 Found 3758 hits with Last Name = 'sim' and Initial = 'j'
Found 3758 hits with Last Name = 'sim' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471255

(Acolbifene | EM-652 | SCH-57068)Show SMILES CC1=C([C@@H](Oc2cc(O)ccc12)c1ccc(OCCN2CCCCC2)cc1)c1ccc(O)cc1 |t:1| Show InChI InChI=1S/C29H31NO4/c1-20-26-14-11-24(32)19-27(26)34-29(28(20)21-5-9-23(31)10-6-21)22-7-12-25(13-8-22)33-18-17-30-15-3-2-4-16-30/h5-14,19,29,31-32H,2-4,15-18H2,1H3/t29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec
Curated by ChEMBL
| Assay Description
Apparent binding affinity against estradiol-stimulated T-47D cell proliferation |
J Med Chem 40: 2117-22 (1997)
Article DOI: 10.1021/jm970095o
BindingDB Entry DOI: 10.7270/Q2474DKC |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471254
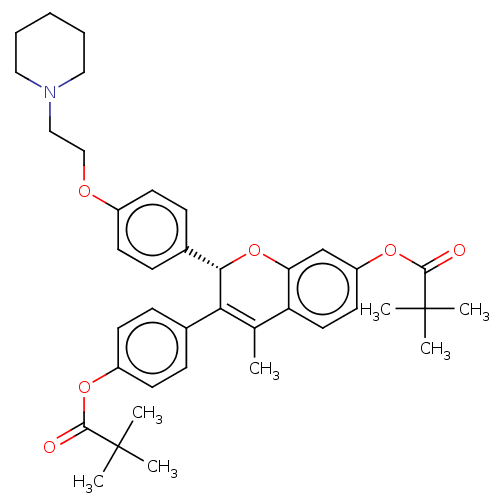
(CHEMBL308234)Show SMILES CC1=C([C@@H](Oc2cc(OC(=O)C(C)(C)C)ccc12)c1ccc(OCCN2CCCCC2)cc1)c1ccc(OC(=O)C(C)(C)C)cc1 |r,t:1| Show InChI InChI=1S/C39H47NO6/c1-26-32-20-19-31(45-37(42)39(5,6)7)25-33(32)46-35(34(26)27-11-17-30(18-12-27)44-36(41)38(2,3)4)28-13-15-29(16-14-28)43-24-23-40-21-9-8-10-22-40/h11-20,25,35H,8-10,21-24H2,1-7H3/t35-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec
Curated by ChEMBL
| Assay Description
Apparent binding affinity against estradiol-stimulated T-47D cell proliferation |
J Med Chem 40: 2117-22 (1997)
Article DOI: 10.1021/jm970095o
BindingDB Entry DOI: 10.7270/Q2474DKC |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471254

(CHEMBL308234)Show SMILES CC1=C([C@@H](Oc2cc(OC(=O)C(C)(C)C)ccc12)c1ccc(OCCN2CCCCC2)cc1)c1ccc(OC(=O)C(C)(C)C)cc1 |r,t:1| Show InChI InChI=1S/C39H47NO6/c1-26-32-20-19-31(45-37(42)39(5,6)7)25-33(32)46-35(34(26)27-11-17-30(18-12-27)44-36(41)38(2,3)4)28-13-15-29(16-14-28)43-24-23-40-21-9-8-10-22-40/h11-20,25,35H,8-10,21-24H2,1-7H3/t35-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec
Curated by ChEMBL
| Assay Description
Stimulation of alkaline phosphatase activity in human endometrial Ishikawa cells with 1 nM E2 estradiol |
J Med Chem 40: 2117-22 (1997)
Article DOI: 10.1021/jm970095o
BindingDB Entry DOI: 10.7270/Q2474DKC |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471256
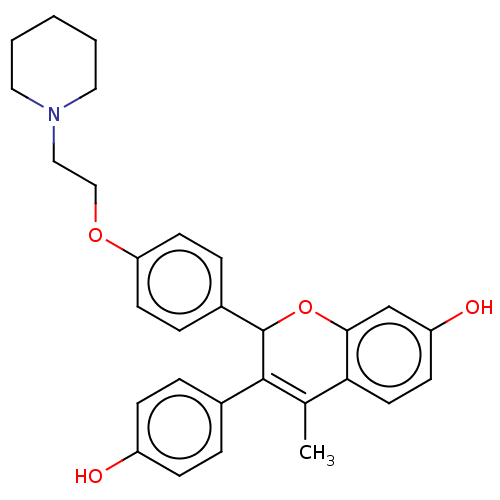
(CHEMBL291808)Show SMILES CC1=C(C(Oc2cc(O)ccc12)c1ccc(OCCN2CCCCC2)cc1)c1ccc(O)cc1 |t:1| Show InChI InChI=1S/C29H31NO4/c1-20-26-14-11-24(32)19-27(26)34-29(28(20)21-5-9-23(31)10-6-21)22-7-12-25(13-8-22)33-18-17-30-15-3-2-4-16-30/h5-14,19,29,31-32H,2-4,15-18H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec
Curated by ChEMBL
| Assay Description
Apparent binding affinity against estradiol-stimulated T-47D cell proliferation |
J Med Chem 40: 2117-22 (1997)
Article DOI: 10.1021/jm970095o
BindingDB Entry DOI: 10.7270/Q2474DKC |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471255

(Acolbifene | EM-652 | SCH-57068)Show SMILES CC1=C([C@@H](Oc2cc(O)ccc12)c1ccc(OCCN2CCCCC2)cc1)c1ccc(O)cc1 |t:1| Show InChI InChI=1S/C29H31NO4/c1-20-26-14-11-24(32)19-27(26)34-29(28(20)21-5-9-23(31)10-6-21)22-7-12-25(13-8-22)33-18-17-30-15-3-2-4-16-30/h5-14,19,29,31-32H,2-4,15-18H2,1H3/t29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec
Curated by ChEMBL
| Assay Description
Apparent inhibition constant for estrogen receptor in Human uterine cytosol in 3.3%ethanol |
J Med Chem 40: 2117-22 (1997)
Article DOI: 10.1021/jm970095o
BindingDB Entry DOI: 10.7270/Q2474DKC |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471255

(Acolbifene | EM-652 | SCH-57068)Show SMILES CC1=C([C@@H](Oc2cc(O)ccc12)c1ccc(OCCN2CCCCC2)cc1)c1ccc(O)cc1 |t:1| Show InChI InChI=1S/C29H31NO4/c1-20-26-14-11-24(32)19-27(26)34-29(28(20)21-5-9-23(31)10-6-21)22-7-12-25(13-8-22)33-18-17-30-15-3-2-4-16-30/h5-14,19,29,31-32H,2-4,15-18H2,1H3/t29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec
Curated by ChEMBL
| Assay Description
Inhibition of estradiol binding to estrogen receptor in Human Breast cancer cytosol (3.3% ethanol) |
J Med Chem 40: 2117-22 (1997)
Article DOI: 10.1021/jm970095o
BindingDB Entry DOI: 10.7270/Q2474DKC |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471255

(Acolbifene | EM-652 | SCH-57068)Show SMILES CC1=C([C@@H](Oc2cc(O)ccc12)c1ccc(OCCN2CCCCC2)cc1)c1ccc(O)cc1 |t:1| Show InChI InChI=1S/C29H31NO4/c1-20-26-14-11-24(32)19-27(26)34-29(28(20)21-5-9-23(31)10-6-21)22-7-12-25(13-8-22)33-18-17-30-15-3-2-4-16-30/h5-14,19,29,31-32H,2-4,15-18H2,1H3/t29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec
Curated by ChEMBL
| Assay Description
Apparent inhibition constant for estrogen receptor in Human uterine cytosol in 2.5%DMF |
J Med Chem 40: 2117-22 (1997)
Article DOI: 10.1021/jm970095o
BindingDB Entry DOI: 10.7270/Q2474DKC |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471255

(Acolbifene | EM-652 | SCH-57068)Show SMILES CC1=C([C@@H](Oc2cc(O)ccc12)c1ccc(OCCN2CCCCC2)cc1)c1ccc(O)cc1 |t:1| Show InChI InChI=1S/C29H31NO4/c1-20-26-14-11-24(32)19-27(26)34-29(28(20)21-5-9-23(31)10-6-21)22-7-12-25(13-8-22)33-18-17-30-15-3-2-4-16-30/h5-14,19,29,31-32H,2-4,15-18H2,1H3/t29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec
Curated by ChEMBL
| Assay Description
Apparent inhibition constant for estrogen receptor in Human Breast cancer cytosol in 2.5%DMF |
J Med Chem 40: 2117-22 (1997)
Article DOI: 10.1021/jm970095o
BindingDB Entry DOI: 10.7270/Q2474DKC |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471255

(Acolbifene | EM-652 | SCH-57068)Show SMILES CC1=C([C@@H](Oc2cc(O)ccc12)c1ccc(OCCN2CCCCC2)cc1)c1ccc(O)cc1 |t:1| Show InChI InChI=1S/C29H31NO4/c1-20-26-14-11-24(32)19-27(26)34-29(28(20)21-5-9-23(31)10-6-21)22-7-12-25(13-8-22)33-18-17-30-15-3-2-4-16-30/h5-14,19,29,31-32H,2-4,15-18H2,1H3/t29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec
Curated by ChEMBL
| Assay Description
Apparent binding affinity against estradiol-stimulated ZR-75-1-cell proliferation |
J Med Chem 40: 2117-22 (1997)
Article DOI: 10.1021/jm970095o
BindingDB Entry DOI: 10.7270/Q2474DKC |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50093175

(CHEMBL311655 | Derivative of APC-2059)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#7]-[#6]-c2ccc(cc2)\[#7]=[#6](/[#7])-[#7])cc1 Show InChI InChI=1S/C34H50N12O4/c35-31(36)41-27-11-7-25(8-12-27)23-39-33(49)45-19-15-43(16-20-45)29(47)5-3-1-2-4-6-30(48)44-17-21-46(22-18-44)34(50)40-24-26-9-13-28(14-10-26)42-32(37)38/h7-14H,1-6,15-24H2,(H,39,49)(H,40,50)(H4,35,36,41)(H4,37,38,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Evaluated for its inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2361-6 (2001)
BindingDB Entry DOI: 10.7270/Q2HX1BWR |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50093157

(CHEMBL431969 | Derivative of piperazine-1-carboxyl...)Show SMILES NCc1ccc(CNC(=O)N2CCN(CC2)C(=O)O[C@H]2CCC[C@H](CCC2)OC(=O)N2CCN(CC2)C(=O)NCc2ccc(CN)cc2)cc1 Show InChI InChI=1S/C36H52N8O6/c37-23-27-7-11-29(12-8-27)25-39-33(45)41-15-19-43(20-16-41)35(47)49-31-3-1-4-32(6-2-5-31)50-36(48)44-21-17-42(18-22-44)34(46)40-26-30-13-9-28(24-38)10-14-30/h7-14,31-32H,1-6,15-26,37-38H2,(H,39,45)(H,40,46)/t31-,32+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Evaluated for its inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2361-6 (2001)
BindingDB Entry DOI: 10.7270/Q2HX1BWR |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec
Curated by ChEMBL
| Assay Description
Apparent inhibition constant for estrogen receptor in Human Breast cancer cytosol in 2.5%DMF |
J Med Chem 40: 2117-22 (1997)
Article DOI: 10.1021/jm970095o
BindingDB Entry DOI: 10.7270/Q2474DKC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec
Curated by ChEMBL
| Assay Description
Apparent inhibition constant for estrogen receptor in Human uterine cytosol in 3.3%ethanol |
J Med Chem 40: 2117-22 (1997)
Article DOI: 10.1021/jm970095o
BindingDB Entry DOI: 10.7270/Q2474DKC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM20625
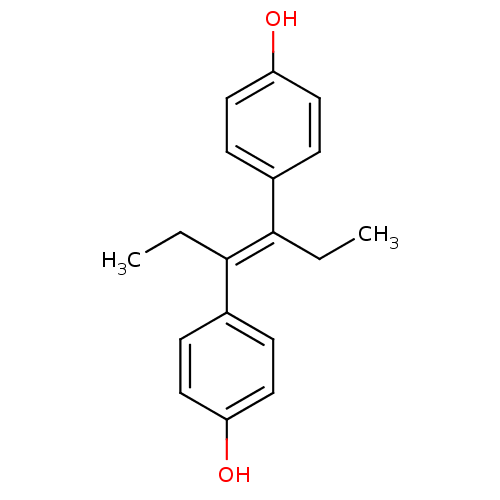
(4-[(3E)-4-(4-hydroxyphenyl)hex-3-en-3-yl]phenol | ...)Show InChI InChI=1S/C18H20O2/c1-3-17(13-5-9-15(19)10-6-13)18(4-2)14-7-11-16(20)12-8-14/h5-12,19-20H,3-4H2,1-2H3/b18-17+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec
Curated by ChEMBL
| Assay Description
Inhibition of estradiol binding to estrogen receptor in Human Breast cancer cytosol (3.3% ethanol) |
J Med Chem 40: 2117-22 (1997)
Article DOI: 10.1021/jm970095o
BindingDB Entry DOI: 10.7270/Q2474DKC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM20625

(4-[(3E)-4-(4-hydroxyphenyl)hex-3-en-3-yl]phenol | ...)Show InChI InChI=1S/C18H20O2/c1-3-17(13-5-9-15(19)10-6-13)18(4-2)14-7-11-16(20)12-8-14/h5-12,19-20H,3-4H2,1-2H3/b18-17+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.128 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec
Curated by ChEMBL
| Assay Description
Apparent inhibition constant for estrogen receptor in Human uterine cytosol in 3.3%ethanol |
J Med Chem 40: 2117-22 (1997)
Article DOI: 10.1021/jm970095o
BindingDB Entry DOI: 10.7270/Q2474DKC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec
Curated by ChEMBL
| Assay Description
Inhibition of estradiol binding to estrogen receptor in Human Breast cancer cytosol (3.3% ethanol) |
J Med Chem 40: 2117-22 (1997)
Article DOI: 10.1021/jm970095o
BindingDB Entry DOI: 10.7270/Q2474DKC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.181 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec
Curated by ChEMBL
| Assay Description
Apparent inhibition constant for estrogen receptor in Human uterine cytosol in 2.5%DMF |
J Med Chem 40: 2117-22 (1997)
Article DOI: 10.1021/jm970095o
BindingDB Entry DOI: 10.7270/Q2474DKC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity towards mu-specific opiate receptor from combinatorial peptoid library |
J Med Chem 37: 2678-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26ZSP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50093192
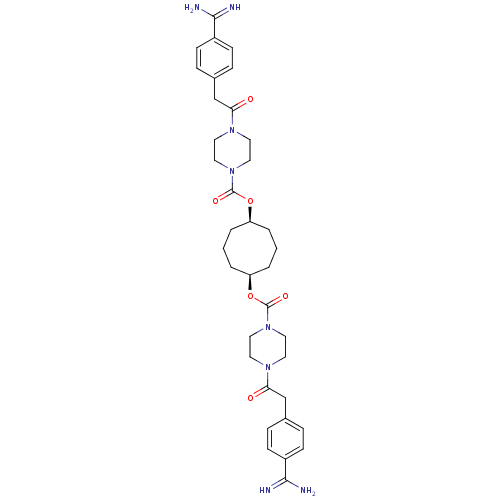
(CHEMBL311482 | Derivative of piperazine-1-carboxyl...)Show SMILES NC(=N)c1ccc(CC(=O)N2CCN(CC2)C(=O)O[C@H]2CCC[C@H](CCC2)OC(=O)N2CCN(CC2)C(=O)Cc2ccc(cc2)C(N)=N)cc1 Show InChI InChI=1S/C36H48N8O6/c37-33(38)27-11-7-25(8-12-27)23-31(45)41-15-19-43(20-16-41)35(47)49-29-3-1-4-30(6-2-5-29)50-36(48)44-21-17-42(18-22-44)32(46)24-26-9-13-28(14-10-26)34(39)40/h7-14,29-30H,1-6,15-24H2,(H3,37,38)(H3,39,40)/t29-,30+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Evaluated for its inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2361-6 (2001)
BindingDB Entry DOI: 10.7270/Q2HX1BWR |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50276802
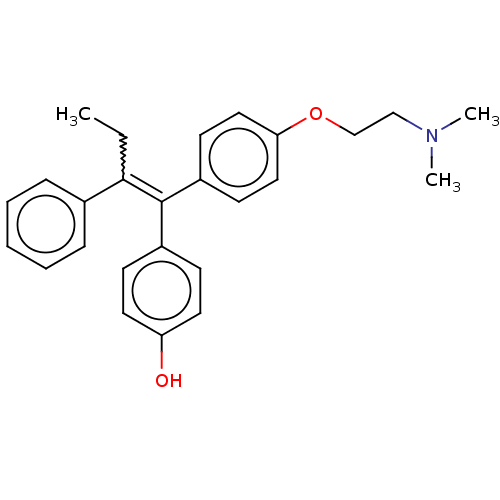
(4-OHT | Afimoxifene | TamoGel)Show SMILES CCC(=C(c1ccc(O)cc1)c1ccc(OCCN(C)C)cc1)c1ccccc1 Show InChI InChI=1S/C26H29NO2/c1-4-25(20-8-6-5-7-9-20)26(21-10-14-23(28)15-11-21)22-12-16-24(17-13-22)29-19-18-27(2)3/h5-17,28H,4,18-19H2,1-3H3/b26-25- | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.249 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec
Curated by ChEMBL
| Assay Description
Inhibition of estradiol binding to estrogen receptor in Human Breast cancer cytosol (3.3% ethanol) |
J Med Chem 40: 2117-22 (1997)
Article DOI: 10.1021/jm970095o
BindingDB Entry DOI: 10.7270/Q2474DKC |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50017233
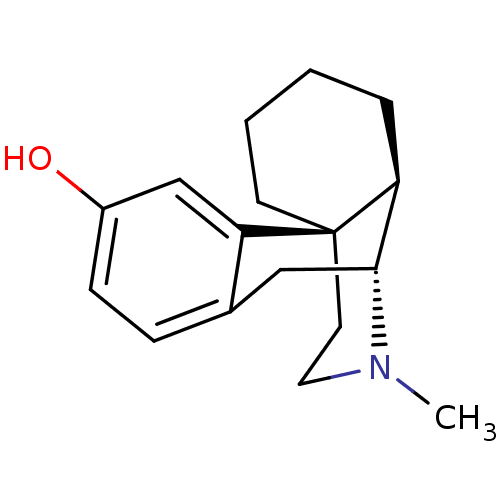
(CHEMBL592 | LEVO-DROMORAN | LEVORPHANOL)Show SMILES CN1CC[C@]23CCCC[C@H]2[C@H]1Cc1ccc(O)cc31 |r,TLB:0:1:12.18.11:9| Show InChI InChI=1S/C17H23NO/c1-18-9-8-17-7-3-2-4-14(17)16(18)10-12-5-6-13(19)11-15(12)17/h5-6,11,14,16,19H,2-4,7-10H2,1H3/t14-,16+,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
Binding affinity for mouse opioid receptor mu |
J Med Chem 32: 2221-6 (1989)
BindingDB Entry DOI: 10.7270/Q2Q240T3 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50276802

(4-OHT | Afimoxifene | TamoGel)Show SMILES CCC(=C(c1ccc(O)cc1)c1ccc(OCCN(C)C)cc1)c1ccccc1 Show InChI InChI=1S/C26H29NO2/c1-4-25(20-8-6-5-7-9-20)26(21-10-14-23(28)15-11-21)22-12-16-24(17-13-22)29-19-18-27(2)3/h5-17,28H,4,18-19H2,1-3H3/b26-25- | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.346 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec
Curated by ChEMBL
| Assay Description
Apparent inhibition constant for estrogen receptor in Human uterine cytosol in 3.3%ethanol |
J Med Chem 40: 2117-22 (1997)
Article DOI: 10.1021/jm970095o
BindingDB Entry DOI: 10.7270/Q2474DKC |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50093154
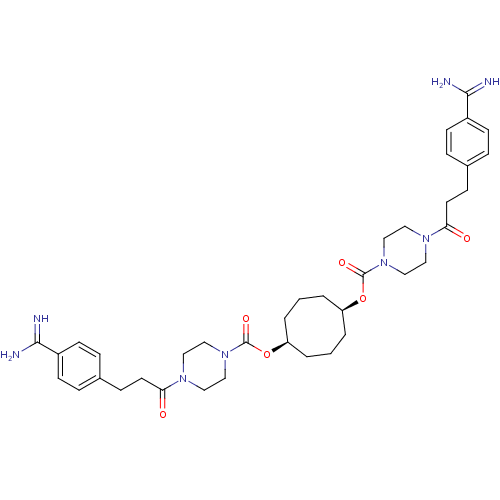
(CHEMBL448786 | Derivative of piperazine-1-carboxyl...)Show SMILES NC(=N)c1ccc(CCC(=O)N2CCN(CC2)C(=O)O[C@H]2CCC[C@H](CCC2)OC(=O)N2CCN(CC2)C(=O)CCc2ccc(cc2)C(N)=N)cc1 Show InChI InChI=1S/C38H52N8O6/c39-35(40)29-13-7-27(8-14-29)11-17-33(47)43-19-23-45(24-20-43)37(49)51-31-3-1-4-32(6-2-5-31)52-38(50)46-25-21-44(22-26-46)34(48)18-12-28-9-15-30(16-10-28)36(41)42/h7-10,13-16,31-32H,1-6,11-12,17-26H2,(H3,39,40)(H3,41,42)/t31-,32+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Evaluated for its inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2361-6 (2001)
BindingDB Entry DOI: 10.7270/Q2HX1BWR |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50093167
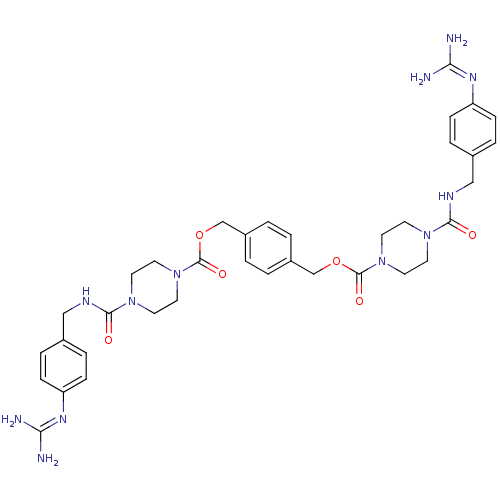
(CHEMBL75750 | Derivative of APC-2059)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#8]-[#6]-c2ccc(-[#6]-[#8]-[#6](=O)-[#7]-3-[#6]-[#6]-[#7](-[#6]-[#6]-3)-[#6](=O)-[#7]-[#6]-c3ccc(cc3)\[#7]=[#6](/[#7])-[#7])cc2)cc1 Show InChI InChI=1S/C36H46N12O6/c37-31(38)43-29-9-5-25(6-10-29)21-41-33(49)45-13-17-47(18-14-45)35(51)53-23-27-1-2-28(4-3-27)24-54-36(52)48-19-15-46(16-20-48)34(50)42-22-26-7-11-30(12-8-26)44-32(39)40/h1-12H,13-24H2,(H,41,49)(H,42,50)(H4,37,38,43)(H4,39,40,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Evaluated for its inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2361-6 (2001)
BindingDB Entry DOI: 10.7270/Q2HX1BWR |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50093156

(CHEMBL432172 | Derivative of APC-2059)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#8]-[#6]C23[#6]-[#6]C([#6]-[#8]-[#6](=O)-[#7]-4-[#6]-[#6]-[#7](-[#6]-[#6]-4)-[#6](=O)-[#7]-[#6]-c4ccc(cc4)\[#7]=[#6](/[#7])-[#7])([#6]-[#6]2)[#6]-[#6]3)cc1 Show InChI InChI=1S/C38H54N12O6/c39-31(40)45-29-5-1-27(2-6-29)23-43-33(51)47-15-19-49(20-16-47)35(53)55-25-37-9-12-38(13-10-37,14-11-37)26-56-36(54)50-21-17-48(18-22-50)34(52)44-24-28-3-7-30(8-4-28)46-32(41)42/h1-8H,9-26H2,(H,43,51)(H,44,52)(H4,39,40,45)(H4,41,42,46) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Evaluated for its inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2361-6 (2001)
BindingDB Entry DOI: 10.7270/Q2HX1BWR |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50202412
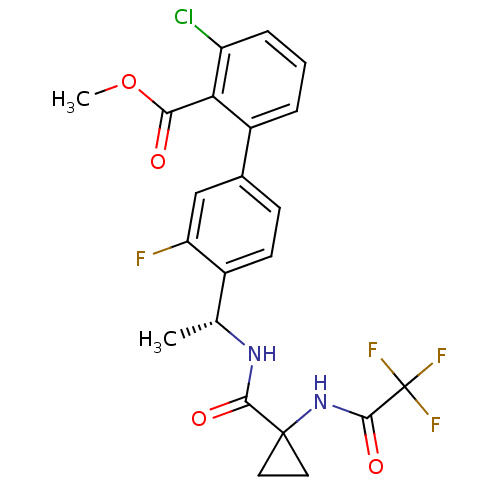
(3-Chloro-3'-fluoro-4'-((R)-1-{[1-(2,2,2-trifluoro-...)Show SMILES COC(=O)c1c(Cl)cccc1-c1ccc([C@@H](C)NC(=O)C2(CC2)NC(=O)C(F)(F)F)c(F)c1 |r| Show InChI InChI=1S/C22H19ClF4N2O4/c1-11(28-19(31)21(8-9-21)29-20(32)22(25,26)27)13-7-6-12(10-16(13)24)14-4-3-5-15(23)17(14)18(30)33-2/h3-7,10-11H,8-9H2,1-2H3,(H,28,31)(H,29,32)/t11-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human bradykinin B1 receptor |
Bioorg Med Chem Lett 18: 5027-31 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.014
BindingDB Entry DOI: 10.7270/Q2NG4QDX |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50093158

(1-{4-[4-amino(imino)methylaminobenzylcarbamoyl]hex...)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#7]-1-[#6]-[#6]-[#7](-[#6]-[#6]-1)-[#6](=O)-[#8]-[#6@H]-1-[#6]-[#6]-[#6]-[#6@H](-[#6]-[#6]-[#6]-1)-[#8]-[#6](=O)-[#7]-1-[#6]-[#6]-[#7](-[#6]-[#6]-1)-[#6](=O)-[#7]-[#6]-c1ccc(cc1)\[#7]=[#6](/[#7])-[#7] Show InChI InChI=1S/C33H54N10O6/c34-14-2-1-3-15-37-30(44)40-16-20-42(21-17-40)32(46)48-27-6-4-8-28(9-5-7-27)49-33(47)43-22-18-41(19-23-43)31(45)38-24-25-10-12-26(13-11-25)39-29(35)36/h10-13,27-28H,1-9,14-24,34H2,(H,37,44)(H,38,45)(H4,35,36,39)/t27-,28+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Evaluated for its inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2361-6 (2001)
BindingDB Entry DOI: 10.7270/Q2HX1BWR |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50093199
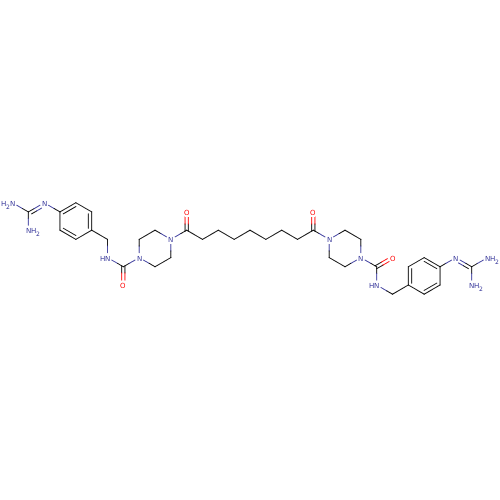
(CHEMBL75972 | Derivative of APC-2059)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#7]-[#6]-c2ccc(cc2)\[#7]=[#6](/[#7])-[#7])cc1 Show InChI InChI=1S/C35H52N12O4/c36-32(37)42-28-12-8-26(9-13-28)24-40-34(50)46-20-16-44(17-21-46)30(48)6-4-2-1-3-5-7-31(49)45-18-22-47(23-19-45)35(51)41-25-27-10-14-29(15-11-27)43-33(38)39/h8-15H,1-7,16-25H2,(H,40,50)(H,41,51)(H4,36,37,42)(H4,38,39,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Evaluated for its inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2361-6 (2001)
BindingDB Entry DOI: 10.7270/Q2HX1BWR |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
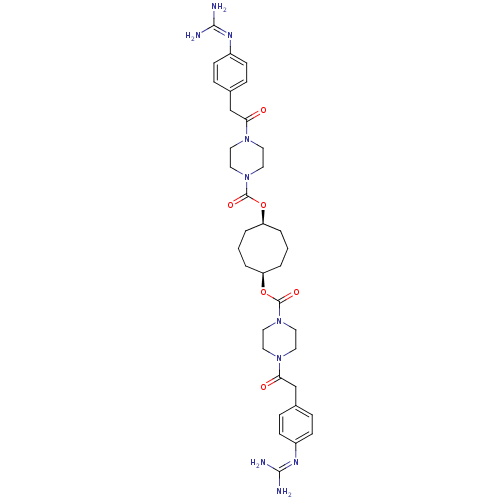
(Homo sapiens (Human)) | BDBM50093173
(CHEMBL309830 | Derivative of piperazine-1-carboxyl...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#8]-[#6@H]-2-[#6]-[#6]-[#6]-[#6@H](-[#6]-[#6]-[#6]-2)-[#8]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#6]-c2ccc(cc2)\[#7]=[#6](\[#7])-[#7])cc1 Show InChI InChI=1S/C36H50N10O6/c37-33(38)41-27-11-7-25(8-12-27)23-31(47)43-15-19-45(20-16-43)35(49)51-29-3-1-4-30(6-2-5-29)52-36(50)46-21-17-44(18-22-46)32(48)24-26-9-13-28(14-10-26)42-34(39)40/h7-14,29-30H,1-6,15-24H2,(H4,37,38,41)(H4,39,40,42)/t29-,30+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Evaluated for its inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2361-6 (2001)
BindingDB Entry DOI: 10.7270/Q2HX1BWR |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471258
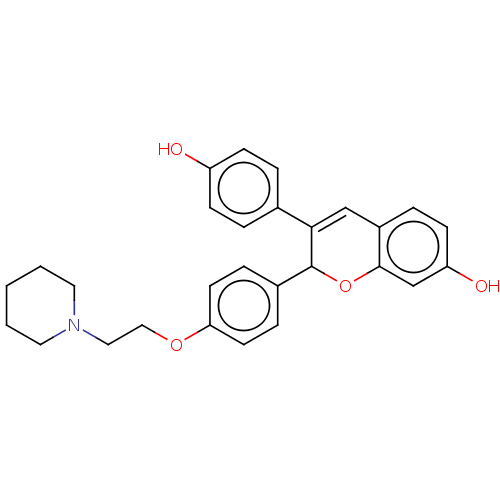
(CHEMBL67783)Show SMILES Oc1ccc(cc1)C1=Cc2ccc(O)cc2OC1c1ccc(OCCN2CCCCC2)cc1 |t:8| Show InChI InChI=1S/C28H29NO4/c30-23-9-4-20(5-10-23)26-18-22-6-11-24(31)19-27(22)33-28(26)21-7-12-25(13-8-21)32-17-16-29-14-2-1-3-15-29/h4-13,18-19,28,30-31H,1-3,14-17H2 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.525 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec
Curated by ChEMBL
| Assay Description
Apparent binding affinity against estradiol-stimulated T-47D cell proliferation |
J Med Chem 40: 2117-22 (1997)
Article DOI: 10.1021/jm970095o
BindingDB Entry DOI: 10.7270/Q2474DKC |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50127438
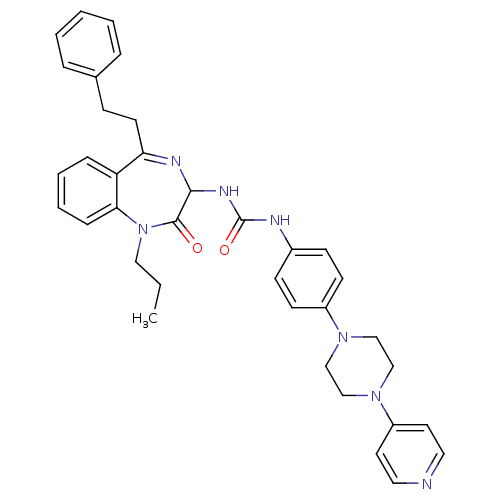
(1-(2-Oxo-5-phenethyl-1-propyl-2,3-dihydro-1H-benzo...)Show SMILES CCCN1c2ccccc2C(CCc2ccccc2)=NC(NC(=O)Nc2ccc(cc2)N2CCN(CC2)c2ccncc2)C1=O |c:20| Show InChI InChI=1S/C36H39N7O2/c1-2-22-43-33-11-7-6-10-31(33)32(17-12-27-8-4-3-5-9-27)39-34(35(43)44)40-36(45)38-28-13-15-29(16-14-28)41-23-25-42(26-24-41)30-18-20-37-21-19-30/h3-11,13-16,18-21,34H,2,12,17,22-26H2,1H3,(H2,38,40,45) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human bradykinin B1 receptor |
Bioorg Med Chem Lett 18: 5027-31 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.014
BindingDB Entry DOI: 10.7270/Q2NG4QDX |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50238741
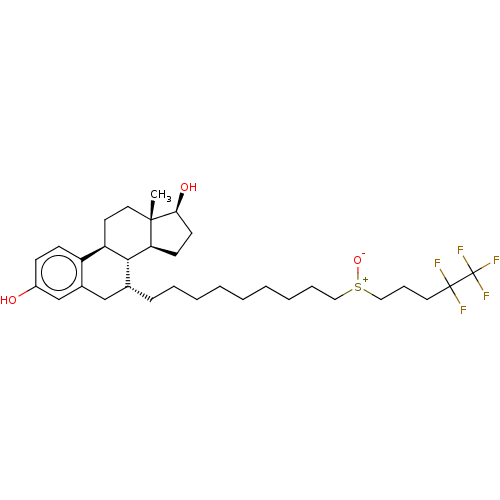
(CHEBI:31638 | Faslodex | Fulvestrant | ICI-182780 ...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3C[C@@H](CCCCCCCCC[S+]([O-])CCCC(F)(F)C(F)(F)F)[C@@]21[H] Show InChI InChI=1S/C32H47F5O3S/c1-30-17-15-26-25-12-11-24(38)21-23(25)20-22(29(26)27(30)13-14-28(30)39)10-7-5-3-2-4-6-8-18-41(40)19-9-16-31(33,34)32(35,36)37/h11-12,21-22,26-29,38-39H,2-10,13-20H2,1H3/t22-,26-,27+,28+,29-,30+,41?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.668 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec
Curated by ChEMBL
| Assay Description
Apparent inhibition constant for estrogen receptor in Human uterine cytosol in 2.5%DMF |
J Med Chem 40: 2117-22 (1997)
Article DOI: 10.1021/jm970095o
BindingDB Entry DOI: 10.7270/Q2474DKC |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50093178
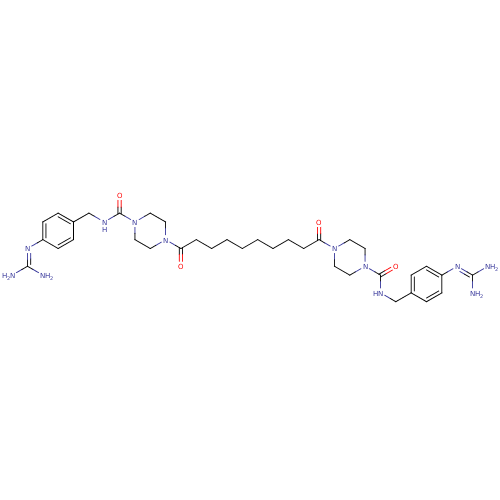
(CHEMBL76883 | Derivative of APC-2059)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#7]-[#6]-c2ccc(cc2)\[#7]=[#6](/[#7])-[#7])cc1 Show InChI InChI=1S/C36H54N12O4/c37-33(38)43-29-13-9-27(10-14-29)25-41-35(51)47-21-17-45(18-22-47)31(49)7-5-3-1-2-4-6-8-32(50)46-19-23-48(24-20-46)36(52)42-26-28-11-15-30(16-12-28)44-34(39)40/h9-16H,1-8,17-26H2,(H,41,51)(H,42,52)(H4,37,38,43)(H4,39,40,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Evaluated for its inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2361-6 (2001)
BindingDB Entry DOI: 10.7270/Q2HX1BWR |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50238741

(CHEBI:31638 | Faslodex | Fulvestrant | ICI-182780 ...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3C[C@@H](CCCCCCCCC[S+]([O-])CCCC(F)(F)C(F)(F)F)[C@@]21[H] Show InChI InChI=1S/C32H47F5O3S/c1-30-17-15-26-25-12-11-24(38)21-23(25)20-22(29(26)27(30)13-14-28(30)39)10-7-5-3-2-4-6-8-18-41(40)19-9-16-31(33,34)32(35,36)37/h11-12,21-22,26-29,38-39H,2-10,13-20H2,1H3/t22-,26-,27+,28+,29-,30+,41?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.755 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec
Curated by ChEMBL
| Assay Description
Apparent inhibition constant for estrogen receptor in Human Breast cancer cytosol in 2.5%DMF |
J Med Chem 40: 2117-22 (1997)
Article DOI: 10.1021/jm970095o
BindingDB Entry DOI: 10.7270/Q2474DKC |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM35723

(CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...)Show SMILES Cc1ccccc1C(=O)Nc1ccc(C(=O)N2CCCC(O)c3cc(Cl)ccc23)c(C)c1 Show InChI InChI=1S/C26H25ClN2O3/c1-16-6-3-4-7-20(16)25(31)28-19-10-11-21(17(2)14-19)26(32)29-13-5-8-24(30)22-15-18(27)9-12-23(22)29/h3-4,6-7,9-12,14-15,24,30H,5,8,13H2,1-2H3,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 301: 322-32 (2002)
Article DOI: 10.1124/jpet.301.1.322
BindingDB Entry DOI: 10.7270/Q2MP51VM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50363283

(CHEMBL1945711)Show SMILES COc1ccc(cc1)C1=[N+]2C(C=C1)=Cc1c(C)c(CCC(=O)NCCCCCC(=O)NCCCn3nc(C(=O)NC4C[C@@H]5CCC[C@@H](C4)N5C)c4ccccc34)c(C)n1[B-]2(F)F |r,c:12,14,t:9,TLB:49:48:43.44.45:47.40.41| Show InChI InChI=1S/C47H59BF2N8O4/c1-31-39(32(2)57-43(31)30-37-19-23-41(58(37)48(57,49)50)33-17-20-38(62-4)21-18-33)22-24-45(60)51-25-9-5-6-16-44(59)52-26-11-27-56-42-15-8-7-14-40(42)46(54-56)47(61)53-34-28-35-12-10-13-36(29-34)55(35)3/h7-8,14-15,17-21,23,30,34-36H,5-6,9-13,16,22,24-29H2,1-4H3,(H,51,60)(H,52,59)(H,53,61)/t35-,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bern
Curated by ChEMBL
| Assay Description
Displacement of [3H]granisetron from human 5HT3A expressed in HEK293 cells after 1 hr by scintillation counting |
Bioorg Med Chem Lett 22: 1151-5 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.097
BindingDB Entry DOI: 10.7270/Q237795K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50017231
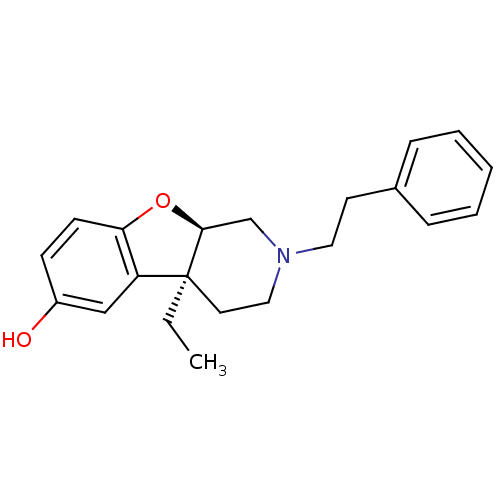
((4aS,9aR)-4a-Ethyl-2-phenethyl-1,2,3,4,4a,9a-hexah...)Show SMILES CC[C@@]12CCN(CCc3ccccc3)C[C@@H]1Oc1ccc(O)cc21 Show InChI InChI=1S/C21H25NO2/c1-2-21-11-13-22(12-10-16-6-4-3-5-7-16)15-20(21)24-19-9-8-17(23)14-18(19)21/h3-9,14,20,23H,2,10-13,15H2,1H3/t20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
Binding affinity for mouse opioid receptor mu |
J Med Chem 32: 2221-6 (1989)
BindingDB Entry DOI: 10.7270/Q2Q240T3 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(RAT) | BDBM50127438

(1-(2-Oxo-5-phenethyl-1-propyl-2,3-dihydro-1H-benzo...)Show SMILES CCCN1c2ccccc2C(CCc2ccccc2)=NC(NC(=O)Nc2ccc(cc2)N2CCN(CC2)c2ccncc2)C1=O |c:20| Show InChI InChI=1S/C36H39N7O2/c1-2-22-43-33-11-7-6-10-31(33)32(17-12-27-8-4-3-5-9-27)39-34(35(43)44)40-36(45)38-28-13-15-29(16-14-28)41-23-25-42(26-24-41)30-18-20-37-21-19-30/h3-11,13-16,18-21,34H,2,12,17,22-26H2,1H3,(H2,38,40,45) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at rat bradykinin B1 receptor |
Bioorg Med Chem Lett 18: 5027-31 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.014
BindingDB Entry DOI: 10.7270/Q2NG4QDX |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50093176

(CHEMBL308763 | Derivative of piperazine-1-carboxyl...)Show SMILES N[C@H]1CC[C@H](CNC(=O)N2CCN(CC2)C(=O)O[C@H]2CCC[C@H](CCC2)OC(=O)N2CCN(CC2)C(=O)NCc2ccc(cc2)N=C(N)N)CC1 |wU:22.27,18.18,4.4,wD:1.0,(30.03,-3.88,;28.7,-3.11,;28.68,-1.56,;27.35,-.8,;26.02,-1.57,;24.69,-.8,;23.36,-1.57,;22.03,-.79,;22.03,.75,;20.7,-1.56,;20.7,-3.09,;19.37,-3.86,;18.04,-3.09,;18.04,-1.55,;19.37,-.78,;16.69,-3.86,;16.69,-5.4,;15.36,-3.09,;13.95,-3.69,;13.95,-5.23,;12.86,-6.32,;11.32,-6.32,;10.23,-5.23,;10.23,-3.69,;11.32,-2.61,;12.86,-2.61,;8.8,-5.82,;7.47,-6.59,;7.47,-8.13,;6.14,-5.82,;4.81,-6.59,;3.48,-5.82,;3.48,-4.28,;4.79,-3.51,;6.14,-4.28,;2.13,-3.52,;2.13,-1.98,;.8,-4.29,;-.53,-3.53,;-1.86,-4.3,;-3.2,-3.53,;-4.53,-4.32,;-4.53,-5.86,;-3.2,-6.63,;-1.86,-5.86,;-5.87,-6.63,;-7.2,-5.86,;-7.2,-4.32,;-8.53,-6.63,;26.02,-3.11,;27.35,-3.88,)| Show InChI InChI=1S/C35H56N10O6/c36-27-11-7-25(8-12-27)23-39-32(46)42-15-19-44(20-16-42)34(48)50-29-3-1-5-30(6-2-4-29)51-35(49)45-21-17-43(18-22-45)33(47)40-24-26-9-13-28(14-10-26)41-31(37)38/h9-10,13-14,25,27,29-30H,1-8,11-12,15-24,36H2,(H,39,46)(H,40,47)(H4,37,38,41)/t25-,27-,29-,30+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Evaluated for its inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2361-6 (2001)
BindingDB Entry DOI: 10.7270/Q2HX1BWR |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Rattus norvegicus (rat)) | BDBM35723

(CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...)Show SMILES Cc1ccccc1C(=O)Nc1ccc(C(=O)N2CCCC(O)c3cc(Cl)ccc23)c(C)c1 Show InChI InChI=1S/C26H25ClN2O3/c1-16-6-3-4-7-20(16)25(31)28-19-10-11-21(17(2)14-19)26(32)29-13-5-8-24(30)22-15-18(27)9-12-23(22)29/h3-4,6-7,9-12,14-15,24,30H,5,8,13H2,1-2H3,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 301: 322-32 (2002)
Article DOI: 10.1124/jpet.301.1.322
BindingDB Entry DOI: 10.7270/Q2MP51VM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50403100
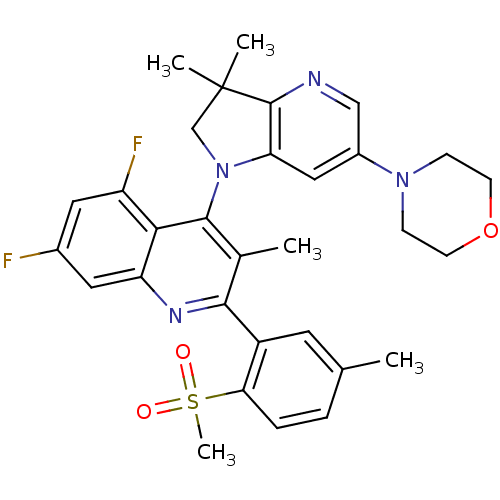
(CHEMBL2216891 | US8765940, 4-(3,3-dimethyl-6-(4-mo...)Show SMILES Cc1ccc(c(c1)-c1nc2cc(F)cc(F)c2c(N2CC(C)(C)c3ncc(cc23)N2CCOCC2)c1C)S(C)(=O)=O Show InChI InChI=1S/C31H32F2N4O3S/c1-18-6-7-26(41(5,38)39)22(12-18)28-19(2)29(27-23(33)13-20(32)14-24(27)35-28)37-17-31(3,4)30-25(37)15-21(16-34-30)36-8-10-40-11-9-36/h6-7,12-16H,8-11,17H2,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 1.05 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Amgen Inc.
US Patent
| Assay Description
A PI3K Alphascreen assay (PerkinElmer, Waltham, Mass.) was used to measure the activity of a panel of four phosphoinositide 3-kinases: PI3Kα, PI... |
US Patent US8765940 (2014)
BindingDB Entry DOI: 10.7270/Q2WW7G94 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50363287
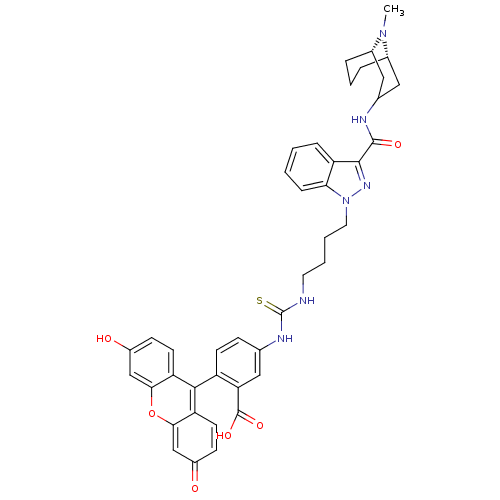
(CHEMBL1945830)Show SMILES CN1[C@H]2CCC[C@H]1CC(C2)NC(=O)c1nn(CCCCNC(=S)Nc2ccc(c(c2)C(O)=O)-c2c3ccc(O)cc3oc3cc(=O)ccc23)c2ccccc12 |r,wU:2.1,6.6,TLB:0:1:3.4.5:7.8.9,(1.85,-18.97,;1.02,-20.27,;.73,-21.74,;.75,-23.57,;-.77,-23.85,;.03,-22.8,;-.02,-21.04,;-1.71,-20.98,;-2,-22.45,;-1.05,-21.7,;-2.48,-23.91,;-3.98,-24.23,;-5.02,-23.09,;-4.46,-25.7,;-3.55,-26.95,;-4.46,-28.21,;-3.69,-29.54,;-2.15,-29.54,;-1.38,-30.88,;.16,-30.88,;.93,-32.21,;2.46,-32.22,;3.19,-30.86,;3.28,-33.53,;2.55,-34.89,;1.02,-34.94,;.29,-36.29,;1.11,-37.6,;2.65,-37.55,;3.37,-36.19,;3.47,-38.85,;5.01,-38.79,;3.93,-40.31,;.39,-38.96,;1.21,-40.26,;2.74,-40.21,;3.55,-41.53,;2.81,-42.89,;3.62,-44.2,;1.27,-42.92,;.47,-41.62,;-1.07,-41.67,;-1.88,-40.36,;-3.4,-40.41,;-4.2,-39.1,;-5.74,-39.14,;-3.48,-37.76,;-1.95,-37.72,;-1.15,-39.02,;-5.94,-27.73,;-7.27,-28.5,;-8.6,-27.73,;-8.6,-26.19,;-7.27,-25.41,;-5.94,-26.18,)| Show InChI InChI=1S/C42H42N6O6S/c1-47-26-7-6-8-27(47)20-25(19-26)44-40(51)39-31-9-2-3-10-35(31)48(46-39)18-5-4-17-43-42(55)45-24-11-14-30(34(21-24)41(52)53)38-32-15-12-28(49)22-36(32)54-37-23-29(50)13-16-33(37)38/h2-3,9-16,21-23,25-27,49H,4-8,17-20H2,1H3,(H,44,51)(H,52,53)(H2,43,45,55)/t26-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bern
Curated by ChEMBL
| Assay Description
Displacement of [3H]granisetron from human 5HT3A expressed in HEK293 cells after 1 hr by scintillation counting |
Bioorg Med Chem Lett 22: 1151-5 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.097
BindingDB Entry DOI: 10.7270/Q237795K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50363291

(CHEMBL1945835)Show SMILES CN(C)c1cccc2c(cccc12)S(=O)(=O)NCCCn1nc(C(=O)NC2C[C@@H]3CCC[C@@H](C2)N3C)c2ccccc12 |r,TLB:35:34:29.30.31:33.26.27| Show InChI InChI=1S/C32H40N6O3S/c1-36(2)28-16-7-14-26-25(28)13-8-17-30(26)42(40,41)33-18-9-19-38-29-15-5-4-12-27(29)31(35-38)32(39)34-22-20-23-10-6-11-24(21-22)37(23)3/h4-5,7-8,12-17,22-24,33H,6,9-11,18-21H2,1-3H3,(H,34,39)/t23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bern
Curated by ChEMBL
| Assay Description
Displacement of [3H]granisetron from human 5HT3A expressed in HEK293 cells after 1 hr by scintillation counting |
Bioorg Med Chem Lett 22: 1151-5 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.097
BindingDB Entry DOI: 10.7270/Q237795K |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(MOUSE) | BDBM35723

(CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...)Show SMILES Cc1ccccc1C(=O)Nc1ccc(C(=O)N2CCCC(O)c3cc(Cl)ccc23)c(C)c1 Show InChI InChI=1S/C26H25ClN2O3/c1-16-6-3-4-7-20(16)25(31)28-19-10-11-21(17(2)14-19)26(32)29-13-5-8-24(30)22-15-18(27)9-12-23(22)29/h3-4,6-7,9-12,14-15,24,30H,5,8,13H2,1-2H3,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 301: 322-32 (2002)
Article DOI: 10.1124/jpet.301.1.322
BindingDB Entry DOI: 10.7270/Q2MP51VM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM124797
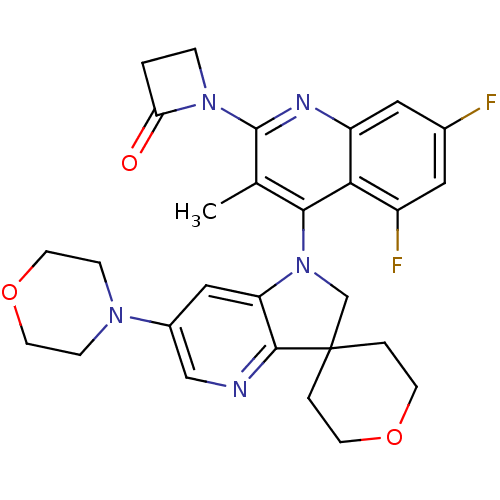
(US8765940, 1-(5,7-difluoro-3-methyl-4-(6'-(4-morph...)Show SMILES Cc1c(nc2cc(F)cc(F)c2c1N1CC2(CCOCC2)c2ncc(cc12)N1CCOCC1)N1CCC1=O Show InChI InChI=1S/C28H29F2N5O3/c1-17-25(24-20(30)12-18(29)13-21(24)32-27(17)34-5-2-23(34)36)35-16-28(3-8-37-9-4-28)26-22(35)14-19(15-31-26)33-6-10-38-11-7-33/h12-15H,2-11,16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.27 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Amgen Inc.
US Patent
| Assay Description
A PI3K Alphascreen assay (PerkinElmer, Waltham, Mass.) was used to measure the activity of a panel of four phosphoinositide 3-kinases: PI3Kα, PI... |
US Patent US8765940 (2014)
BindingDB Entry DOI: 10.7270/Q2WW7G94 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50017232
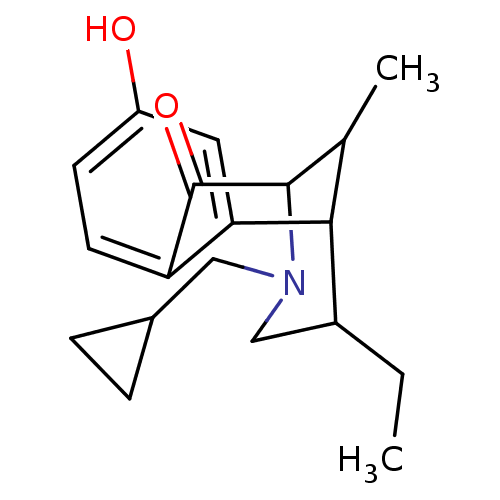
(3-Cyclopropylmethyl-5-ethyl-8-hydroxy-11-methyl-3,...)Show SMILES CCC1CN(CC2CC2)C2C(C)C1c1cc(O)ccc1C2=O |TLB:11:10:20.13.19:4.2.3| Show InChI InChI=1S/C19H25NO2/c1-3-13-10-20(9-12-4-5-12)18-11(2)17(13)16-8-14(21)6-7-15(16)19(18)22/h6-8,11-13,17-18,21H,3-5,9-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
Binding affinity for mouse opioid receptor mu |
J Med Chem 32: 2221-6 (1989)
BindingDB Entry DOI: 10.7270/Q2Q240T3 |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 1122-30 (2002)
Article DOI: 10.1124/jpet.300.3.1122
BindingDB Entry DOI: 10.7270/Q20V8BBW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM124781
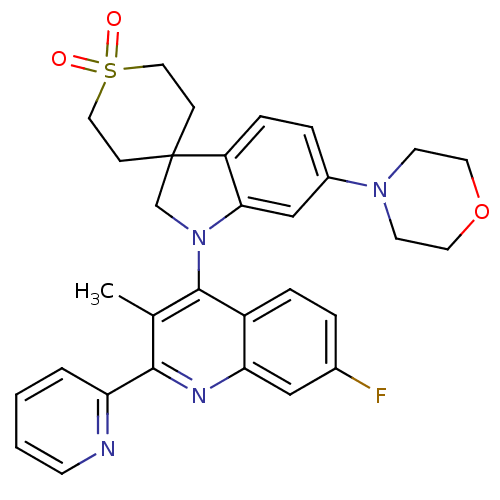
(US8765940, 1-(7-fluoro-3-methyl-2-(2-pyridinyl)-4-...)Show SMILES Cc1c(nc2cc(F)ccc2c1N1CC2(CCS(=O)(=O)CC2)c2ccc(cc12)N1CCOCC1)-c1ccccn1 Show InChI InChI=1S/C31H31FN4O3S/c1-21-29(26-4-2-3-11-33-26)34-27-18-22(32)5-7-24(27)30(21)36-20-31(9-16-40(37,38)17-10-31)25-8-6-23(19-28(25)36)35-12-14-39-15-13-35/h2-8,11,18-19H,9-10,12-17,20H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.37 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Amgen Inc.
US Patent
| Assay Description
A PI3K Alphascreen assay (PerkinElmer, Waltham, Mass.) was used to measure the activity of a panel of four phosphoinositide 3-kinases: PI3Kα, PI... |
US Patent US8765940 (2014)
BindingDB Entry DOI: 10.7270/Q2WW7G94 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM198694
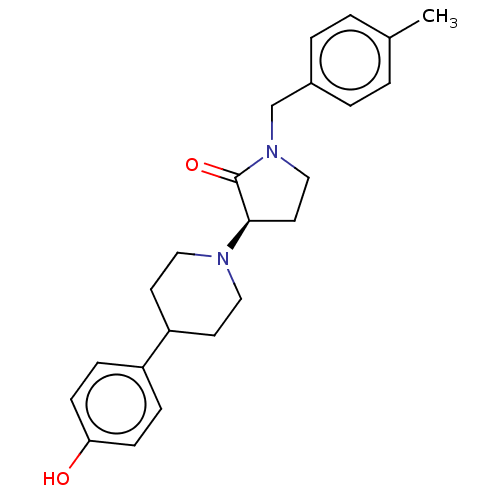
(US9221796, 23b)Show SMILES Cc1ccc(CN2CC[C@@H](N3CCC(CC3)c3ccc(O)cc3)C2=O)cc1 |r| Show InChI InChI=1S/C23H28N2O2/c1-17-2-4-18(5-3-17)16-25-15-12-22(23(25)27)24-13-10-20(11-14-24)19-6-8-21(26)9-7-19/h2-9,20,22,26H,10-16H2,1H3/t22-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM198665
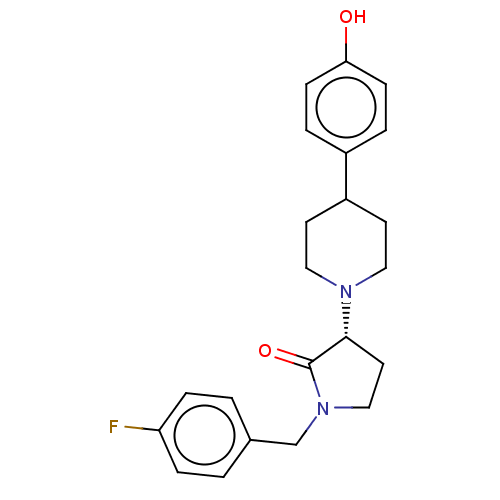
(US9221796, 2b)Show SMILES Oc1ccc(cc1)C1CCN(CC1)[C@@H]1CCN(Cc2ccc(F)cc2)C1=O |r| Show InChI InChI=1S/C22H25FN2O2/c23-19-5-1-16(2-6-19)15-25-14-11-21(22(25)27)24-12-9-18(10-13-24)17-3-7-20(26)8-4-17/h1-8,18,21,26H,9-15H2/t21-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data