

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020771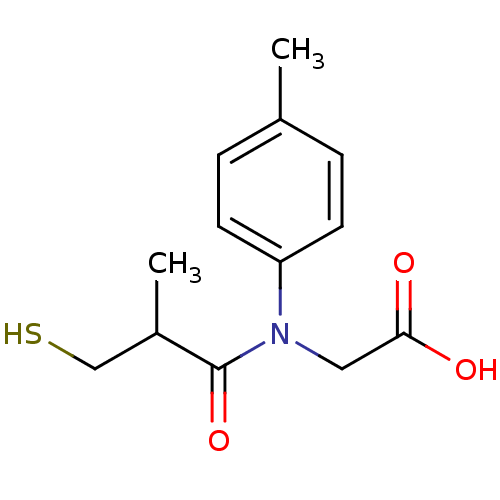 (CHEMBL160361 | [(3-Mercapto-2-methyl-propionyl)-p-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50015471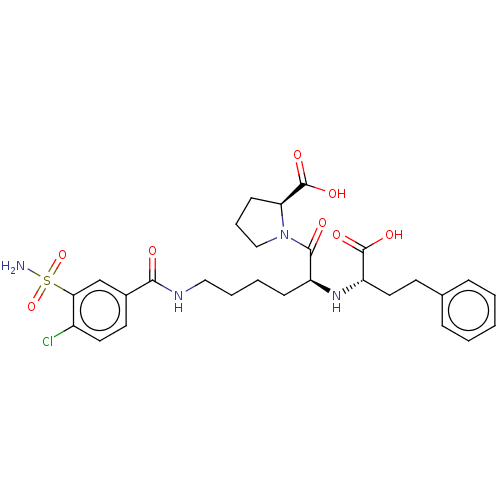 (1-[2-(1-Carboxy-3-phenyl-propylamino)-6-(4-chloro-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme (ACE) | J Med Chem 33: 1600-6 (1990) BindingDB Entry DOI: 10.7270/Q2H41S2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50015473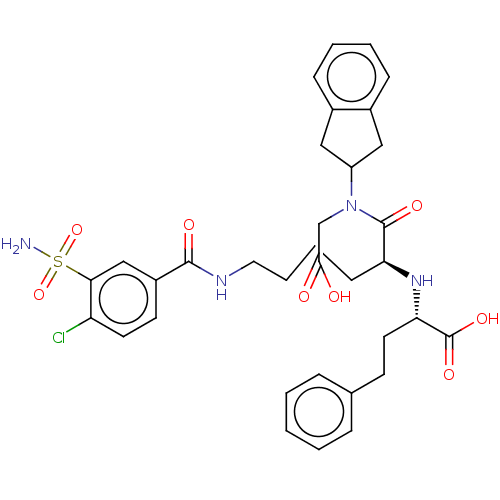 (2-[1-(Carboxymethyl-indan-2-yl-carbamoyl)-5-(4-chl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme (ACE) | J Med Chem 33: 1600-6 (1990) BindingDB Entry DOI: 10.7270/Q2H41S2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50367217 (CHEMBL1907938) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM21642 ((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020794 (CHEMBL348040 | [Cyclobutyl-(3-mercapto-2-methyl-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020766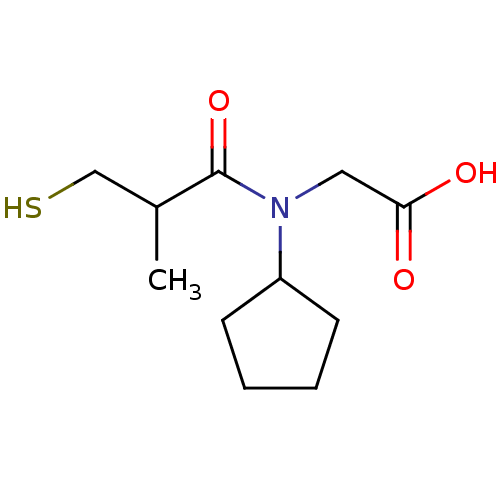 ((R+S)-[Cyclopentyl-(3-mercapto-2-methyl-propionyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020761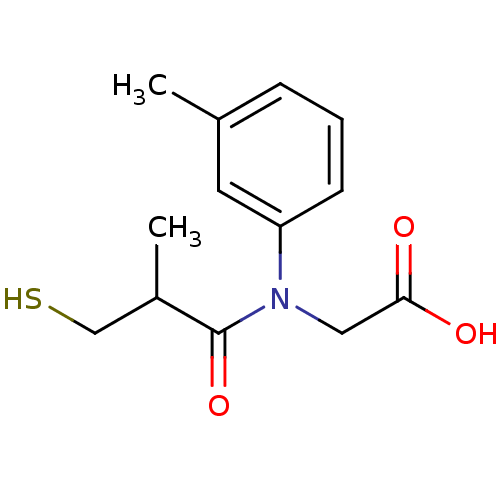 (CHEMBL346255 | [(3-Mercapto-2-methyl-propionyl)-m-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020791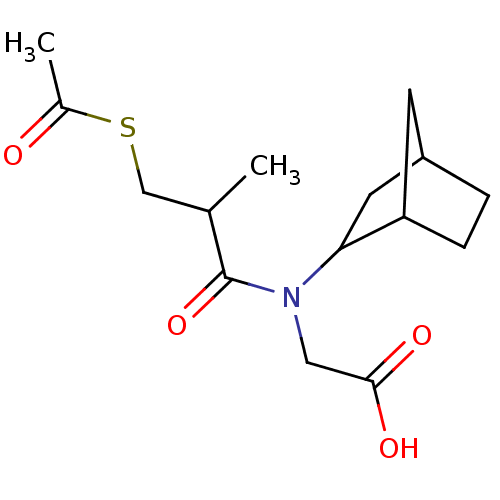 (CHEMBL346561 | [(3-Acetylsulfanyl-2-methyl-propion...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 8.3 | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020791 (CHEMBL346561 | [(3-Acetylsulfanyl-2-methyl-propion...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM21642 ((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM21642 ((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020793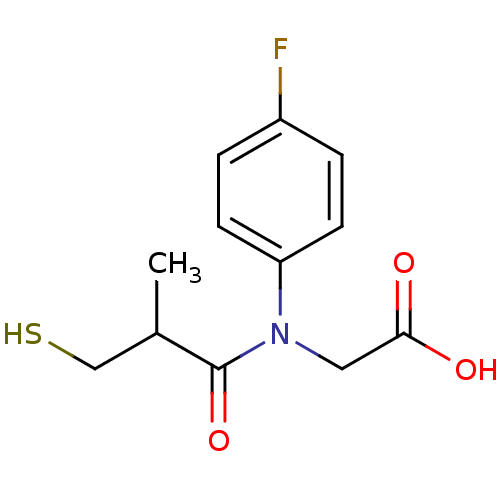 (CHEMBL23727 | [(4-Fluoro-phenyl)-(3-mercapto-2-met...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021340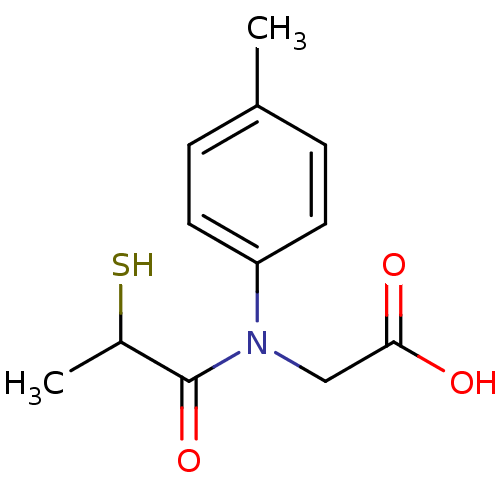 (CHEMBL166743 | [(2-Mercapto-propionyl)-p-tolyl-ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of the activity of rabbit lung Angiotensin I converting enzyme | J Med Chem 29: 784-96 (1986) BindingDB Entry DOI: 10.7270/Q2F47N4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005159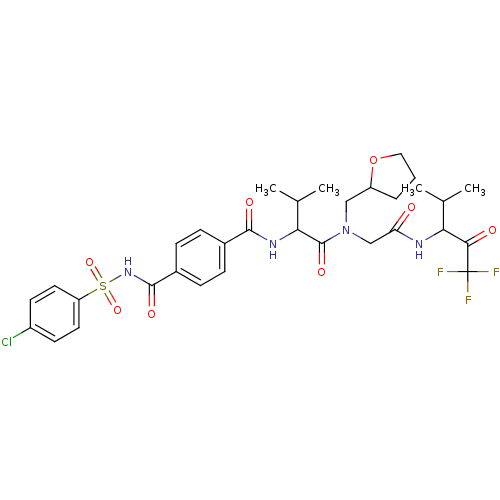 (4-(4-Chloro-benzenesulfonylaminocarbonyl)-N-(2-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020779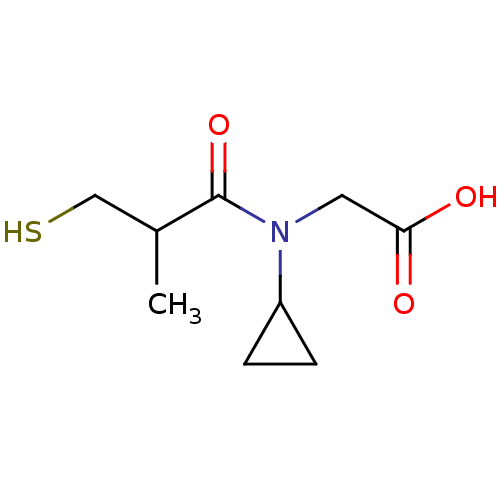 (CHEMBL158962 | [Cyclopropyl-(3-mercapto-2-methyl-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020808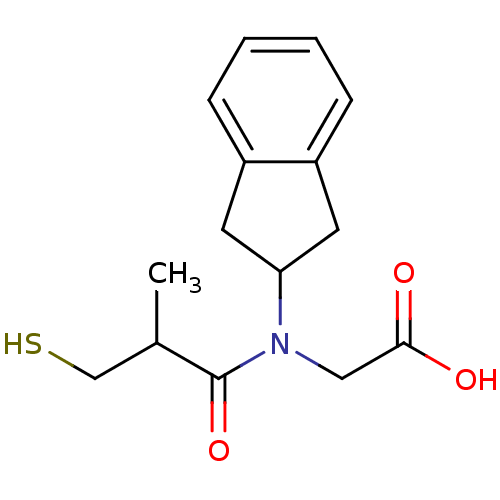 (CHEMBL1788147 | CHEMBL278348 | [Indan-2-yl-(3-merc...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020798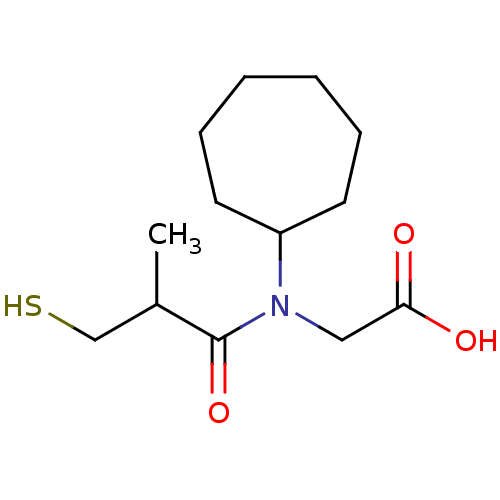 (CHEMBL1788148 | CHEMBL23841 | [Cycloheptyl-(3-merc...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020769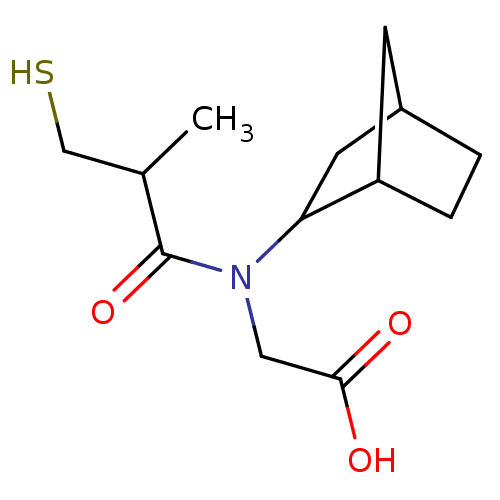 (CHEMBL345858 | [Bicyclo[2.2.1]hept-2-yl-(3-mercapt...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020801 (CHEMBL23641 | [Indan-5-yl-(3-mercapto-2-methyl-pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020768 (CHEMBL1788152 | CHEMBL23518 | [Cyclohexyl-(3-merca...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005147 ((S)-1-{(S)-2-[4-(4-Bromo-benzenesulfonylaminocarbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50015477 (2-[1-(Carboxymethyl-indan-2-yl-carbamoyl)-5-(4-chl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme (ACE) | J Med Chem 33: 1600-6 (1990) BindingDB Entry DOI: 10.7270/Q2H41S2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020763 (CHEMBL422013 | [(3-Acetylsulfanyl-2-methyl-propion...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020803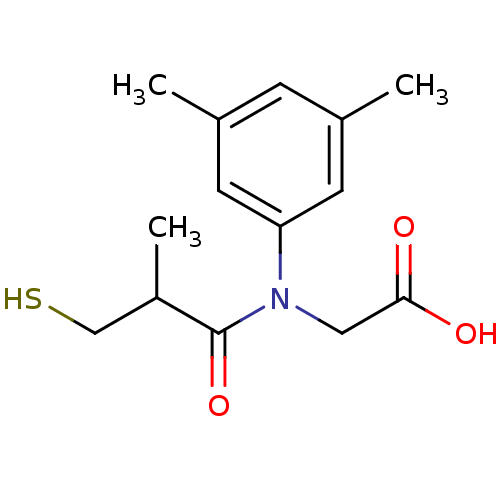 (CHEMBL350484 | [(3,5-Dimethyl-phenyl)-(3-mercapto-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020786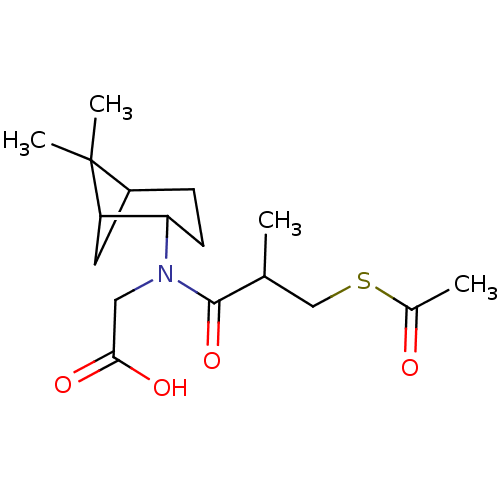 (CHEMBL406430 | [(3-Acetylsulfanyl-2-methyl-propion...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020792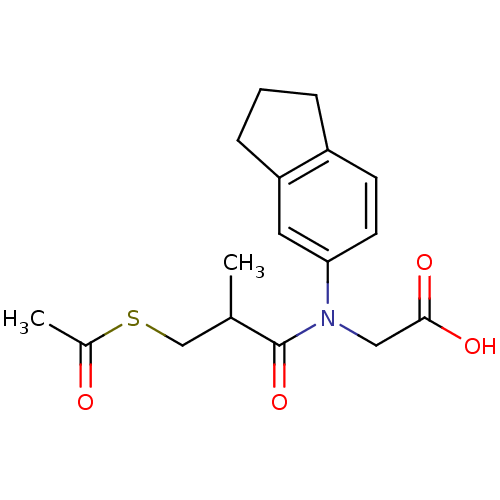 (CHEMBL347259 | [(3-Acetylsulfanyl-2-methyl-propion...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020778 (CHEMBL159075 | [(3-Acetylsulfanyl-2-methyl-propion...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005166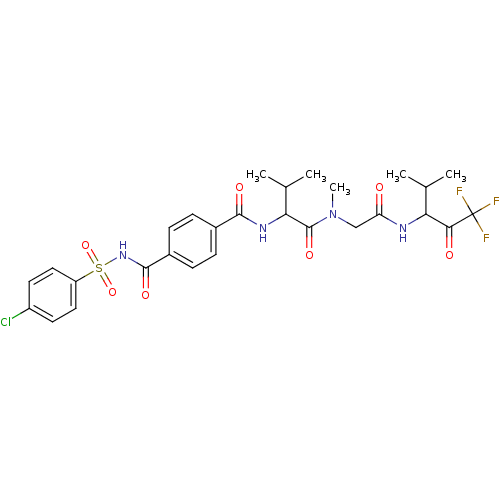 (4-(4-Chloro-benzenesulfonylaminocarbonyl)-N-(2-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020810 (CHEMBL346588 | [(3-Mercapto-2-methyl-propionyl)-(3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020780 (CHEMBL422535 | [(3-Mercapto-2-methyl-propionyl)-th...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021332 (CHEMBL165952 | [Cyclopentyl-(2-mercapto-propionyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of the activity of rabbit lung Angiotensin I converting enzyme | J Med Chem 29: 784-96 (1986) BindingDB Entry DOI: 10.7270/Q2F47N4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50004182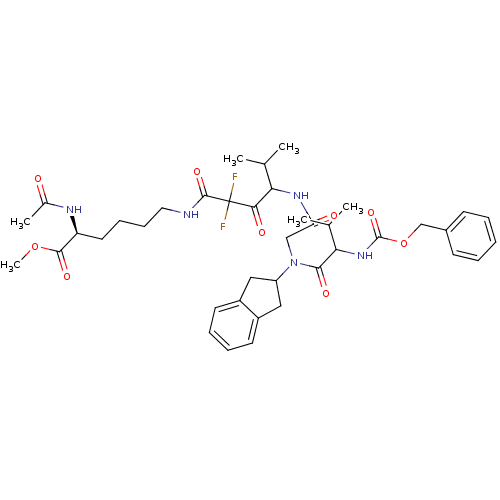 (2-Acetylamino-6-(4-{2-[(2-benzyloxycarbonylamino-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 | J Med Chem 35: 4795-808 (1993) BindingDB Entry DOI: 10.7270/Q2HX1BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005176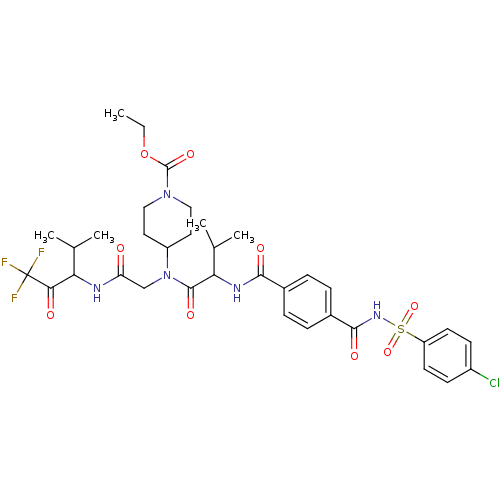 (4-{{2-[4-(4-Chloro-benzenesulfonylaminocarbonyl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020807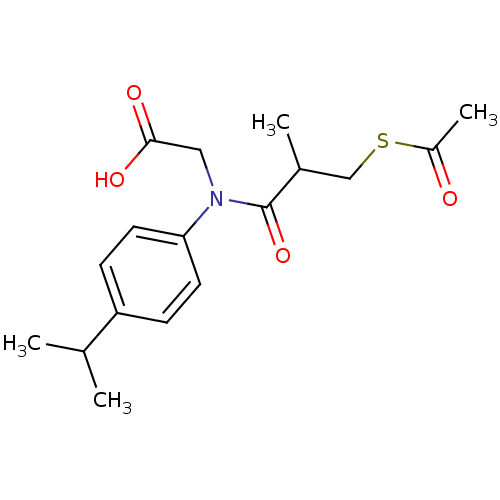 (CHEMBL350258 | [(3-Acetylsulfanyl-2-methyl-propion...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005174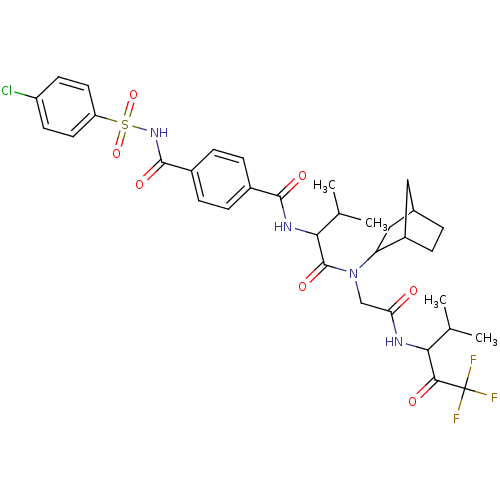 (CHEMBL435649 | N-(1-{Bicyclo[2.2.1]hept-2-yl-[(3,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020783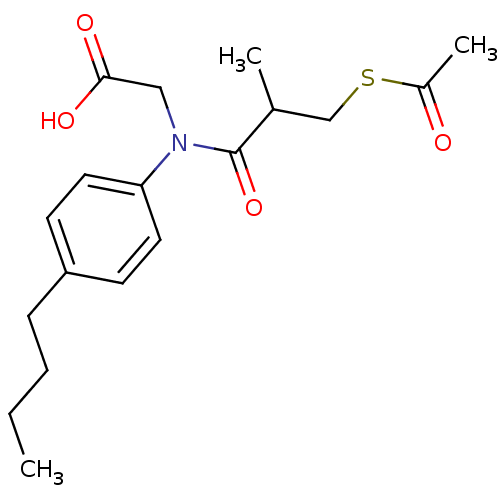 (CHEMBL347447 | [(3-Acetylsulfanyl-2-methyl-propion...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005154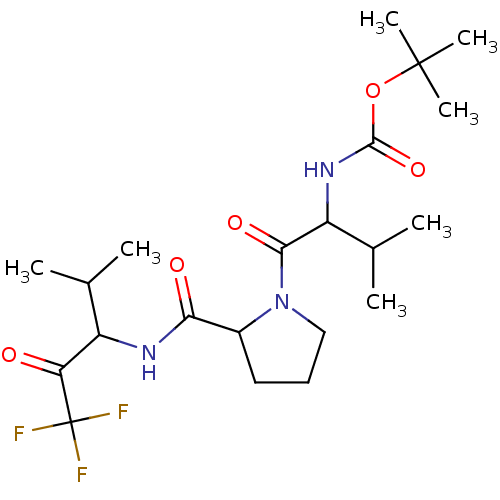 (CHEMBL423067 | {2-Methyl-1-[2-(3,3,3-trifluoro-1-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005156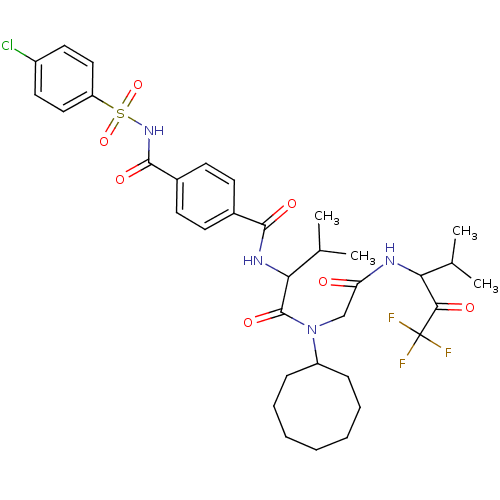 (4-(4-Chloro-benzenesulfonylaminocarbonyl)-N-(1-{cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50004192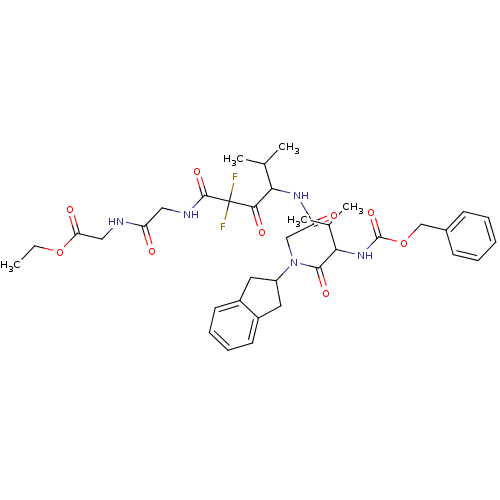 (CHEMBL344981 | [2-(4-{2-[(2-Benzyloxycarbonylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 | J Med Chem 35: 4795-808 (1993) BindingDB Entry DOI: 10.7270/Q2HX1BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020784 (CHEMBL346495 | [Isopropyl-(3-mercapto-2-methyl-pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005178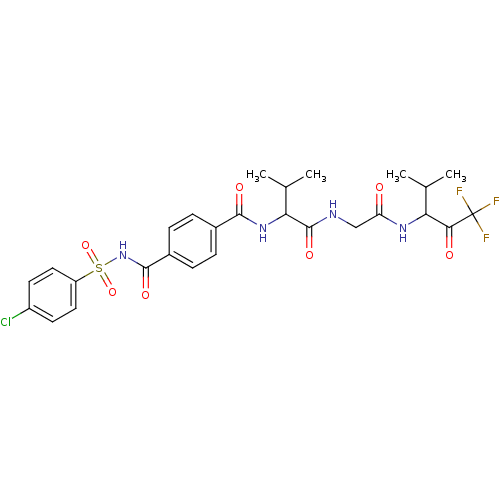 (4-(4-Chloro-benzenesulfonylaminocarbonyl)-N-(2-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005152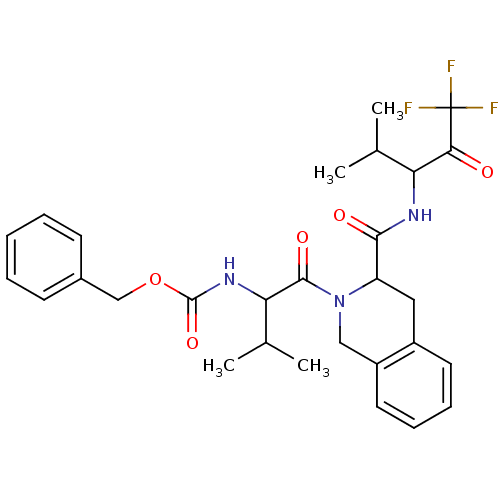 (CHEMBL160365 | {2-Methyl-1-[3-(3,3,3-trifluoro-1-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005152 (CHEMBL160365 | {2-Methyl-1-[3-(3,3,3-trifluoro-1-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020756 (CHEMBL348043 | [(3-Acetylsulfanyl-2-methyl-propion...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020774 (CHEMBL160689 | [Ethyl-(3-mercapto-2-methyl-propion...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020787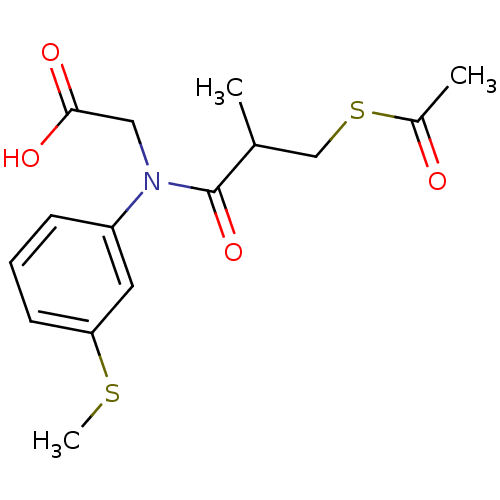 (CHEMBL345619 | [(3-Acetylsulfanyl-2-methyl-propion...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020760 (2-[Cyclopropyl-(3-mercapto-2-methyl-propionyl)-ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020799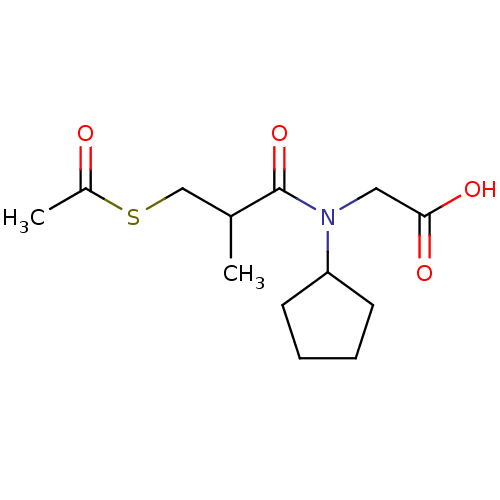 (CHEMBL160770 | [(3-Acetylsulfanyl-2-methyl-propion...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rabbit lung Angiotensin I converting enzyme at pH 8.3 | J Med Chem 28: 57-66 (1985) BindingDB Entry DOI: 10.7270/Q2M90975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50004184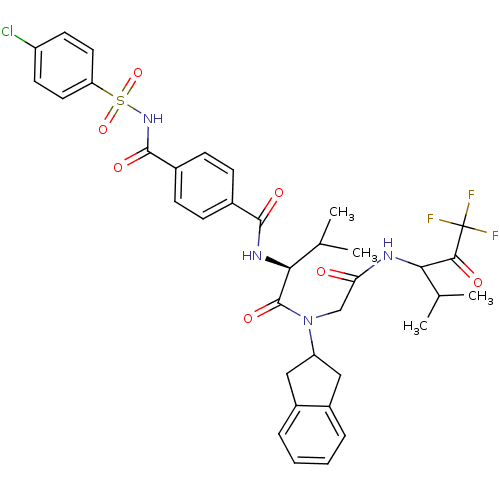 (4-(4-Chloro-benzenesulfonylaminocarbonyl)-N-((S)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 158 total ) | Next | Last >> |