-14H,15-16H2,1-4H3,(H2,28,30,33) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rat leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 2265-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.030
BindingDB Entry DOI: 10.7270/Q2GB23K1 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50590532
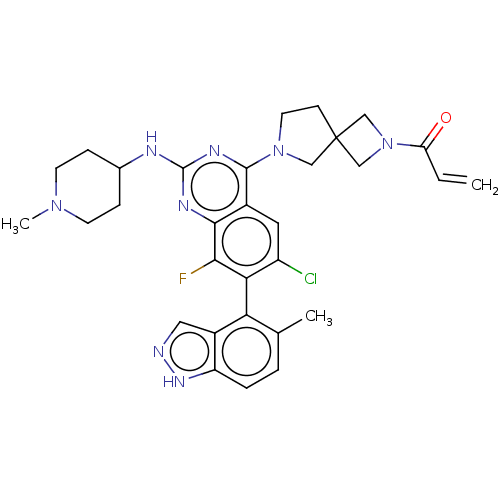
(CHEMBL5208546)Show SMILES CN1CCC(CC1)Nc1nc(N2CCC3(CN(C3)C(=O)C=C)C2)c2cc(Cl)c(c(F)c2n1)-c1c(C)ccc2[nH]ncc12 |(8.57,-1.53,;7.23,-2.3,;5.9,-1.53,;4.56,-2.3,;4.56,-3.84,;5.9,-4.61,;7.23,-3.84,;3.23,-4.61,;1.9,-3.84,;1.89,-2.32,;.56,-1.55,;.56,-.01,;-.68,.89,;-.21,2.36,;1.33,2.36,;.93,3.87,;2.41,4.27,;2.82,2.75,;3.18,5.6,;2.41,6.94,;4.72,5.6,;5.49,6.94,;1.81,.89,;-.76,-2.32,;-2.09,-1.55,;-3.43,-2.31,;-4.76,-1.54,;-3.43,-3.86,;-2.09,-4.63,;-2.09,-6.17,;-.76,-3.85,;.58,-4.62,;-4.76,-4.62,;-4.76,-6.17,;-3.43,-6.94,;-6.08,-6.93,;-7.42,-6.17,;-7.42,-4.63,;-8.57,-3.6,;-7.94,-2.19,;-6.41,-2.35,;-6.09,-3.86,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116949
BindingDB Entry DOI: 10.7270/Q23T9N5B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data