 Found 5660 hits with Last Name = 'smith' and Initial = 'e'
Found 5660 hits with Last Name = 'smith' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384817
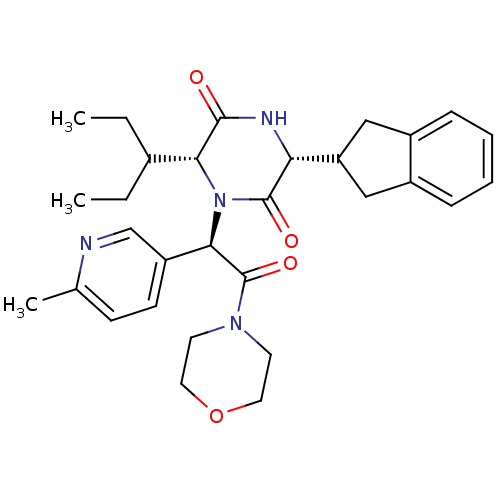
(CHEMBL2037514)Show SMILES CCC(CC)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C30H38N4O4/c1-4-20(5-2)26-28(35)32-25(24-16-21-8-6-7-9-22(21)17-24)29(36)34(26)27(23-11-10-19(3)31-18-23)30(37)33-12-14-38-15-13-33/h6-11,18,20,24-27H,4-5,12-17H2,1-3H3,(H,32,35)/t25-,26-,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384800
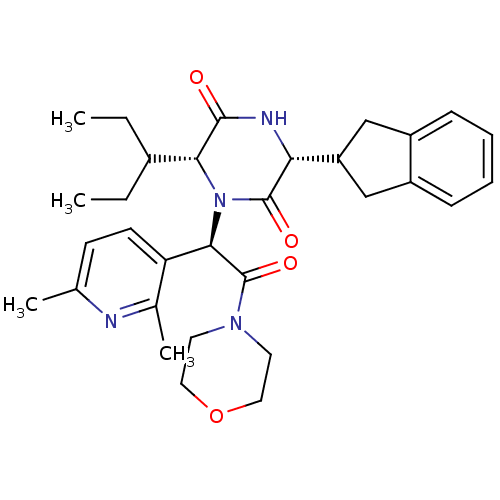
(CHEMBL2037517)Show SMILES CCC(CC)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2ccc(C)nc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C31H40N4O4/c1-5-21(6-2)27-29(36)33-26(24-17-22-9-7-8-10-23(22)18-24)30(37)35(27)28(25-12-11-19(3)32-20(25)4)31(38)34-13-15-39-16-14-34/h7-12,21,24,26-28H,5-6,13-18H2,1-4H3,(H,33,36)/t26-,27-,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384816

(CHEMBL2037516)Show SMILES CCC(CC)[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(C)nc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C29H38N4O3/c1-7-19(8-2)25-27(34)31-24(22-15-20-11-9-10-12-21(20)16-22)28(35)33(25)26(29(36)32(5)6)23-14-13-17(3)30-18(23)4/h9-14,19,22,24-26H,7-8,15-16H2,1-6H3,(H,31,34)/t24-,25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384823
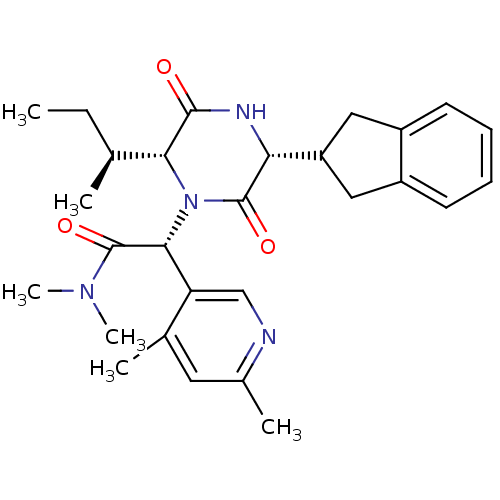
(CHEMBL2037507)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N(C)C)c2cnc(C)cc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C28H36N4O3/c1-7-16(2)24-26(33)30-23(21-13-19-10-8-9-11-20(19)14-21)27(34)32(24)25(28(35)31(5)6)22-15-29-18(4)12-17(22)3/h8-12,15-16,21,23-25H,7,13-14H2,1-6H3,(H,30,33)/t16-,23+,24+,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384822
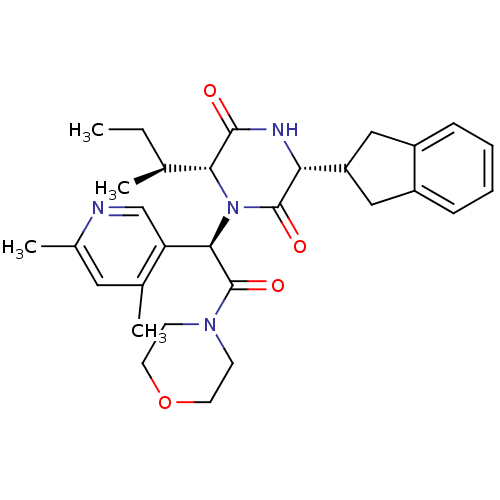
(CHEMBL2037508)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2cnc(C)cc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C30H38N4O4/c1-5-18(2)26-28(35)32-25(23-15-21-8-6-7-9-22(21)16-23)29(36)34(26)27(24-17-31-20(4)14-19(24)3)30(37)33-10-12-38-13-11-33/h6-9,14,17-18,23,25-27H,5,10-13,15-16H2,1-4H3,(H,32,35)/t18-,25+,26+,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384818
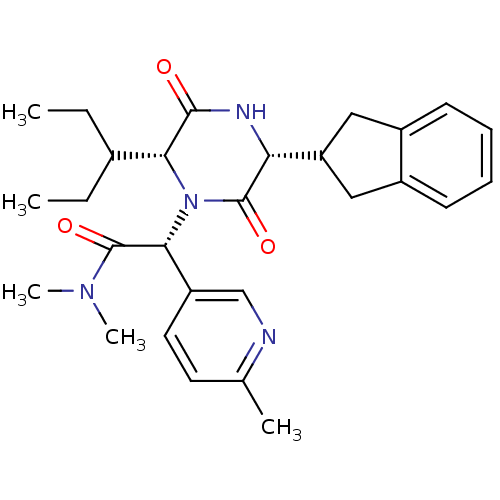
(CHEMBL2037513)Show SMILES CCC(CC)[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C28H36N4O3/c1-6-18(7-2)24-26(33)30-23(22-14-19-10-8-9-11-20(19)15-22)27(34)32(24)25(28(35)31(4)5)21-13-12-17(3)29-16-21/h8-13,16,18,22-25H,6-7,14-15H2,1-5H3,(H,30,33)/t23-,24-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384837

(CHEMBL2037515)Show SMILES CCC(CC)[C@H]1N([C@@H](C(=O)NC)c2ccc(C)nc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C28H36N4O3/c1-6-18(7-2)24-27(34)31-23(21-14-19-10-8-9-11-20(19)15-21)28(35)32(24)25(26(33)29-5)22-13-12-16(3)30-17(22)4/h8-13,18,21,23-25H,6-7,14-15H2,1-5H3,(H,29,33)(H,31,34)/t23-,24-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384824
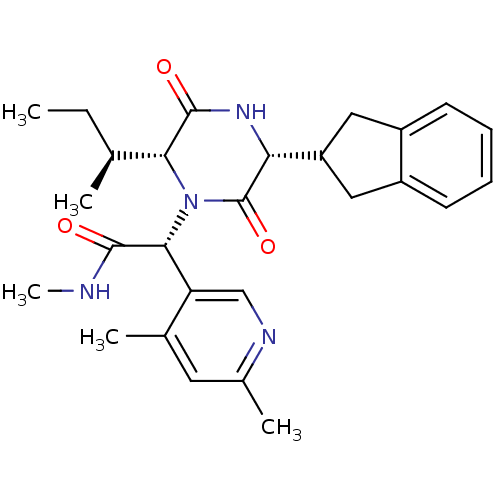
(CHEMBL2037506)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)NC)c2cnc(C)cc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C27H34N4O3/c1-6-15(2)23-26(33)30-22(20-12-18-9-7-8-10-19(18)13-20)27(34)31(23)24(25(32)28-5)21-14-29-17(4)11-16(21)3/h7-11,14-15,20,22-24H,6,12-13H2,1-5H3,(H,28,32)(H,30,33)/t15-,22+,23+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384815
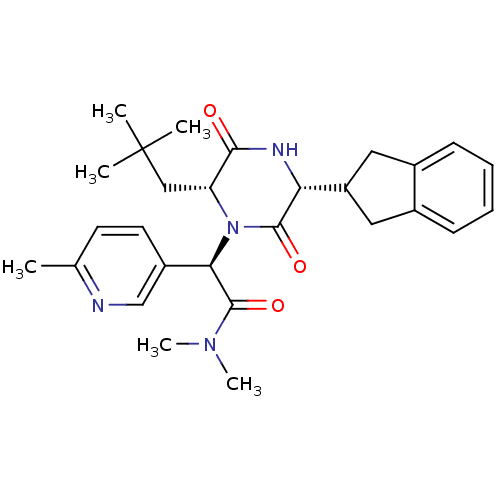
(CHEMBL2037496)Show SMILES CN(C)C(=O)[C@H](N1[C@H](CC(C)(C)C)C(=O)N[C@H](C2Cc3ccccc3C2)C1=O)c1ccc(C)nc1 |r| Show InChI InChI=1S/C28H36N4O3/c1-17-11-12-20(16-29-17)24(27(35)31(5)6)32-22(15-28(2,3)4)25(33)30-23(26(32)34)21-13-18-9-7-8-10-19(18)14-21/h7-12,16,21-24H,13-15H2,1-6H3,(H,30,33)/t22-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384834
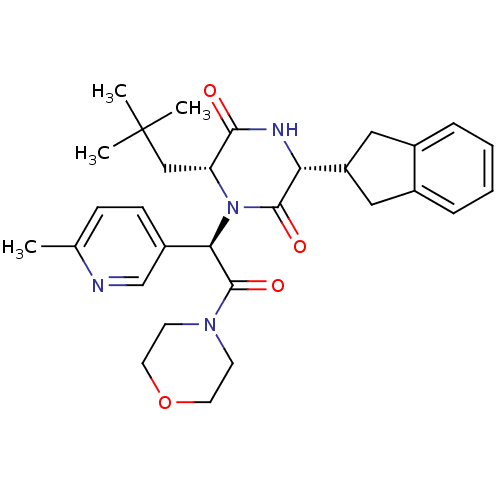
(CHEMBL2037497)Show SMILES Cc1ccc(cn1)[C@@H](N1[C@H](CC(C)(C)C)C(=O)N[C@H](C2Cc3ccccc3C2)C1=O)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C30H38N4O4/c1-19-9-10-22(18-31-19)26(29(37)33-11-13-38-14-12-33)34-24(17-30(2,3)4)27(35)32-25(28(34)36)23-15-20-7-5-6-8-21(20)16-23/h5-10,18,23-26H,11-17H2,1-4H3,(H,32,35)/t24-,25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384838
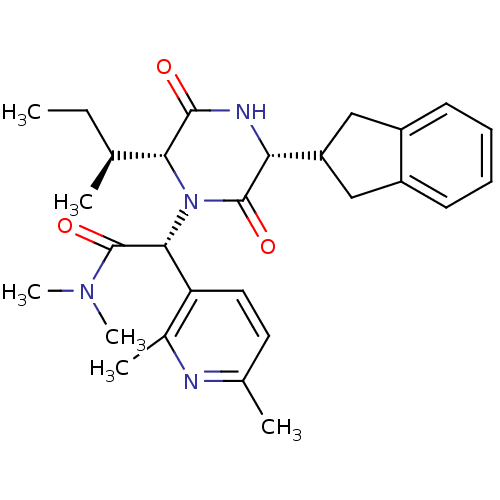
(CHEMBL2037510)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(C)nc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C28H36N4O3/c1-7-16(2)24-26(33)30-23(21-14-19-10-8-9-11-20(19)15-21)27(34)32(24)25(28(35)31(5)6)22-13-12-17(3)29-18(22)4/h8-13,16,21,23-25H,7,14-15H2,1-6H3,(H,30,33)/t16-,23+,24+,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50190528
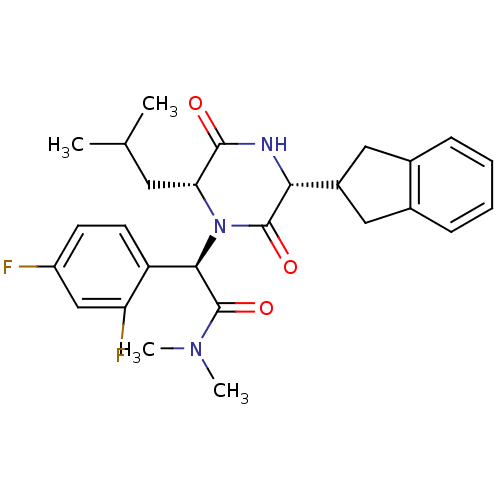
((2R)-2-(2,4-difluorophenyl)-2-[(3R,6R)-3-(2,3-dihy...)Show SMILES CC(C)C[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(F)cc2F)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 Show InChI InChI=1S/C27H31F2N3O3/c1-15(2)11-22-25(33)30-23(18-12-16-7-5-6-8-17(16)13-18)26(34)32(22)24(27(35)31(3)4)20-10-9-19(28)14-21(20)29/h5-10,14-15,18,22-24H,11-13H2,1-4H3,(H,30,33)/t22-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384819
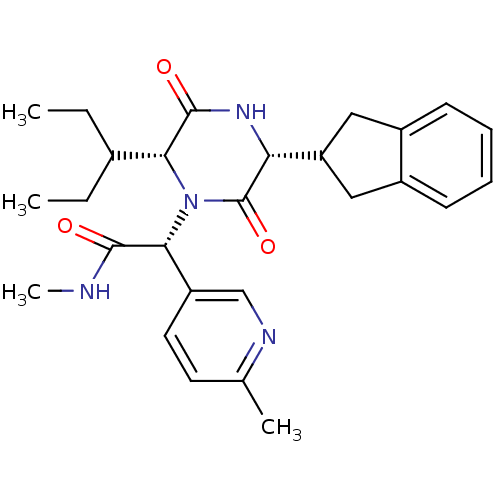
(CHEMBL2037512)Show SMILES CCC(CC)[C@H]1N([C@@H](C(=O)NC)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C27H34N4O3/c1-5-17(6-2)23-26(33)30-22(21-13-18-9-7-8-10-19(18)14-21)27(34)31(23)24(25(32)28-4)20-12-11-16(3)29-15-20/h7-12,15,17,21-24H,5-6,13-14H2,1-4H3,(H,28,32)(H,30,33)/t22-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Galanin receptor type 1
(Homo sapiens (Human)) | BDBM50273370
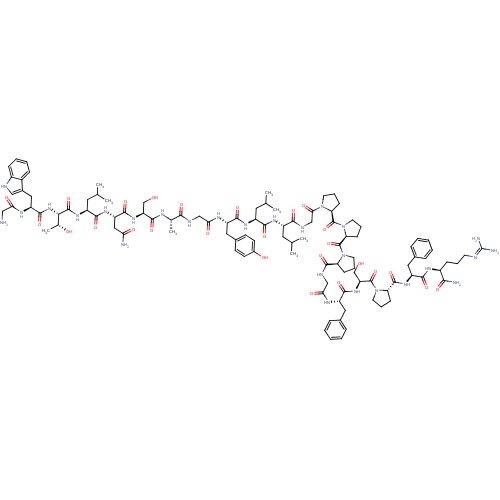
(CHEMBL503473 | GWTLNSAGYLLGPPPGFSPFR-CONH2 | Galan...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CN)[C@@H](C)O)C(=O)NCC(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCN=C(N)N)C(N)=O |r,wU:106.112,4.4,8.12,32.33,37.40,43.48,51.56,63.76,wD:149.157,138.145,134.142,125.131,114.119,99.104,92.96,16.25,59.60,79.83,(16.53,-9.13,;15.19,-9.9,;15.2,-11.44,;13.86,-9.13,;12.52,-9.9,;11.19,-9.13,;11.19,-7.59,;12.53,-6.82,;9.86,-6.82,;9.86,-5.28,;11.2,-4.51,;12.52,-5.28,;11.2,-2.97,;8.13,-7.82,;6.39,-6.82,;6.39,-5.28,;4.66,-7.83,;4.66,-9.36,;5.99,-10.13,;5.99,-11.67,;7.32,-12.44,;8.66,-11.67,;9.99,-12.44,;8.66,-10.13,;7.33,-9.36,;2.92,-6.82,;2.92,-5.28,;4.26,-4.52,;1.19,-4.28,;1.19,-2.75,;2.52,-1.97,;3.86,-2.74,;2.52,-.43,;3.86,.34,;1.2,.34,;1.19,1.88,;2.53,2.65,;-.14,2.65,;-.14,4.19,;1.19,4.96,;-1.48,1.88,;-2.81,2.65,;-2.81,4.19,;-4.14,1.88,;-5.48,2.64,;-5.47,4.19,;-6.81,4.96,;-4.14,4.96,;-4.15,.34,;-5.48,-.43,;-6.81,.34,;-5.48,-1.97,;-6.81,-2.74,;-8.14,-1.97,;-8.14,-.43,;-9.47,-2.74,;-4.14,-2.74,;-4.14,-4.28,;-5.48,-5.05,;-2.81,-5.05,;-2.81,-6.59,;-1.48,-7.36,;-.15,-6.59,;-1.48,-8.9,;-2.85,-9.59,;-4.14,-8.76,;-4.22,-7.22,;-5.71,-6.82,;-6.55,-8.12,;-8.07,-8.35,;-8.62,-9.79,;-7.65,-10.99,;-6.13,-10.75,;-5.58,-9.31,;-.14,-9.67,;1.19,-8.9,;1.19,-7.36,;2.53,-9.67,;2.53,-11.21,;-1.47,-4.28,;-.15,-5.05,;-1.47,-2.74,;12.53,-11.44,;13.86,-12.21,;11.19,-12.21,;11.19,-13.75,;12.53,-14.52,;13.86,-13.75,;12.53,-16.06,;11.28,-16.96,;11.76,-18.43,;13.29,-18.43,;13.77,-16.97,;15.24,-16.49,;15.64,-15,;16.65,-17.91,;16.41,-19.42,;17.78,-20.13,;18.87,-19.04,;18.18,-17.67,;19.08,-15.88,;20.62,-15.8,;18.31,-14.55,;16.78,-14.38,;16.46,-12.88,;17.8,-12.12,;18.94,-13.14,;20.31,-12.44,;21.65,-13.21,;20.31,-10.9,;21.64,-10.13,;22.97,-10.89,;24.3,-10.12,;22.98,-12.43,;24.31,-13.2,;24.32,-14.74,;25.65,-15.5,;25.66,-17.04,;26.99,-17.81,;28.33,-17.04,;28.32,-15.5,;26.99,-14.73,;25.64,-12.43,;25.64,-10.89,;26.98,-13.19,;28.31,-12.42,;29.65,-13.18,;30.98,-12.41,;28.31,-10.88,;26.97,-10.11,;29.64,-10.1,;31.05,-10.72,;32.07,-9.57,;31.3,-8.24,;29.8,-8.57,;28.51,-7.72,;27.17,-8.47,;28.53,-6.17,;29.88,-5.42,;31.2,-6.21,;32.54,-5.45,;32.56,-3.91,;33.91,-3.16,;35.23,-3.95,;35.21,-5.49,;33.87,-6.24,;29.9,-3.88,;31.24,-3.12,;28.57,-3.1,;28.59,-1.55,;29.93,-.8,;29.95,.74,;28.63,1.53,;28.65,3.07,;29.99,3.82,;30.01,5.36,;31.32,3.03,;27.27,-.77,;27.29,.77,;25.92,-1.52,)| Show InChI InChI=1S/C107H153N27O26/c1-57(2)42-70(123-93(147)71(43-58(3)4)124-95(149)74(47-64-33-35-66(138)36-34-64)121-86(141)52-115-91(145)60(7)118-100(154)78(55-135)128-98(152)77(49-84(109)139)125-94(148)72(44-59(5)6)127-103(157)89(61(8)137)130-99(153)76(119-85(140)50-108)48-65-51-114-68-27-16-15-26-67(65)68)92(146)117-54-88(143)131-38-20-31-82(131)105(159)134-41-21-32-83(134)106(160)133-40-18-29-80(133)101(155)116-53-87(142)120-73(45-62-22-11-9-12-23-62)96(150)129-79(56-136)104(158)132-39-19-30-81(132)102(156)126-75(46-63-24-13-10-14-25-63)97(151)122-69(90(110)144)28-17-37-113-107(111)112/h9-16,22-27,33-36,51,57-61,69-83,89,114,135-138H,17-21,28-32,37-50,52-56,108H2,1-8H3,(H2,109,139)(H2,110,144)(H,115,145)(H,116,155)(H,117,146)(H,118,154)(H,119,140)(H,120,142)(H,121,141)(H,122,151)(H,123,147)(H,124,149)(H,125,148)(H,126,156)(H,127,157)(H,128,152)(H,129,150)(H,130,153)(H4,111,112,113)/t60-,61+,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,89-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation
Curated by PDSP Ki Database
| |
Peptides 19: 1771-81 (1998)
Article DOI: 10.1016/s0196-9781(98)00133-8
BindingDB Entry DOI: 10.7270/Q2NC5ZQ6 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(RAT) | BDBM50302273
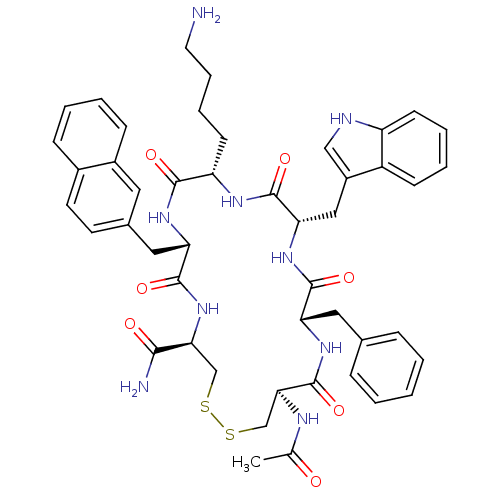
((4R,7S,10S,13S,16S,19R)-13-((1H-indol-3-yl)methyl)...)Show SMILES CC(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccc3ccccc3c2)NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC1=O)C(N)=O |r| Show InChI InChI=1S/C47H55N9O7S2/c1-28(57)51-41-27-65-64-26-40(42(49)58)56-45(61)38(23-30-18-19-31-13-5-6-14-32(31)21-30)53-43(59)36(17-9-10-20-48)52-46(62)39(24-33-25-50-35-16-8-7-15-34(33)35)55-44(60)37(54-47(41)63)22-29-11-3-2-4-12-29/h2-8,11-16,18-19,21,25,36-41,50H,9-10,17,20,22-24,26-27,48H2,1H3,(H2,49,58)(H,51,57)(H,52,62)(H,53,59)(H,54,63)(H,55,60)(H,56,61)/t36-,37-,38-,39-,40-,41-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]urotensin 2 from rat urotensin 2 receptor expressed in CHOK1 cells by scintillation proximity assay |
J Med Chem 52: 7432-45 (2009)
Article DOI: 10.1021/jm900683d
BindingDB Entry DOI: 10.7270/Q2S75GD7 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384820
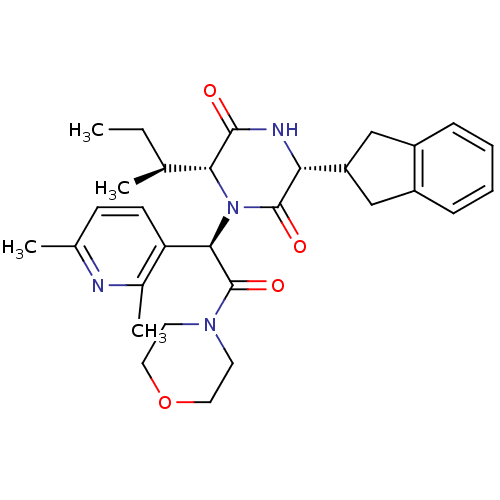
(EPELSIBAN | GSK557296B)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2ccc(C)nc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C30H38N4O4/c1-5-18(2)26-28(35)32-25(23-16-21-8-6-7-9-22(21)17-23)29(36)34(26)27(24-11-10-19(3)31-20(24)4)30(37)33-12-14-38-15-13-33/h6-11,18,23,25-27H,5,12-17H2,1-4H3,(H,32,35)/t18-,25+,26+,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384803
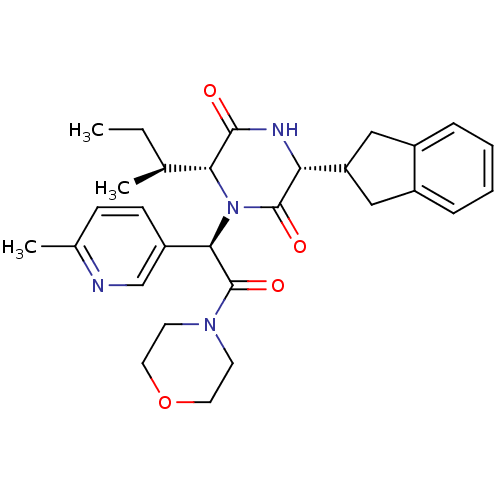
(CHEMBL2037501)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C29H36N4O4/c1-4-18(2)25-27(34)31-24(23-15-20-7-5-6-8-21(20)16-23)28(35)33(25)26(22-10-9-19(3)30-17-22)29(36)32-11-13-37-14-12-32/h5-10,17-18,23-26H,4,11-16H2,1-3H3,(H,31,34)/t18-,24+,25+,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Ability to inhibit the binding of iodine-125-labelled lysergic acid diethylamide([125I]-LSD) to the S-2A serotonin receptor. |
J Med Chem 38: 708-14 (1995)
BindingDB Entry DOI: 10.7270/Q2PG1SCG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442142
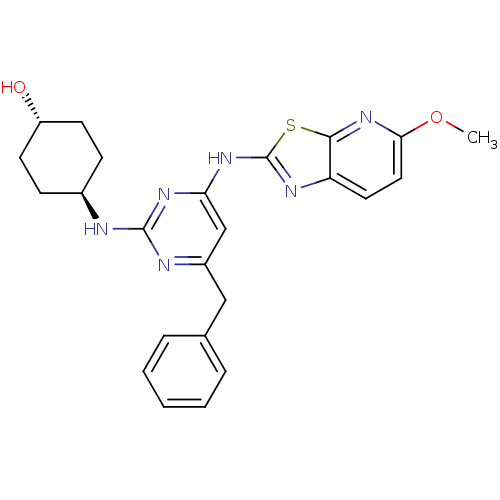
(CHEMBL2441275)Show SMILES COc1ccc2nc(Nc3cc(Cc4ccccc4)nc(N[C@H]4CC[C@H](O)CC4)n3)sc2n1 |r,wU:25.26,wD:22.22,(34.29,-5.26,;33.51,-3.93,;31.98,-3.93,;31.04,-2.71,;29.52,-2.9,;28.92,-4.33,;27.47,-4.83,;27.5,-6.37,;26.29,-7.31,;26.32,-8.85,;25,-9.64,;25.03,-11.18,;23.69,-11.97,;22.36,-11.2,;22.37,-9.66,;21.03,-8.89,;19.69,-9.66,;19.69,-11.2,;21.03,-11.97,;26.37,-11.93,;27.69,-11.14,;29.03,-11.88,;30.36,-11.09,;31.7,-11.84,;33.02,-11.06,;33,-9.52,;34.33,-8.73,;31.66,-8.77,;30.34,-9.56,;27.67,-9.6,;28.98,-6.82,;29.86,-5.55,;31.38,-5.35,)| Show InChI InChI=1S/C24H26N6O2S/c1-32-21-12-11-19-22(30-21)33-24(27-19)29-20-14-17(13-15-5-3-2-4-6-15)26-23(28-20)25-16-7-9-18(31)10-8-16/h2-6,11-12,14,16,18,31H,7-10,13H2,1H3,(H2,25,26,27,28,29)/t16-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384805
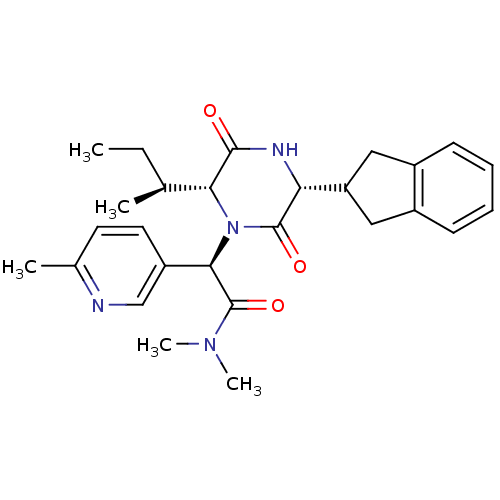
(CHEMBL2037499)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C27H34N4O3/c1-6-16(2)23-25(32)29-22(21-13-18-9-7-8-10-19(18)14-21)26(33)31(23)24(27(34)30(4)5)20-12-11-17(3)28-15-20/h7-12,15-16,21-24H,6,13-14H2,1-5H3,(H,29,32)/t16-,22+,23+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50007518

((S)-3-chloro-5-ethyl-N-((1-ethylpyrrolidin-2-yl)me...)Show InChI InChI=1S/C17H25ClN2O3/c1-4-11-9-13(18)16(23-3)14(15(11)21)17(22)19-10-12-7-6-8-20(12)5-2/h9,12,21H,4-8,10H2,1-3H3,(H,19,22)/t12-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 264: 1002-11 (1993)
BindingDB Entry DOI: 10.7270/Q2T1525C |
More data for this
Ligand-Target Pair | |
Galanin receptor type 1
(Homo sapiens (Human)) | BDBM85070
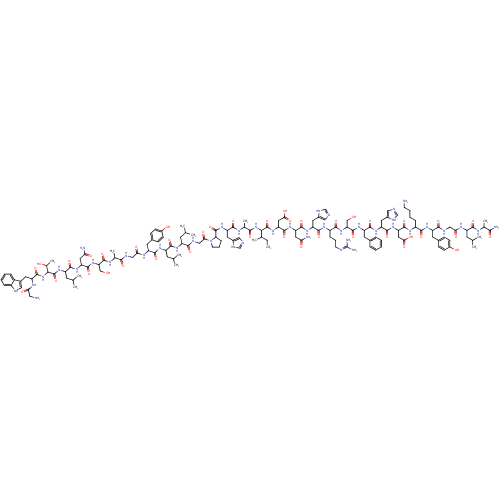
(Galanin, Porcine)Show SMILES CCC(C)C(NC(=O)C(C)NC(=O)C(Cc1cnc[nH]1)NC(=O)C1CCCN1C(=O)CNC(=O)C(CC(C)C)NC(=O)C(CC(C)C)NC(=O)C(Cc1ccc(O)cc1)NC(=O)CNC(=O)C(C)NC(=O)C(CO)NC(=O)C(CC(N)=O)NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(Cc1c[nH]c2ccccc12)NC(=O)CN)C(C)O)C(=O)NC(CC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(Cc1cnc[nH]1)C(=O)NC(CCCN=C(N)N)C(=O)NC(CO)C(=O)NC(Cc1ccccc1)C(=O)NC(Cc1cnc[nH]1)C(=O)NC(CC(O)=O)C(=O)NC(CCCCN)C(=O)NC(Cc1ccc(O)cc1)C(=O)NCC(=O)NC(CC(C)C)C(=O)NC(C)C(N)=O |(22.15,12.84,;20.71,12.32,;20.44,10.8,;21.61,9.81,;18.99,10.28,;18.72,8.76,;19.9,7.77,;21.34,8.3,;19.63,6.25,;18.18,5.73,;20.8,5.26,;20.53,3.75,;19.09,3.22,;21.71,2.75,;21.44,1.24,;19.99,.71,;18.72,1.58,;17.5,.63,;18.03,-.82,;19.57,-.77,;23.16,3.28,;24.34,2.29,;24.07,.77,;25.79,2.81,;26.21,4.29,;27.75,4.34,;28.28,2.89,;27.06,1.95,;27.11,.41,;25.81,-.41,;28.47,-.32,;28.52,-1.86,;29.88,-2.58,;31.19,-1.77,;29.93,-4.12,;31.29,-4.85,;32.6,-4.03,;33.96,-4.76,;32.55,-2.49,;28.62,-4.93,;28.68,-6.47,;30.2,-6.69,;27.73,-7.69,;26.22,-8,;25.19,-6.86,;23.68,-7.17,;25.67,-5.39,;28.3,-9.11,;27.35,-10.33,;25.82,-10.11,;27.92,-11.76,;26.97,-12.97,;25.45,-12.75,;24.87,-11.32,;23.35,-11.1,;22.4,-12.32,;20.88,-12.1,;22.97,-13.75,;24.5,-13.96,;29.45,-11.97,;30.02,-13.4,;29.07,-14.61,;31.55,-13.62,;32.12,-15.05,;33.65,-15.26,;34.6,-14.05,;34.22,-16.69,;33.27,-17.9,;35.75,-16.91,;36.32,-18.34,;35.37,-19.55,;37.85,-18.56,;38.8,-17.34,;38.22,-15.91,;38.42,-19.98,;39.94,-20.2,;40.89,-18.99,;40.52,-21.63,;42.04,-21.85,;42.99,-20.64,;44.52,-20.85,;42.42,-19.21,;39.57,-22.84,;40.14,-24.27,;41.65,-23.95,;39.66,-25.74,;38.36,-26.55,;37,-25.82,;35.69,-26.64,;36.95,-24.28,;40.69,-26.88,;40.21,-28.35,;38.71,-28.66,;41.24,-29.49,;40.76,-30.96,;41.79,-32.1,;43.3,-31.78,;41.31,-33.57,;39.81,-33.88,;39.33,-35.35,;40.23,-36.59,;39.33,-37.83,;37.87,-37.36,;36.54,-38.13,;35.2,-37.36,;35.2,-35.82,;36.54,-35.05,;37.87,-35.82,;42.34,-34.71,;43.85,-34.39,;44.33,-32.93,;44.88,-35.54,;46.39,-35.22,;42.75,-29.17,;43.78,-30.32,;43.23,-27.71,;17.81,11.27,;16.36,10.75,;18.08,12.79,;16.9,13.78,;15.45,13.25,;14.28,14.25,;12.83,13.72,;14.55,15.76,;17.17,15.3,;15.99,16.29,;18.62,15.82,;18.89,17.34,;17.37,17.61,;16.85,19.05,;15.33,19.32,;17.84,20.23,;20.34,17.86,;21.52,16.87,;21.33,19.04,;22.85,18.77,;23.37,17.32,;24.89,17.05,;26,18.12,;27.36,17.39,;27.09,15.88,;25.56,15.66,;23.84,19.95,;25.35,19.68,;23.31,21.39,;24.31,22.57,;25.82,22.3,;26.81,23.48,;28.33,23.21,;29.32,24.39,;30.84,24.12,;31.83,25.3,;31.36,22.67,;23.78,24.02,;22.27,24.29,;24.77,25.2,;24.25,26.65,;22.73,26.92,;22.21,28.36,;25.24,27.82,;26.76,27.55,;24.72,29.27,;25.71,30.45,;27.23,30.18,;28.22,31.36,;29.73,31.09,;30.73,32.27,;30.2,33.71,;28.69,33.98,;27.69,32.81,;25.19,31.9,;26.18,33.08,;23.67,32.17,;23.14,33.62,;24.14,34.79,;23.61,36.24,;22.13,36.67,;22.08,38.21,;23.53,38.73,;24.48,37.52,;21.63,33.89,;21.1,35.33,;20.64,32.71,;19.12,32.98,;18.6,34.43,;17.42,35.42,;17.69,36.93,;15.97,34.89,;18.13,31.8,;18.65,30.35,;16.61,32.07,;15.62,30.89,;16.14,29.44,;15.15,28.27,;15.68,26.82,;14.69,25.64,;15.21,24.19,;14.1,31.16,;13.58,32.61,;13.11,29.98,;11.6,30.25,;11.07,31.7,;9.56,31.97,;9.03,33.42,;7.52,33.69,;6.52,32.51,;5.01,32.78,;7.05,31.06,;8.56,30.79,;10.6,29.08,;9.09,29.35,;11.13,27.63,;10.14,26.45,;8.62,26.72,;8.1,28.17,;7.63,25.54,;6.11,25.81,;5.59,27.26,;4.07,27.53,;3.55,28.98,;3.08,26.35,;5.12,24.63,;3.6,24.9,;5.64,23.19,;4.65,22.01,;3.14,22.28,;5.18,20.56,;4.19,19.38,;6.69,20.29,)| Show InChI InChI=1S/C146H213N43O40/c1-15-75(10)119(187-123(207)78(13)167-129(213)101(51-84-60-154-68-162-84)182-143(227)110-31-24-42-189(110)116(200)65-161-124(208)93(43-71(2)3)173-130(214)95(45-73(6)7)174-132(216)98(49-82-34-38-88(194)39-35-82)170-114(198)63-159-122(206)77(12)166-141(225)108(66-190)186-137(221)105(55-112(150)196)179-131(215)96(46-74(8)9)183-145(229)120(79(14)192)188-140(224)100(168-113(197)58-148)50-83-59-158-90-28-20-19-27-89(83)90)144(228)184-107(57-118(203)204)139(223)180-104(54-111(149)195)136(220)178-102(52-85-61-155-69-163-85)134(218)172-92(30-23-41-157-146(152)153)127(211)185-109(67-191)142(226)176-99(47-80-25-17-16-18-26-80)133(217)177-103(53-86-62-156-70-164-86)135(219)181-106(56-117(201)202)138(222)171-91(29-21-22-40-147)126(210)175-97(48-81-32-36-87(193)37-33-81)125(209)160-64-115(199)169-94(44-72(4)5)128(212)165-76(11)121(151)205/h16-20,25-28,32-39,59-62,68-79,91-110,119-120,158,190-194H,15,21-24,29-31,40-58,63-67,147-148H2,1-14H3,(H2,149,195)(H2,150,196)(H2,151,205)(H,154,162)(H,155,163)(H,156,164)(H,159,206)(H,160,209)(H,161,208)(H,165,212)(H,166,225)(H,167,213)(H,168,197)(H,169,199)(H,170,198)(H,171,222)(H,172,218)(H,173,214)(H,174,216)(H,175,210)(H,176,226)(H,177,217)(H,178,220)(H,179,215)(H,180,223)(H,181,219)(H,182,227)(H,183,229)(H,184,228)(H,185,211)(H,186,221)(H,187,207)(H,188,224)(H,201,202)(H,203,204)(H4,152,153,157) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation
Curated by PDSP Ki Database
| |
Peptides 19: 1771-81 (1998)
Article DOI: 10.1016/s0196-9781(98)00133-8
BindingDB Entry DOI: 10.7270/Q2NC5ZQ6 |
More data for this
Ligand-Target Pair | |
Galanin receptor type 1
(Homo sapiens (Human)) | BDBM85073
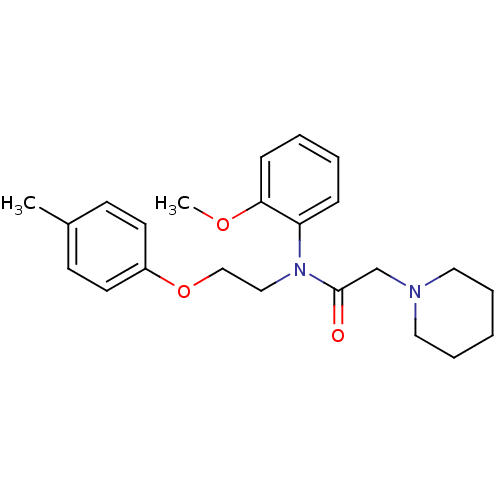
(CAS_3043476 | Galantide (M15) | NSC_3043476)Show InChI InChI=1S/C23H30N2O3/c1-19-10-12-20(13-11-19)28-17-16-25(21-8-4-5-9-22(21)27-2)23(26)18-24-14-6-3-7-15-24/h4-5,8-13H,3,6-7,14-18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation
Curated by PDSP Ki Database
| |
Peptides 19: 1771-81 (1998)
Article DOI: 10.1016/s0196-9781(98)00133-8
BindingDB Entry DOI: 10.7270/Q2NC5ZQ6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442143
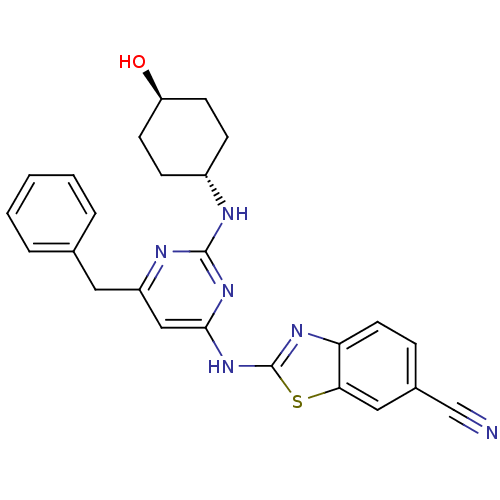
(CHEMBL2441274)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1nc(Cc2ccccc2)cc(Nc2nc3ccc(cc3s2)C#N)n1 |r,wU:1.0,wD:4.7,(23.68,-13.98,;22.35,-14.78,;22.37,-16.32,;21.05,-17.1,;19.71,-16.35,;19.69,-14.81,;21.01,-14.02,;18.39,-17.14,;17.04,-16.39,;15.72,-17.18,;14.38,-16.44,;13.04,-17.23,;11.71,-16.46,;11.71,-14.92,;10.38,-14.14,;9.04,-14.92,;9.04,-16.46,;10.38,-17.22,;14.35,-14.9,;15.67,-14.1,;15.65,-12.56,;16.85,-11.63,;16.82,-10.09,;18.27,-9.58,;18.87,-8.16,;20.4,-7.96,;21.33,-9.19,;20.73,-10.61,;19.2,-10.8,;18.33,-12.08,;22.85,-8.99,;24.37,-8.8,;17.02,-14.86,)| Show InChI InChI=1S/C25H24N6OS/c26-15-17-6-11-21-22(13-17)33-25(29-21)31-23-14-19(12-16-4-2-1-3-5-16)28-24(30-23)27-18-7-9-20(32)10-8-18/h1-6,11,13-14,18,20,32H,7-10,12H2,(H2,27,28,29,30,31)/t18-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442141

(CHEMBL2441276)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1nc(Cc2ccccc2)cc(Nc2nc3cccnc3s2)n1 |r,wU:1.0,wD:4.7,(34.33,-8.73,;33,-9.52,;33.02,-11.06,;31.7,-11.84,;30.36,-11.09,;30.34,-9.56,;31.66,-8.77,;29.03,-11.88,;27.69,-11.14,;26.37,-11.93,;25.03,-11.18,;23.69,-11.97,;22.36,-11.2,;22.36,-9.66,;21.03,-8.89,;19.69,-9.66,;19.69,-11.2,;21.03,-11.97,;25,-9.64,;26.32,-8.85,;26.29,-7.31,;27.5,-6.37,;27.47,-4.83,;28.92,-4.33,;29.52,-2.9,;31.04,-2.71,;31.98,-3.93,;31.38,-5.35,;29.86,-5.55,;28.98,-6.82,;27.67,-9.6,)| Show InChI InChI=1S/C23H24N6OS/c30-18-10-8-16(9-11-18)25-22-26-17(13-15-5-2-1-3-6-15)14-20(28-22)29-23-27-19-7-4-12-24-21(19)31-23/h1-7,12,14,16,18,30H,8-11,13H2,(H2,25,26,27,28,29)/t16-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384836
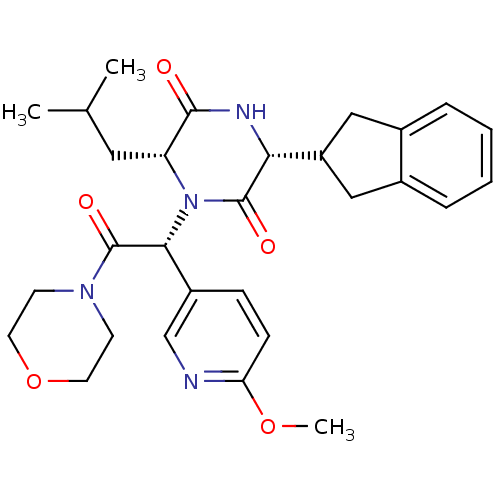
(CHEMBL2037487)Show SMILES COc1ccc(cn1)[C@@H](N1[C@H](CC(C)C)C(=O)N[C@H](C2Cc3ccccc3C2)C1=O)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C29H36N4O5/c1-18(2)14-23-27(34)31-25(22-15-19-6-4-5-7-20(19)16-22)28(35)33(23)26(21-8-9-24(37-3)30-17-21)29(36)32-10-12-38-13-11-32/h4-9,17-18,22-23,25-26H,10-16H2,1-3H3,(H,31,34)/t23-,25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384812
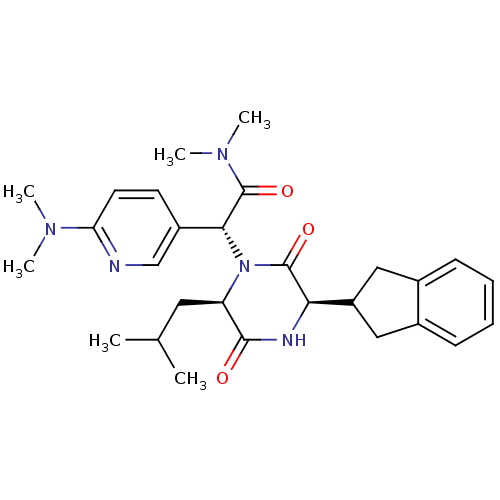
(CHEMBL2037489)Show SMILES CC(C)C[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(nc2)N(C)C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C28H37N5O3/c1-17(2)13-22-26(34)30-24(21-14-18-9-7-8-10-19(18)15-21)27(35)33(22)25(28(36)32(5)6)20-11-12-23(29-16-20)31(3)4/h7-12,16-17,21-22,24-25H,13-15H2,1-6H3,(H,30,34)/t22-,24-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Galanin receptor type 1
(Homo sapiens (Human)) | BDBM85168

(M32)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H]1CCCN1C(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CN)[C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |r,wU:111.115,131.138,95.103,84.88,68.80,52.59,20.28,4.4,2.2,145.150,161.166,162.169,179.184,188.193,199.204,wD:115.131,103.111,89.95,60.67,41.42,30.39,8.17,137.142,153.158,154.160,168.173,(13.69,11.51,;13.72,9.97,;12.46,9.14,;11.11,9.89,;12.54,7.63,;11.23,6.8,;9.89,7.48,;9.85,9.02,;8.62,6.65,;8.66,5.14,;10.01,4.43,;10.12,2.93,;11.51,2.18,;12.74,3.05,;14.16,2.37,;12.66,4.59,;11.27,5.26,;7.28,7.36,;5.97,6.57,;5.97,4.98,;4.67,7.32,;4.63,8.82,;5.93,9.65,;7.36,9.02,;8.39,10.12,;7.71,11.47,;6.13,11.19,;3.36,6.53,;2.02,7.16,;2.02,8.62,;.63,6.37,;.59,4.82,;1.94,3.99,;1.94,2.45,;3.24,1.66,;3.2,.08,;4.55,-.67,;1.86,-.67,;-.71,7.2,;-2.06,6.49,;-3.56,7.12,;-2.06,5.02,;-.75,4.11,;-1.19,2.65,;-2.69,2.61,;-3.16,4.03,;-4.63,4.43,;-4.98,5.93,;-5.77,3.36,;-7.24,3.84,;-8.27,2.73,;-7.91,1.19,;-9.73,3.08,;-10.12,4.59,;-9.02,5.7,;-9.41,7.2,;-7.51,5.26,;-10.84,2.06,;-12.34,2.37,;-12.66,3.8,;-13.41,1.27,;-13.05,-.2,;-11.59,-.59,;-10.48,.51,;-11.15,-2.06,;-14.87,1.7,;-15.98,.67,;-15.62,-.87,;-17.56,1.07,;-17.92,2.61,;-16.85,3.64,;-15.35,3.28,;-14.24,4.39,;-14.6,5.85,;-13.49,6.96,;-16.1,6.29,;-17.24,5.22,;-18.59,0,;-20.05,.44,;-20.45,1.9,;-21.12,-.67,;-22.54,-.28,;-23.65,-1.34,;-23.26,-2.81,;-25.19,-.99,;-25.55,.55,;-26.26,-2.02,;-27.73,-1.62,;-28.08,-.16,;-28.83,-2.69,;-28.44,-4.15,;-26.97,-4.63,;-30.3,-2.25,;-31.44,-3.28,;-31.09,-4.71,;-32.95,-2.81,;-33.3,-1.34,;-32.19,-.28,;-30.73,-.71,;-32.55,1.23,;-34.05,-3.92,;-35.56,-3.56,;-35.87,-2.14,;-36.63,-4.71,;-36.27,-6.13,;-34.8,-6.57,;-33.7,-5.5,;-34.41,-8.03,;-38.09,-4.23,;-39.23,-5.3,;-38.84,-6.76,;-40.7,-4.87,;-41.85,-5.93,;-43.27,-5.54,;-43.66,-4.03,;-44.46,-6.6,;-44.18,-8.11,;-42.64,-8.54,;-41.45,-7.59,;-40.11,-8.39,;-40.54,-9.93,;-39.71,-11.27,;-40.46,-12.66,;-41.96,-12.66,;-42.87,-11.35,;-42.12,-10.05,;-45.92,-6.13,;-47.07,-7.2,;-46.71,-8.66,;-48.57,-6.76,;-49.68,-7.83,;-41.05,-3.36,;-42.6,-2.89,;-39.91,-2.29,;13.88,6.88,;13.88,5.42,;15.19,7.67,;16.53,6.88,;16.53,5.38,;17.92,4.67,;19.18,5.46,;17.96,3.12,;17.84,7.71,;17.84,9.18,;19.18,7.04,;20.45,7.75,;20.49,9.25,;21.79,10.09,;21.83,11.59,;23.14,9.25,;21.79,7.04,;21.79,5.46,;23.14,7.75,;24.44,7,;24.48,5.5,;23.18,4.71,;25.79,4.75,;25.83,3.24,;25.71,7.75,;25.71,9.29,;27.01,6.92,;28.32,7.71,;28.32,9.25,;27.01,10.05,;29.66,10.01,;29.66,6.96,;29.66,5.46,;30.97,7.67,;32.31,6.88,;32.27,5.38,;33.62,4.59,;33.62,3.08,;34.88,2.37,;34.92,.83,;36.27,.04,;33.62,0,;33.66,7.63,;33.66,9.1,;35,6.88,;36.27,7.67,;36.27,9.18,;37.53,9.97,;37.53,11.47,;38.8,12.26,;36.19,12.26,;37.61,6.92,;37.61,5.38,;38.88,7.75,;40.18,7.04,;40.26,5.5,;41.61,4.71,;41.69,3.16,;42.99,2.49,;43.07,.95,;44.46,.16,;41.77,.04,;41.53,7.87,;41.49,9.25,;42.91,7.24,;44.22,7.99,;44.22,9.57,;45.56,10.32,;45.6,11.87,;46.99,12.66,;48.33,11.83,;49.68,12.58,;48.29,10.32,;46.91,9.53,;45.52,7.24,;46.79,8.07,;45.52,5.66,)| Show InChI InChI=1S/C136H209N41O34/c1-16-70(11)108(130(208)171-99(58-104(140)186)123(201)166-93(51-69(9)10)125(203)174-109(71(12)17-2)131(209)176-111(74(15)180)132(210)162-87(28-22-46-151-136(146)147)115(193)160-88(42-43-102(138)184)118(196)159-85(26-20-44-149-134(142)143)116(194)163-89(112(141)190)52-75-30-36-80(181)37-31-75)173-126(204)95(54-77-34-40-82(183)41-35-77)167-122(200)97(56-79-61-148-65-155-79)168-117(195)86(27-21-45-150-135(144)145)161-129(207)101-29-23-47-177(101)107(189)63-154-114(192)90(48-66(3)4)164-119(197)91(49-67(5)6)165-121(199)94(53-76-32-38-81(182)39-33-76)158-106(188)62-153-113(191)72(13)156-128(206)100(64-178)172-124(202)98(57-103(139)185)169-120(198)92(50-68(7)8)170-133(211)110(73(14)179)175-127(205)96(157-105(187)59-137)55-78-60-152-84-25-19-18-24-83(78)84/h18-19,24-25,30-41,60-61,65-74,85-101,108-111,152,178-183H,16-17,20-23,26-29,42-59,62-64,137H2,1-15H3,(H2,138,184)(H2,139,185)(H2,140,186)(H2,141,190)(H,148,155)(H,153,191)(H,154,192)(H,156,206)(H,157,187)(H,158,188)(H,159,196)(H,160,193)(H,161,207)(H,162,210)(H,163,194)(H,164,197)(H,165,199)(H,166,201)(H,167,200)(H,168,195)(H,169,198)(H,170,211)(H,171,208)(H,172,202)(H,173,204)(H,174,203)(H,175,205)(H,176,209)(H4,142,143,149)(H4,144,145,150)(H4,146,147,151)/t70-,71-,72-,73+,74+,85+,86-,87-,88-,89-,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,108-,109-,110-,111-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation
Curated by PDSP Ki Database
| |
Peptides 19: 1771-81 (1998)
Article DOI: 10.1016/s0196-9781(98)00133-8
BindingDB Entry DOI: 10.7270/Q2NC5ZQ6 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM84746

(BRL 34778 | BRL-34778)Show SMILES COc1cc(N)c(Cl)cc1C(=O)N[C@H]1C[C@@H]2CCC[C@H](C1)N2Cc1ccc(F)cc1 |THB:12:13:21:16.18.17| Show InChI InChI=1S/C23H27ClFN3O2/c1-30-22-12-21(26)20(24)11-19(22)23(29)27-16-9-17-3-2-4-18(10-16)28(17)13-14-5-7-15(25)8-6-14/h5-8,11-12,16-18H,2-4,9-10,13,26H2,1H3,(H,27,29)/t16-,17-,18+ | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 264: 1002-11 (1993)
BindingDB Entry DOI: 10.7270/Q2T1525C |
More data for this
Ligand-Target Pair | |
Galanin receptor type 1
(Homo sapiens (Human)) | BDBM85200
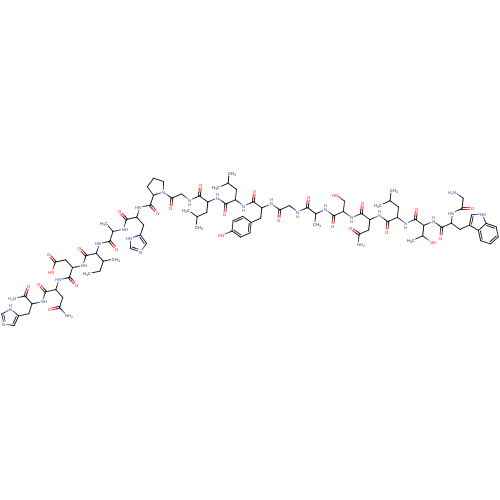
(Galanin (1-19), rat | Galanin rat)Show SMILES CCC(C)C(NC(=O)C(C)NC(=O)C(Cc1cnc[nH]1)NC(=O)C1CCCN1C(=O)CNC(=O)C(CC(C)C)NC(=O)C(CC(C)C)NC(=O)C(Cc1ccc(O)cc1)NC(=O)CNC(=O)C(C)NC(=O)C(CO)NC(=O)C(CC(N)=O)NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(Cc1c[nH]c2ccccc12)NC(=O)CN)C(C)O)C(=O)NC(CC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(Cc1cnc[nH]1)C(N)=O Show InChI InChI=1S/C92H135N27O26/c1-12-46(8)75(91(144)115-66(33-74(128)129)87(140)112-64(31-69(94)123)85(138)108-57(77(96)130)29-52-36-97-41-102-52)117-79(132)48(10)105-81(134)63(30-53-37-98-42-103-53)113-90(143)68-18-15-23-119(68)73(127)39-101-80(133)58(24-43(2)3)109-82(135)59(25-44(4)5)110-84(137)61(27-50-19-21-54(122)22-20-50)107-72(126)38-100-78(131)47(9)104-89(142)67(40-120)116-86(139)65(32-70(95)124)111-83(136)60(26-45(6)7)114-92(145)76(49(11)121)118-88(141)62(106-71(125)34-93)28-51-35-99-56-17-14-13-16-55(51)56/h13-14,16-17,19-22,35-37,41-49,57-68,75-76,99,120-122H,12,15,18,23-34,38-40,93H2,1-11H3,(H2,94,123)(H2,95,124)(H2,96,130)(H,97,102)(H,98,103)(H,100,131)(H,101,133)(H,104,142)(H,105,134)(H,106,125)(H,107,126)(H,108,138)(H,109,135)(H,110,137)(H,111,136)(H,112,140)(H,113,143)(H,114,145)(H,115,144)(H,116,139)(H,117,132)(H,118,141)(H,128,129) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation
Curated by PDSP Ki Database
| |
Peptides 19: 1771-81 (1998)
Article DOI: 10.1016/s0196-9781(98)00133-8
BindingDB Entry DOI: 10.7270/Q2NC5ZQ6 |
More data for this
Ligand-Target Pair | |
Galanin receptor type 1
(RAT) | BDBM50273370

(CHEMBL503473 | GWTLNSAGYLLGPPPGFSPFR-CONH2 | Galan...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CN)[C@@H](C)O)C(=O)NCC(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCN=C(N)N)C(N)=O |r,wU:106.112,4.4,8.12,32.33,37.40,43.48,51.56,63.76,wD:149.157,138.145,134.142,125.131,114.119,99.104,92.96,16.25,59.60,79.83,(16.53,-9.13,;15.19,-9.9,;15.2,-11.44,;13.86,-9.13,;12.52,-9.9,;11.19,-9.13,;11.19,-7.59,;12.53,-6.82,;9.86,-6.82,;9.86,-5.28,;11.2,-4.51,;12.52,-5.28,;11.2,-2.97,;8.13,-7.82,;6.39,-6.82,;6.39,-5.28,;4.66,-7.83,;4.66,-9.36,;5.99,-10.13,;5.99,-11.67,;7.32,-12.44,;8.66,-11.67,;9.99,-12.44,;8.66,-10.13,;7.33,-9.36,;2.92,-6.82,;2.92,-5.28,;4.26,-4.52,;1.19,-4.28,;1.19,-2.75,;2.52,-1.97,;3.86,-2.74,;2.52,-.43,;3.86,.34,;1.2,.34,;1.19,1.88,;2.53,2.65,;-.14,2.65,;-.14,4.19,;1.19,4.96,;-1.48,1.88,;-2.81,2.65,;-2.81,4.19,;-4.14,1.88,;-5.48,2.64,;-5.47,4.19,;-6.81,4.96,;-4.14,4.96,;-4.15,.34,;-5.48,-.43,;-6.81,.34,;-5.48,-1.97,;-6.81,-2.74,;-8.14,-1.97,;-8.14,-.43,;-9.47,-2.74,;-4.14,-2.74,;-4.14,-4.28,;-5.48,-5.05,;-2.81,-5.05,;-2.81,-6.59,;-1.48,-7.36,;-.15,-6.59,;-1.48,-8.9,;-2.85,-9.59,;-4.14,-8.76,;-4.22,-7.22,;-5.71,-6.82,;-6.55,-8.12,;-8.07,-8.35,;-8.62,-9.79,;-7.65,-10.99,;-6.13,-10.75,;-5.58,-9.31,;-.14,-9.67,;1.19,-8.9,;1.19,-7.36,;2.53,-9.67,;2.53,-11.21,;-1.47,-4.28,;-.15,-5.05,;-1.47,-2.74,;12.53,-11.44,;13.86,-12.21,;11.19,-12.21,;11.19,-13.75,;12.53,-14.52,;13.86,-13.75,;12.53,-16.06,;11.28,-16.96,;11.76,-18.43,;13.29,-18.43,;13.77,-16.97,;15.24,-16.49,;15.64,-15,;16.65,-17.91,;16.41,-19.42,;17.78,-20.13,;18.87,-19.04,;18.18,-17.67,;19.08,-15.88,;20.62,-15.8,;18.31,-14.55,;16.78,-14.38,;16.46,-12.88,;17.8,-12.12,;18.94,-13.14,;20.31,-12.44,;21.65,-13.21,;20.31,-10.9,;21.64,-10.13,;22.97,-10.89,;24.3,-10.12,;22.98,-12.43,;24.31,-13.2,;24.32,-14.74,;25.65,-15.5,;25.66,-17.04,;26.99,-17.81,;28.33,-17.04,;28.32,-15.5,;26.99,-14.73,;25.64,-12.43,;25.64,-10.89,;26.98,-13.19,;28.31,-12.42,;29.65,-13.18,;30.98,-12.41,;28.31,-10.88,;26.97,-10.11,;29.64,-10.1,;31.05,-10.72,;32.07,-9.57,;31.3,-8.24,;29.8,-8.57,;28.51,-7.72,;27.17,-8.47,;28.53,-6.17,;29.88,-5.42,;31.2,-6.21,;32.54,-5.45,;32.56,-3.91,;33.91,-3.16,;35.23,-3.95,;35.21,-5.49,;33.87,-6.24,;29.9,-3.88,;31.24,-3.12,;28.57,-3.1,;28.59,-1.55,;29.93,-.8,;29.95,.74,;28.63,1.53,;28.65,3.07,;29.99,3.82,;30.01,5.36,;31.32,3.03,;27.27,-.77,;27.29,.77,;25.92,-1.52,)| Show InChI InChI=1S/C107H153N27O26/c1-57(2)42-70(123-93(147)71(43-58(3)4)124-95(149)74(47-64-33-35-66(138)36-34-64)121-86(141)52-115-91(145)60(7)118-100(154)78(55-135)128-98(152)77(49-84(109)139)125-94(148)72(44-59(5)6)127-103(157)89(61(8)137)130-99(153)76(119-85(140)50-108)48-65-51-114-68-27-16-15-26-67(65)68)92(146)117-54-88(143)131-38-20-31-82(131)105(159)134-41-21-32-83(134)106(160)133-40-18-29-80(133)101(155)116-53-87(142)120-73(45-62-22-11-9-12-23-62)96(150)129-79(56-136)104(158)132-39-19-30-81(132)102(156)126-75(46-63-24-13-10-14-25-63)97(151)122-69(90(110)144)28-17-37-113-107(111)112/h9-16,22-27,33-36,51,57-61,69-83,89,114,135-138H,17-21,28-32,37-50,52-56,108H2,1-8H3,(H2,109,139)(H2,110,144)(H,115,145)(H,116,155)(H,117,146)(H,118,154)(H,119,140)(H,120,142)(H,121,141)(H,122,151)(H,123,147)(H,124,149)(H,125,148)(H,126,156)(H,127,157)(H,128,152)(H,129,150)(H,130,153)(H4,111,112,113)/t60-,61+,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,89-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation
Curated by PDSP Ki Database
| |
J Biol Chem 272: 24612-6 (1997)
Article DOI: 10.1074/jbc.272.39.24612
BindingDB Entry DOI: 10.7270/Q2BC3X2Q |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384811
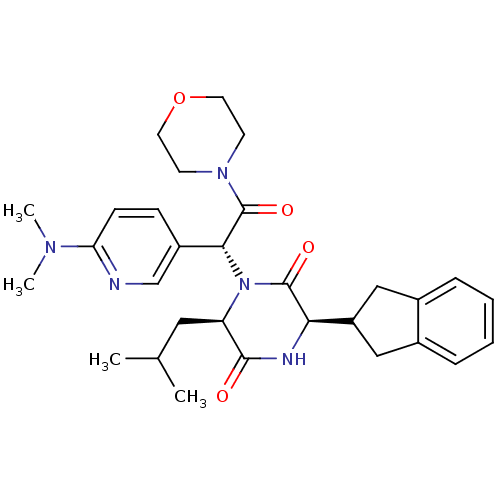
(CHEMBL2037490)Show SMILES CC(C)C[C@H]1N([C@@H](C(=O)N2CCOCC2)c2ccc(nc2)N(C)C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C30H39N5O4/c1-19(2)15-24-28(36)32-26(23-16-20-7-5-6-8-21(20)17-23)29(37)35(24)27(30(38)34-11-13-39-14-12-34)22-9-10-25(31-18-22)33(3)4/h5-10,18-19,23-24,26-27H,11-17H2,1-4H3,(H,32,36)/t24-,26-,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442145

(CHEMBL2441271)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1nc(Cc2ccccc2)cc(Nc2nc3ccc(Cl)cc3s2)n1 |r,wU:1.0,wD:4.7,(34.33,-8.73,;33,-9.52,;33.02,-11.06,;31.7,-11.84,;30.36,-11.09,;30.34,-9.56,;31.66,-8.77,;29.03,-11.88,;27.69,-11.14,;26.37,-11.93,;25.03,-11.18,;23.69,-11.97,;22.36,-11.2,;22.37,-9.66,;21.03,-8.89,;19.69,-9.66,;19.69,-11.2,;21.03,-11.97,;25,-9.64,;26.32,-8.85,;26.29,-7.31,;27.5,-6.37,;27.47,-4.83,;28.92,-4.33,;29.52,-2.9,;31.04,-2.71,;31.98,-3.93,;33.51,-3.74,;31.38,-5.35,;29.86,-5.55,;28.98,-6.82,;27.67,-9.6,)| Show InChI InChI=1S/C24H24ClN5OS/c25-16-6-11-20-21(13-16)32-24(28-20)30-22-14-18(12-15-4-2-1-3-5-15)27-23(29-22)26-17-7-9-19(31)10-8-17/h1-6,11,13-14,17,19,31H,7-10,12H2,(H2,26,27,28,29,30)/t17-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Galanin receptor type 1
(RAT) | BDBM50273370

(CHEMBL503473 | GWTLNSAGYLLGPPPGFSPFR-CONH2 | Galan...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CN)[C@@H](C)O)C(=O)NCC(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCN=C(N)N)C(N)=O |r,wU:106.112,4.4,8.12,32.33,37.40,43.48,51.56,63.76,wD:149.157,138.145,134.142,125.131,114.119,99.104,92.96,16.25,59.60,79.83,(16.53,-9.13,;15.19,-9.9,;15.2,-11.44,;13.86,-9.13,;12.52,-9.9,;11.19,-9.13,;11.19,-7.59,;12.53,-6.82,;9.86,-6.82,;9.86,-5.28,;11.2,-4.51,;12.52,-5.28,;11.2,-2.97,;8.13,-7.82,;6.39,-6.82,;6.39,-5.28,;4.66,-7.83,;4.66,-9.36,;5.99,-10.13,;5.99,-11.67,;7.32,-12.44,;8.66,-11.67,;9.99,-12.44,;8.66,-10.13,;7.33,-9.36,;2.92,-6.82,;2.92,-5.28,;4.26,-4.52,;1.19,-4.28,;1.19,-2.75,;2.52,-1.97,;3.86,-2.74,;2.52,-.43,;3.86,.34,;1.2,.34,;1.19,1.88,;2.53,2.65,;-.14,2.65,;-.14,4.19,;1.19,4.96,;-1.48,1.88,;-2.81,2.65,;-2.81,4.19,;-4.14,1.88,;-5.48,2.64,;-5.47,4.19,;-6.81,4.96,;-4.14,4.96,;-4.15,.34,;-5.48,-.43,;-6.81,.34,;-5.48,-1.97,;-6.81,-2.74,;-8.14,-1.97,;-8.14,-.43,;-9.47,-2.74,;-4.14,-2.74,;-4.14,-4.28,;-5.48,-5.05,;-2.81,-5.05,;-2.81,-6.59,;-1.48,-7.36,;-.15,-6.59,;-1.48,-8.9,;-2.85,-9.59,;-4.14,-8.76,;-4.22,-7.22,;-5.71,-6.82,;-6.55,-8.12,;-8.07,-8.35,;-8.62,-9.79,;-7.65,-10.99,;-6.13,-10.75,;-5.58,-9.31,;-.14,-9.67,;1.19,-8.9,;1.19,-7.36,;2.53,-9.67,;2.53,-11.21,;-1.47,-4.28,;-.15,-5.05,;-1.47,-2.74,;12.53,-11.44,;13.86,-12.21,;11.19,-12.21,;11.19,-13.75,;12.53,-14.52,;13.86,-13.75,;12.53,-16.06,;11.28,-16.96,;11.76,-18.43,;13.29,-18.43,;13.77,-16.97,;15.24,-16.49,;15.64,-15,;16.65,-17.91,;16.41,-19.42,;17.78,-20.13,;18.87,-19.04,;18.18,-17.67,;19.08,-15.88,;20.62,-15.8,;18.31,-14.55,;16.78,-14.38,;16.46,-12.88,;17.8,-12.12,;18.94,-13.14,;20.31,-12.44,;21.65,-13.21,;20.31,-10.9,;21.64,-10.13,;22.97,-10.89,;24.3,-10.12,;22.98,-12.43,;24.31,-13.2,;24.32,-14.74,;25.65,-15.5,;25.66,-17.04,;26.99,-17.81,;28.33,-17.04,;28.32,-15.5,;26.99,-14.73,;25.64,-12.43,;25.64,-10.89,;26.98,-13.19,;28.31,-12.42,;29.65,-13.18,;30.98,-12.41,;28.31,-10.88,;26.97,-10.11,;29.64,-10.1,;31.05,-10.72,;32.07,-9.57,;31.3,-8.24,;29.8,-8.57,;28.51,-7.72,;27.17,-8.47,;28.53,-6.17,;29.88,-5.42,;31.2,-6.21,;32.54,-5.45,;32.56,-3.91,;33.91,-3.16,;35.23,-3.95,;35.21,-5.49,;33.87,-6.24,;29.9,-3.88,;31.24,-3.12,;28.57,-3.1,;28.59,-1.55,;29.93,-.8,;29.95,.74,;28.63,1.53,;28.65,3.07,;29.99,3.82,;30.01,5.36,;31.32,3.03,;27.27,-.77,;27.29,.77,;25.92,-1.52,)| Show InChI InChI=1S/C107H153N27O26/c1-57(2)42-70(123-93(147)71(43-58(3)4)124-95(149)74(47-64-33-35-66(138)36-34-64)121-86(141)52-115-91(145)60(7)118-100(154)78(55-135)128-98(152)77(49-84(109)139)125-94(148)72(44-59(5)6)127-103(157)89(61(8)137)130-99(153)76(119-85(140)50-108)48-65-51-114-68-27-16-15-26-67(65)68)92(146)117-54-88(143)131-38-20-31-82(131)105(159)134-41-21-32-83(134)106(160)133-40-18-29-80(133)101(155)116-53-87(142)120-73(45-62-22-11-9-12-23-62)96(150)129-79(56-136)104(158)132-39-19-30-81(132)102(156)126-75(46-63-24-13-10-14-25-63)97(151)122-69(90(110)144)28-17-37-113-107(111)112/h9-16,22-27,33-36,51,57-61,69-83,89,114,135-138H,17-21,28-32,37-50,52-56,108H2,1-8H3,(H2,109,139)(H2,110,144)(H,115,145)(H,116,155)(H,117,146)(H,118,154)(H,119,140)(H,120,142)(H,121,141)(H,122,151)(H,123,147)(H,124,149)(H,125,148)(H,126,156)(H,127,157)(H,128,152)(H,129,150)(H,130,153)(H4,111,112,113)/t60-,61+,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,89-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation
Curated by PDSP Ki Database
| |
J Biol Chem 273: 23321-6 (1998)
Article DOI: 10.1074/jbc.273.36.23321
BindingDB Entry DOI: 10.7270/Q22N50TF |
More data for this
Ligand-Target Pair | |
Galanin receptor type 1
(RAT) | BDBM85070

(Galanin, Porcine)Show SMILES CCC(C)C(NC(=O)C(C)NC(=O)C(Cc1cnc[nH]1)NC(=O)C1CCCN1C(=O)CNC(=O)C(CC(C)C)NC(=O)C(CC(C)C)NC(=O)C(Cc1ccc(O)cc1)NC(=O)CNC(=O)C(C)NC(=O)C(CO)NC(=O)C(CC(N)=O)NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(Cc1c[nH]c2ccccc12)NC(=O)CN)C(C)O)C(=O)NC(CC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(Cc1cnc[nH]1)C(=O)NC(CCCN=C(N)N)C(=O)NC(CO)C(=O)NC(Cc1ccccc1)C(=O)NC(Cc1cnc[nH]1)C(=O)NC(CC(O)=O)C(=O)NC(CCCCN)C(=O)NC(Cc1ccc(O)cc1)C(=O)NCC(=O)NC(CC(C)C)C(=O)NC(C)C(N)=O |(22.15,12.84,;20.71,12.32,;20.44,10.8,;21.61,9.81,;18.99,10.28,;18.72,8.76,;19.9,7.77,;21.34,8.3,;19.63,6.25,;18.18,5.73,;20.8,5.26,;20.53,3.75,;19.09,3.22,;21.71,2.75,;21.44,1.24,;19.99,.71,;18.72,1.58,;17.5,.63,;18.03,-.82,;19.57,-.77,;23.16,3.28,;24.34,2.29,;24.07,.77,;25.79,2.81,;26.21,4.29,;27.75,4.34,;28.28,2.89,;27.06,1.95,;27.11,.41,;25.81,-.41,;28.47,-.32,;28.52,-1.86,;29.88,-2.58,;31.19,-1.77,;29.93,-4.12,;31.29,-4.85,;32.6,-4.03,;33.96,-4.76,;32.55,-2.49,;28.62,-4.93,;28.68,-6.47,;30.2,-6.69,;27.73,-7.69,;26.22,-8,;25.19,-6.86,;23.68,-7.17,;25.67,-5.39,;28.3,-9.11,;27.35,-10.33,;25.82,-10.11,;27.92,-11.76,;26.97,-12.97,;25.45,-12.75,;24.87,-11.32,;23.35,-11.1,;22.4,-12.32,;20.88,-12.1,;22.97,-13.75,;24.5,-13.96,;29.45,-11.97,;30.02,-13.4,;29.07,-14.61,;31.55,-13.62,;32.12,-15.05,;33.65,-15.26,;34.6,-14.05,;34.22,-16.69,;33.27,-17.9,;35.75,-16.91,;36.32,-18.34,;35.37,-19.55,;37.85,-18.56,;38.8,-17.34,;38.22,-15.91,;38.42,-19.98,;39.94,-20.2,;40.89,-18.99,;40.52,-21.63,;42.04,-21.85,;42.99,-20.64,;44.52,-20.85,;42.42,-19.21,;39.57,-22.84,;40.14,-24.27,;41.65,-23.95,;39.66,-25.74,;38.36,-26.55,;37,-25.82,;35.69,-26.64,;36.95,-24.28,;40.69,-26.88,;40.21,-28.35,;38.71,-28.66,;41.24,-29.49,;40.76,-30.96,;41.79,-32.1,;43.3,-31.78,;41.31,-33.57,;39.81,-33.88,;39.33,-35.35,;40.23,-36.59,;39.33,-37.83,;37.87,-37.36,;36.54,-38.13,;35.2,-37.36,;35.2,-35.82,;36.54,-35.05,;37.87,-35.82,;42.34,-34.71,;43.85,-34.39,;44.33,-32.93,;44.88,-35.54,;46.39,-35.22,;42.75,-29.17,;43.78,-30.32,;43.23,-27.71,;17.81,11.27,;16.36,10.75,;18.08,12.79,;16.9,13.78,;15.45,13.25,;14.28,14.25,;12.83,13.72,;14.55,15.76,;17.17,15.3,;15.99,16.29,;18.62,15.82,;18.89,17.34,;17.37,17.61,;16.85,19.05,;15.33,19.32,;17.84,20.23,;20.34,17.86,;21.52,16.87,;21.33,19.04,;22.85,18.77,;23.37,17.32,;24.89,17.05,;26,18.12,;27.36,17.39,;27.09,15.88,;25.56,15.66,;23.84,19.95,;25.35,19.68,;23.31,21.39,;24.31,22.57,;25.82,22.3,;26.81,23.48,;28.33,23.21,;29.32,24.39,;30.84,24.12,;31.83,25.3,;31.36,22.67,;23.78,24.02,;22.27,24.29,;24.77,25.2,;24.25,26.65,;22.73,26.92,;22.21,28.36,;25.24,27.82,;26.76,27.55,;24.72,29.27,;25.71,30.45,;27.23,30.18,;28.22,31.36,;29.73,31.09,;30.73,32.27,;30.2,33.71,;28.69,33.98,;27.69,32.81,;25.19,31.9,;26.18,33.08,;23.67,32.17,;23.14,33.62,;24.14,34.79,;23.61,36.24,;22.13,36.67,;22.08,38.21,;23.53,38.73,;24.48,37.52,;21.63,33.89,;21.1,35.33,;20.64,32.71,;19.12,32.98,;18.6,34.43,;17.42,35.42,;17.69,36.93,;15.97,34.89,;18.13,31.8,;18.65,30.35,;16.61,32.07,;15.62,30.89,;16.14,29.44,;15.15,28.27,;15.68,26.82,;14.69,25.64,;15.21,24.19,;14.1,31.16,;13.58,32.61,;13.11,29.98,;11.6,30.25,;11.07,31.7,;9.56,31.97,;9.03,33.42,;7.52,33.69,;6.52,32.51,;5.01,32.78,;7.05,31.06,;8.56,30.79,;10.6,29.08,;9.09,29.35,;11.13,27.63,;10.14,26.45,;8.62,26.72,;8.1,28.17,;7.63,25.54,;6.11,25.81,;5.59,27.26,;4.07,27.53,;3.55,28.98,;3.08,26.35,;5.12,24.63,;3.6,24.9,;5.64,23.19,;4.65,22.01,;3.14,22.28,;5.18,20.56,;4.19,19.38,;6.69,20.29,)| Show InChI InChI=1S/C146H213N43O40/c1-15-75(10)119(187-123(207)78(13)167-129(213)101(51-84-60-154-68-162-84)182-143(227)110-31-24-42-189(110)116(200)65-161-124(208)93(43-71(2)3)173-130(214)95(45-73(6)7)174-132(216)98(49-82-34-38-88(194)39-35-82)170-114(198)63-159-122(206)77(12)166-141(225)108(66-190)186-137(221)105(55-112(150)196)179-131(215)96(46-74(8)9)183-145(229)120(79(14)192)188-140(224)100(168-113(197)58-148)50-83-59-158-90-28-20-19-27-89(83)90)144(228)184-107(57-118(203)204)139(223)180-104(54-111(149)195)136(220)178-102(52-85-61-155-69-163-85)134(218)172-92(30-23-41-157-146(152)153)127(211)185-109(67-191)142(226)176-99(47-80-25-17-16-18-26-80)133(217)177-103(53-86-62-156-70-164-86)135(219)181-106(56-117(201)202)138(222)171-91(29-21-22-40-147)126(210)175-97(48-81-32-36-87(193)37-33-81)125(209)160-64-115(199)169-94(44-72(4)5)128(212)165-76(11)121(151)205/h16-20,25-28,32-39,59-62,68-79,91-110,119-120,158,190-194H,15,21-24,29-31,40-58,63-67,147-148H2,1-14H3,(H2,149,195)(H2,150,196)(H2,151,205)(H,154,162)(H,155,163)(H,156,164)(H,159,206)(H,160,209)(H,161,208)(H,165,212)(H,166,225)(H,167,213)(H,168,197)(H,169,199)(H,170,198)(H,171,222)(H,172,218)(H,173,214)(H,174,216)(H,175,210)(H,176,226)(H,177,217)(H,178,220)(H,179,215)(H,180,223)(H,181,219)(H,182,227)(H,183,229)(H,184,228)(H,185,211)(H,186,221)(H,187,207)(H,188,224)(H,201,202)(H,203,204)(H4,152,153,157) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation
Curated by PDSP Ki Database
| |
J Biol Chem 273: 23321-6 (1998)
Article DOI: 10.1074/jbc.273.36.23321
BindingDB Entry DOI: 10.7270/Q22N50TF |
More data for this
Ligand-Target Pair | |
Galanin receptor type 1
(RAT) | BDBM85200

(Galanin (1-19), rat | Galanin rat)Show SMILES CCC(C)C(NC(=O)C(C)NC(=O)C(Cc1cnc[nH]1)NC(=O)C1CCCN1C(=O)CNC(=O)C(CC(C)C)NC(=O)C(CC(C)C)NC(=O)C(Cc1ccc(O)cc1)NC(=O)CNC(=O)C(C)NC(=O)C(CO)NC(=O)C(CC(N)=O)NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(Cc1c[nH]c2ccccc12)NC(=O)CN)C(C)O)C(=O)NC(CC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(Cc1cnc[nH]1)C(N)=O Show InChI InChI=1S/C92H135N27O26/c1-12-46(8)75(91(144)115-66(33-74(128)129)87(140)112-64(31-69(94)123)85(138)108-57(77(96)130)29-52-36-97-41-102-52)117-79(132)48(10)105-81(134)63(30-53-37-98-42-103-53)113-90(143)68-18-15-23-119(68)73(127)39-101-80(133)58(24-43(2)3)109-82(135)59(25-44(4)5)110-84(137)61(27-50-19-21-54(122)22-20-50)107-72(126)38-100-78(131)47(9)104-89(142)67(40-120)116-86(139)65(32-70(95)124)111-83(136)60(26-45(6)7)114-92(145)76(49(11)121)118-88(141)62(106-71(125)34-93)28-51-35-99-56-17-14-13-16-55(51)56/h13-14,16-17,19-22,35-37,41-49,57-68,75-76,99,120-122H,12,15,18,23-34,38-40,93H2,1-11H3,(H2,94,123)(H2,95,124)(H2,96,130)(H,97,102)(H,98,103)(H,100,131)(H,101,133)(H,104,142)(H,105,134)(H,106,125)(H,107,126)(H,108,138)(H,109,135)(H,110,137)(H,111,136)(H,112,140)(H,113,143)(H,114,145)(H,115,144)(H,116,139)(H,117,132)(H,118,141)(H,128,129) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation
Curated by PDSP Ki Database
| |
J Biol Chem 273: 23321-6 (1998)
Article DOI: 10.1074/jbc.273.36.23321
BindingDB Entry DOI: 10.7270/Q22N50TF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442146
(CHEMBL2441270)Show SMILES Cc1ccc2nc(Nc3cc(Cc4ccccc4)nc(N[C@H]4CC[C@H](O)CC4)n3)sc2c1 |r,wU:24.25,wD:21.21,(33.51,-3.74,;31.98,-3.93,;31.04,-2.71,;29.52,-2.9,;28.92,-4.33,;27.47,-4.83,;27.5,-6.37,;26.29,-7.31,;26.32,-8.85,;25,-9.64,;25.03,-11.18,;23.69,-11.97,;22.36,-11.2,;22.37,-9.66,;21.03,-8.89,;19.69,-9.66,;19.69,-11.2,;21.03,-11.97,;26.37,-11.93,;27.69,-11.14,;29.03,-11.88,;30.36,-11.09,;31.7,-11.84,;33.02,-11.06,;33,-9.52,;34.33,-8.73,;31.66,-8.77,;30.34,-9.56,;27.67,-9.6,;28.98,-6.82,;29.86,-5.55,;31.38,-5.35,)| Show InChI InChI=1S/C25H27N5OS/c1-16-7-12-21-22(13-16)32-25(28-21)30-23-15-19(14-17-5-3-2-4-6-17)27-24(29-23)26-18-8-10-20(31)11-9-18/h2-7,12-13,15,18,20,31H,8-11,14H2,1H3,(H2,26,27,28,29,30)/t18-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384821
(CHEMBL2037509)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)NC)c2ccc(C)nc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C27H34N4O3/c1-6-15(2)23-26(33)30-22(20-13-18-9-7-8-10-19(18)14-20)27(34)31(23)24(25(32)28-5)21-12-11-16(3)29-17(21)4/h7-12,15,20,22-24H,6,13-14H2,1-5H3,(H,28,32)(H,30,33)/t15-,22+,23+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384814
(CHEMBL2037486)Show SMILES COc1ccc(cn1)[C@@H](N1[C@H](CC(C)C)C(=O)N[C@H](C2Cc3ccccc3C2)C1=O)C(=O)N(C)C |r| Show InChI InChI=1S/C27H34N4O4/c1-16(2)12-21-25(32)29-23(20-13-17-8-6-7-9-18(17)14-20)26(33)31(21)24(27(34)30(3)4)19-10-11-22(35-5)28-15-19/h6-11,15-16,20-21,23-24H,12-14H2,1-5H3,(H,29,32)/t21-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384835
(CHEMBL2037492)Show SMILES CC(C)C[C@H]1N([C@@H](C(=O)N2CCOCC2)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C29H36N4O4/c1-18(2)14-24-27(34)31-25(23-15-20-6-4-5-7-21(20)16-23)28(35)33(24)26(22-9-8-19(3)30-17-22)29(36)32-10-12-37-13-11-32/h4-9,17-18,23-26H,10-16H2,1-3H3,(H,31,34)/t24-,25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50316677
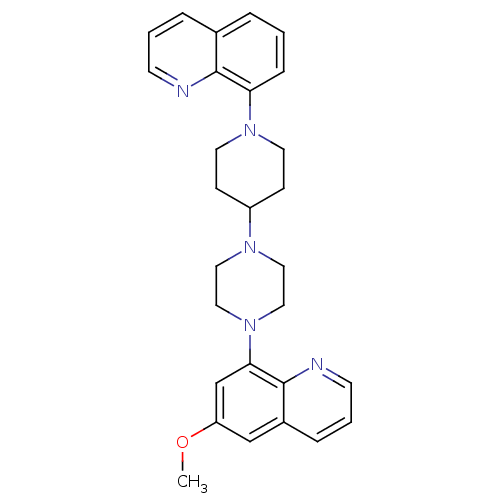
(6-Methoxy-8-{4-[1-(8-quinolinyl)-4-piperidinyl]-1-...)Show SMILES COc1cc(N2CCN(CC2)C2CCN(CC2)c2cccc3cccnc23)c2ncccc2c1 Show InChI InChI=1S/C28H31N5O/c1-34-24-19-22-7-4-12-30-28(22)26(20-24)33-17-15-31(16-18-33)23-9-13-32(14-10-23)25-8-2-5-21-6-3-11-29-27(21)25/h2-8,11-12,19-20,23H,9-10,13-18H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells |
J Med Chem 53: 4066-84 (2010)
Article DOI: 10.1021/jm1000908
BindingDB Entry DOI: 10.7270/Q28P60P7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50316673
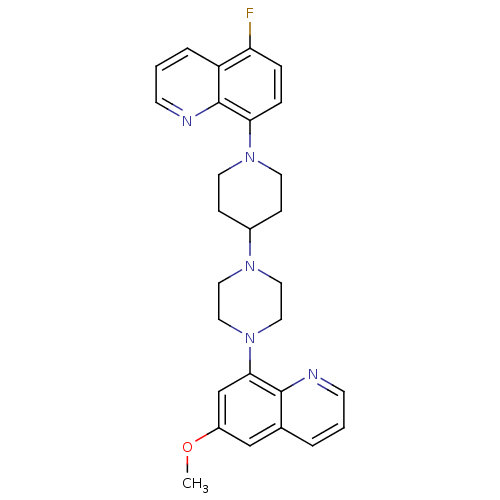
(5-Fluoro-8-{4-[4-(6-methoxyquinolin-8-yl)piperazin...)Show SMILES COc1cc(N2CCN(CC2)C2CCN(CC2)c2ccc(F)c3cccnc23)c2ncccc2c1 Show InChI InChI=1S/C28H30FN5O/c1-35-22-18-20-4-2-10-30-27(20)26(19-22)34-16-14-32(15-17-34)21-8-12-33(13-9-21)25-7-6-24(29)23-5-3-11-31-28(23)25/h2-7,10-11,18-19,21H,8-9,12-17H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells |
J Med Chem 53: 4066-84 (2010)
Article DOI: 10.1021/jm1000908
BindingDB Entry DOI: 10.7270/Q28P60P7 |
More data for this
Ligand-Target Pair | |
Galanin receptor type 1
(Homo sapiens (Human)) | BDBM85072
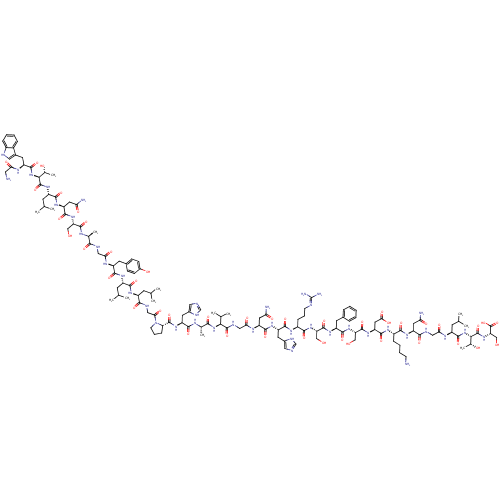
(Galanin, Human)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCCN1C(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CN)[C@@H](C)O)C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(O)=O |wU:212.220,131.139,139.152,71.79,166.175,182.187,54.57,211.217,20.25,37.39,112.114,97.99,155.160,wD:202.210,102.111,123.131,93.95,81.87,174.183,160.167,60.68,186.203,4.4,12.16,43.51,29.33,218.224,(13.66,18.44,;13.66,16.9,;12.32,16.13,;14.99,16.13,;14.99,14.59,;16.32,13.82,;17.86,14.59,;17.86,16.13,;19.2,13.82,;20.53,14.59,;22.07,13.82,;22.07,12.28,;23.4,14.59,;23.4,16.13,;22.07,16.9,;22.07,18.44,;20.74,16.13,;24.74,13.82,;26.28,14.59,;26.28,16.13,;27.61,13.82,;27.61,12.28,;28.95,11.51,;28.95,9.97,;30.28,9.2,;31.61,9.97,;28.95,14.59,;30.49,13.82,;30.49,12.28,;31.82,14.59,;31.82,16.13,;33.15,16.9,;33.15,18.44,;34.49,16.13,;33.15,13.82,;32.43,3.03,;32.43,1.49,;31.1,3.8,;31.1,5.34,;32.43,6.11,;29.76,3.03,;28.22,3.8,;28.22,5.34,;26.89,3.03,;26.89,1.49,;28.22,.72,;28.22,-.82,;29.56,-1.59,;30.89,-.82,;30.89,.72,;29.56,1.49,;25.56,3.8,;24.02,3.03,;24.02,1.49,;22.68,3.8,;22.68,5.34,;24.02,6.11,;21.35,3.03,;19.81,3.8,;19.81,5.34,;18.47,3.03,;18.47,1.49,;19.81,.72,;19.81,-.82,;21.14,-1.59,;22.48,-.82,;23.81,-1.59,;22.48,.72,;17.14,3.8,;15.6,3.03,;15.6,1.49,;14.27,3.8,;14.27,5.34,;15.6,6.11,;17.01,5.49,;18.04,6.63,;17.27,7.97,;15.76,7.65,;12.93,3.03,;11.39,3.8,;11.39,5.34,;10.06,3.03,;10.06,1.49,;11.39,.72,;11.39,-.82,;12.73,1.49,;8.73,3.8,;7.19,3.03,;7.19,1.49,;5.85,3.8,;4.52,3.03,;5.24,-7.75,;5.24,-9.29,;6.57,-6.98,;7.91,-7.75,;9.45,-6.98,;9.45,-5.44,;10.78,-7.75,;10.78,-9.29,;12.12,-6.98,;13.66,-7.75,;13.66,-9.29,;14.99,-6.98,;14.99,-5.44,;13.66,-4.67,;12.25,-5.29,;11.22,-4.15,;11.99,-2.81,;13.49,-3.13,;16.32,-7.75,;17.66,-6.98,;17.66,-5.44,;18.99,-7.75,;19.15,-9.28,;20.66,-9.6,;21.43,-8.26,;20.4,-7.12,;20.72,-5.61,;19.57,-4.58,;22.18,-5.14,;23.33,-6.17,;24.79,-5.69,;25.11,-4.19,;25.94,-6.72,;27.4,-6.25,;27.72,-4.74,;29.19,-4.26,;26.58,-3.71,;25.62,-8.23,;26.76,-9.26,;28.22,-8.78,;26.44,-10.77,;24.98,-11.24,;24.65,-12.75,;23.19,-13.22,;25.8,-13.78,;27.58,-11.8,;29.05,-11.32,;29.37,-9.81,;30.19,-12.35,;29.87,-13.86,;31.02,-14.89,;30.7,-16.39,;31.84,-17.42,;33.31,-16.95,;34.45,-17.98,;33.63,-15.44,;32.48,-14.41,;31.66,-11.87,;31.98,-10.37,;30.83,-9.34,;33.44,-9.89,;33.76,-8.39,;35.23,-7.91,;35.55,-6.4,;36.37,-8.94,;36.05,-10.45,;37.84,-8.46,;38.98,-9.49,;38.66,-11,;40.45,-9.02,;40.77,-7.51,;42.23,-7.04,;41.59,-10.05,;43.06,-9.57,;43.38,-8.07,;44.2,-10.6,;43.88,-12.11,;45.02,-13.14,;44.7,-14.65,;46.49,-12.66,;45.66,-10.13,;45.98,-8.62,;44.84,-7.59,;47.45,-8.15,;47.77,-6.64,;46.62,-5.61,;46.94,-4.1,;45.16,-6.08,;48.59,-9.18,;50.06,-8.7,;50.38,-7.19,;51.2,-9.73,;52.67,-9.25,;53.81,-10.29,;53.49,-11.79,;55.28,-9.81,;55.6,-8.3,;57.06,-7.83,;58.3,-8.73,;59.54,-7.83,;59.07,-6.37,;59.85,-5.04,;59.08,-3.71,;57.54,-3.7,;56.77,-5.04,;57.53,-6.37,;56.42,-10.84,;56.1,-12.35,;54.64,-12.82,;57.25,-13.38,;56.93,-14.88,;50.88,-11.24,;49.42,-11.71,;52.03,-12.27,;6.57,-5.44,;5.24,-4.67,;7.91,-4.67,;13.66,13.82,;13.66,12.28,;12.12,14.59,;10.78,13.82,;10.78,12.28,;12.12,11.51,;9.45,11.51,;9.45,14.59,;9.45,16.13,;7.91,13.82,;6.57,14.59,;6.57,16.13,;5.24,16.9,;5.24,13.82,;3.91,14.59,;5.24,12.28,)| Show InChI InChI=1S/C139H210N42O43/c1-65(2)38-84(165-121(206)86(40-67(5)6)166-123(208)88(43-75-31-33-79(188)34-32-75)161-106(193)55-151-114(199)70(11)157-131(216)97(59-182)175-127(212)95(49-104(144)191)170-122(207)87(41-68(7)8)173-136(221)112(72(13)186)180-130(215)90(159-105(192)51-141)44-76-52-150-81-27-19-18-26-80(76)81)116(201)154-58-109(196)181-37-23-30-101(181)134(219)172-91(45-77-53-147-63-155-77)120(205)158-71(12)115(200)178-111(69(9)10)135(220)153-57-108(195)162-94(48-103(143)190)126(211)168-92(46-78-54-148-64-156-78)125(210)164-83(29-22-36-149-139(145)146)119(204)174-98(60-183)132(217)167-89(42-74-24-16-15-17-25-74)124(209)176-99(61-184)133(218)171-96(50-110(197)198)128(213)163-82(28-20-21-35-140)118(203)169-93(47-102(142)189)117(202)152-56-107(194)160-85(39-66(3)4)129(214)179-113(73(14)187)137(222)177-100(62-185)138(223)224/h15-19,24-27,31-34,52-54,63-73,82-101,111-113,150,182-188H,20-23,28-30,35-51,55-62,140-141H2,1-14H3,(H2,142,189)(H2,143,190)(H2,144,191)(H,147,155)(H,148,156)(H,151,199)(H,152,202)(H,153,220)(H,154,201)(H,157,216)(H,158,205)(H,159,192)(H,160,194)(H,161,193)(H,162,195)(H,163,213)(H,164,210)(H,165,206)(H,166,208)(H,167,217)(H,168,211)(H,169,203)(H,170,207)(H,171,218)(H,172,219)(H,173,221)(H,174,204)(H,175,212)(H,176,209)(H,177,222)(H,178,200)(H,179,214)(H,180,215)(H,197,198)(H,223,224)(H4,145,146,149)/t70-,71-,72+,73+,82-,83-,84-,85-,86-,87-,88-,89-,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,111-,112-,113-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation
Curated by PDSP Ki Database
| |
Peptides 19: 1771-81 (1998)
Article DOI: 10.1016/s0196-9781(98)00133-8
BindingDB Entry DOI: 10.7270/Q2NC5ZQ6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50316680
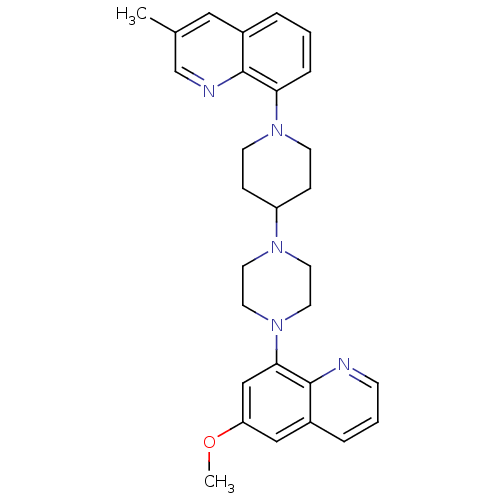
(8-{4-[4-(6-Methoxyquinolin-8-yl)piperazin-1-yl]pip...)Show SMILES COc1cc(N2CCN(CC2)C2CCN(CC2)c2cccc3cc(C)cnc23)c2ncccc2c1 Show InChI InChI=1S/C29H33N5O/c1-21-17-22-5-3-7-26(28(22)31-20-21)33-11-8-24(9-12-33)32-13-15-34(16-14-32)27-19-25(35-2)18-23-6-4-10-30-29(23)27/h3-7,10,17-20,24H,8-9,11-16H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells |
J Med Chem 53: 4066-84 (2010)
Article DOI: 10.1021/jm1000908
BindingDB Entry DOI: 10.7270/Q28P60P7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50316684
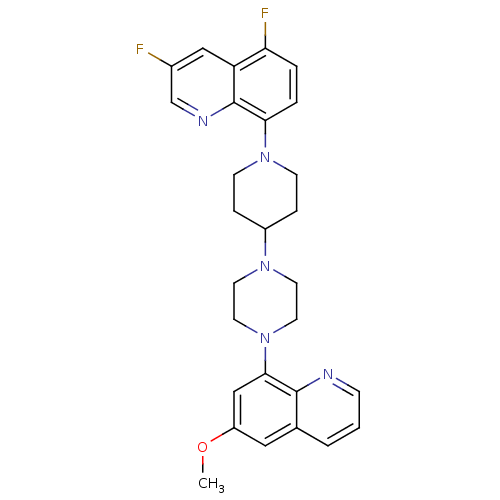
(3,5-Difluoro-8-{4-[4-(6-methoxyquinolin-8-yl)piper...)Show SMILES COc1cc(N2CCN(CC2)C2CCN(CC2)c2ccc(F)c3cc(F)cnc23)c2ncccc2c1 Show InChI InChI=1S/C28H29F2N5O/c1-36-22-15-19-3-2-8-31-27(19)26(17-22)35-13-11-33(12-14-35)21-6-9-34(10-7-21)25-5-4-24(30)23-16-20(29)18-32-28(23)25/h2-5,8,15-18,21H,6-7,9-14H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells |
J Med Chem 53: 4066-84 (2010)
Article DOI: 10.1021/jm1000908
BindingDB Entry DOI: 10.7270/Q28P60P7 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50362726
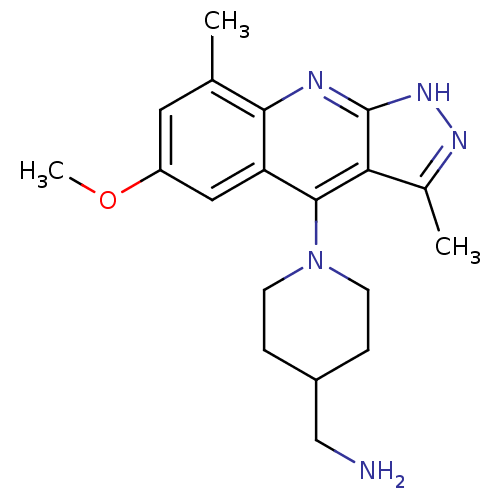
(CHEMBL1939796)Show SMILES COc1cc(C)c2nc3[nH]nc(C)c3c(N3CCC(CN)CC3)c2c1 Show InChI InChI=1S/C19H25N5O/c1-11-8-14(25-3)9-15-17(11)21-19-16(12(2)22-23-19)18(15)24-6-4-13(10-20)5-7-24/h8-9,13H,4-7,10,20H2,1-3H3,(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PDE10A using [3H]cAMP as substrate by scintillation proximity assay |
Bioorg Med Chem Lett 22: 1019-22 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.127
BindingDB Entry DOI: 10.7270/Q2BV7H3J |
More data for this
Ligand-Target Pair | |
Galanin receptor type 2
(RAT) | BDBM85169
(Galanin (1-29), porcine | Galanin [D-Trp2], porcin...)Show SMILES CCC(C)C(NC(=O)C(C)NC(=O)C(Cc1cnc[nH]1)NC(=O)C1CCCN1C(=O)CNC(=O)C(CC(C)C)NC(=O)C(CC(C)C)NC(=O)C(Cc1ccc(O)cc1)NC(=O)CNC(=O)C(C)NC(=O)C(CO)NC(=O)C(CC(N)=O)NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(Cc1c[nH]c2ccccc12)NC(=O)CN)C(C)O)C(=O)NC(CC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(Cc1cnc[nH]1)C(=O)NC(CCCN=C(N)N)C(=O)NC(CO)C(=O)NC(Cc1ccccc1)C(=O)NC(Cc1cnc[nH]1)C(=O)NC(CC(O)=O)C(=O)NC(CCCCN)C(=O)NC(CCc1ccc(O)cc1)C(=O)NCC(=O)NC(CC(C)C)C(N)=O |(19.65,4.85,;18.2,4.33,;17.93,2.81,;19.11,1.82,;16.48,2.29,;16.21,.77,;17.39,-.22,;18.84,.3,;17.12,-1.74,;15.67,-2.26,;18.3,-2.73,;18.03,-4.24,;16.58,-4.77,;19.21,-5.24,;18.94,-6.75,;17.49,-7.28,;16.22,-6.41,;15,-7.36,;15.52,-8.81,;17.06,-8.76,;20.66,-4.71,;21.83,-5.7,;21.56,-7.22,;23.28,-5.18,;23.71,-3.7,;25.25,-3.65,;25.77,-5.1,;24.56,-6.04,;24.61,-7.58,;23.3,-8.4,;25.97,-8.31,;26.02,-9.85,;27.38,-10.57,;28.68,-9.76,;27.43,-12.11,;28.79,-12.84,;30.09,-12.02,;31.45,-12.75,;30.04,-10.48,;26.12,-12.93,;26.17,-14.46,;27.7,-14.68,;25.22,-15.68,;23.72,-15.99,;22.69,-14.85,;21.18,-15.17,;23.16,-13.38,;25.8,-17.11,;24.85,-18.32,;23.32,-18.1,;25.42,-19.75,;24.47,-20.96,;22.95,-20.74,;22,-21.95,;20.47,-21.74,;19.9,-20.31,;18.37,-20.09,;20.85,-19.1,;22.37,-19.31,;26.95,-19.96,;27.52,-21.39,;26.57,-22.6,;29.04,-21.61,;29.62,-23.04,;31.14,-23.26,;32.09,-22.04,;31.72,-24.68,;30.77,-25.9,;33.24,-24.9,;33.82,-26.33,;32.87,-27.54,;35.34,-26.55,;36.29,-25.34,;35.72,-23.91,;35.92,-27.98,;37.44,-28.19,;38.39,-26.98,;38.02,-29.62,;39.54,-29.84,;40.49,-28.63,;42.01,-28.84,;39.92,-27.2,;37.06,-30.83,;37.64,-32.26,;39.15,-31.95,;37.16,-33.73,;35.85,-34.54,;34.5,-33.82,;33.19,-34.63,;34.44,-32.28,;38.19,-34.87,;37.71,-36.34,;36.2,-36.65,;38.74,-37.48,;38.26,-38.95,;39.29,-40.09,;40.8,-39.78,;38.81,-41.56,;37.3,-41.87,;36.83,-43.34,;37.72,-44.58,;36.83,-45.82,;35.37,-45.35,;34.03,-46.12,;32.7,-45.35,;32.7,-43.81,;34.03,-43.04,;35.37,-43.81,;39.84,-42.7,;41.33,-42.3,;41.73,-40.82,;42.42,-43.39,;43.9,-42.99,;40.25,-37.17,;41.27,-38.31,;40.72,-35.7,;15.31,3.28,;13.86,2.76,;15.58,4.8,;14.4,5.79,;12.95,5.26,;11.77,6.26,;10.32,5.73,;12.04,7.77,;14.67,7.3,;13.49,8.3,;16.12,7.83,;16.39,9.34,;14.94,9.87,;14.68,11.39,;13.23,11.92,;15.86,12.38,;17.84,9.87,;19.01,8.88,;18.61,11.2,;20.15,11.2,;20.92,9.86,;22.46,9.86,;23.36,11.1,;24.83,10.63,;24.82,9.09,;23.36,8.61,;20.92,12.53,;22.46,12.53,;20.15,13.87,;20.92,15.2,;22.46,15.2,;23.24,16.53,;24.78,16.52,;25.55,17.86,;27.09,17.85,;27.86,19.19,;27.86,16.52,;20.16,16.53,;18.62,16.54,;20.93,17.87,;20.16,19.2,;18.62,19.2,;17.85,20.54,;20.93,20.53,;22.47,20.53,;20.17,21.87,;20.94,23.2,;22.48,23.2,;23.25,24.53,;22.48,25.86,;23.26,27.2,;24.8,27.19,;25.56,25.86,;24.79,24.53,;20.17,24.54,;20.94,25.87,;18.63,24.54,;17.86,25.87,;18.64,27.21,;18.21,28.48,;16.67,28.48,;16.19,29.94,;17.44,30.84,;18.68,29.94,;16.32,25.88,;15.55,24.54,;15.56,27.21,;14.02,27.21,;13.24,25.88,;11.7,25.88,;10.93,24.55,;10.93,27.22,;13.25,28.55,;11.71,28.55,;14.02,29.88,;13.25,31.22,;11.71,31.22,;10.94,32.55,;9.4,32.56,;8.64,33.89,;7.1,33.89,;14.02,32.55,;15.56,32.55,;13.26,33.88,;14.03,35.22,;15.57,35.21,;16.34,36.55,;16.17,37.95,;14.84,38.72,;14.84,40.26,;16.17,41.03,;16.17,42.57,;17.5,40.26,;17.5,38.72,;13.26,36.55,;14.03,37.88,;11.72,36.55,;10.95,37.89,;9.41,37.89,;8.64,36.56,;8.65,39.23,;7.11,39.23,;6.33,37.9,;4.79,37.9,;4.02,36.57,;4.03,39.23,;6.34,40.56,;7.11,41.9,;4.8,40.57,)| Show InChI InChI=1S/C144H210N42O39/c1-14-75(10)118(142(224)181-106(57-117(200)201)137(219)177-103(54-110(147)192)134(216)175-101(52-84-61-153-69-161-84)132(214)169-91(29-22-42-155-144(150)151)126(208)182-108(67-188)140(222)173-98(48-80-24-16-15-17-25-80)131(213)174-102(53-85-62-154-70-162-85)133(215)178-105(56-116(198)199)136(218)168-90(28-20-21-41-145)125(207)170-92(40-35-79-31-36-86(190)37-32-79)123(205)158-64-113(195)165-93(120(149)202)44-71(2)3)184-122(204)77(12)164-127(209)100(51-83-60-152-68-160-83)179-141(223)109-30-23-43-186(109)115(197)65-159-124(206)94(45-72(4)5)171-128(210)95(46-73(6)7)172-130(212)97(49-81-33-38-87(191)39-34-81)167-114(196)63-157-121(203)76(11)163-139(221)107(66-187)183-135(217)104(55-111(148)193)176-129(211)96(47-74(8)9)180-143(225)119(78(13)189)185-138(220)99(166-112(194)58-146)50-82-59-156-89-27-19-18-26-88(82)89/h15-19,24-27,31-34,36-39,59-62,68-78,90-109,118-119,156,187-191H,14,20-23,28-30,35,40-58,63-67,145-146H2,1-13H3,(H2,147,192)(H2,148,193)(H2,149,202)(H,152,160)(H,153,161)(H,154,162)(H,157,203)(H,158,205)(H,159,206)(H,163,221)(H,164,209)(H,165,195)(H,166,194)(H,167,196)(H,168,218)(H,169,214)(H,170,207)(H,171,210)(H,172,212)(H,173,222)(H,174,213)(H,175,216)(H,176,211)(H,177,219)(H,178,215)(H,179,223)(H,180,225)(H,181,224)(H,182,208)(H,183,217)(H,184,204)(H,185,220)(H,198,199)(H,200,201)(H4,150,151,155) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation
Curated by PDSP Ki Database
| |
J Biol Chem 272: 24612-6 (1997)
Article DOI: 10.1074/jbc.272.39.24612
BindingDB Entry DOI: 10.7270/Q2BC3X2Q |
More data for this
Ligand-Target Pair | |
Galanin receptor type 1
(RAT) | BDBM85169

(Galanin (1-29), porcine | Galanin [D-Trp2], porcin...)Show SMILES CCC(C)C(NC(=O)C(C)NC(=O)C(Cc1cnc[nH]1)NC(=O)C1CCCN1C(=O)CNC(=O)C(CC(C)C)NC(=O)C(CC(C)C)NC(=O)C(Cc1ccc(O)cc1)NC(=O)CNC(=O)C(C)NC(=O)C(CO)NC(=O)C(CC(N)=O)NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(Cc1c[nH]c2ccccc12)NC(=O)CN)C(C)O)C(=O)NC(CC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(Cc1cnc[nH]1)C(=O)NC(CCCN=C(N)N)C(=O)NC(CO)C(=O)NC(Cc1ccccc1)C(=O)NC(Cc1cnc[nH]1)C(=O)NC(CC(O)=O)C(=O)NC(CCCCN)C(=O)NC(CCc1ccc(O)cc1)C(=O)NCC(=O)NC(CC(C)C)C(N)=O |(19.65,4.85,;18.2,4.33,;17.93,2.81,;19.11,1.82,;16.48,2.29,;16.21,.77,;17.39,-.22,;18.84,.3,;17.12,-1.74,;15.67,-2.26,;18.3,-2.73,;18.03,-4.24,;16.58,-4.77,;19.21,-5.24,;18.94,-6.75,;17.49,-7.28,;16.22,-6.41,;15,-7.36,;15.52,-8.81,;17.06,-8.76,;20.66,-4.71,;21.83,-5.7,;21.56,-7.22,;23.28,-5.18,;23.71,-3.7,;25.25,-3.65,;25.77,-5.1,;24.56,-6.04,;24.61,-7.58,;23.3,-8.4,;25.97,-8.31,;26.02,-9.85,;27.38,-10.57,;28.68,-9.76,;27.43,-12.11,;28.79,-12.84,;30.09,-12.02,;31.45,-12.75,;30.04,-10.48,;26.12,-12.93,;26.17,-14.46,;27.7,-14.68,;25.22,-15.68,;23.72,-15.99,;22.69,-14.85,;21.18,-15.17,;23.16,-13.38,;25.8,-17.11,;24.85,-18.32,;23.32,-18.1,;25.42,-19.75,;24.47,-20.96,;22.95,-20.74,;22,-21.95,;20.47,-21.74,;19.9,-20.31,;18.37,-20.09,;20.85,-19.1,;22.37,-19.31,;26.95,-19.96,;27.52,-21.39,;26.57,-22.6,;29.04,-21.61,;29.62,-23.04,;31.14,-23.26,;32.09,-22.04,;31.72,-24.68,;30.77,-25.9,;33.24,-24.9,;33.82,-26.33,;32.87,-27.54,;35.34,-26.55,;36.29,-25.34,;35.72,-23.91,;35.92,-27.98,;37.44,-28.19,;38.39,-26.98,;38.02,-29.62,;39.54,-29.84,;40.49,-28.63,;42.01,-28.84,;39.92,-27.2,;37.06,-30.83,;37.64,-32.26,;39.15,-31.95,;37.16,-33.73,;35.85,-34.54,;34.5,-33.82,;33.19,-34.63,;34.44,-32.28,;38.19,-34.87,;37.71,-36.34,;36.2,-36.65,;38.74,-37.48,;38.26,-38.95,;39.29,-40.09,;40.8,-39.78,;38.81,-41.56,;37.3,-41.87,;36.83,-43.34,;37.72,-44.58,;36.83,-45.82,;35.37,-45.35,;34.03,-46.12,;32.7,-45.35,;32.7,-43.81,;34.03,-43.04,;35.37,-43.81,;39.84,-42.7,;41.33,-42.3,;41.73,-40.82,;42.42,-43.39,;43.9,-42.99,;40.25,-37.17,;41.27,-38.31,;40.72,-35.7,;15.31,3.28,;13.86,2.76,;15.58,4.8,;14.4,5.79,;12.95,5.26,;11.77,6.26,;10.32,5.73,;12.04,7.77,;14.67,7.3,;13.49,8.3,;16.12,7.83,;16.39,9.34,;14.94,9.87,;14.68,11.39,;13.23,11.92,;15.86,12.38,;17.84,9.87,;19.01,8.88,;18.61,11.2,;20.15,11.2,;20.92,9.86,;22.46,9.86,;23.36,11.1,;24.83,10.63,;24.82,9.09,;23.36,8.61,;20.92,12.53,;22.46,12.53,;20.15,13.87,;20.92,15.2,;22.46,15.2,;23.24,16.53,;24.78,16.52,;25.55,17.86,;27.09,17.85,;27.86,19.19,;27.86,16.52,;20.16,16.53,;18.62,16.54,;20.93,17.87,;20.16,19.2,;18.62,19.2,;17.85,20.54,;20.93,20.53,;22.47,20.53,;20.17,21.87,;20.94,23.2,;22.48,23.2,;23.25,24.53,;22.48,25.86,;23.26,27.2,;24.8,27.19,;25.56,25.86,;24.79,24.53,;20.17,24.54,;20.94,25.87,;18.63,24.54,;17.86,25.87,;18.64,27.21,;18.21,28.48,;16.67,28.48,;16.19,29.94,;17.44,30.84,;18.68,29.94,;16.32,25.88,;15.55,24.54,;15.56,27.21,;14.02,27.21,;13.24,25.88,;11.7,25.88,;10.93,24.55,;10.93,27.22,;13.25,28.55,;11.71,28.55,;14.02,29.88,;13.25,31.22,;11.71,31.22,;10.94,32.55,;9.4,32.56,;8.64,33.89,;7.1,33.89,;14.02,32.55,;15.56,32.55,;13.26,33.88,;14.03,35.22,;15.57,35.21,;16.34,36.55,;16.17,37.95,;14.84,38.72,;14.84,40.26,;16.17,41.03,;16.17,42.57,;17.5,40.26,;17.5,38.72,;13.26,36.55,;14.03,37.88,;11.72,36.55,;10.95,37.89,;9.41,37.89,;8.64,36.56,;8.65,39.23,;7.11,39.23,;6.33,37.9,;4.79,37.9,;4.02,36.57,;4.03,39.23,;6.34,40.56,;7.11,41.9,;4.8,40.57,)| Show InChI InChI=1S/C144H210N42O39/c1-14-75(10)118(142(224)181-106(57-117(200)201)137(219)177-103(54-110(147)192)134(216)175-101(52-84-61-153-69-161-84)132(214)169-91(29-22-42-155-144(150)151)126(208)182-108(67-188)140(222)173-98(48-80-24-16-15-17-25-80)131(213)174-102(53-85-62-154-70-162-85)133(215)178-105(56-116(198)199)136(218)168-90(28-20-21-41-145)125(207)170-92(40-35-79-31-36-86(190)37-32-79)123(205)158-64-113(195)165-93(120(149)202)44-71(2)3)184-122(204)77(12)164-127(209)100(51-83-60-152-68-160-83)179-141(223)109-30-23-43-186(109)115(197)65-159-124(206)94(45-72(4)5)171-128(210)95(46-73(6)7)172-130(212)97(49-81-33-38-87(191)39-34-81)167-114(196)63-157-121(203)76(11)163-139(221)107(66-187)183-135(217)104(55-111(148)193)176-129(211)96(47-74(8)9)180-143(225)119(78(13)189)185-138(220)99(166-112(194)58-146)50-82-59-156-89-27-19-18-26-88(82)89/h15-19,24-27,31-34,36-39,59-62,68-78,90-109,118-119,156,187-191H,14,20-23,28-30,35,40-58,63-67,145-146H2,1-13H3,(H2,147,192)(H2,148,193)(H2,149,202)(H,152,160)(H,153,161)(H,154,162)(H,157,203)(H,158,205)(H,159,206)(H,163,221)(H,164,209)(H,165,195)(H,166,194)(H,167,196)(H,168,218)(H,169,214)(H,170,207)(H,171,210)(H,172,212)(H,173,222)(H,174,213)(H,175,216)(H,176,211)(H,177,219)(H,178,215)(H,179,223)(H,180,225)(H,181,224)(H,182,208)(H,183,217)(H,184,204)(H,185,220)(H,198,199)(H,200,201)(H4,150,151,155) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation
Curated by PDSP Ki Database
| |
J Biol Chem 272: 24612-6 (1997)
Article DOI: 10.1074/jbc.272.39.24612
BindingDB Entry DOI: 10.7270/Q2BC3X2Q |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384809

(CHEMBL2037493)Show SMILES CC(C)C[C@H]1N([C@@H](C(=O)N2CCCC2)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C29H36N4O3/c1-18(2)14-24-27(34)31-25(23-15-20-8-4-5-9-21(20)16-23)28(35)33(24)26(22-11-10-19(3)30-17-22)29(36)32-12-6-7-13-32/h4-5,8-11,17-18,23-26H,6-7,12-16H2,1-3H3,(H,31,34)/t24-,25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384804
(CHEMBL2037500)Show SMILES CC[C@@H](C)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C29H36N4O4/c1-4-18(2)25-27(34)31-24(23-15-20-7-5-6-8-21(20)16-23)28(35)33(25)26(22-10-9-19(3)30-17-22)29(36)32-11-13-37-14-12-32/h5-10,17-18,23-26H,4,11-16H2,1-3H3,(H,31,34)/t18-,24-,25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data