

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50583383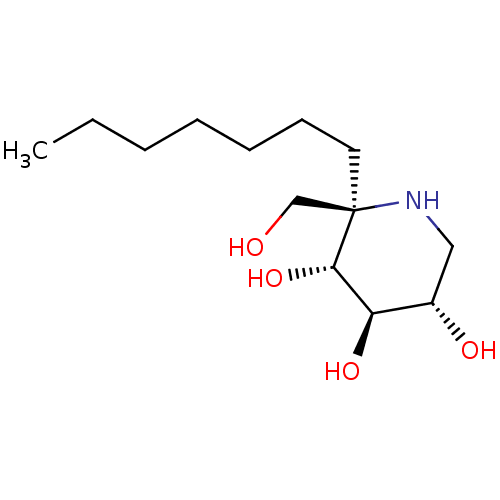 (CHEMBL5028005 | US20230339856, Compound (IIb3)) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50583382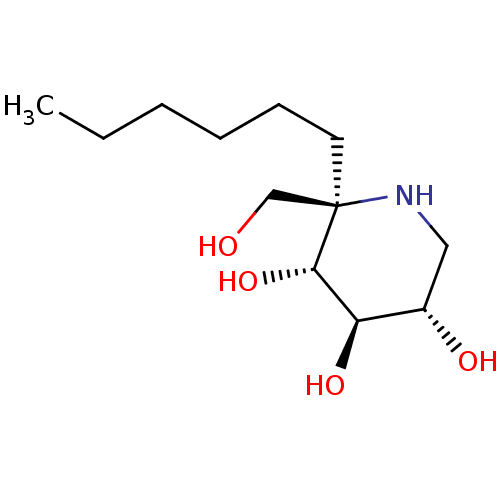 (CHEMBL5028265) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50583381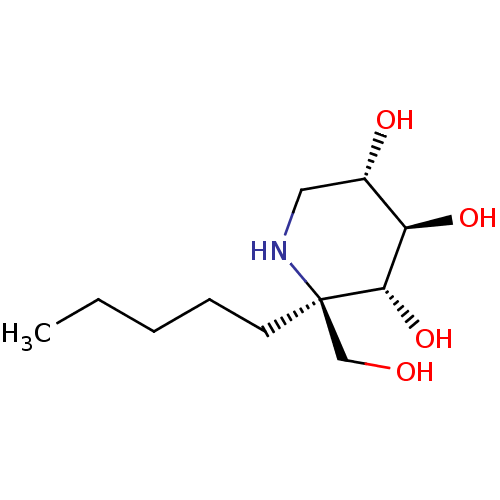 (CHEMBL5029066 | US20230339856, Compound (IIb1)) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50583380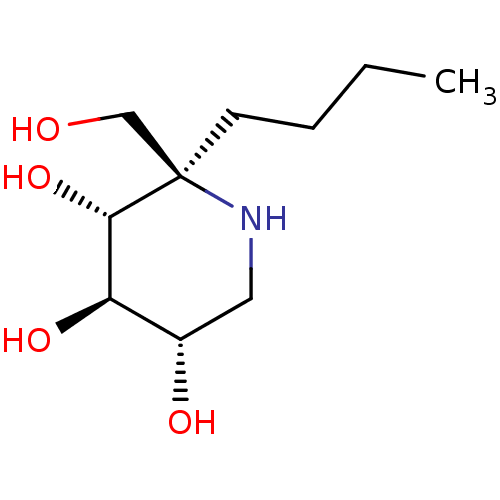 (CHEMBL5027974) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM18353 ((2R,3R,4R,5S)-2-(hydroxymethyl)-1-methylpiperidine...) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50583379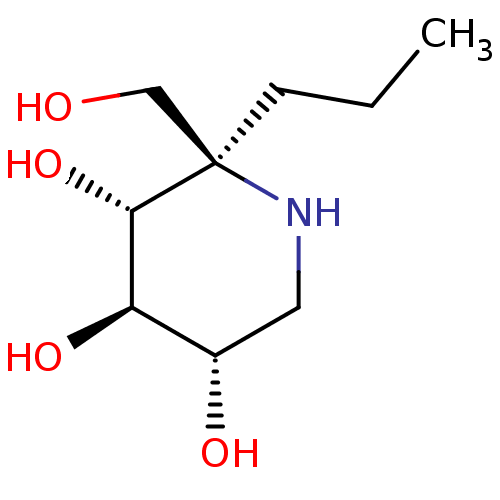 (CHEMBL5028138 | US20230339856, Compound (IIb)) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50335398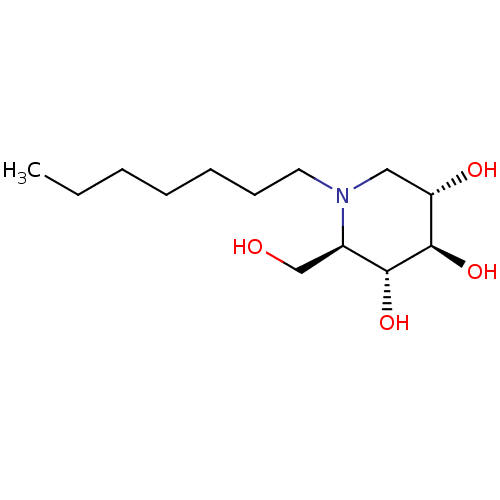 (CHEMBL1651551 | N-Heptyl-1-deoxynojirimycin) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM18356 ((2R,3R,4R,5S)-1-hexyl-2-(hydroxymethyl)piperidine-...) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM18355 ((2R,3R,4R,5S)-1-butyl-2-(hydroxymethyl)piperidine-...) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50335399 (CHEMBL1651549 | N-Pentyl-1-deoxynojirimycin) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50583384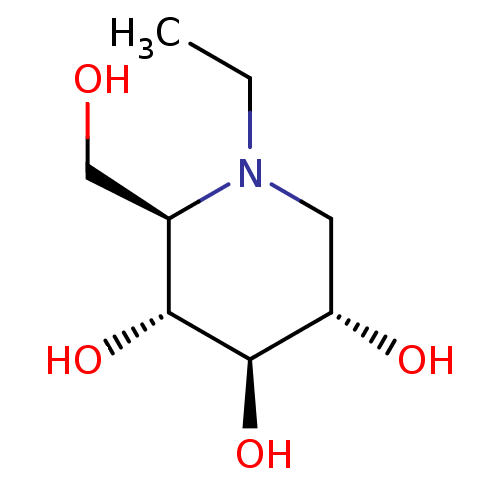 (CHEMBL5080975) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM18354 ((2R,3R,4R,5S)-2-(hydroxymethyl)-1-propylpiperidine...) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50583385 (CHEMBL5028105) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50583378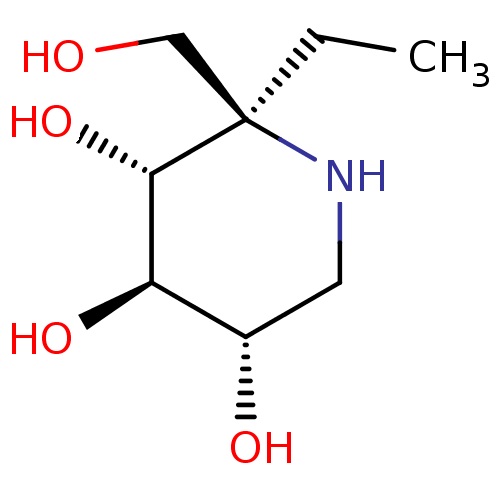 (CHEMBL5028084 | US20230339856, Compound (IIa)) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50583377 (CHEMBL5028072) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Bos taurus) | BDBM50036731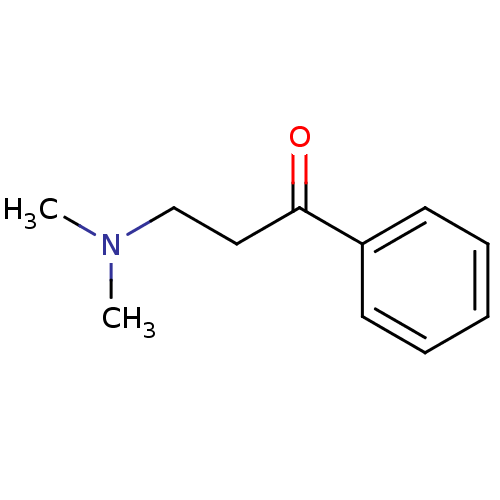 (3-Dimethylamino-1-phenyl-propan-1-one | CHEMBL5011...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 3.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of Monoamine oxidase-B from Bovine liver | J Med Chem 36: 1711-5 (1993) BindingDB Entry DOI: 10.7270/Q2DN443D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Bos taurus) | BDBM50047897 (CHEMBL289199 | Dimethyl-(3-phenyl-propyl)-amine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of Monoamine oxidase-B from Bovine liver | J Med Chem 36: 1711-5 (1993) BindingDB Entry DOI: 10.7270/Q2DN443D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Bos taurus) | BDBM50046768 (2-Pentylamino-acetamide | 2-Pentylamino-acetamide(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | 9.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Kinetic constant (KI) was calculated by inhibition of monoamino oxidase B (MAO B) | J Med Chem 36: 446-8 (1993) BindingDB Entry DOI: 10.7270/Q24B30CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Bos taurus) | BDBM50046768 (2-Pentylamino-acetamide | 2-Pentylamino-acetamide(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | 1.08E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Kinetic constant was calculated by inhibition of monoamino oxidase B (MAO B) | J Med Chem 36: 446-8 (1993) BindingDB Entry DOI: 10.7270/Q24B30CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Bos taurus) | BDBM50046767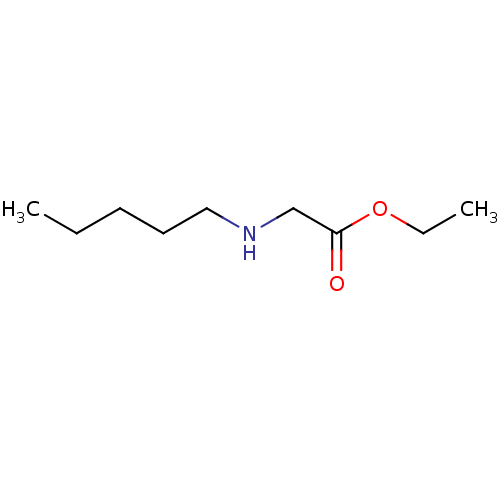 (CHEMBL344189 | Pentylamino-acetic acid ethyl ester) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | 2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Kinetic constant was calculated by inhibition of monoamino oxidase B (MAO B) | J Med Chem 36: 446-8 (1993) BindingDB Entry DOI: 10.7270/Q24B30CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Bos taurus) | BDBM50047898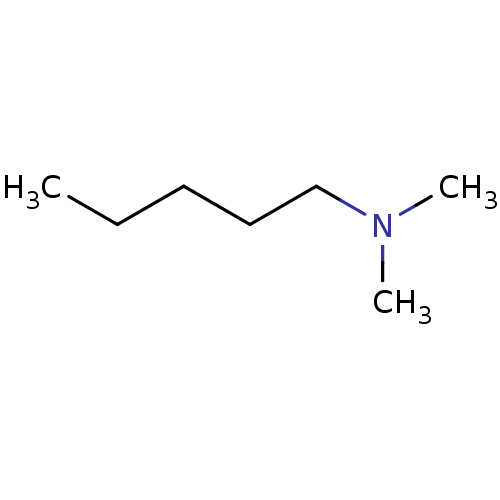 (CHEMBL47794 | Dimethyl-pentyl-amine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of Monoamine oxidase-B from Bovine liver | J Med Chem 36: 1711-5 (1993) BindingDB Entry DOI: 10.7270/Q2DN443D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Bos taurus) | BDBM50047896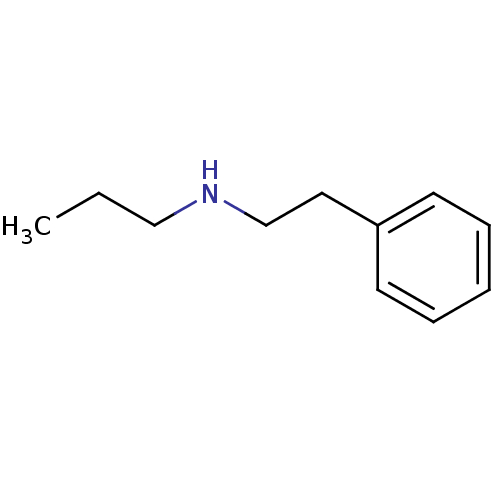 (CHEMBL46232 | Phenethyl-propyl-amine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of Monoamine oxidase-B from Bovine liver | J Med Chem 36: 1711-5 (1993) BindingDB Entry DOI: 10.7270/Q2DN443D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Bos taurus) | BDBM50046769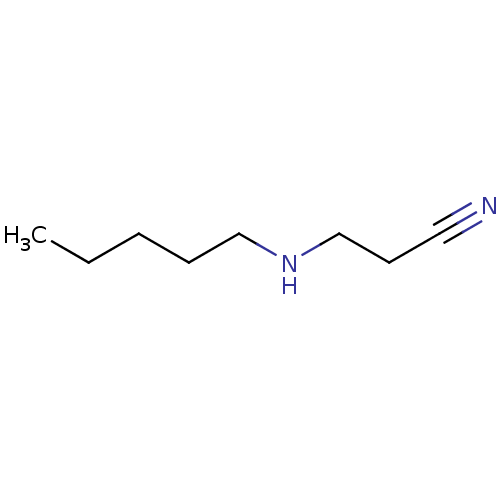 (3-Pentylamino-propionitrile | CHEMBL139492) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | 4.94E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Kinetic constant was calculated by inhibition of monoamino oxidase B (MAO B) | J Med Chem 36: 446-8 (1993) BindingDB Entry DOI: 10.7270/Q24B30CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Bos taurus) | BDBM50046766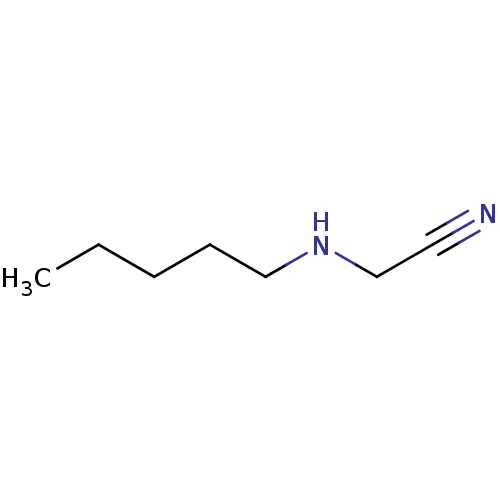 (CHEMBL139751 | Pentylamino-acetonitrile) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 4.94E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Kinetic constant was calculated by inhibition of monoamino oxidase B (MAO B) | J Med Chem 36: 446-8 (1993) BindingDB Entry DOI: 10.7270/Q24B30CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Bos taurus) | BDBM50046769 (3-Pentylamino-propionitrile | CHEMBL139492) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | 5.79E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Kinetic constant (KI) was calculated by inhibition of monoamino oxidase B (MAO B) | J Med Chem 36: 446-8 (1993) BindingDB Entry DOI: 10.7270/Q24B30CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Bos taurus) | BDBM50018453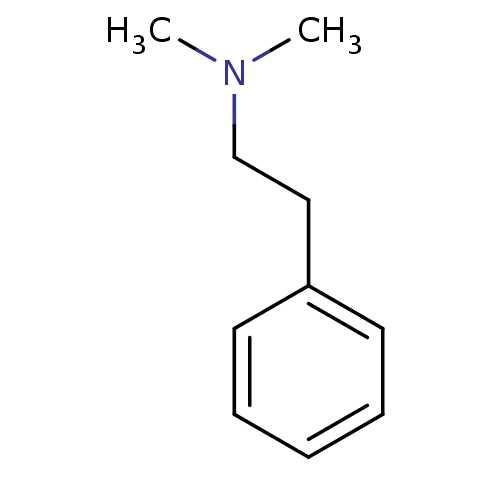 (CHEMBL46278 | Dimethyl-phenethyl-amine | N,N-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of Monoamine oxidase-B from Bovine liver | J Med Chem 36: 1711-5 (1993) BindingDB Entry DOI: 10.7270/Q2DN443D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Bos taurus) | BDBM50016907 (CHEMBL46841 | Dimethyl-(4-phenyl-butyl)-amine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of Monoamine oxidase-B from Bovine liver | J Med Chem 36: 1711-5 (1993) BindingDB Entry DOI: 10.7270/Q2DN443D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Bos taurus) | BDBM50046766 (CHEMBL139751 | Pentylamino-acetonitrile) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.40E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Kinetic constant (KI) was calculated by inhibition of monoamino oxidase B (MAO B) | J Med Chem 36: 446-8 (1993) BindingDB Entry DOI: 10.7270/Q24B30CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Bos taurus) | BDBM50046767 (CHEMBL344189 | Pentylamino-acetic acid ethyl ester) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | 1.42E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Kinetic constant (KI) was calculated by inhibition of monoamino oxidase B (MAO B) | J Med Chem 36: 446-8 (1993) BindingDB Entry DOI: 10.7270/Q24B30CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50285779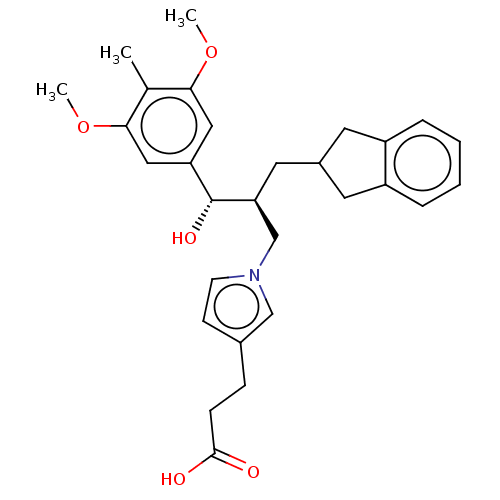 (CHEMBL4165205) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
ONO Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Antagonist activity at recombinant human LPA1 expressed in CHO cell membranes pretreated for 24 hrs prior to Fura-2-AM dye addition for 1 hr followed... | ACS Med Chem Lett 8: 1281-1286 (2017) Article DOI: 10.1021/acsmedchemlett.7b00383 BindingDB Entry DOI: 10.7270/Q2PG1V8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50285779 (CHEMBL4165205) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
ONO Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Antagonist activity at recombinant human LPA1 expressed in CHO cell membranes pretreated for 24 hrs prior to Fura-2-AM dye addition for 1 hr followed... | ACS Med Chem Lett 8: 1281-1286 (2017) Article DOI: 10.1021/acsmedchemlett.7b00383 BindingDB Entry DOI: 10.7270/Q2PG1V8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM249335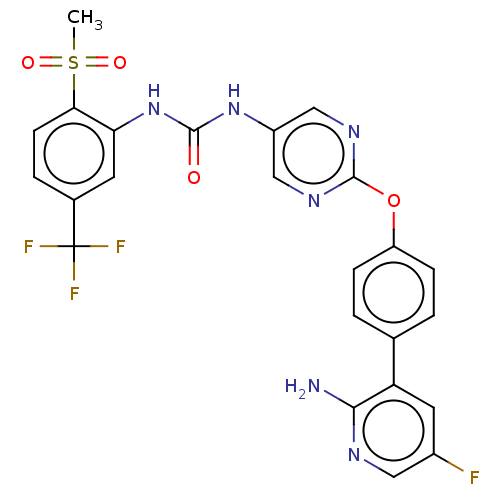 (US10300060, Example 15-96 | US10765676, Example 15...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
ONO PHARMACEUTICAL CO., LTD. US Patent | Assay Description On the day before the assay, CellSenser TrkA-NFAT-bla CHO-K1 cells were suspended in an assay medium (Opti-MEM1 Reduced Serum Medium (Invitrogen) con... | US Patent US9763943 (2017) BindingDB Entry DOI: 10.7270/Q2ZW1P0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM249335 (US10300060, Example 15-96 | US10765676, Example 15...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
ONO PHARMACEUTICAL CO., LTD. US Patent | Assay Description On the day before the assay, CellSenser TrkA-NFAT-bla CHO-K1 cells were suspended in an assay medium (Opti-MEM1 Reduced Serum Medium (Invitrogen) con... | US Patent US10765676 (2020) BindingDB Entry DOI: 10.7270/Q2VX0KKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM249335 (US10300060, Example 15-96 | US10765676, Example 15...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd | Assay Description On the day before the assay, CellSenserô TrkA-NFAT-b1a CHO-K1 cells were suspended in an assay medium (Opti-MEM1 Reduced Serum Medium (Invitrogen) co... | Bioorg Med Chem Lett 19: 1654-7 (2009) BindingDB Entry DOI: 10.7270/Q2JH3PGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM249335 (US10300060, Example 15-96 | US10765676, Example 15...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. | Assay Description TrkA kinase-inhibiting activity in cell systems was measured using CHO-K1 cells expressing human TrkA and NFAT-bla (CellSenser TrkA-NFAT-bla CHO-K1 c... | Bioorg Med Chem 17: 2017-29 (2009) BindingDB Entry DOI: 10.7270/Q2WD42WT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM249335 (US10300060, Example 15-96 | US10765676, Example 15...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | 37 |
ONO PHARMACEUTICAL CO., LTD. US Patent | Assay Description On the day before the assay, CellSenser TrkA-NFAT-bla CHO-K1 cells were suspended in an assay medium (Opti-MEM1 Reduced Serum Medium (Invitrogen) con... | US Patent US9463192 (2016) BindingDB Entry DOI: 10.7270/Q22Z14FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50287290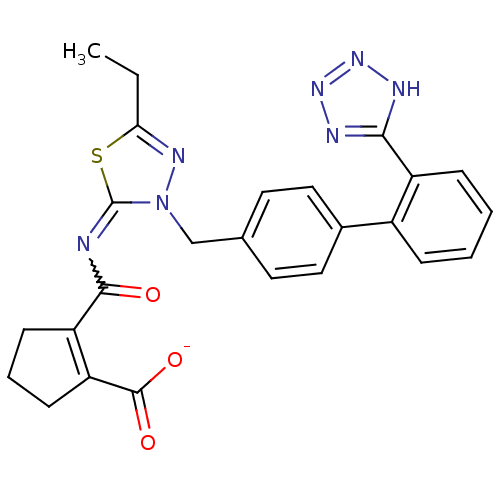 (CHEMBL34866 | KRH-594 | Potassium; 2-[5-ethyl-3-[2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested in vitro for the ability to displace the specific binding of [125I]-A II from receptors in rat liver membrane(type 1 receptor) | Bioorg Med Chem Lett 6: 1469-1474 (1996) Article DOI: 10.1016/S0960-894X(96)00250-8 BindingDB Entry DOI: 10.7270/Q2SN08XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50287294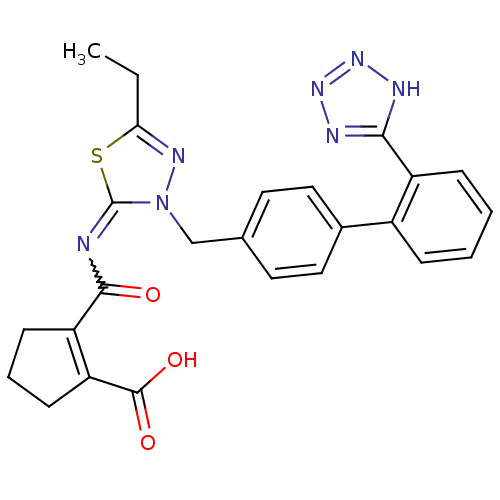 (2-[5-Ethyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested in vitro for the ability to displace the specific binding of [125I]-A II from receptors in rat liver membrane(type 1 receptor) | Bioorg Med Chem Lett 6: 1469-1474 (1996) Article DOI: 10.1016/S0960-894X(96)00250-8 BindingDB Entry DOI: 10.7270/Q2SN08XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM249337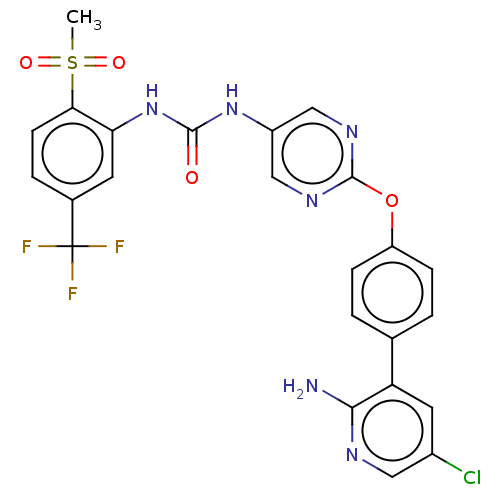 (US10300060, Example 15-104 | US10765676, Example 1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ONO PHARMACEUTICAL CO., LTD. US Patent | Assay Description On the day before the assay, CellSenser TrkA-NFAT-bla CHO-K1 cells were suspended in an assay medium (Opti-MEM1 Reduced Serum Medium (Invitrogen) con... | US Patent US9763943 (2017) BindingDB Entry DOI: 10.7270/Q2ZW1P0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM249337 (US10300060, Example 15-104 | US10765676, Example 1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. | Assay Description TrkA kinase-inhibiting activity in cell systems was measured using CHO-K1 cells expressing human TrkA and NFAT-bla (CellSenser TrkA-NFAT-bla CHO-K1 c... | Bioorg Med Chem 17: 2017-29 (2009) BindingDB Entry DOI: 10.7270/Q2WD42WT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM249337 (US10300060, Example 15-104 | US10765676, Example 1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ONO PHARMACEUTICAL CO., LTD. US Patent | Assay Description On the day before the assay, CellSenser TrkA-NFAT-bla CHO-K1 cells were suspended in an assay medium (Opti-MEM1 Reduced Serum Medium (Invitrogen) con... | US Patent US10765676 (2020) BindingDB Entry DOI: 10.7270/Q2VX0KKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM249337 (US10300060, Example 15-104 | US10765676, Example 1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | 37 |
ONO PHARMACEUTICAL CO., LTD. US Patent | Assay Description On the day before the assay, CellSenser TrkA-NFAT-bla CHO-K1 cells were suspended in an assay medium (Opti-MEM1 Reduced Serum Medium (Invitrogen) con... | US Patent US9463192 (2016) BindingDB Entry DOI: 10.7270/Q22Z14FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM249337 (US10300060, Example 15-104 | US10765676, Example 1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd | Assay Description On the day before the assay, CellSenserô TrkA-NFAT-b1a CHO-K1 cells were suspended in an assay medium (Opti-MEM1 Reduced Serum Medium (Invitrogen) co... | Bioorg Med Chem Lett 19: 1654-7 (2009) BindingDB Entry DOI: 10.7270/Q2JH3PGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50287291 (CHEMBL35381 | N-[5-Ethyl-3-[2'-(1H-tetrazol-5-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested in vitro for the ability to displace the specific binding of [125I]-A II from receptors in rat liver membrane(type 1 receptor) | Bioorg Med Chem Lett 6: 1469-1474 (1996) Article DOI: 10.1016/S0960-894X(96)00250-8 BindingDB Entry DOI: 10.7270/Q2SN08XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM249345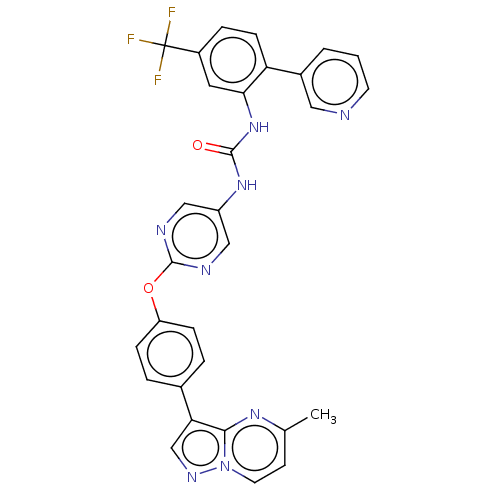 (US10300060, Example 21-65 | US10765676, Example 21...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd | Assay Description On the day before the assay, CellSenserô TrkA-NFAT-b1a CHO-K1 cells were suspended in an assay medium (Opti-MEM1 Reduced Serum Medium (Invitrogen) co... | Bioorg Med Chem Lett 19: 1654-7 (2009) BindingDB Entry DOI: 10.7270/Q2JH3PGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM249345 (US10300060, Example 21-65 | US10765676, Example 21...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. | Assay Description TrkA kinase-inhibiting activity in cell systems was measured using CHO-K1 cells expressing human TrkA and NFAT-bla (CellSenser TrkA-NFAT-bla CHO-K1 c... | Bioorg Med Chem 17: 2017-29 (2009) BindingDB Entry DOI: 10.7270/Q2WD42WT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM249345 (US10300060, Example 21-65 | US10765676, Example 21...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | 37 |
ONO PHARMACEUTICAL CO., LTD. US Patent | Assay Description On the day before the assay, CellSenser TrkA-NFAT-bla CHO-K1 cells were suspended in an assay medium (Opti-MEM1 Reduced Serum Medium (Invitrogen) con... | US Patent US9463192 (2016) BindingDB Entry DOI: 10.7270/Q22Z14FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM249345 (US10300060, Example 21-65 | US10765676, Example 21...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
ONO PHARMACEUTICAL CO., LTD. US Patent | Assay Description On the day before the assay, CellSenser TrkA-NFAT-bla CHO-K1 cells were suspended in an assay medium (Opti-MEM1 Reduced Serum Medium (Invitrogen) con... | US Patent US10765676 (2020) BindingDB Entry DOI: 10.7270/Q2VX0KKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM249345 (US10300060, Example 21-65 | US10765676, Example 21...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
ONO PHARMACEUTICAL CO., LTD. US Patent | Assay Description On the day before the assay, CellSenser TrkA-NFAT-bla CHO-K1 cells were suspended in an assay medium (Opti-MEM1 Reduced Serum Medium (Invitrogen) con... | US Patent US9763943 (2017) BindingDB Entry DOI: 10.7270/Q2ZW1P0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 638 total ) | Next | Last >> |