

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50346873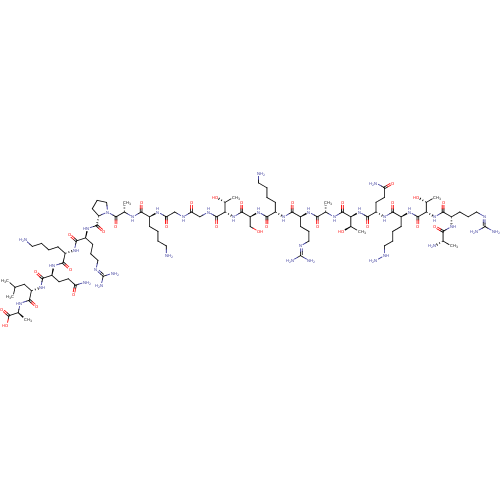 (CHEMBL1797652) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (178 to 831) (unknown origin) expressed in baculovirus infected insect Sf9 cells using diMeK4H3-21 as substrate by per... | ACS Med Chem Lett 5: 29-33 (2014) Article DOI: 10.1021/ml4002997 BindingDB Entry DOI: 10.7270/Q2HT2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50446141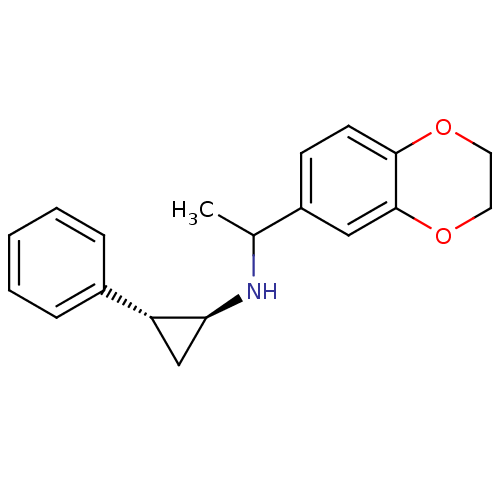 (CHEMBL3108901) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Inhibition of human recombinant LSD1 using di-methylated H3-K4 peptide as substrate assessed as release of H2O2 preincubated for 15 mins followed by ... | ACS Med Chem Lett 5: 29-33 (2014) Article DOI: 10.1021/ml4002997 BindingDB Entry DOI: 10.7270/Q2HT2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM101302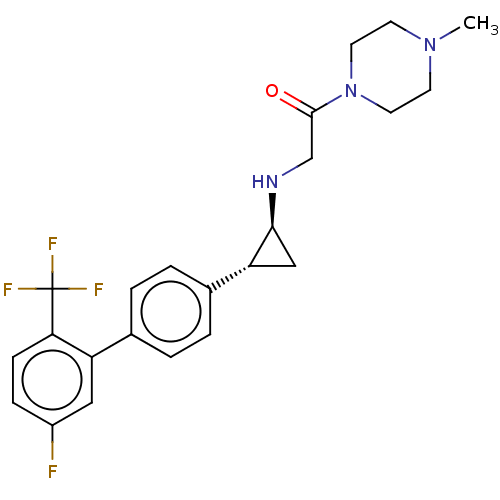 (CHEMBL3108900 | US8524717, 57) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Inhibition of human recombinant LSD1 using di-methylated H3-K4 peptide as substrate assessed as release of H2O2 preincubated for 15 mins followed by ... | ACS Med Chem Lett 5: 29-33 (2014) Article DOI: 10.1021/ml4002997 BindingDB Entry DOI: 10.7270/Q2HT2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50346870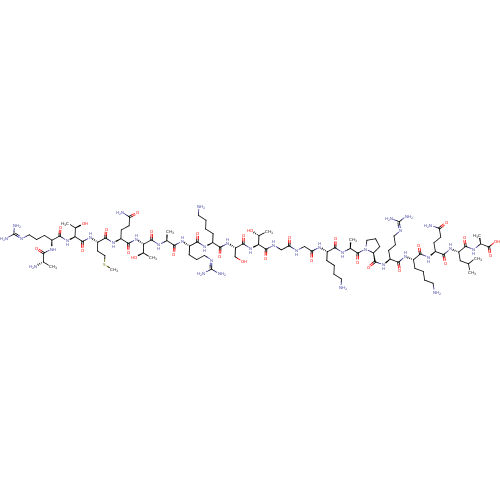 (CHEMBL1797647) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Inhibition of recombinant human LSD1 expressed in Escherichia coli using methylated H3-K4 peptide as substrate by peroxidase-coupled assay | ACS Med Chem Lett 5: 29-33 (2014) Article DOI: 10.1021/ml4002997 BindingDB Entry DOI: 10.7270/Q2HT2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| REST corepressor 1 (Homo sapiens (Human)) | BDBM50458057 (CHEMBL4210908) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Inhibition of LSD1/CoREST (unknown origin) | Eur J Med Chem 148: 210-220 (2018) Article DOI: 10.1016/j.ejmech.2018.01.098 BindingDB Entry DOI: 10.7270/Q2N0195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A/REST corepressor 1 [4-485] (Homo sapiens (Human)) | BDBM50346870 (CHEMBL1797647) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Inhibition of recombinant human LSD1/CoREST complex expressed in Escherichia coli using methylated H3-K4 peptide as substrate by peroxidase-coupled a... | ACS Med Chem Lett 5: 29-33 (2014) Article DOI: 10.1021/ml4002997 BindingDB Entry DOI: 10.7270/Q2HT2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50346875 (CHEMBL1797648) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (178 to 831) (unknown origin) expressed in baculovirus infected insect Sf9 cells using diMeK4H3-21 as substrate by per... | ACS Med Chem Lett 5: 29-33 (2014) Article DOI: 10.1021/ml4002997 BindingDB Entry DOI: 10.7270/Q2HT2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50446142 (CHEMBL3108892) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (unknown origin) using di-methylated H3-K4 peptide as substrate incubated for 5 mins prior to substrate addition by fl... | ACS Med Chem Lett 5: 29-33 (2014) Article DOI: 10.1021/ml4002997 BindingDB Entry DOI: 10.7270/Q2HT2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50446143 (CHEMBL3108897) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 385 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Competitive inhibition of recombinant LSD1 (unknown origin) using H3K4Me2 peptide as substrate preincubated for 30 mins followed by substrate additio... | ACS Med Chem Lett 5: 29-33 (2014) Article DOI: 10.1021/ml4002997 BindingDB Entry DOI: 10.7270/Q2HT2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50346869 (CHEMBL1797646) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Competitive inhibition of recombinant LSD1 (unknown origin) expressed in Escherichia coli BL21 (DE3) cells using H3K4 peptide as substrate by Dixon p... | ACS Med Chem Lett 5: 29-33 (2014) Article DOI: 10.1021/ml4002997 BindingDB Entry DOI: 10.7270/Q2HT2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6348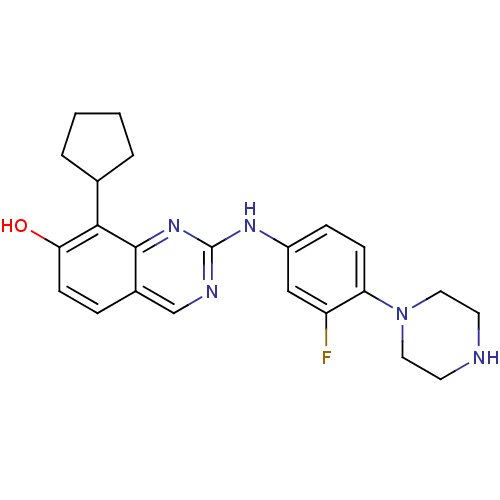 (2-Aminoquinazoline 27 | 8-cyclopentyl-2-{[3-fluoro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
NAEJA Pharmaceutical Inc. | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 25 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | Bioorg Med Chem Lett 15: 3881-5 (2005) Article DOI: 10.1016/j.bmcl.2005.05.131 BindingDB Entry DOI: 10.7270/Q2WW7FVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6341 (2-Aminoquinazoline 20 | 8-cyclopentyl-7-methoxy-N-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
NAEJA Pharmaceutical Inc. | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 25 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | Bioorg Med Chem Lett 15: 3881-5 (2005) Article DOI: 10.1016/j.bmcl.2005.05.131 BindingDB Entry DOI: 10.7270/Q2WW7FVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6347 (2-Aminoquinazoline 26 | 8-cyclopentyl-2-{[4-(piper...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
NAEJA Pharmaceutical Inc. | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 25 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | Bioorg Med Chem Lett 15: 3881-5 (2005) Article DOI: 10.1016/j.bmcl.2005.05.131 BindingDB Entry DOI: 10.7270/Q2WW7FVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6344 (2-Aminoquinazoline 23 | 2-amino-1-(4-{4-[(8-cyclop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
NAEJA Pharmaceutical Inc. | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 25 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | Bioorg Med Chem Lett 15: 3881-5 (2005) Article DOI: 10.1016/j.bmcl.2005.05.131 BindingDB Entry DOI: 10.7270/Q2WW7FVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6343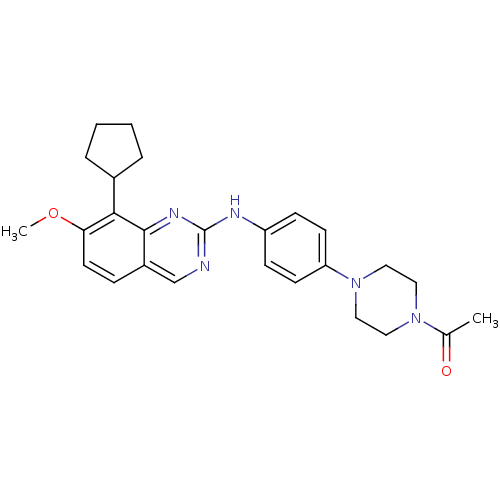 (1-(4-{4-[(8-cyclopentyl-7-methoxyquinazolin-2-yl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
NAEJA Pharmaceutical Inc. | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 25 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | Bioorg Med Chem Lett 15: 3881-5 (2005) Article DOI: 10.1016/j.bmcl.2005.05.131 BindingDB Entry DOI: 10.7270/Q2WW7FVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6346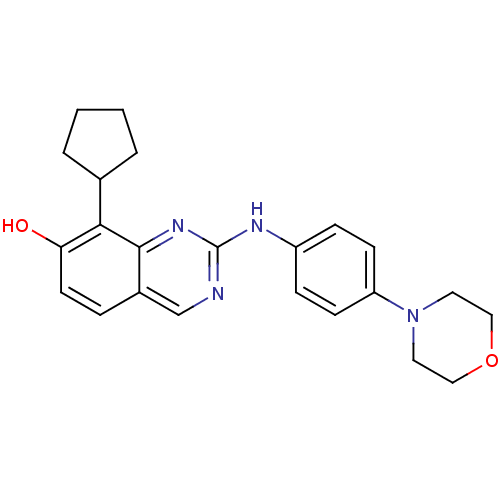 (2-Aminoquinazoline 25 | 8-cyclopentyl-2-{[4-(morph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
NAEJA Pharmaceutical Inc. | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 25 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | Bioorg Med Chem Lett 15: 3881-5 (2005) Article DOI: 10.1016/j.bmcl.2005.05.131 BindingDB Entry DOI: 10.7270/Q2WW7FVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6342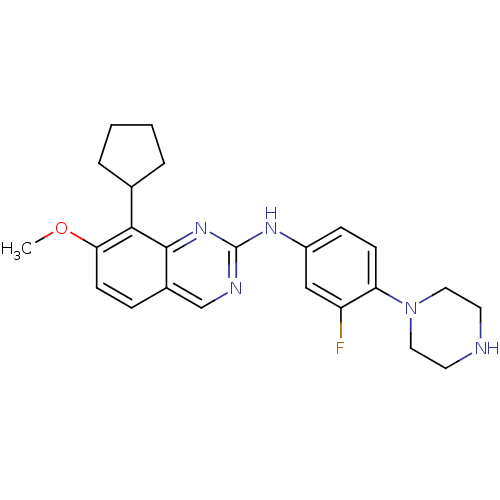 (2-Aminoquinazoline 21 | 8-cyclopentyl-N-[3-fluoro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
NAEJA Pharmaceutical Inc. | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 25 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | Bioorg Med Chem Lett 15: 3881-5 (2005) Article DOI: 10.1016/j.bmcl.2005.05.131 BindingDB Entry DOI: 10.7270/Q2WW7FVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6334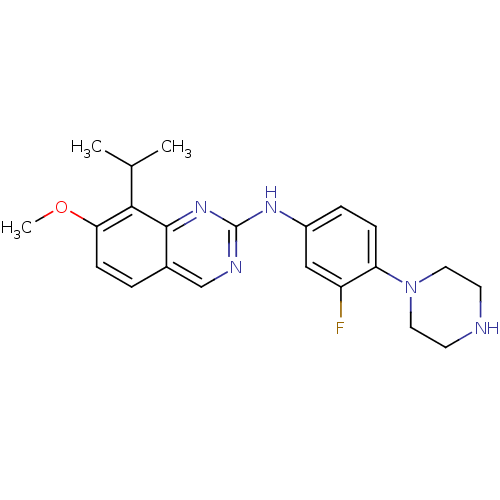 (2-Aminoquinazoline 12 | N-[3-fluoro-4-(piperazin-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
NAEJA Pharmaceutical Inc. | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 25 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | Bioorg Med Chem Lett 15: 3881-5 (2005) Article DOI: 10.1016/j.bmcl.2005.05.131 BindingDB Entry DOI: 10.7270/Q2WW7FVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6335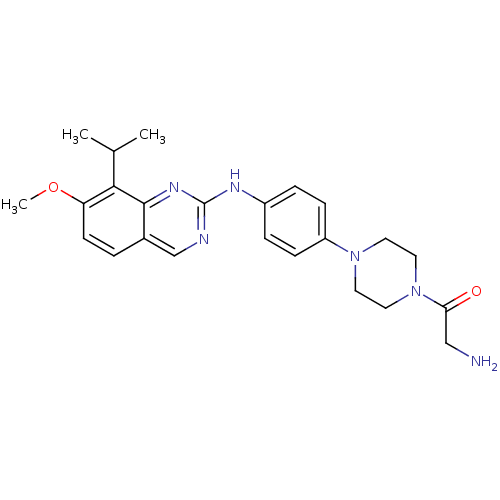 (2-Aminoquinazoline 14 | 2-amino-1-[4-(4-{[7-methox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
NAEJA Pharmaceutical Inc. | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 25 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | Bioorg Med Chem Lett 15: 3881-5 (2005) Article DOI: 10.1016/j.bmcl.2005.05.131 BindingDB Entry DOI: 10.7270/Q2WW7FVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6339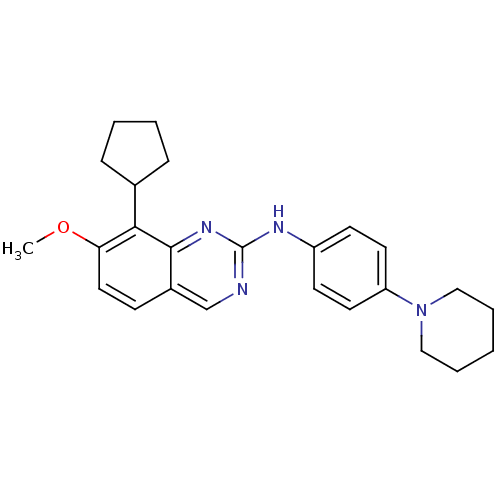 (2-Aminoquinazoline 18 | 8-cyclopentyl-7-methoxy-N-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
NAEJA Pharmaceutical Inc. | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 25 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | Bioorg Med Chem Lett 15: 3881-5 (2005) Article DOI: 10.1016/j.bmcl.2005.05.131 BindingDB Entry DOI: 10.7270/Q2WW7FVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6330 (2-Aminoquinazoline 8 | N-[4-(3-aminopyrrolidin-1-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
NAEJA Pharmaceutical Inc. | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 25 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | Bioorg Med Chem Lett 15: 3881-5 (2005) Article DOI: 10.1016/j.bmcl.2005.05.131 BindingDB Entry DOI: 10.7270/Q2WW7FVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6352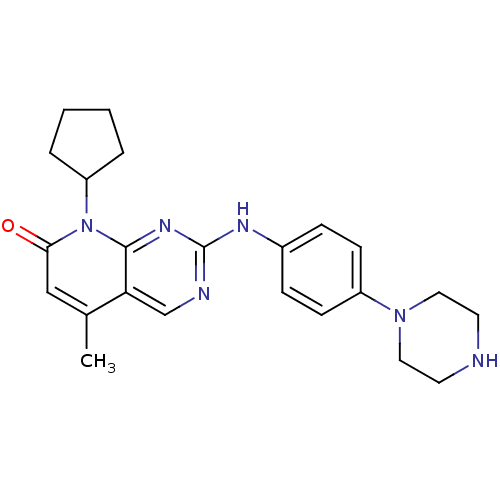 (2-Aminoquinazoline 31 | 8-cyclopentyl-5-methyl-2-{...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
NAEJA Pharmaceutical Inc. | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 25 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | Bioorg Med Chem Lett 15: 3881-5 (2005) Article DOI: 10.1016/j.bmcl.2005.05.131 BindingDB Entry DOI: 10.7270/Q2WW7FVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6333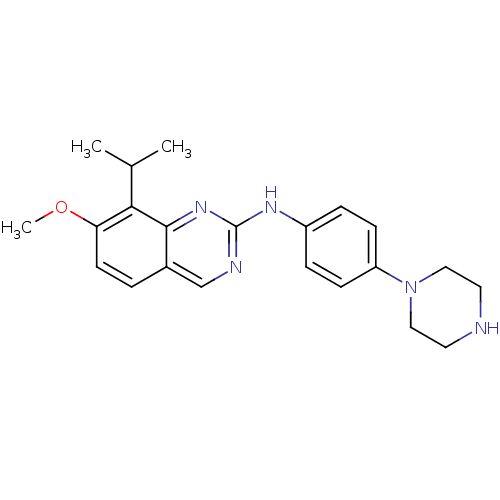 (2-Aminoquinazoline 11 | 7-methoxy-N-[4-(piperazin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
NAEJA Pharmaceutical Inc. | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 25 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | Bioorg Med Chem Lett 15: 3881-5 (2005) Article DOI: 10.1016/j.bmcl.2005.05.131 BindingDB Entry DOI: 10.7270/Q2WW7FVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6340 (2-Aminoquinazoline 19 | 8-cyclopentyl-7-methoxy-N-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
NAEJA Pharmaceutical Inc. | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 25 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | Bioorg Med Chem Lett 15: 3881-5 (2005) Article DOI: 10.1016/j.bmcl.2005.05.131 BindingDB Entry DOI: 10.7270/Q2WW7FVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6351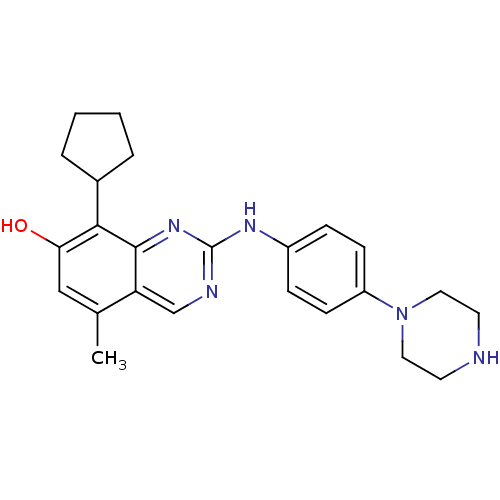 (2-Aminoquinazoline 30 | 8-cyclopentyl-5-methyl-2-{...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
NAEJA Pharmaceutical Inc. | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 25 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | Bioorg Med Chem Lett 15: 3881-5 (2005) Article DOI: 10.1016/j.bmcl.2005.05.131 BindingDB Entry DOI: 10.7270/Q2WW7FVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6353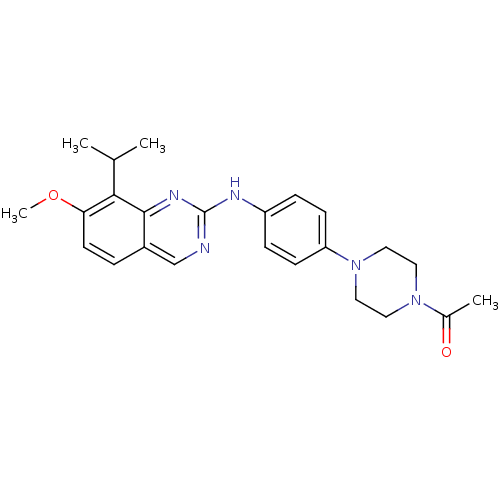 (1-[4-(4-{[7-methoxy-8-(propan-2-yl)quinazolin-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
NAEJA Pharmaceutical Inc. | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 25 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | Bioorg Med Chem Lett 15: 3881-5 (2005) Article DOI: 10.1016/j.bmcl.2005.05.131 BindingDB Entry DOI: 10.7270/Q2WW7FVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM6341 (2-Aminoquinazoline 20 | 8-cyclopentyl-7-methoxy-N-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
NAEJA Pharmaceutical Inc. | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 12 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | Bioorg Med Chem Lett 15: 3881-5 (2005) Article DOI: 10.1016/j.bmcl.2005.05.131 BindingDB Entry DOI: 10.7270/Q2WW7FVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1 (Homo sapiens (Human)) | BDBM6347 (2-Aminoquinazoline 26 | 8-cyclopentyl-2-{[4-(piper...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
NAEJA Pharmaceutical Inc. | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 12 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | Bioorg Med Chem Lett 15: 3881-5 (2005) Article DOI: 10.1016/j.bmcl.2005.05.131 BindingDB Entry DOI: 10.7270/Q2WW7FVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Inhibition of COX1-mediated PGH2 production by enzyme immunoassay | Bioorg Med Chem 17: 5044-53 (2009) Article DOI: 10.1016/j.bmc.2009.05.065 BindingDB Entry DOI: 10.7270/Q2X92BCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6331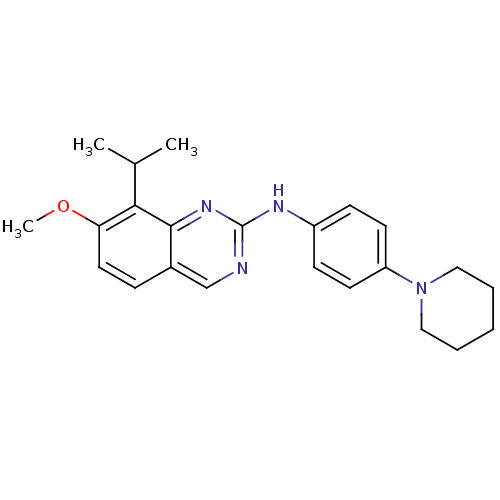 (2-Aminoquinazoline 9 | 7-methoxy-N-[4-(piperidin-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
NAEJA Pharmaceutical Inc. | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 25 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | Bioorg Med Chem Lett 15: 3881-5 (2005) Article DOI: 10.1016/j.bmcl.2005.05.131 BindingDB Entry DOI: 10.7270/Q2WW7FVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6332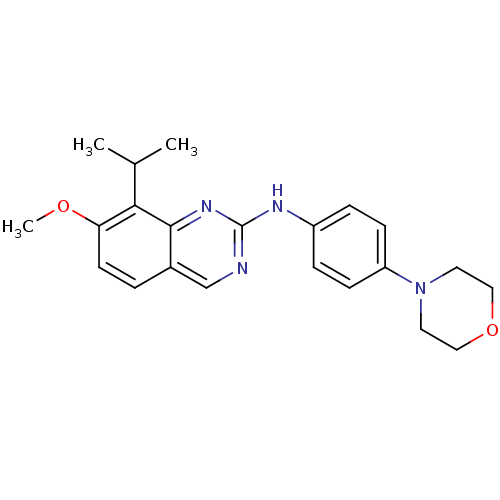 (2-Aminoquinazoline 10 | 7-methoxy-N-[4-(morpholin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
NAEJA Pharmaceutical Inc. | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 25 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | Bioorg Med Chem Lett 15: 3881-5 (2005) Article DOI: 10.1016/j.bmcl.2005.05.131 BindingDB Entry DOI: 10.7270/Q2WW7FVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6349 (2-Aminoquinazoline 28 | 8-cyclopentyl-7-methoxy-5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
NAEJA Pharmaceutical Inc. | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 25 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | Bioorg Med Chem Lett 15: 3881-5 (2005) Article DOI: 10.1016/j.bmcl.2005.05.131 BindingDB Entry DOI: 10.7270/Q2WW7FVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM6342 (2-Aminoquinazoline 21 | 8-cyclopentyl-N-[3-fluoro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
NAEJA Pharmaceutical Inc. | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 12 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | Bioorg Med Chem Lett 15: 3881-5 (2005) Article DOI: 10.1016/j.bmcl.2005.05.131 BindingDB Entry DOI: 10.7270/Q2WW7FVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM6347 (2-Aminoquinazoline 26 | 8-cyclopentyl-2-{[4-(piper...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
NAEJA Pharmaceutical Inc. | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 12 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | Bioorg Med Chem Lett 15: 3881-5 (2005) Article DOI: 10.1016/j.bmcl.2005.05.131 BindingDB Entry DOI: 10.7270/Q2WW7FVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM6344 (2-Aminoquinazoline 23 | 2-amino-1-(4-{4-[(8-cyclop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
NAEJA Pharmaceutical Inc. | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 12 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | Bioorg Med Chem Lett 15: 3881-5 (2005) Article DOI: 10.1016/j.bmcl.2005.05.131 BindingDB Entry DOI: 10.7270/Q2WW7FVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| REST corepressor 1 (Homo sapiens (Human)) | BDBM50458056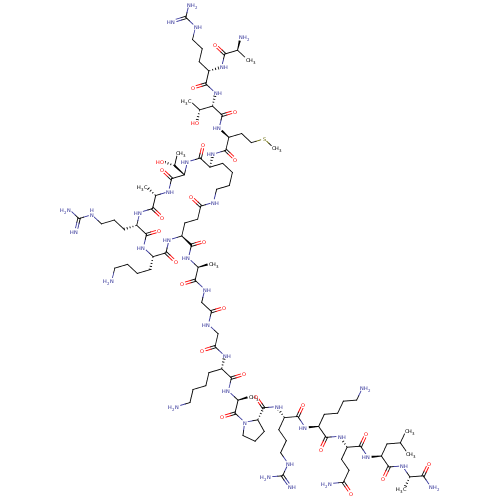 (CHEMBL4208031) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Inhibition of recombinant human LSD1/CoREST preincubated for 5 mins followed by ART-(N,N-dimethyl-K)-QTARKSTGGKAPRKQLA substrate addition measured af... | Eur J Med Chem 148: 210-220 (2018) Article DOI: 10.1016/j.ejmech.2018.01.098 BindingDB Entry DOI: 10.7270/Q2N0195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| REST corepressor 1 (Homo sapiens (Human)) | BDBM50458056 (CHEMBL4208031) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Inhibition of recombinant human LSD1/CoREST preincubated for 5 mins followed by ART-(N,N-dimethyl-K)-QTARKSTGGKAPRKQLA substrate addition measured af... | Eur J Med Chem 148: 210-220 (2018) Article DOI: 10.1016/j.ejmech.2018.01.098 BindingDB Entry DOI: 10.7270/Q2N0195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM6348 (2-Aminoquinazoline 27 | 8-cyclopentyl-2-{[3-fluoro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
NAEJA Pharmaceutical Inc. | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 12 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | Bioorg Med Chem Lett 15: 3881-5 (2005) Article DOI: 10.1016/j.bmcl.2005.05.131 BindingDB Entry DOI: 10.7270/Q2WW7FVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6338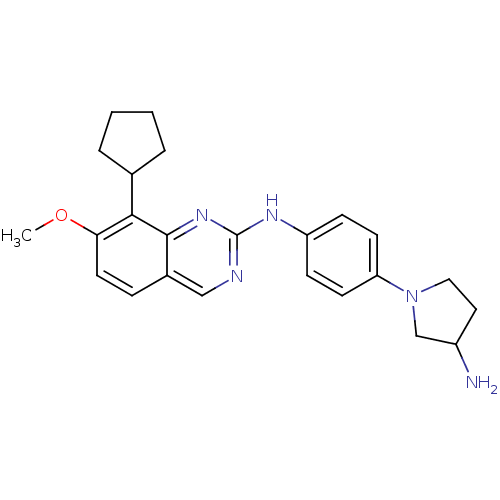 (2-Aminoquinazoline 17 | N-[4-(3-aminopyrrolidin-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
NAEJA Pharmaceutical Inc. | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 25 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | Bioorg Med Chem Lett 15: 3881-5 (2005) Article DOI: 10.1016/j.bmcl.2005.05.131 BindingDB Entry DOI: 10.7270/Q2WW7FVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6328 (2-Aminoquinazoline 6 | 7-methoxy-N-phenyl-8-(propa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
NAEJA Pharmaceutical Inc. | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 25 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | Bioorg Med Chem Lett 15: 3881-5 (2005) Article DOI: 10.1016/j.bmcl.2005.05.131 BindingDB Entry DOI: 10.7270/Q2WW7FVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1 (Homo sapiens (Human)) | BDBM6341 (2-Aminoquinazoline 20 | 8-cyclopentyl-7-methoxy-N-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 132 | n/a | n/a | n/a | n/a | 7.4 | 25 |
NAEJA Pharmaceutical Inc. | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 12 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | Bioorg Med Chem Lett 15: 3881-5 (2005) Article DOI: 10.1016/j.bmcl.2005.05.131 BindingDB Entry DOI: 10.7270/Q2WW7FVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| REST corepressor 1 (Homo sapiens (Human)) | BDBM50458055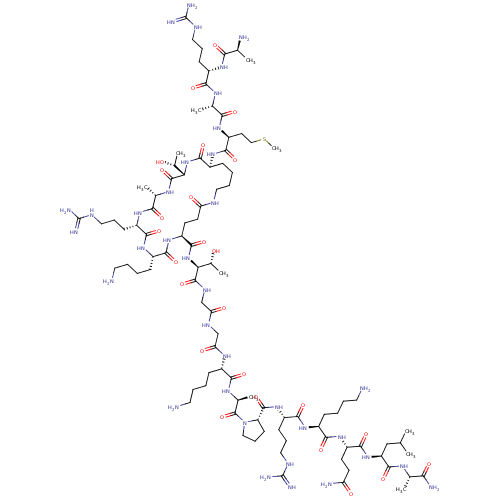 (CHEMBL4214605) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 136 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Inhibition of recombinant human LSD1/CoREST preincubated for 5 mins followed by ART-(N,N-dimethyl-K)-QTARKSTGGKAPRKQLA substrate addition measured af... | Eur J Med Chem 148: 210-220 (2018) Article DOI: 10.1016/j.ejmech.2018.01.098 BindingDB Entry DOI: 10.7270/Q2N0195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| REST corepressor 1 (Homo sapiens (Human)) | BDBM50458055 (CHEMBL4214605) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 136 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Inhibition of recombinant human LSD1/CoREST preincubated for 5 mins followed by ART-(N,N-dimethyl-K)-QTARKSTGGKAPRKQLA substrate addition measured af... | Eur J Med Chem 148: 210-220 (2018) Article DOI: 10.1016/j.ejmech.2018.01.098 BindingDB Entry DOI: 10.7270/Q2N0195V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM6334 (2-Aminoquinazoline 12 | N-[3-fluoro-4-(piperazin-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a | n/a |
NAEJA Pharmaceutical Inc. | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 12 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | Bioorg Med Chem Lett 15: 3881-5 (2005) Article DOI: 10.1016/j.bmcl.2005.05.131 BindingDB Entry DOI: 10.7270/Q2WW7FVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6336 (2-Aminoquinazoline 15 | 8-cyclopentyl-7-methoxy-N-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 166 | n/a | n/a | n/a | n/a | n/a | n/a |
NAEJA Pharmaceutical Inc. | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 25 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | Bioorg Med Chem Lett 15: 3881-5 (2005) Article DOI: 10.1016/j.bmcl.2005.05.131 BindingDB Entry DOI: 10.7270/Q2WW7FVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM6346 (2-Aminoquinazoline 25 | 8-cyclopentyl-2-{[4-(morph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
NAEJA Pharmaceutical Inc. | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 12 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | Bioorg Med Chem Lett 15: 3881-5 (2005) Article DOI: 10.1016/j.bmcl.2005.05.131 BindingDB Entry DOI: 10.7270/Q2WW7FVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM6333 (2-Aminoquinazoline 11 | 7-methoxy-N-[4-(piperazin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 185 | n/a | n/a | n/a | n/a | n/a | n/a |
NAEJA Pharmaceutical Inc. | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 12 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | Bioorg Med Chem Lett 15: 3881-5 (2005) Article DOI: 10.1016/j.bmcl.2005.05.131 BindingDB Entry DOI: 10.7270/Q2WW7FVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM6335 (2-Aminoquinazoline 14 | 2-amino-1-[4-(4-{[7-methox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 191 | n/a | n/a | n/a | n/a | n/a | n/a |
NAEJA Pharmaceutical Inc. | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 12 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | Bioorg Med Chem Lett 15: 3881-5 (2005) Article DOI: 10.1016/j.bmcl.2005.05.131 BindingDB Entry DOI: 10.7270/Q2WW7FVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM6336 (2-Aminoquinazoline 15 | 8-cyclopentyl-7-methoxy-N-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 202 | n/a | n/a | n/a | n/a | n/a | n/a |
NAEJA Pharmaceutical Inc. | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 12 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | Bioorg Med Chem Lett 15: 3881-5 (2005) Article DOI: 10.1016/j.bmcl.2005.05.131 BindingDB Entry DOI: 10.7270/Q2WW7FVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM6338 (2-Aminoquinazoline 17 | N-[4-(3-aminopyrrolidin-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 203 | n/a | n/a | n/a | n/a | n/a | n/a |
NAEJA Pharmaceutical Inc. | Assay Description The enzyme was assayed with substrate GST- retinoblastoma in the presence of 12 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which in... | Bioorg Med Chem Lett 15: 3881-5 (2005) Article DOI: 10.1016/j.bmcl.2005.05.131 BindingDB Entry DOI: 10.7270/Q2WW7FVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 147 total ) | Next | Last >> |