

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50010096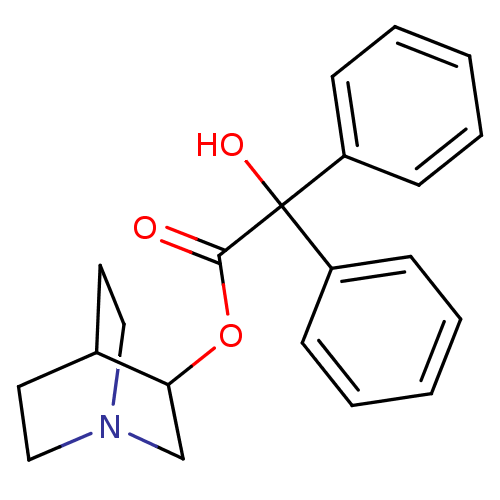 (CHEMBL12980 | Hydroxy-diphenyl-acetic acid 1-aza-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description In vitro binding affinity against rat heart membrane using [3H]-AF-DX-384 | J Med Chem 38: 1711-9 (1995) BindingDB Entry DOI: 10.7270/Q2PV6JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50034872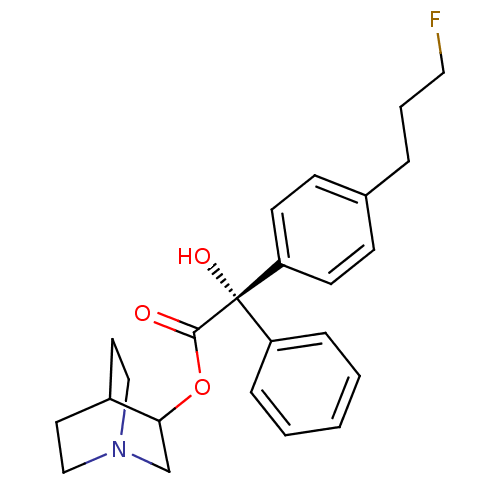 ((S)-[4-(3-Fluoro-propyl)-phenyl]-hydroxy-phenyl-ac...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description In vitro binding affinity against bovine striatal membrane using [3H]-pirenzepine | J Med Chem 38: 1711-9 (1995) BindingDB Entry DOI: 10.7270/Q2PV6JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50010096 (CHEMBL12980 | Hydroxy-diphenyl-acetic acid 1-aza-b...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description In vitro binding affinity against bovine striatal membrane using [3H]-pirenzepine | J Med Chem 38: 1711-9 (1995) BindingDB Entry DOI: 10.7270/Q2PV6JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50034875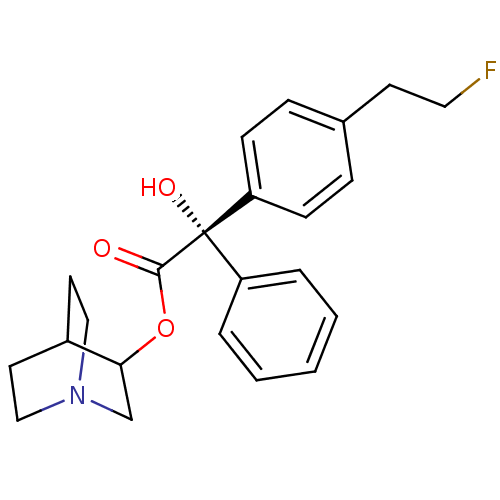 ((S)-[4-(2-Fluoro-ethyl)-phenyl]-hydroxy-phenyl-ace...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description In vitro binding affinity against bovine striatal membrane using [3H]-pirenzepine | J Med Chem 38: 1711-9 (1995) BindingDB Entry DOI: 10.7270/Q2PV6JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50034875 ((S)-[4-(2-Fluoro-ethyl)-phenyl]-hydroxy-phenyl-ace...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description In vitro binding affinity against rat heart membrane using [3H]-AF-DX-384 | J Med Chem 38: 1711-9 (1995) BindingDB Entry DOI: 10.7270/Q2PV6JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50034874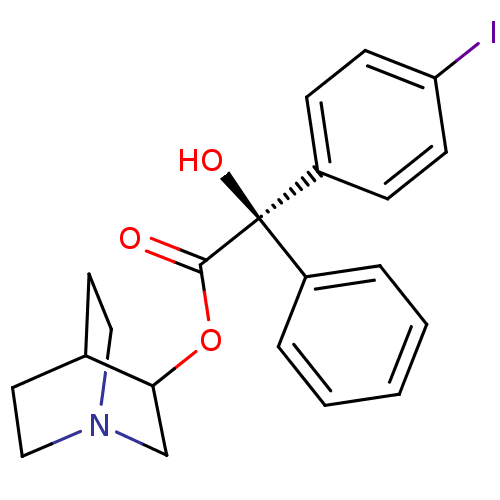 ((R)-Hydroxy-(4-iodo-phenyl)-phenyl-acetic acid 1-a...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description In vitro binding affinity against bovine striatal membrane using [3H]-pirenzepine | J Med Chem 38: 1711-9 (1995) BindingDB Entry DOI: 10.7270/Q2PV6JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50034872 ((S)-[4-(3-Fluoro-propyl)-phenyl]-hydroxy-phenyl-ac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description In vitro binding affinity against rat heart membrane using [3H]-AF-DX-384 | J Med Chem 38: 1711-9 (1995) BindingDB Entry DOI: 10.7270/Q2PV6JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50034871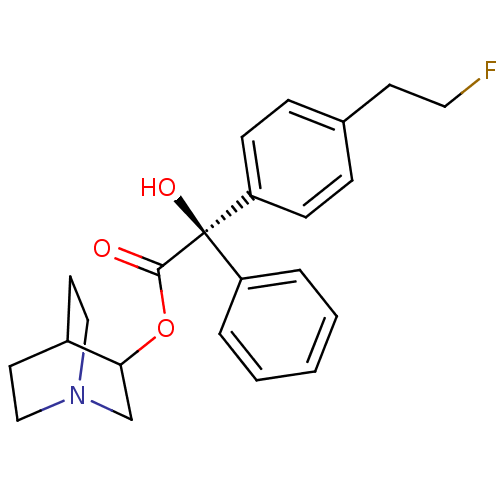 ((R)-[4-(2-Fluoro-ethyl)-phenyl]-hydroxy-phenyl-ace...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description In vitro binding affinity against bovine striatal membrane using [3H]-pirenzepine | J Med Chem 38: 1711-9 (1995) BindingDB Entry DOI: 10.7270/Q2PV6JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50011771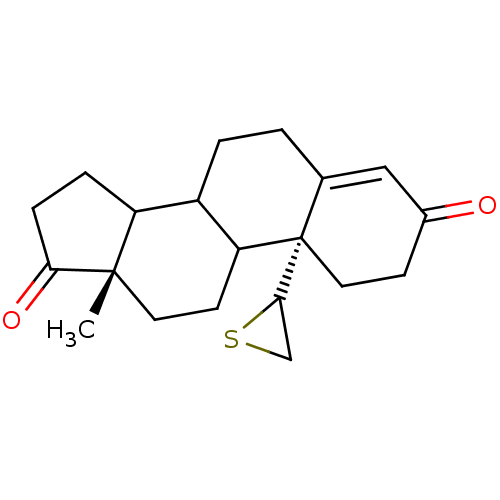 (13-Methyl-10-thiiranyl-1,6,7,8,9,10,11,12,13,14,15...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human placental Cytochrome P450 19A1 | J Med Chem 34: 1344-9 (1991) BindingDB Entry DOI: 10.7270/Q2M044C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50367061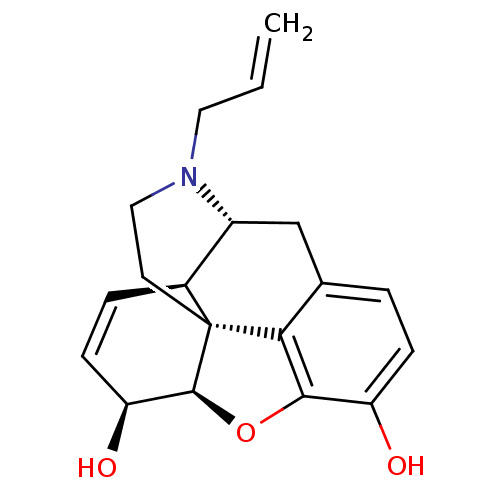 (NALORPHINE | NALORPHINE HYDROCHLORIDE) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound administered subcutaneously was evaluated for inhibition constant measured as the displacement of [3H]DAGO from rat brain | J Med Chem 30: 947-50 (1987) BindingDB Entry DOI: 10.7270/Q2TD9XXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50034873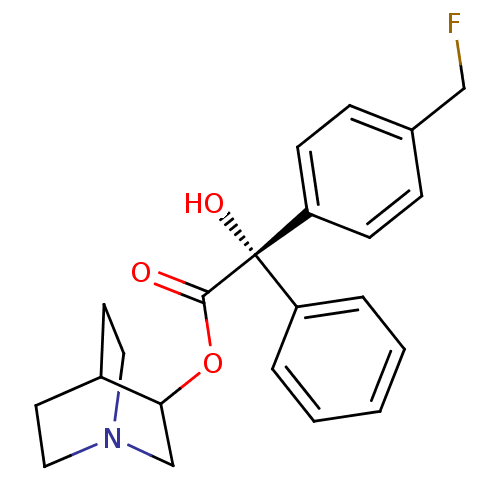 ((S)-(4-Fluoromethyl-phenyl)-hydroxy-phenyl-acetic ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description In vitro binding affinity against rat heart membrane using [3H]-AF-DX-384 | J Med Chem 38: 1711-9 (1995) BindingDB Entry DOI: 10.7270/Q2PV6JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50034873 ((S)-(4-Fluoromethyl-phenyl)-hydroxy-phenyl-acetic ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description In vitro binding affinity against rat heart membrane using [3H]-AF-DX-384 | J Med Chem 38: 1711-9 (1995) BindingDB Entry DOI: 10.7270/Q2PV6JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50034874 ((R)-Hydroxy-(4-iodo-phenyl)-phenyl-acetic acid 1-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description In vitro binding affinity against rat heart membrane using [3H]-AF-DX-384 | J Med Chem 38: 1711-9 (1995) BindingDB Entry DOI: 10.7270/Q2PV6JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50034870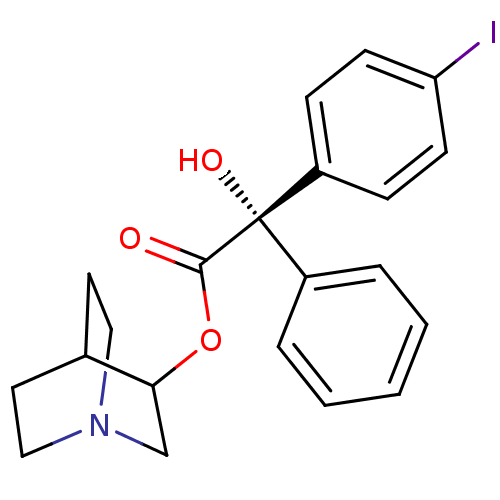 ((S)-Hydroxy-(4-iodo-phenyl)-phenyl-acetic acid 1-a...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description In vitro binding affinity against bovine striatal membrane using [3H]-pirenzepine | J Med Chem 38: 1711-9 (1995) BindingDB Entry DOI: 10.7270/Q2PV6JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor (Cavia porcellus) | BDBM50010096 (CHEMBL12980 | Hydroxy-diphenyl-acetic acid 1-aza-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description In vitro binding affinity against against guinea pig ileum using [3H]-N-methylscopolamine | J Med Chem 38: 1711-9 (1995) BindingDB Entry DOI: 10.7270/Q2PV6JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50034873 ((S)-(4-Fluoromethyl-phenyl)-hydroxy-phenyl-acetic ...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description In vitro binding affinity against bovine striatal membrane using [3H]-pirenzepine | J Med Chem 38: 1711-9 (1995) BindingDB Entry DOI: 10.7270/Q2PV6JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50034873 ((S)-(4-Fluoromethyl-phenyl)-hydroxy-phenyl-acetic ...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description In vitro binding affinity against bovine striatal membrane using [3H]-pirenzepine | J Med Chem 38: 1711-9 (1995) BindingDB Entry DOI: 10.7270/Q2PV6JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50011774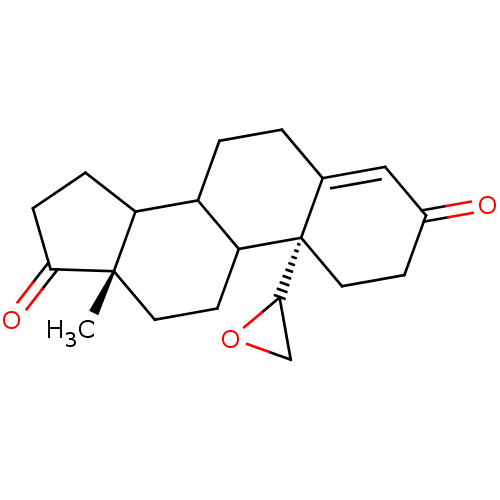 (13-Methyl-10-oxiranyl-1,6,7,8,9,10,11,12,13,14,15,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human placental Cytochrome P450 19A1 | J Med Chem 34: 1344-9 (1991) BindingDB Entry DOI: 10.7270/Q2M044C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50034871 ((R)-[4-(2-Fluoro-ethyl)-phenyl]-hydroxy-phenyl-ace...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description In vitro binding affinity against rat heart membrane using [3H]-AF-DX-384 | J Med Chem 38: 1711-9 (1995) BindingDB Entry DOI: 10.7270/Q2PV6JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor (Cavia porcellus) | BDBM50034874 ((R)-Hydroxy-(4-iodo-phenyl)-phenyl-acetic acid 1-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description In vitro binding affinity against guinea pig ileum using [3H]-N-methylscopolamine | J Med Chem 38: 1711-9 (1995) BindingDB Entry DOI: 10.7270/Q2PV6JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50034870 ((S)-Hydroxy-(4-iodo-phenyl)-phenyl-acetic acid 1-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description In vitro binding affinity against rat heart membrane using [3H]-AF-DX-384 | J Med Chem 38: 1711-9 (1995) BindingDB Entry DOI: 10.7270/Q2PV6JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50034869 ((R)-(4-Fluoromethyl-phenyl)-hydroxy-phenyl-acetic ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description In vitro binding affinity against rat heart membrane using [3H]-AF-DX-384 | J Med Chem 38: 1711-9 (1995) BindingDB Entry DOI: 10.7270/Q2PV6JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50034869 ((R)-(4-Fluoromethyl-phenyl)-hydroxy-phenyl-acetic ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description In vitro binding affinity against rat heart membrane using [3H]-AF-DX-384 | J Med Chem 38: 1711-9 (1995) BindingDB Entry DOI: 10.7270/Q2PV6JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50034869 ((R)-(4-Fluoromethyl-phenyl)-hydroxy-phenyl-acetic ...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 17.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description In vitro binding affinity against bovine striatal membrane using [3H]-pirenzepine | J Med Chem 38: 1711-9 (1995) BindingDB Entry DOI: 10.7270/Q2PV6JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50034869 ((R)-(4-Fluoromethyl-phenyl)-hydroxy-phenyl-acetic ...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 17.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description In vitro binding affinity against bovine striatal membrane using [3H]-pirenzepine | J Med Chem 38: 1711-9 (1995) BindingDB Entry DOI: 10.7270/Q2PV6JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50406525 (CHEMBL2113272) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human placental Cytochrome P450 19A1 | J Med Chem 34: 1344-9 (1991) BindingDB Entry DOI: 10.7270/Q2M044C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor (Cavia porcellus) | BDBM50034873 ((S)-(4-Fluoromethyl-phenyl)-hydroxy-phenyl-acetic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description In vitro binding affinity against guinea pig ileum using [3H]-N-methylscopolamine | J Med Chem 38: 1711-9 (1995) BindingDB Entry DOI: 10.7270/Q2PV6JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor (Cavia porcellus) | BDBM50034873 ((S)-(4-Fluoromethyl-phenyl)-hydroxy-phenyl-acetic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description In vitro binding affinity against guinea pig ileum using [3H]-N-methylscopolamine | J Med Chem 38: 1711-9 (1995) BindingDB Entry DOI: 10.7270/Q2PV6JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor (Cavia porcellus) | BDBM50034870 ((S)-Hydroxy-(4-iodo-phenyl)-phenyl-acetic acid 1-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description In vitro binding affinity against guinea pig ileum using [3H]-N-methylscopolamine | J Med Chem 38: 1711-9 (1995) BindingDB Entry DOI: 10.7270/Q2PV6JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor (Cavia porcellus) | BDBM50034872 ((S)-[4-(3-Fluoro-propyl)-phenyl]-hydroxy-phenyl-ac...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description In vitro binding affinity against guinea pig ileum using [3H]-N-methylscopolamine | J Med Chem 38: 1711-9 (1995) BindingDB Entry DOI: 10.7270/Q2PV6JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50011770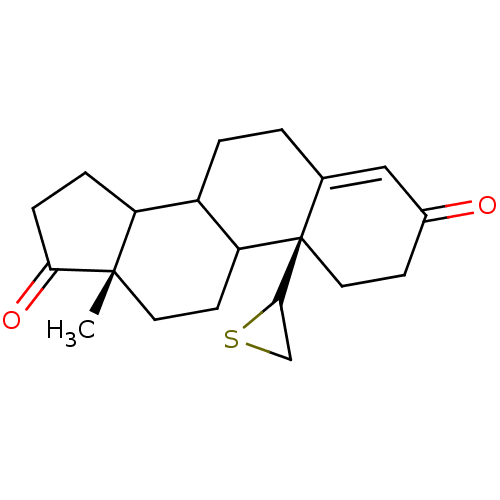 (13-Methyl-10-thiiranyl-1,6,7,8,9,10,11,12,13,14,15...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human placental Cytochrome P450 19A1 | J Med Chem 34: 1344-9 (1991) BindingDB Entry DOI: 10.7270/Q2M044C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50011775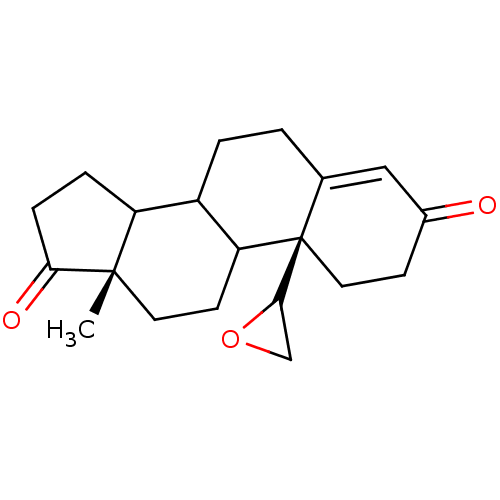 (13-Methyl-10-oxiranyl-1,6,7,8,9,10,11,12,13,14,15,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human placental Cytochrome P450 19A1 | J Med Chem 34: 1344-9 (1991) BindingDB Entry DOI: 10.7270/Q2M044C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50021778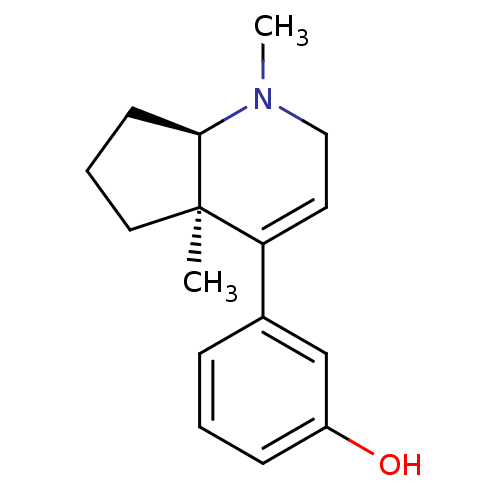 (3-(1,4a-Dimethyl-2,4a,5,6,7,7a-hexahydro-1H-[1]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound administered subcutaneously was evaluated for inhibition constant measured as the displacement of [3H]DAGO from rat brain | J Med Chem 30: 947-50 (1987) BindingDB Entry DOI: 10.7270/Q2TD9XXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50021779 (3-(4a-Ethyl-1-methyl-2,4a,5,6,7,7a-hexahydro-1H-[1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound administered subcutaneously was evaluated for inhibition constant measured as the displacement of [3H]DAGO from rat brain | J Med Chem 30: 947-50 (1987) BindingDB Entry DOI: 10.7270/Q2TD9XXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor (Cavia porcellus) | BDBM50034875 ((S)-[4-(2-Fluoro-ethyl)-phenyl]-hydroxy-phenyl-ace...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description In vitro binding affinity against guinea pig ileum using [3H]-N-methylscopolamine | J Med Chem 38: 1711-9 (1995) BindingDB Entry DOI: 10.7270/Q2PV6JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50406524 (CHEMBL2113269) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human placental Cytochrome P450 19A1 | J Med Chem 34: 1344-9 (1991) BindingDB Entry DOI: 10.7270/Q2M044C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50406523 (CHEMBL2113276) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 206 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human placental Cytochrome P450 19A1 | J Med Chem 34: 1344-9 (1991) BindingDB Entry DOI: 10.7270/Q2M044C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50406522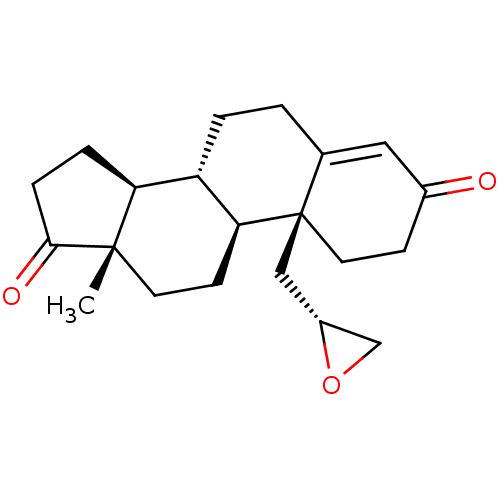 (CHEMBL2113268) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 753 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human placental Cytochrome P450 19A1 | J Med Chem 34: 1344-9 (1991) BindingDB Entry DOI: 10.7270/Q2M044C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor (Cavia porcellus) | BDBM50034871 ((R)-[4-(2-Fluoro-ethyl)-phenyl]-hydroxy-phenyl-ace...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description In vitro binding affinity against guinea pig ileum using [3H]-N-methylscopolamine | J Med Chem 38: 1711-9 (1995) BindingDB Entry DOI: 10.7270/Q2PV6JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor (Cavia porcellus) | BDBM50034869 ((R)-(4-Fluoromethyl-phenyl)-hydroxy-phenyl-acetic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description In vitro binding affinity against guinea pig ileum using [3H]-N-methylscopolamine | J Med Chem 38: 1711-9 (1995) BindingDB Entry DOI: 10.7270/Q2PV6JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor (Cavia porcellus) | BDBM50034869 ((R)-(4-Fluoromethyl-phenyl)-hydroxy-phenyl-acetic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description In vitro binding affinity against guinea pig ileum using [3H]-N-methylscopolamine | J Med Chem 38: 1711-9 (1995) BindingDB Entry DOI: 10.7270/Q2PV6JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1 (Rattus norvegicus (rat)-RAT) | BDBM50021347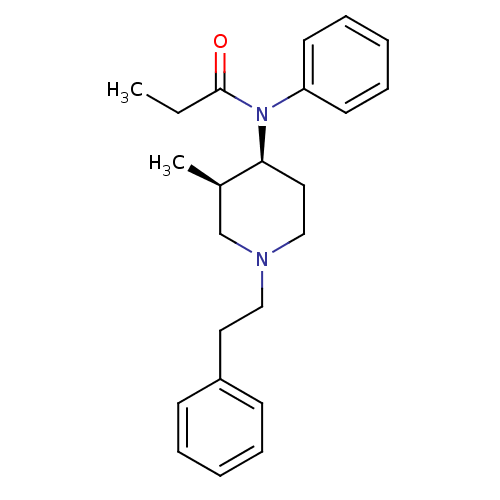 (CHEMBL12391 | N-(3-Methyl-1-phenethyl-piperidin-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vivo binding affinity to opioid receptor preparations from rat brain | J Med Chem 29: 1087-93 (1986) BindingDB Entry DOI: 10.7270/Q2JQ1364 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1 (Rattus norvegicus (rat)-RAT) | BDBM50021347 (CHEMBL12391 | N-(3-Methyl-1-phenethyl-piperidin-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vivo binding affinity to opioid receptor preparations from rat brain | J Med Chem 29: 1087-93 (1986) BindingDB Entry DOI: 10.7270/Q2JQ1364 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1 (Rattus norvegicus (rat)-RAT) | BDBM50008984 (4-(4-Chloro-benzyl)-2-(1-methyl-azepan-4-yl)-2H-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vivo binding affinity to opioid receptor preparations from rat brain | J Med Chem 29: 1087-93 (1986) BindingDB Entry DOI: 10.7270/Q2JQ1364 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1 (Rattus norvegicus (rat)-RAT) | BDBM50226247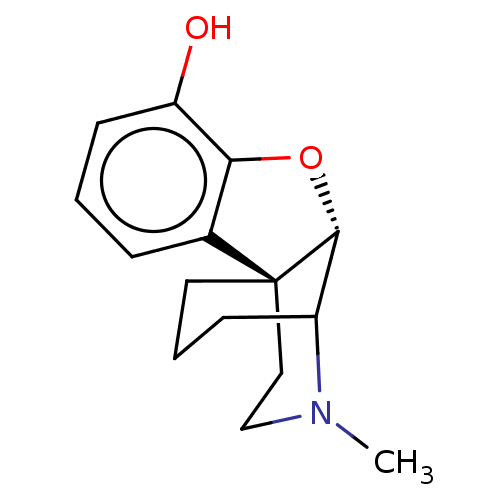 (CHEMBL423449) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards opioid receptor | J Med Chem 29: 748-51 (1986) BindingDB Entry DOI: 10.7270/Q2PG1TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1 (Rattus norvegicus (rat)-RAT) | BDBM50226268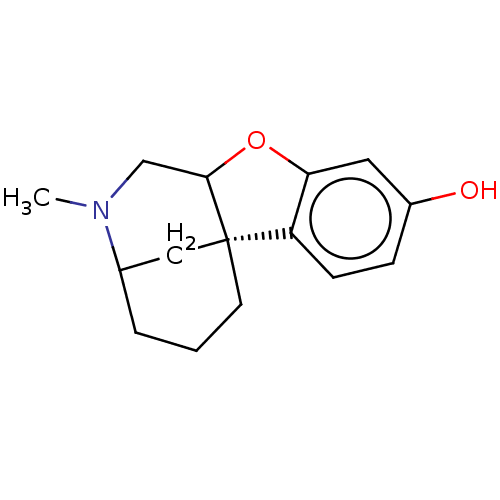 (CHEMBL348954) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards opioid receptor | J Med Chem 29: 748-51 (1986) BindingDB Entry DOI: 10.7270/Q2PG1TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1 (Rattus norvegicus (rat)-RAT) | BDBM50226243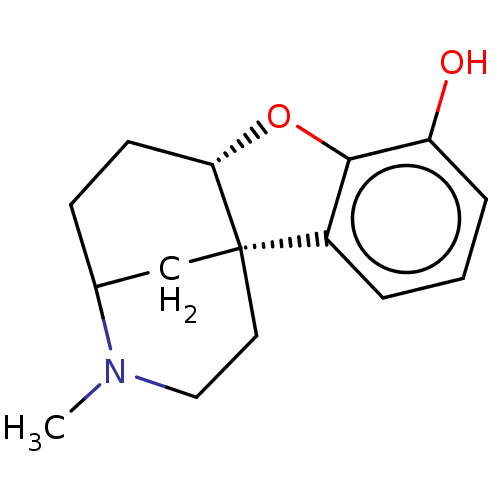 (CHEMBL353357) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards opioid receptor | J Med Chem 29: 748-51 (1986) BindingDB Entry DOI: 10.7270/Q2PG1TX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||