 Found 306 hits with Last Name = 'sinha-roy' and Initial = 'r'
Found 306 hits with Last Name = 'sinha-roy' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50003020
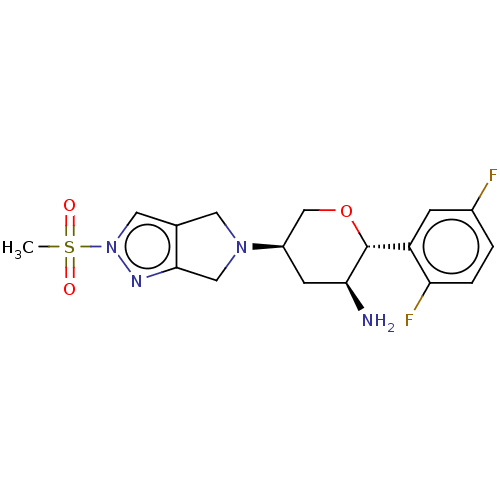
(MK-3102 | OMARIGLIPTIN | US10155775, Omarigliptin ...)Show SMILES CS(=O)(=O)n1cc2CN(Cc2n1)[C@H]1CO[C@@H]([C@@H](N)C1)c1cc(F)ccc1F |r| Show InChI InChI=1S/C17H20F2N4O3S/c1-27(24,25)23-7-10-6-22(8-16(10)21-23)12-5-15(20)17(26-9-12)13-4-11(18)2-3-14(13)19/h2-4,7,12,15,17H,5-6,8-9,20H2,1H3/t12-,15+,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Competitive reversible inhibition of DPP4 (unknown origin) |
J Med Chem 57: 3205-12 (2014)
Article DOI: 10.1021/jm401992e
BindingDB Entry DOI: 10.7270/Q2WD423H |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50170939
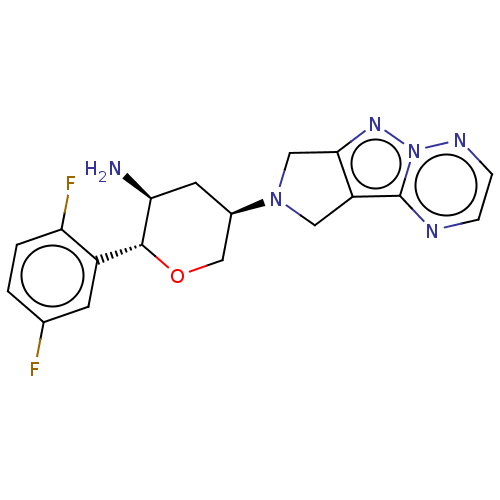
(CHEMBL3806052)Show SMILES N[C@H]1C[C@H](CO[C@@H]1c1cc(F)ccc1F)N1Cc2nn3nccnc3c2C1 |r| Show InChI InChI=1S/C18H18F2N6O/c19-10-1-2-14(20)12(5-10)17-15(21)6-11(9-27-17)25-7-13-16(8-25)24-26-18(13)22-3-4-23-26/h1-5,11,15,17H,6-9,21H2/t11-,15+,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem Lett 26: 2622-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.020
BindingDB Entry DOI: 10.7270/Q2Z321JP |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50170919
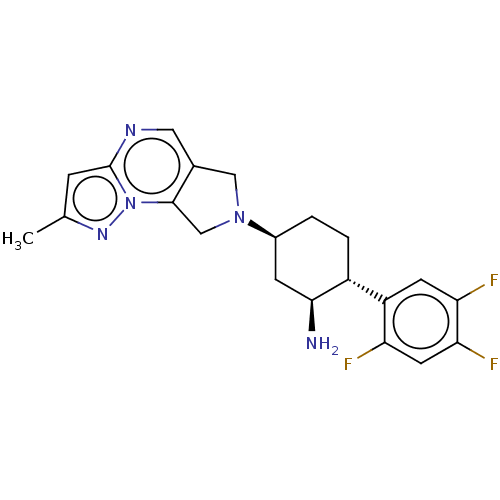
(CHEMBL3805966)Show SMILES Cc1cc2ncc3CN(Cc3n2n1)[C@H]1CC[C@@H]([C@@H](N)C1)c1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C21H22F3N5/c1-11-4-21-26-8-12-9-28(10-20(12)29(21)27-11)13-2-3-14(19(25)5-13)15-6-17(23)18(24)7-16(15)22/h4,6-8,13-14,19H,2-3,5,9-10,25H2,1H3/t13-,14+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem Lett 26: 2622-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.020
BindingDB Entry DOI: 10.7270/Q2Z321JP |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50170922

(CHEMBL3805629)Show SMILES Cc1nc2ncc3CN(Cc3n2n1)[C@H]1CC[C@@H]([C@@H](N)C1)c1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C20H21F3N6/c1-10-26-20-25-7-11-8-28(9-19(11)29(20)27-10)12-2-3-13(18(24)4-12)14-5-16(22)17(23)6-15(14)21/h5-7,12-13,18H,2-4,8-9,24H2,1H3/t12-,13+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem Lett 26: 2622-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.020
BindingDB Entry DOI: 10.7270/Q2Z321JP |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50170923
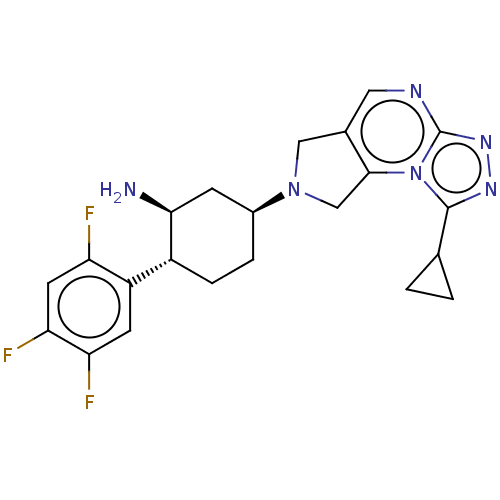
(CHEMBL3806041)Show SMILES N[C@H]1C[C@H](CC[C@@H]1c1cc(F)c(F)cc1F)N1Cc2cnc3nnc(C4CC4)n3c2C1 |r| Show InChI InChI=1S/C22H23F3N6/c23-16-7-18(25)17(24)6-15(16)14-4-3-13(5-19(14)26)30-9-12-8-27-22-29-28-21(11-1-2-11)31(22)20(12)10-30/h6-8,11,13-14,19H,1-5,9-10,26H2/t13-,14+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem Lett 26: 2622-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.020
BindingDB Entry DOI: 10.7270/Q2Z321JP |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM13528
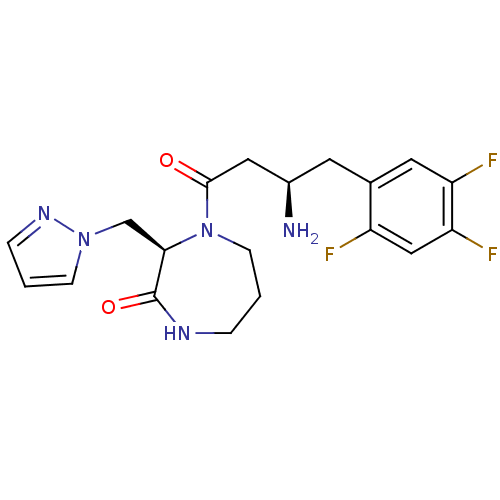
((3R)-4-[(3R)-3-amino-4-(2,4,5-trifluorophenyl)buta...)Show SMILES N[C@@H](CC(=O)N1CCCNC(=O)[C@H]1Cn1cccn1)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C19H22F3N5O2/c20-14-10-16(22)15(21)8-12(14)7-13(23)9-18(28)27-6-1-3-24-19(29)17(27)11-26-5-2-4-25-26/h2,4-5,8,10,13,17H,1,3,6-7,9,11,23H2,(H,24,29)/t13-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Research Laboratories
| Assay Description
The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... |
Bioorg Med Chem Lett 17: 49-52 (2007)
Article DOI: 10.1016/j.bmcl.2006.09.099
BindingDB Entry DOI: 10.7270/Q2F47MC2 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM13527
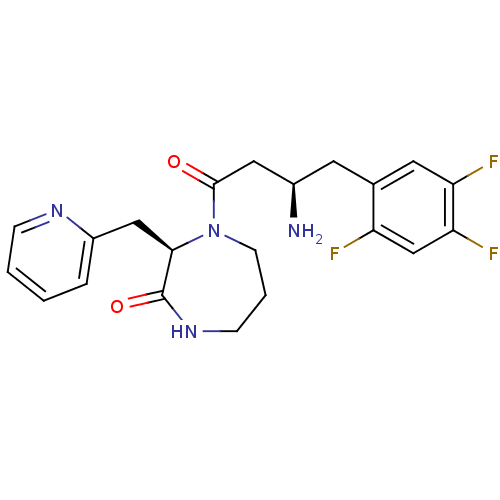
((3R)-4-[(3R)-3-amino-4-(2,4,5-trifluorophenyl)buta...)Show SMILES N[C@@H](CC(=O)N1CCCNC(=O)[C@H]1Cc1ccccn1)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C21H23F3N4O2/c22-16-12-18(24)17(23)9-13(16)8-14(25)10-20(29)28-7-3-6-27-21(30)19(28)11-15-4-1-2-5-26-15/h1-2,4-5,9,12,14,19H,3,6-8,10-11,25H2,(H,27,30)/t14-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Research Laboratories
| Assay Description
The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... |
Bioorg Med Chem Lett 17: 49-52 (2007)
Article DOI: 10.1016/j.bmcl.2006.09.099
BindingDB Entry DOI: 10.7270/Q2F47MC2 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50170929

(CHEMBL3805751)Show SMILES Cc1ccn2nc3CN(Cc3c2n1)[C@H]1CO[C@@H]([C@@H](N)C1)c1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C20H20F3N5O/c1-10-2-3-28-20(25-10)13-7-27(8-18(13)26-28)11-4-17(24)19(29-9-11)12-5-15(22)16(23)6-14(12)21/h2-3,5-6,11,17,19H,4,7-9,24H2,1H3/t11-,17+,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem Lett 26: 2622-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.020
BindingDB Entry DOI: 10.7270/Q2Z321JP |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50170953

(CHEMBL3805154)Show SMILES Cc1cc(O)n2nc3CN(Cc3c2n1)[C@H]1CO[C@@H]([C@@H](N)C1)c1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C20H20F3N5O2/c1-9-2-18(29)28-20(25-9)12-6-27(7-17(12)26-28)10-3-16(24)19(30-8-10)11-4-14(22)15(23)5-13(11)21/h2,4-5,10,16,19,29H,3,6-8,24H2,1H3/t10-,16+,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem Lett 26: 2622-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.020
BindingDB Entry DOI: 10.7270/Q2Z321JP |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50170921
(CHEMBL3806023)Show SMILES Cc1nnc2ncc3CN(Cc3n12)[C@H]1CC[C@@H]([C@@H](N)C1)c1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C20H21F3N6/c1-10-26-27-20-25-7-11-8-28(9-19(11)29(10)20)12-2-3-13(18(24)4-12)14-5-16(22)17(23)6-15(14)21/h5-7,12-13,18H,2-4,8-9,24H2,1H3/t12-,13+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem Lett 26: 2622-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.020
BindingDB Entry DOI: 10.7270/Q2Z321JP |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50170957
(CHEMBL3806157)Show SMILES Cc1cc2ncc3CN(Cc3n2n1)[C@H]1CC[C@@H]([C@@H](N)C1)c1cc(F)ccc1F |r| Show InChI InChI=1S/C21H23F2N5/c1-12-6-21-25-9-13-10-27(11-20(13)28(21)26-12)15-3-4-16(19(24)8-15)17-7-14(22)2-5-18(17)23/h2,5-7,9,15-16,19H,3-4,8,10-11,24H2,1H3/t15-,16+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem Lett 26: 2622-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.020
BindingDB Entry DOI: 10.7270/Q2Z321JP |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50170952
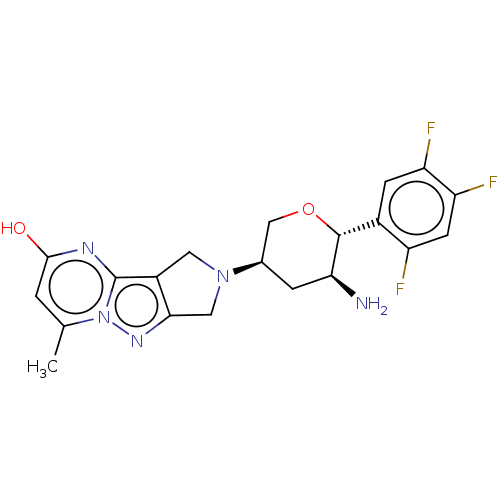
(CHEMBL3806310)Show SMILES Cc1cc(O)nc2c3CN(Cc3nn12)[C@H]1CO[C@@H]([C@@H](N)C1)c1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C20H20F3N5O2/c1-9-2-18(29)25-20-12-6-27(7-17(12)26-28(9)20)10-3-16(24)19(30-8-10)11-4-14(22)15(23)5-13(11)21/h2,4-5,10,16,19H,3,6-8,24H2,1H3,(H,25,29)/t10-,16+,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem Lett 26: 2622-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.020
BindingDB Entry DOI: 10.7270/Q2Z321JP |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50170934
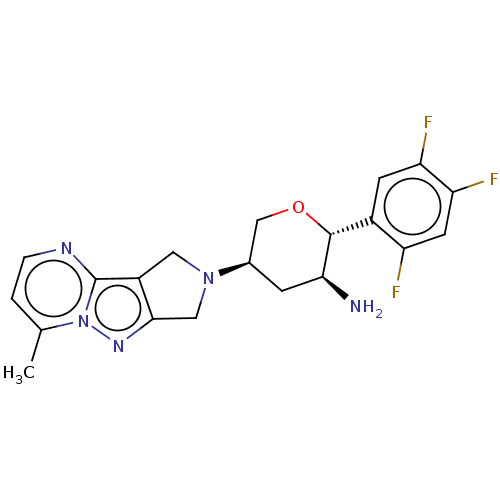
(CHEMBL3806021)Show SMILES Cc1ccnc2c3CN(Cc3nn12)[C@H]1CO[C@@H]([C@@H](N)C1)c1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C20H20F3N5O/c1-10-2-3-25-20-13-7-27(8-18(13)26-28(10)20)11-4-17(24)19(29-9-11)12-5-15(22)16(23)6-14(12)21/h2-3,5-6,11,17,19H,4,7-9,24H2,1H3/t11-,17+,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem Lett 26: 2622-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.020
BindingDB Entry DOI: 10.7270/Q2Z321JP |
More data for this
Ligand-Target Pair | |
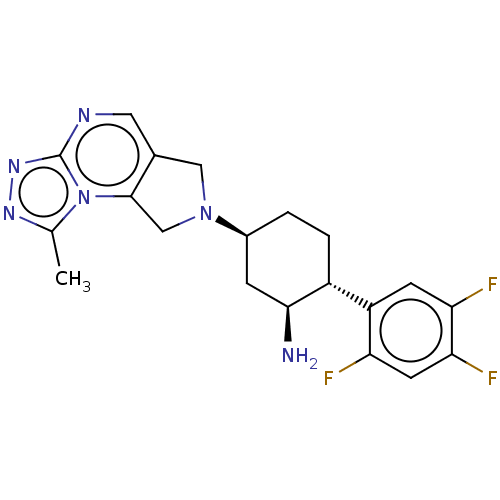
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50212925
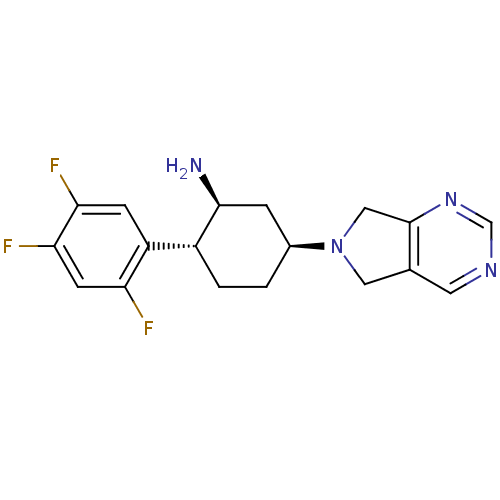
((1S,2R,5S)-5-(5H-pyrrolo[3,4-d]pyrimidin-6(7H)-yl)...)Show SMILES N[C@H]1C[C@H](CC[C@@H]1c1cc(F)c(F)cc1F)N1Cc2cncnc2C1 Show InChI InChI=1S/C18H19F3N4/c19-14-5-16(21)15(20)4-13(14)12-2-1-11(3-17(12)22)25-7-10-6-23-9-24-18(10)8-25/h4-6,9,11-12,17H,1-3,7-8,22H2/t11-,12+,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 17: 3877-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.106
BindingDB Entry DOI: 10.7270/Q20K2884 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50170920

(CHEMBL3806029)Show SMILES Cc1cc2ncc3CN(Cc3n2n1)[C@H]1CO[C@@H]([C@@H](N)C1)c1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C20H20F3N5O/c1-10-2-19-25-6-11-7-27(8-18(11)28(19)26-10)12-3-17(24)20(29-9-12)13-4-15(22)16(23)5-14(13)21/h2,4-6,12,17,20H,3,7-9,24H2,1H3/t12-,17+,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem Lett 26: 2622-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.020
BindingDB Entry DOI: 10.7270/Q2Z321JP |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50170956
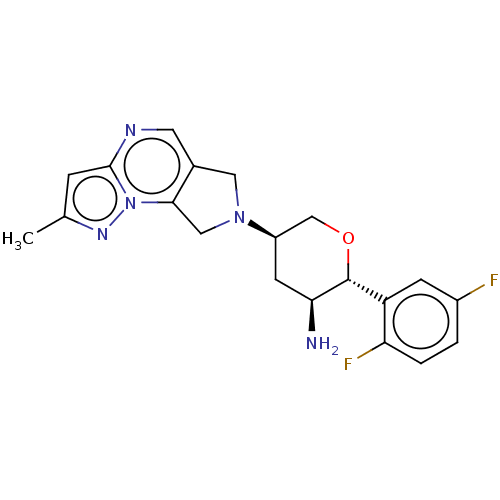
(CHEMBL3805294)Show SMILES Cc1cc2ncc3CN(Cc3n2n1)[C@H]1CO[C@@H]([C@@H](N)C1)c1cc(F)ccc1F |r| Show InChI InChI=1S/C20H21F2N5O/c1-11-4-19-24-7-12-8-26(9-18(12)27(19)25-11)14-6-17(23)20(28-10-14)15-5-13(21)2-3-16(15)22/h2-5,7,14,17,20H,6,8-10,23H2,1H3/t14-,17+,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem Lett 26: 2622-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.020
BindingDB Entry DOI: 10.7270/Q2Z321JP |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50170927
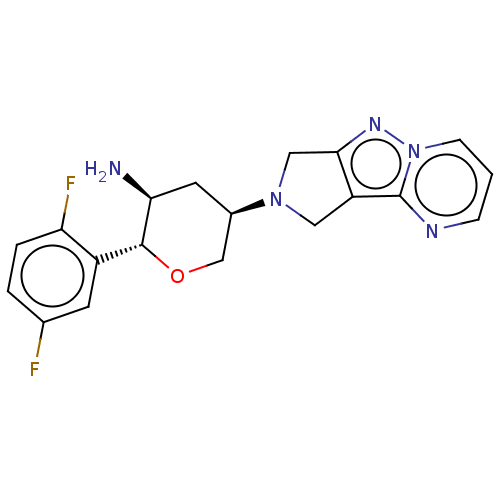
(CHEMBL3806216)Show SMILES N[C@H]1C[C@H](CO[C@@H]1c1cc(F)ccc1F)N1Cc2nn3cccnc3c2C1 |r| Show InChI InChI=1S/C19H19F2N5O/c20-11-2-3-15(21)13(6-11)18-16(22)7-12(10-27-18)25-8-14-17(9-25)24-26-5-1-4-23-19(14)26/h1-6,12,16,18H,7-10,22H2/t12-,16+,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem Lett 26: 2622-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.020
BindingDB Entry DOI: 10.7270/Q2Z321JP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM13526
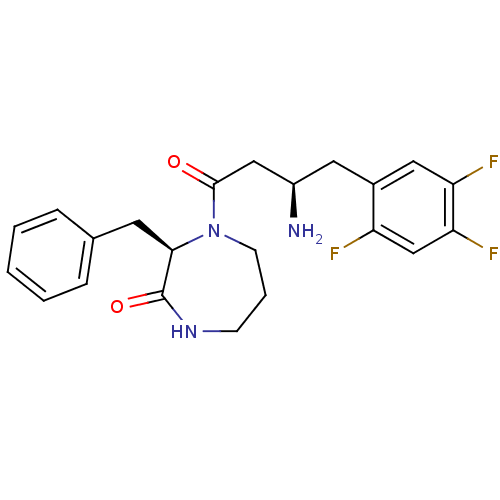
((3R)-4-[(3R)-3-amino-4-(2,4,5-trifluorophenyl)buta...)Show SMILES N[C@@H](CC(=O)N1CCCNC(=O)[C@H]1Cc1ccccc1)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C22H24F3N3O2/c23-17-13-19(25)18(24)11-15(17)10-16(26)12-21(29)28-8-4-7-27-22(30)20(28)9-14-5-2-1-3-6-14/h1-3,5-6,11,13,16,20H,4,7-10,12,26H2,(H,27,30)/t16-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Research Laboratories
| Assay Description
The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... |
Bioorg Med Chem Lett 17: 49-52 (2007)
Article DOI: 10.1016/j.bmcl.2006.09.099
BindingDB Entry DOI: 10.7270/Q2F47MC2 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50170936

(CHEMBL3805179)Show SMILES N[C@H]1C[C@H](CC[C@@H]1c1cc(F)c(F)cc1F)N1Cc2nn3c(ccnc3c2C1)C(F)(F)F |r| Show InChI InChI=1S/C21H19F6N5/c22-14-7-16(24)15(23)6-12(14)11-2-1-10(5-17(11)28)31-8-13-18(9-31)30-32-19(21(25,26)27)3-4-29-20(13)32/h3-4,6-7,10-11,17H,1-2,5,8-9,28H2/t10-,11+,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem Lett 26: 2622-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.020
BindingDB Entry DOI: 10.7270/Q2Z321JP |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50170959
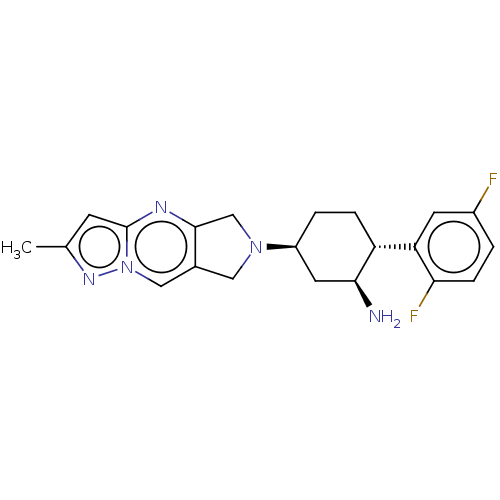
(CHEMBL3805226)Show SMILES Cc1cc2nc3CN(Cc3cn2n1)[C@H]1CC[C@@H]([C@@H](N)C1)c1cc(F)ccc1F |r| Show InChI InChI=1S/C21H23F2N5/c1-12-6-21-25-20-11-27(9-13(20)10-28(21)26-12)15-3-4-16(19(24)8-15)17-7-14(22)2-5-18(17)23/h2,5-7,10,15-16,19H,3-4,8-9,11,24H2,1H3/t15-,16+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem Lett 26: 2622-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.020
BindingDB Entry DOI: 10.7270/Q2Z321JP |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50170931
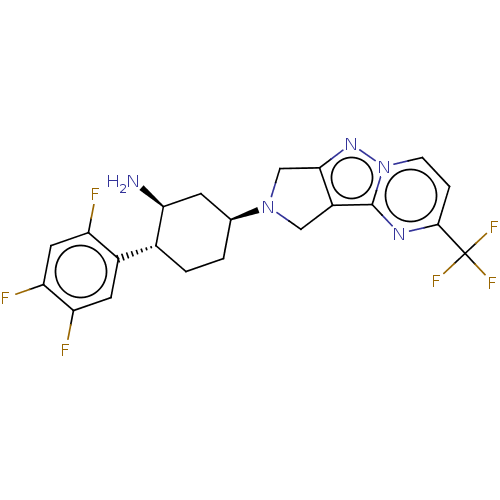
(CHEMBL3805871)Show SMILES N[C@H]1C[C@H](CC[C@@H]1c1cc(F)c(F)cc1F)N1Cc2nn3ccc(nc3c2C1)C(F)(F)F |r| Show InChI InChI=1S/C21H19F6N5/c22-14-7-16(24)15(23)6-12(14)11-2-1-10(5-17(11)28)31-8-13-18(9-31)30-32-4-3-19(21(25,26)27)29-20(13)32/h3-4,6-7,10-11,17H,1-2,5,8-9,28H2/t10-,11+,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem Lett 26: 2622-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.020
BindingDB Entry DOI: 10.7270/Q2Z321JP |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50170955

(CHEMBL3805937)Show SMILES N[C@H]1C[C@H](CO[C@@H]1c1cc(F)c(F)cc1F)N1Cc2nn3c(ccnc3c2C1)C(F)(F)F |r| Show InChI InChI=1S/C20H17F6N5O/c21-12-5-14(23)13(22)4-10(12)18-15(27)3-9(8-32-18)30-6-11-16(7-30)29-31-17(20(24,25)26)1-2-28-19(11)31/h1-2,4-5,9,15,18H,3,6-8,27H2/t9-,15+,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem Lett 26: 2622-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.020
BindingDB Entry DOI: 10.7270/Q2Z321JP |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM15553

((3R,7R)-4-[(3R)-3-amino-4-(2,4,5-trifluorophenyl)b...)Show SMILES C[C@@H]1CCN([C@H](Cc2ccccc2C)C(=O)N1)C(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C24H28F3N3O2/c1-14-5-3-4-6-16(14)11-22-24(32)29-15(2)7-8-30(22)23(31)12-18(28)9-17-10-20(26)21(27)13-19(17)25/h3-6,10,13,15,18,22H,7-9,11-12,28H2,1-2H3,(H,29,32)/t15-,18-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.49 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... |
Bioorg Med Chem Lett 17: 1903-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.039
BindingDB Entry DOI: 10.7270/Q2QZ2870 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50170930
(CHEMBL3805400)Show SMILES Cc1ccn2nc3CN(Cc3c2n1)[C@H]1CO[C@@H]([C@@H](N)C1)c1cc(F)ccc1F |r| Show InChI InChI=1S/C20H21F2N5O/c1-11-4-5-27-20(24-11)15-8-26(9-18(15)25-27)13-7-17(23)19(28-10-13)14-6-12(21)2-3-16(14)22/h2-6,13,17,19H,7-10,23H2,1H3/t13-,17+,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem Lett 26: 2622-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.020
BindingDB Entry DOI: 10.7270/Q2Z321JP |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50170932
(CHEMBL3805502)Show SMILES N[C@H]1C[C@H](CO[C@@H]1c1cc(F)c(F)cc1F)N1Cc2nn3ccc(nc3c2C1)C(F)(F)F |r| Show InChI InChI=1S/C20H17F6N5O/c21-12-5-14(23)13(22)4-10(12)18-15(27)3-9(8-32-18)30-6-11-16(7-30)29-31-2-1-17(20(24,25)26)28-19(11)31/h1-2,4-5,9,15,18H,3,6-8,27H2/t9-,15+,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem Lett 26: 2622-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.020
BindingDB Entry DOI: 10.7270/Q2Z321JP |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50003020

(MK-3102 | OMARIGLIPTIN | US10155775, Omarigliptin ...)Show SMILES CS(=O)(=O)n1cc2CN(Cc2n1)[C@H]1CO[C@@H]([C@@H](N)C1)c1cc(F)ccc1F |r| Show InChI InChI=1S/C17H20F2N4O3S/c1-27(24,25)23-7-10-6-22(8-16(10)21-23)12-5-15(20)17(26-9-12)13-4-11(18)2-3-14(13)19/h2-4,7,12,15,17H,5-6,8-9,20H2,1H3/t12-,15+,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Competitive reversible inhibition of DPP4 (unknown origin) |
J Med Chem 57: 3205-12 (2014)
Article DOI: 10.1021/jm401992e
BindingDB Entry DOI: 10.7270/Q2WD423H |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50170924
(CHEMBL3805346)Show SMILES N[C@H]1C[C@H](CC[C@@H]1c1cc(F)c(F)cc1F)N1Cc2cnc3nc(nn3c2C1)C1CC1 |r| Show InChI InChI=1S/C22H23F3N6/c23-16-7-18(25)17(24)6-15(16)14-4-3-13(5-19(14)26)30-9-12-8-27-22-28-21(11-1-2-11)29-31(22)20(12)10-30/h6-8,11,13-14,19H,1-5,9-10,26H2/t13-,14+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem Lett 26: 2622-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.020
BindingDB Entry DOI: 10.7270/Q2Z321JP |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50170954
(CHEMBL3804996)Show SMILES Cc1cc(nc2c3CN(Cc3nn12)[C@H]1CC[C@@H]([C@@H](N)C1)c1cc(F)c(F)cc1F)C(F)(F)F |r| Show InChI InChI=1S/C22H21F6N5/c1-10-4-20(22(26,27)28)30-21-14-8-32(9-19(14)31-33(10)21)11-2-3-12(18(29)5-11)13-6-16(24)17(25)7-15(13)23/h4,6-7,11-12,18H,2-3,5,8-9,29H2,1H3/t11-,12+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem Lett 26: 2622-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.020
BindingDB Entry DOI: 10.7270/Q2Z321JP |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM13525

((3R)-4-[(3R)-3-amino-4-(2,4,5-trifluorophenyl)buta...)Show SMILES N[C@@H](CC(=O)N1CCCNC(=O)[C@H]1CC(F)(F)F)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C17H19F6N3O2/c18-11-7-13(20)12(19)5-9(11)4-10(24)6-15(27)26-3-1-2-25-16(28)14(26)8-17(21,22)23/h5,7,10,14H,1-4,6,8,24H2,(H,25,28)/t10-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Research Laboratories
| Assay Description
The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... |
Bioorg Med Chem Lett 17: 49-52 (2007)
Article DOI: 10.1016/j.bmcl.2006.09.099
BindingDB Entry DOI: 10.7270/Q2F47MC2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM15554
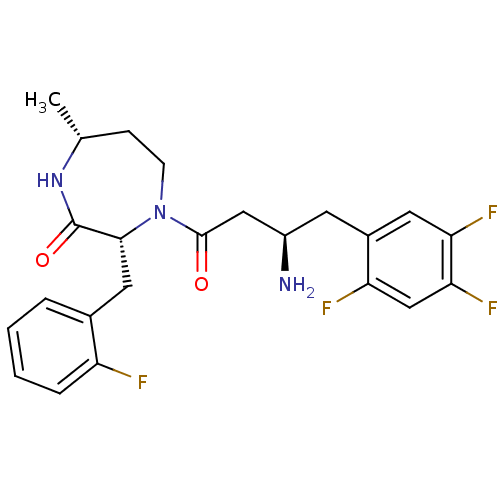
((3R,7R)-4-[(3R)-3-amino-4-(2,4,5-trifluorophenyl)b...)Show SMILES C[C@@H]1CCN([C@H](Cc2ccccc2F)C(=O)N1)C(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C23H25F4N3O2/c1-13-6-7-30(21(23(32)29-13)10-14-4-2-3-5-17(14)24)22(31)11-16(28)8-15-9-19(26)20(27)12-18(15)25/h2-5,9,12-13,16,21H,6-8,10-11,28H2,1H3,(H,29,32)/t13-,16-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.77 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... |
Bioorg Med Chem Lett 17: 1903-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.039
BindingDB Entry DOI: 10.7270/Q2QZ2870 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50170935
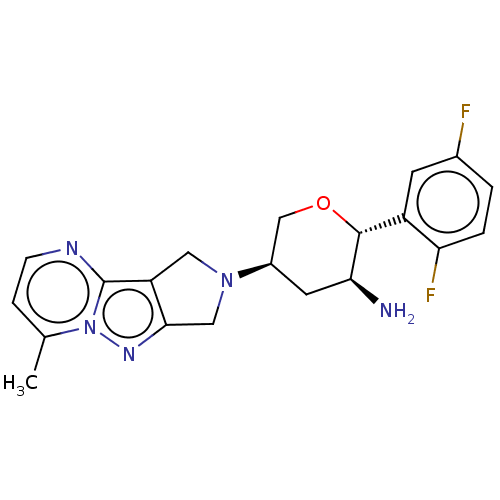
(CHEMBL3804950)Show SMILES Cc1ccnc2c3CN(Cc3nn12)[C@H]1CO[C@@H]([C@@H](N)C1)c1cc(F)ccc1F |r| Show InChI InChI=1S/C20H21F2N5O/c1-11-4-5-24-20-15-8-26(9-18(15)25-27(11)20)13-7-17(23)19(28-10-13)14-6-12(21)2-3-16(14)22/h2-6,13,17,19H,7-10,23H2,1H3/t13-,17+,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem Lett 26: 2622-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.020
BindingDB Entry DOI: 10.7270/Q2Z321JP |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50170940
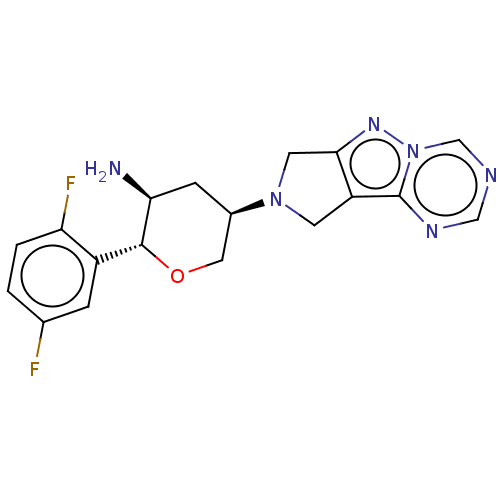
(CHEMBL3805353)Show SMILES N[C@H]1C[C@H](CO[C@@H]1c1cc(F)ccc1F)N1Cc2nn3cncnc3c2C1 |r| Show InChI InChI=1S/C18H18F2N6O/c19-10-1-2-14(20)12(3-10)17-15(21)4-11(7-27-17)25-5-13-16(6-25)24-26-9-22-8-23-18(13)26/h1-3,8-9,11,15,17H,4-7,21H2/t11-,15+,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem Lett 26: 2622-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.020
BindingDB Entry DOI: 10.7270/Q2Z321JP |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50170926
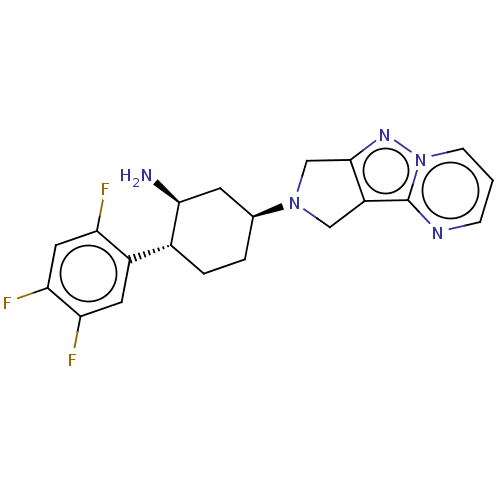
(CHEMBL3806003)Show SMILES N[C@H]1C[C@H](CC[C@@H]1c1cc(F)c(F)cc1F)N1Cc2nn3cccnc3c2C1 |r| Show InChI InChI=1S/C20H20F3N5/c21-15-8-17(23)16(22)7-13(15)12-3-2-11(6-18(12)24)27-9-14-19(10-27)26-28-5-1-4-25-20(14)28/h1,4-5,7-8,11-12,18H,2-3,6,9-10,24H2/t11-,12+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem Lett 26: 2622-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.020
BindingDB Entry DOI: 10.7270/Q2Z321JP |
More data for this
Ligand-Target Pair | |
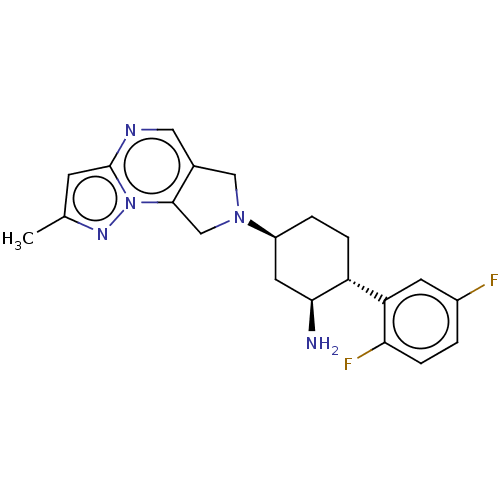
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50212928
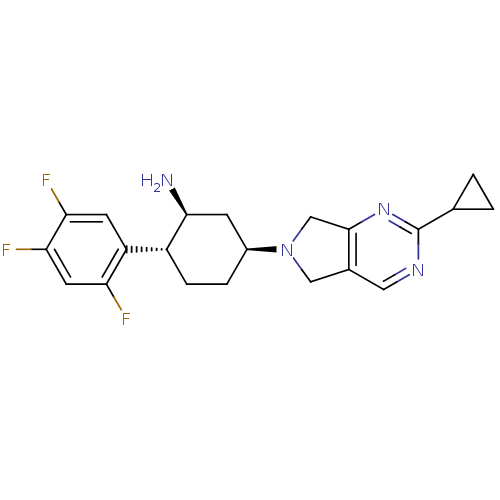
((1S,2R,5S)-5-(2-cyclopropyl-5H-pyrrolo[3,4-d]pyrim...)Show SMILES N[C@H]1C[C@H](CC[C@@H]1c1cc(F)c(F)cc1F)N1Cc2cnc(nc2C1)C1CC1 Show InChI InChI=1S/C21H23F3N4/c22-16-7-18(24)17(23)6-15(16)14-4-3-13(5-19(14)25)28-9-12-8-26-21(11-1-2-11)27-20(12)10-28/h6-8,11,13-14,19H,1-5,9-10,25H2/t13-,14+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 17: 3877-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.106
BindingDB Entry DOI: 10.7270/Q20K2884 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM15534

((3R)-4-[(3R)-3-amino-4-(2,4,5-trifluorophenyl)buta...)Show SMILES C[C@H]1N(CCCN(C)C1=O)C(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C17H22F3N3O2/c1-10-17(25)22(2)4-3-5-23(10)16(24)8-12(21)6-11-7-14(19)15(20)9-13(11)18/h7,9-10,12H,3-6,8,21H2,1-2H3/t10-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Research Laboratories
| Assay Description
The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... |
Bioorg Med Chem Lett 17: 1903-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.039
BindingDB Entry DOI: 10.7270/Q2QZ2870 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM13519
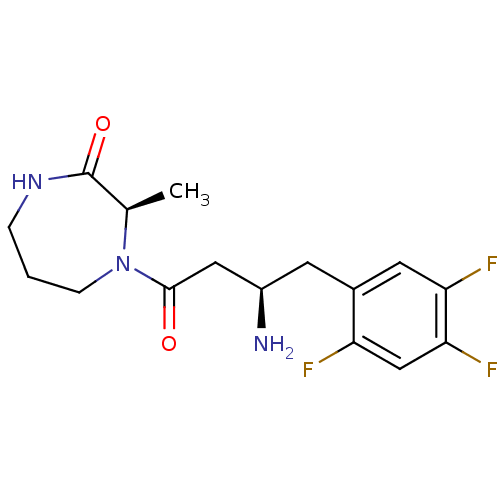
((3R)-4-[(3R)-3-amino-4-(2,4,5-trifluorophenyl)buta...)Show SMILES C[C@H]1N(CCCNC1=O)C(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C16H20F3N3O2/c1-9-16(24)21-3-2-4-22(9)15(23)7-11(20)5-10-6-13(18)14(19)8-12(10)17/h6,8-9,11H,2-5,7,20H2,1H3,(H,21,24)/t9-,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Research Laboratories
| Assay Description
The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... |
Bioorg Med Chem Lett 17: 49-52 (2007)
Article DOI: 10.1016/j.bmcl.2006.09.099
BindingDB Entry DOI: 10.7270/Q2F47MC2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM13519

((3R)-4-[(3R)-3-amino-4-(2,4,5-trifluorophenyl)buta...)Show SMILES C[C@H]1N(CCCNC1=O)C(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C16H20F3N3O2/c1-9-16(24)21-3-2-4-22(9)15(23)7-11(20)5-10-6-13(18)14(19)8-12(10)17/h6,8-9,11H,2-5,7,20H2,1H3,(H,21,24)/t9-,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Research Laboratories
| Assay Description
The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... |
Bioorg Med Chem Lett 17: 1903-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.039
BindingDB Entry DOI: 10.7270/Q2QZ2870 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50212926
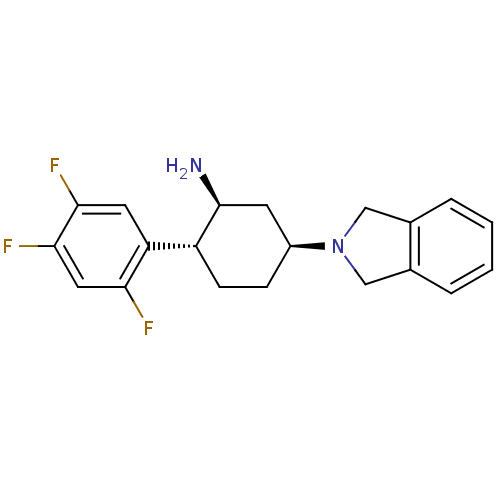
((1S,2R,5S)-5-(isoindolin-2-yl)-2-(2,4,5-trifluorop...)Show SMILES N[C@H]1C[C@H](CC[C@@H]1c1cc(F)c(F)cc1F)N1Cc2ccccc2C1 Show InChI InChI=1S/C20H21F3N2/c21-17-9-19(23)18(22)8-16(17)15-6-5-14(7-20(15)24)25-10-12-3-1-2-4-13(12)11-25/h1-4,8-9,14-15,20H,5-7,10-11,24H2/t14-,15+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 17: 3877-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.106
BindingDB Entry DOI: 10.7270/Q20K2884 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM13515
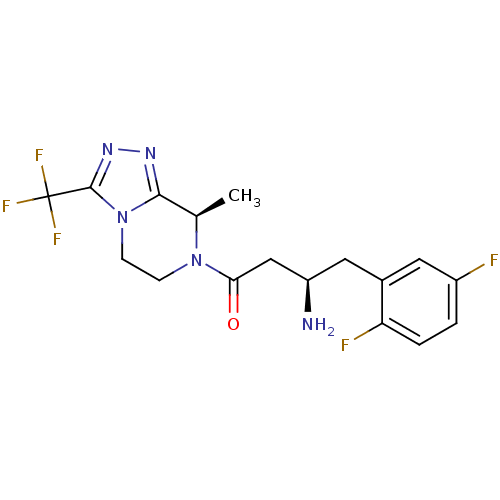
((3R)-3-amino-4-(2,5-difluorophenyl)-1-[(8R)-8-meth...)Show SMILES C[C@H]1N(CCn2c1nnc2C(F)(F)F)C(=O)C[C@H](N)Cc1cc(F)ccc1F |r| Show InChI InChI=1S/C17H18F5N5O/c1-9-15-24-25-16(17(20,21)22)27(15)5-4-26(9)14(28)8-12(23)7-10-6-11(18)2-3-13(10)19/h2-3,6,9,12H,4-5,7-8,23H2,1H3/t9-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Research Laboratories
| Assay Description
The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... |
Bioorg Med Chem Lett 17: 49-52 (2007)
Article DOI: 10.1016/j.bmcl.2006.09.099
BindingDB Entry DOI: 10.7270/Q2F47MC2 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50170925

(CHEMBL3806026)Show SMILES N[C@H]1C[C@H](CC[C@@H]1c1cc(F)ccc1F)N1Cc2nn3cccnc3c2C1 |r| Show InChI InChI=1S/C20H21F2N5/c21-12-2-5-17(22)15(8-12)14-4-3-13(9-18(14)23)26-10-16-19(11-26)25-27-7-1-6-24-20(16)27/h1-2,5-8,13-14,18H,3-4,9-11,23H2/t13-,14+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem Lett 26: 2622-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.020
BindingDB Entry DOI: 10.7270/Q2Z321JP |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Mus musculus) | BDBM50210527
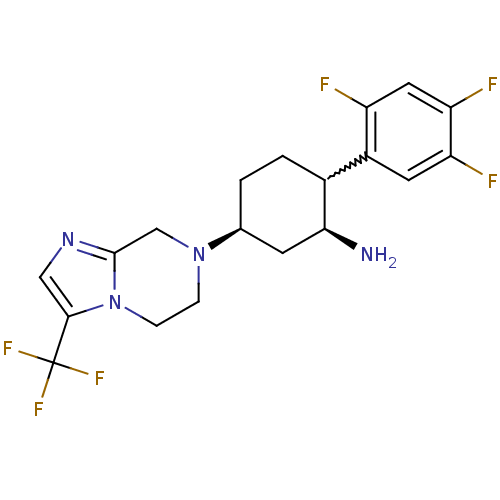
((1S,2R,5S)-5-(3-(trifluoromethyl)-5,6-dihydroimida...)Show SMILES N[C@H]1C[C@H](CCC1c1cc(F)c(F)cc1F)N1CCn2c(C1)ncc2C(F)(F)F |w:6.7| Show InChI InChI=1S/C19H20F6N4/c20-13-7-15(22)14(21)6-12(13)11-2-1-10(5-16(11)26)28-3-4-29-17(19(23,24)25)8-27-18(29)9-28/h6-8,10-11,16H,1-5,9,26H2/t10-,11?,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse DPP4 |
Bioorg Med Chem Lett 17: 3384-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.095
BindingDB Entry DOI: 10.7270/Q2NK3DQW |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM15538

((3R)-4-[(3R)-3-amino-4-(2,4,5-trifluorophenyl)buta...)Show SMILES C[C@H]1N(CCCN(C2CC2)C1=O)C(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C19H24F3N3O2/c1-11-19(27)25(14-3-4-14)6-2-5-24(11)18(26)9-13(23)7-12-8-16(21)17(22)10-15(12)20/h8,10-11,13-14H,2-7,9,23H2,1H3/t11-,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Research Laboratories
| Assay Description
The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... |
Bioorg Med Chem Lett 17: 1903-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.039
BindingDB Entry DOI: 10.7270/Q2QZ2870 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50170960
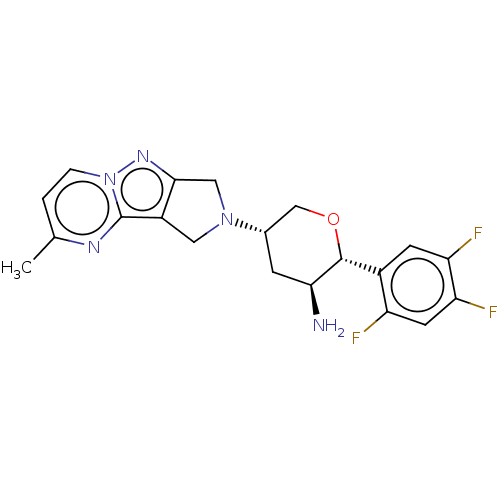
(CHEMBL3804940)Show SMILES Cc1ccn2nc3CN(Cc3c2n1)[C@@H]1CO[C@@H]([C@@H](N)C1)c1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C20H20F3N5O/c1-10-2-3-28-20(25-10)13-7-27(8-18(13)26-28)11-4-17(24)19(29-9-11)12-5-15(22)16(23)6-14(12)21/h2-3,5-6,11,17,19H,4,7-9,24H2,1H3/t11-,17-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem Lett 26: 2622-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.020
BindingDB Entry DOI: 10.7270/Q2Z321JP |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM15532

((3R)-4-[(3R)-3-amino-4-(2,5-difluorophenyl)butanoy...)Show SMILES C[C@H]1N(CCCN(C2CC2)C1=O)C(=O)C[C@H](N)Cc1cc(F)ccc1F |r| Show InChI InChI=1S/C19H25F2N3O2/c1-12-19(26)24(16-4-5-16)8-2-7-23(12)18(25)11-15(22)10-13-9-14(20)3-6-17(13)21/h3,6,9,12,15-16H,2,4-5,7-8,10-11,22H2,1H3/t12-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Research Laboratories
| Assay Description
The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... |
Bioorg Med Chem Lett 17: 1903-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.039
BindingDB Entry DOI: 10.7270/Q2QZ2870 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM15539

((3R)-4-[(3R)-3-amino-4-(2,4,5-trifluorophenyl)buta...)Show SMILES C[C@H]1N(CCCN(C1=O)C(C)(C)C)C(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C20H28F3N3O2/c1-12-19(28)26(20(2,3)4)7-5-6-25(12)18(27)10-14(24)8-13-9-16(22)17(23)11-15(13)21/h9,11-12,14H,5-8,10,24H2,1-4H3/t12-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Research Laboratories
| Assay Description
The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... |
Bioorg Med Chem Lett 17: 1903-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.039
BindingDB Entry DOI: 10.7270/Q2QZ2870 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM15543

((3R,6S)-4-[(3R)-3-amino-4-(2,4,5-trifluorophenyl)b...)Show SMILES C[C@H]1CNC(=O)[C@@H](C)N(C1)C(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C17H22F3N3O2/c1-9-7-22-17(25)10(2)23(8-9)16(24)5-12(21)3-11-4-14(19)15(20)6-13(11)18/h4,6,9-10,12H,3,5,7-8,21H2,1-2H3,(H,22,25)/t9-,10+,12+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Research Laboratories
| Assay Description
The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... |
Bioorg Med Chem Lett 17: 1903-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.039
BindingDB Entry DOI: 10.7270/Q2QZ2870 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM15536
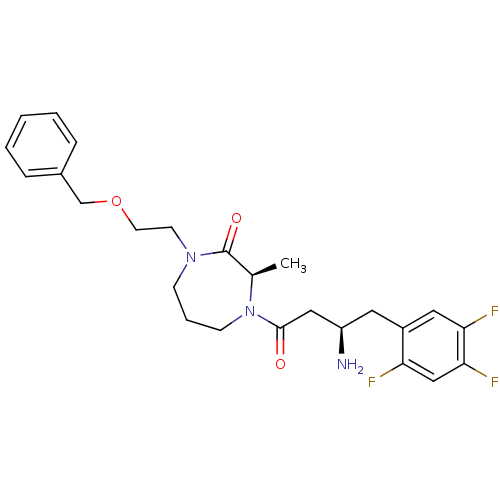
((3R)-4-[(3R)-3-amino-4-(2,4,5-trifluorophenyl)buta...)Show SMILES C[C@H]1N(CCCN(CCOCc2ccccc2)C1=O)C(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C25H30F3N3O3/c1-17-25(33)30(10-11-34-16-18-6-3-2-4-7-18)8-5-9-31(17)24(32)14-20(29)12-19-13-22(27)23(28)15-21(19)26/h2-4,6-7,13,15,17,20H,5,8-12,14,16,29H2,1H3/t17-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10.5 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Research Laboratories
| Assay Description
The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... |
Bioorg Med Chem Lett 17: 1903-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.039
BindingDB Entry DOI: 10.7270/Q2QZ2870 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM15555

((3R,7R)-4-[(3R)-3-amino-4-(2,4,5-trifluorophenyl)b...)Show SMILES C[C@@H]1CCN([C@H](Cc2ccccn2)C(=O)N1)C(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C22H25F3N4O2/c1-13-5-7-29(20(22(31)28-13)11-16-4-2-3-6-27-16)21(30)10-15(26)8-14-9-18(24)19(25)12-17(14)23/h2-4,6,9,12-13,15,20H,5,7-8,10-11,26H2,1H3,(H,28,31)/t13-,15-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... |
Bioorg Med Chem Lett 17: 1903-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.039
BindingDB Entry DOI: 10.7270/Q2QZ2870 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM13518
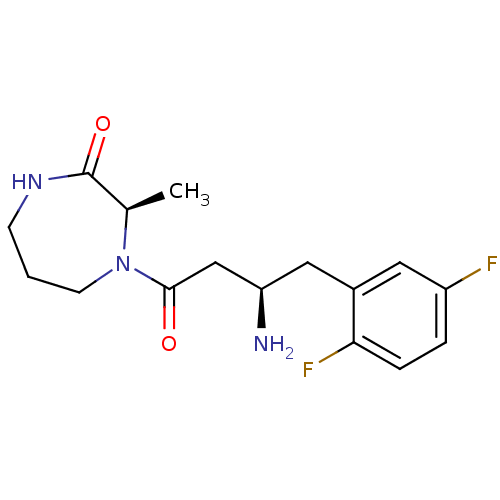
((3R)-4-[(3R)-3-amino-4-(2,5-difluorophenyl)butanoy...)Show SMILES C[C@H]1N(CCCNC1=O)C(=O)C[C@H](N)Cc1cc(F)ccc1F |r| Show InChI InChI=1S/C16H21F2N3O2/c1-10-16(23)20-5-2-6-21(10)15(22)9-13(19)8-11-7-12(17)3-4-14(11)18/h3-4,7,10,13H,2,5-6,8-9,19H2,1H3,(H,20,23)/t10-,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11.5 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Research Laboratories
| Assay Description
The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... |
Bioorg Med Chem Lett 17: 1903-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.039
BindingDB Entry DOI: 10.7270/Q2QZ2870 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM15530

(1-substituted diazepanone 9b | methyl 2-[(3R)-4-[(...)Show SMILES COC(=O)CN1CCCN([C@H](C)C1=O)C(=O)C[C@H](N)Cc1cc(F)ccc1F |r| Show InChI InChI=1S/C19H25F2N3O4/c1-12-19(27)23(11-18(26)28-2)6-3-7-24(12)17(25)10-15(22)9-13-8-14(20)4-5-16(13)21/h4-5,8,12,15H,3,6-7,9-11,22H2,1-2H3/t12-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11.6 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Research Laboratories
| Assay Description
The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... |
Bioorg Med Chem Lett 17: 1903-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.039
BindingDB Entry DOI: 10.7270/Q2QZ2870 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data