

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidylate synthase (Mus musculus) | BDBM50008294 (2-(4-(((2-amino-4-oxo-1,4-dihydroquinazolin-6-yl)m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity was evaluated against thymidylate synthase | J Med Chem 28: 1468-76 (1985) BindingDB Entry DOI: 10.7270/Q2348JDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50008294 (2-(4-(((2-amino-4-oxo-1,4-dihydroquinazolin-6-yl)m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity was evaluated against dihydrofolate reductase | J Med Chem 28: 1468-76 (1985) BindingDB Entry DOI: 10.7270/Q2348JDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM14239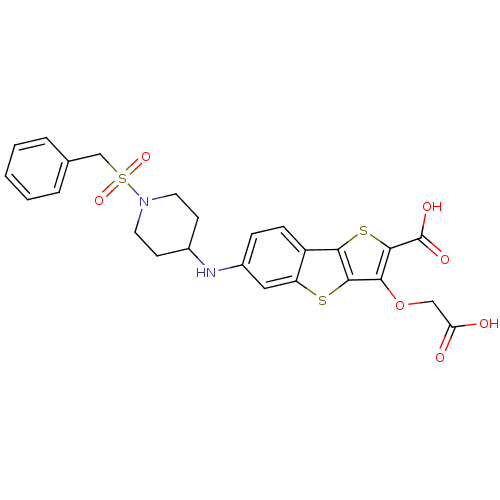 (5-(carboxymethoxy)-10-{[1-(phenylmethane)sulfonylp...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 370 | -36.7 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Wyeth Research | Assay Description The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate... | Bioorg Med Chem 14: 2162-77 (2006) Article DOI: 10.1016/j.bmc.2005.11.005 BindingDB Entry DOI: 10.7270/Q289143K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM14239 (5-(carboxymethoxy)-10-{[1-(phenylmethane)sulfonylp...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 380 | -36.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Wyeth Research | Assay Description The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate... | Bioorg Med Chem 14: 2162-77 (2006) Article DOI: 10.1016/j.bmc.2005.11.005 BindingDB Entry DOI: 10.7270/Q289143K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM14234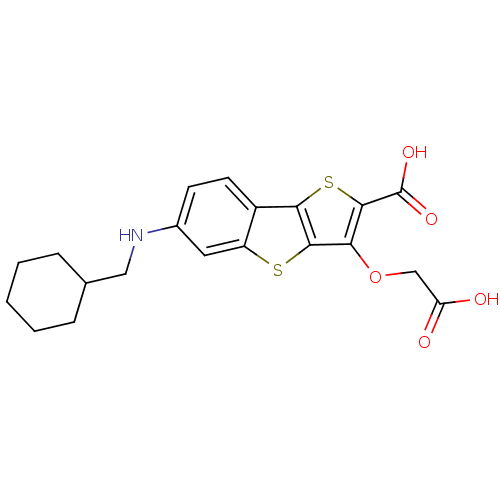 (5-(carboxymethoxy)-10-[(cyclohexylmethyl)amino]-3,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 680 | -35.2 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Wyeth Research | Assay Description The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate... | Bioorg Med Chem 14: 2162-77 (2006) Article DOI: 10.1016/j.bmc.2005.11.005 BindingDB Entry DOI: 10.7270/Q289143K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM14236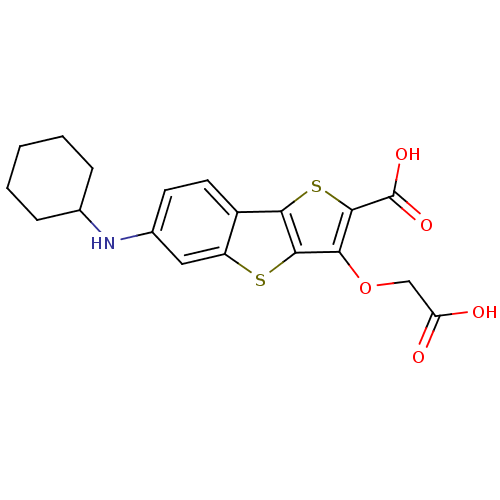 (5-(carboxymethoxy)-10-(cyclohexylamino)-3,7-dithia...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 740 | -35.0 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Wyeth Research | Assay Description The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate... | Bioorg Med Chem 14: 2162-77 (2006) Article DOI: 10.1016/j.bmc.2005.11.005 BindingDB Entry DOI: 10.7270/Q289143K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM14233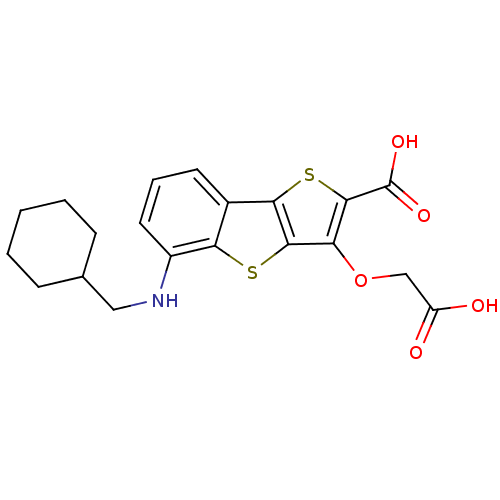 (5-(carboxymethoxy)-9-[(cyclohexylmethyl)amino]-3,7...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 920 | -34.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Wyeth Research | Assay Description The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate... | Bioorg Med Chem 14: 2162-77 (2006) Article DOI: 10.1016/j.bmc.2005.11.005 BindingDB Entry DOI: 10.7270/Q289143K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM14238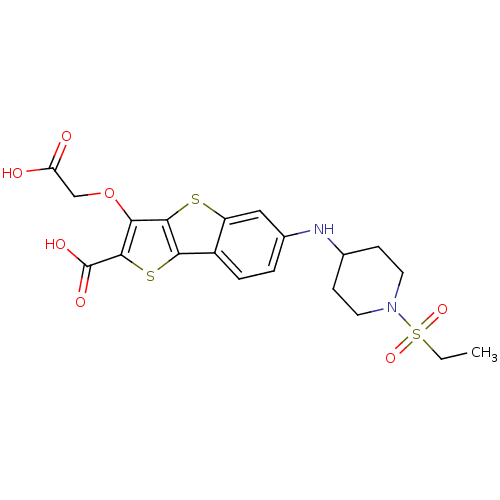 (5-(carboxymethoxy)-10-{[1-(ethanesulfonyl)piperidi...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.60E+3 | -33.1 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Wyeth Research | Assay Description The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate... | Bioorg Med Chem 14: 2162-77 (2006) Article DOI: 10.1016/j.bmc.2005.11.005 BindingDB Entry DOI: 10.7270/Q289143K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM14235 (5-(carboxymethoxy)-9-(cyclohexylamino)-3,7-dithiat...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | -32.9 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Wyeth Research | Assay Description The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate... | Bioorg Med Chem 14: 2162-77 (2006) Article DOI: 10.1016/j.bmc.2005.11.005 BindingDB Entry DOI: 10.7270/Q289143K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM14237 (5-(carboxymethoxy)-10-(oxan-4-ylamino)-3,7-dithiat...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.40E+3 | -32.1 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Wyeth Research | Assay Description The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate... | Bioorg Med Chem 14: 2162-77 (2006) Article DOI: 10.1016/j.bmc.2005.11.005 BindingDB Entry DOI: 10.7270/Q289143K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM14232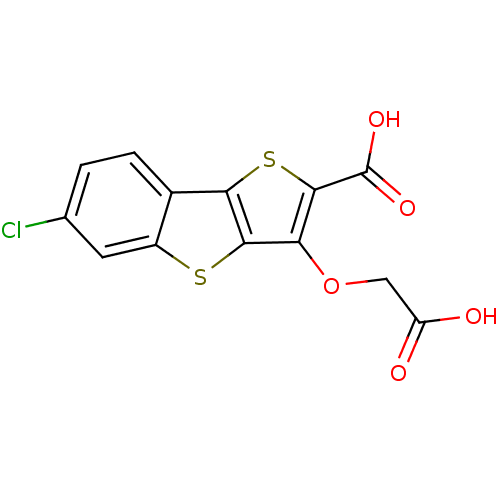 (5-(carboxymethoxy)-10-chloro-3,7-dithiatricyclo[6....) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.50E+3 | -31.1 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Wyeth Research | Assay Description The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate... | Bioorg Med Chem 14: 2162-77 (2006) Article DOI: 10.1016/j.bmc.2005.11.005 BindingDB Entry DOI: 10.7270/Q289143K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM14230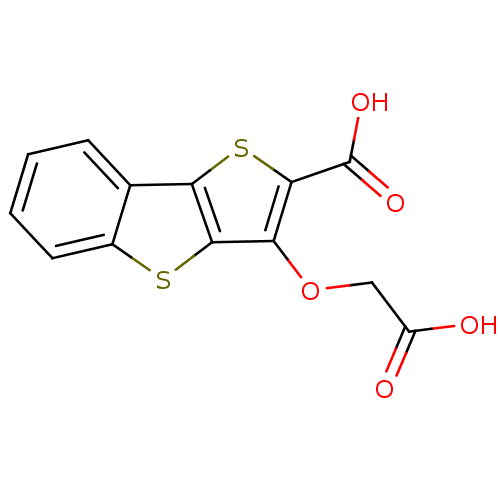 (5-(carboxymethoxy)-3,7-dithiatricyclo[6.4.0.0^{2,6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.10E+3 | -30.7 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Wyeth Research | Assay Description The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate... | Bioorg Med Chem 14: 2162-77 (2006) Article DOI: 10.1016/j.bmc.2005.11.005 BindingDB Entry DOI: 10.7270/Q289143K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM14230 (5-(carboxymethoxy)-3,7-dithiatricyclo[6.4.0.0^{2,6...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9.20E+3 | -28.7 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Wyeth Research | Assay Description The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate... | Bioorg Med Chem 14: 2162-77 (2006) Article DOI: 10.1016/j.bmc.2005.11.005 BindingDB Entry DOI: 10.7270/Q289143K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM14231 (5-(carboxymethoxy)-9-chloro-3,7-dithiatricyclo[6.4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.00E+4 | -28.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Wyeth Research | Assay Description The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate... | Bioorg Med Chem 14: 2162-77 (2006) Article DOI: 10.1016/j.bmc.2005.11.005 BindingDB Entry DOI: 10.7270/Q289143K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM14228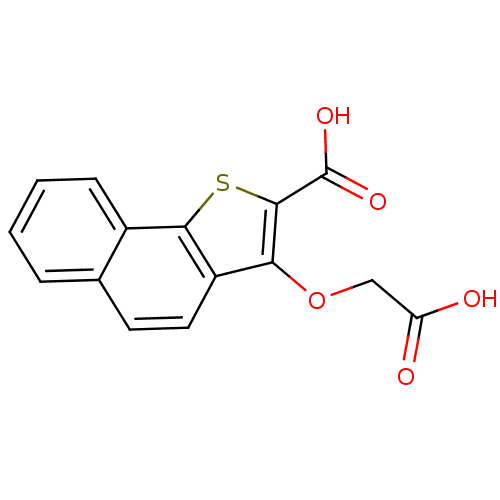 (5-(carboxymethoxy)-3-thiatricyclo[7.4.0.0^{2,6}]tr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.10E+4 | -28.3 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Wyeth Research | Assay Description The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate... | Bioorg Med Chem 14: 2162-77 (2006) Article DOI: 10.1016/j.bmc.2005.11.005 BindingDB Entry DOI: 10.7270/Q289143K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM14225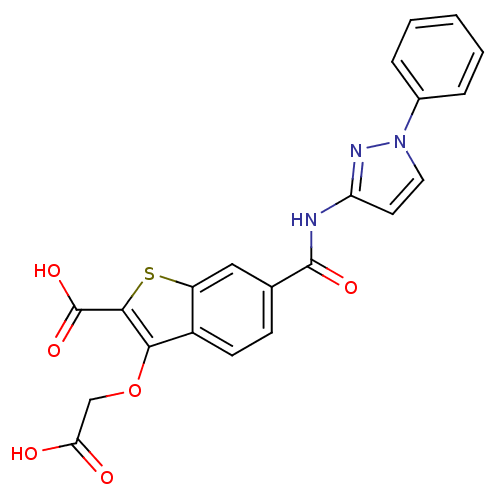 (3-(carboxymethoxy)-6-[(1-phenyl-1H-pyrazol-3-yl)ca...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.00E+4 | -26.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Wyeth Research | Assay Description The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate... | Bioorg Med Chem 14: 2162-77 (2006) Article DOI: 10.1016/j.bmc.2005.11.005 BindingDB Entry DOI: 10.7270/Q289143K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM14223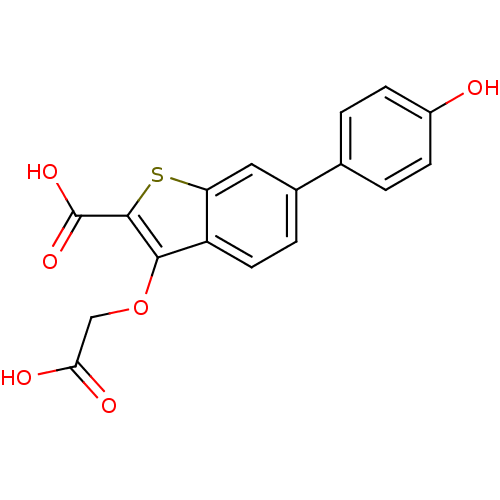 (3-(carboxymethoxy)-6-(4-hydroxyphenyl)-1-benzothio...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.60E+4 | -25.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Wyeth Research | Assay Description The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate... | Bioorg Med Chem 14: 2162-77 (2006) Article DOI: 10.1016/j.bmc.2005.11.005 BindingDB Entry DOI: 10.7270/Q289143K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM14224 (3-(carboxymethoxy)-6-(thiophen-2-yl)-1-benzothioph...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.00E+4 | -25.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Wyeth Research | Assay Description The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate... | Bioorg Med Chem 14: 2162-77 (2006) Article DOI: 10.1016/j.bmc.2005.11.005 BindingDB Entry DOI: 10.7270/Q289143K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM14227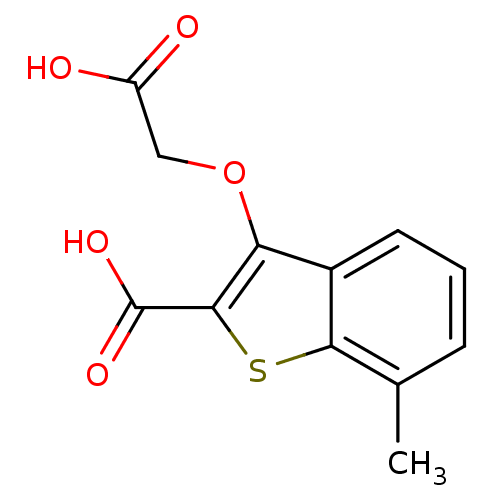 (3-(carboxymethoxy)-7-methyl-1-benzothiophene-2-car...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.70E+4 | -25.3 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Wyeth Research | Assay Description The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate... | Bioorg Med Chem 14: 2162-77 (2006) Article DOI: 10.1016/j.bmc.2005.11.005 BindingDB Entry DOI: 10.7270/Q289143K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM14221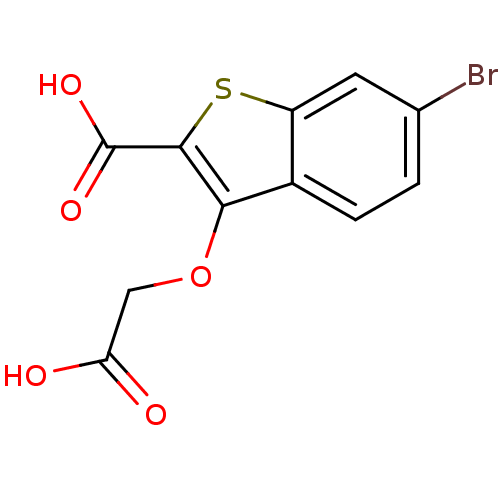 (6-bromo-3-(carboxymethoxy)-1-benzothiophene-2-carb...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.20E+4 | -24.7 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Wyeth Research | Assay Description The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate... | Bioorg Med Chem 14: 2162-77 (2006) Article DOI: 10.1016/j.bmc.2005.11.005 BindingDB Entry DOI: 10.7270/Q289143K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM14220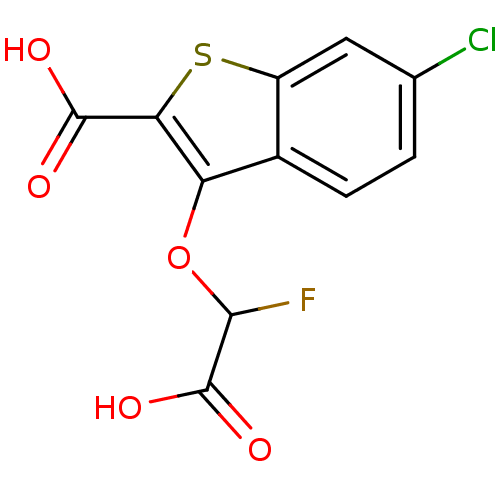 (3-[carboxy(fluoro)methoxy]-6-chloro-1-benzothiophe...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.20E+4 | -24.2 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Wyeth Research | Assay Description The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate... | Bioorg Med Chem 14: 2162-77 (2006) Article DOI: 10.1016/j.bmc.2005.11.005 BindingDB Entry DOI: 10.7270/Q289143K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM14218 (3-(carboxymethoxy)-6-chloro-1-benzothiophene-2-car...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.10E+4 | -23.8 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Wyeth Research | Assay Description The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate... | Bioorg Med Chem 14: 2162-77 (2006) Article DOI: 10.1016/j.bmc.2005.11.005 BindingDB Entry DOI: 10.7270/Q289143K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM14216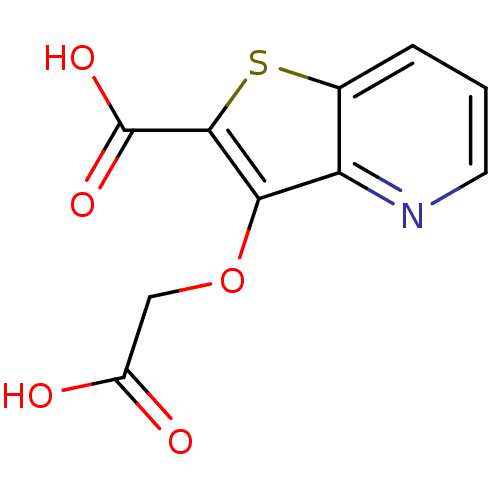 (3-(carboxymethoxy)thieno[3,2-b]pyridine-2-carboxyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 7.70E+4 | -23.2 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Wyeth Research | Assay Description The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate... | Bioorg Med Chem 14: 2162-77 (2006) Article DOI: 10.1016/j.bmc.2005.11.005 BindingDB Entry DOI: 10.7270/Q289143K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM14226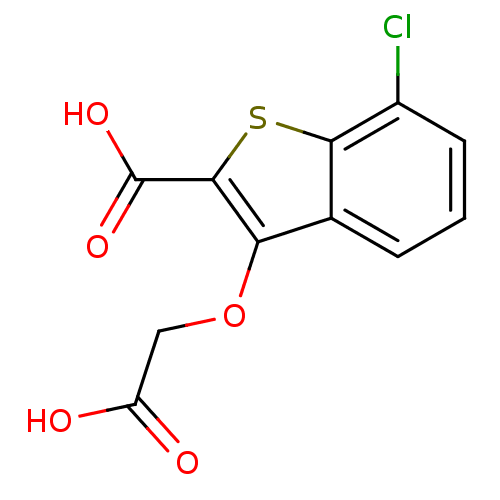 (3-(carboxymethoxy)-7-chloro-1-benzothiophene-2-car...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.19E+5 | -22.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Wyeth Research | Assay Description The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate... | Bioorg Med Chem 14: 2162-77 (2006) Article DOI: 10.1016/j.bmc.2005.11.005 BindingDB Entry DOI: 10.7270/Q289143K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM14222 (3-(carboxymethoxy)-6-phenyl-1-benzothiophene-2-car...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.28E+5 | -22.0 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Wyeth Research | Assay Description The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate... | Bioorg Med Chem 14: 2162-77 (2006) Article DOI: 10.1016/j.bmc.2005.11.005 BindingDB Entry DOI: 10.7270/Q289143K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM14214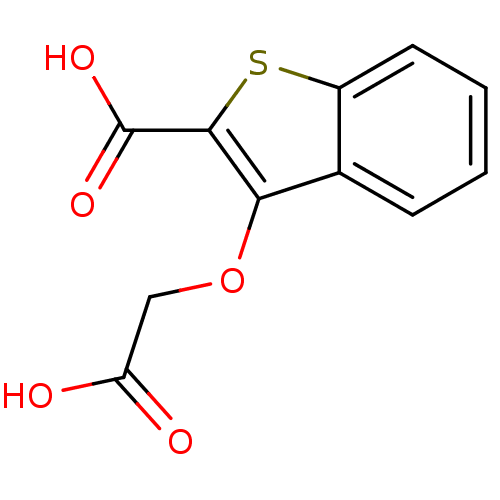 (3-(carboxymethoxy)-1-benzothiophene-2-carboxylic a...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.60E+5 | -21.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Wyeth Research | Assay Description The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate... | Bioorg Med Chem 14: 2162-77 (2006) Article DOI: 10.1016/j.bmc.2005.11.005 BindingDB Entry DOI: 10.7270/Q289143K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM14211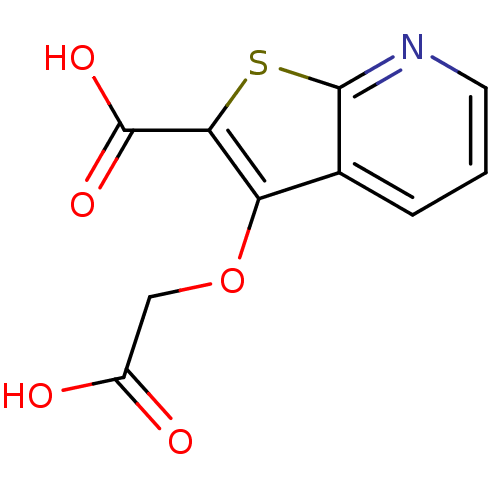 (3-(carboxymethoxy)thieno[2,3-b]pyridine-2-carboxyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents | DrugBank MMDB PDB Article PubMed | 2.30E+5 | -20.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate... | Bioorg Med Chem 14: 2162-77 (2006) Article DOI: 10.1016/j.bmc.2005.11.005 BindingDB Entry DOI: 10.7270/Q289143K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM14217 (3-(carboxymethoxy)thieno[3,2-b]thiophene-2-carboxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.80E+5 | -20.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Wyeth Research | Assay Description The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate... | Bioorg Med Chem 14: 2162-77 (2006) Article DOI: 10.1016/j.bmc.2005.11.005 BindingDB Entry DOI: 10.7270/Q289143K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM14229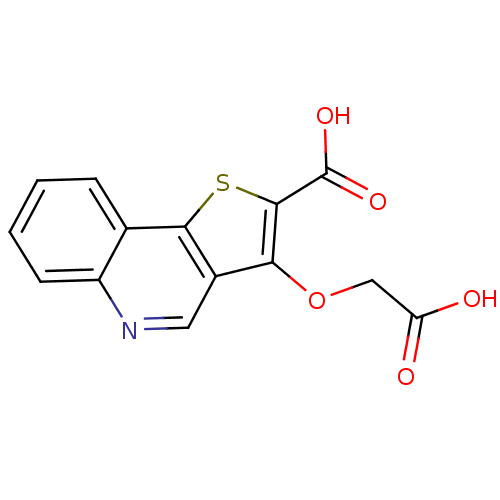 (5-(carboxymethoxy)-3-thia-8-azatricyclo[7.4.0.0^{2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >5.00E+5 | >-18.8 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Wyeth Research | Assay Description The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate... | Bioorg Med Chem 14: 2162-77 (2006) Article DOI: 10.1016/j.bmc.2005.11.005 BindingDB Entry DOI: 10.7270/Q289143K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM14212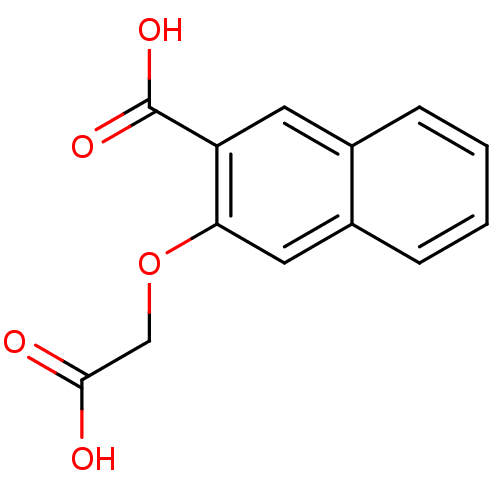 (3-(carboxymethoxy)naphthalene-2-carboxylic acid | ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 8.60E+5 | -17.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Wyeth Research | Assay Description The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate... | Bioorg Med Chem 14: 2162-77 (2006) Article DOI: 10.1016/j.bmc.2005.11.005 BindingDB Entry DOI: 10.7270/Q289143K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM14213 (3-(carboxymethoxy)furo[2,3-b]pyridine-2-carboxylic...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >2.50E+6 | >-14.7 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Wyeth Research | Assay Description The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate... | Bioorg Med Chem 14: 2162-77 (2006) Article DOI: 10.1016/j.bmc.2005.11.005 BindingDB Entry DOI: 10.7270/Q289143K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM14219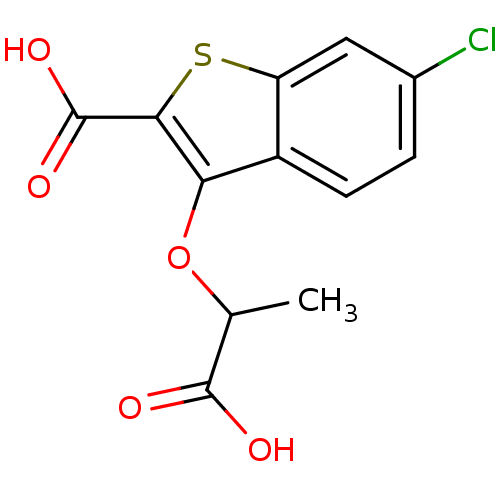 (3-(1-carboxyethoxy)-6-chloro-1-benzothiophene-2-ca...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >2.50E+6 | >-14.7 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Wyeth Research | Assay Description The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate... | Bioorg Med Chem 14: 2162-77 (2006) Article DOI: 10.1016/j.bmc.2005.11.005 BindingDB Entry DOI: 10.7270/Q289143K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM14215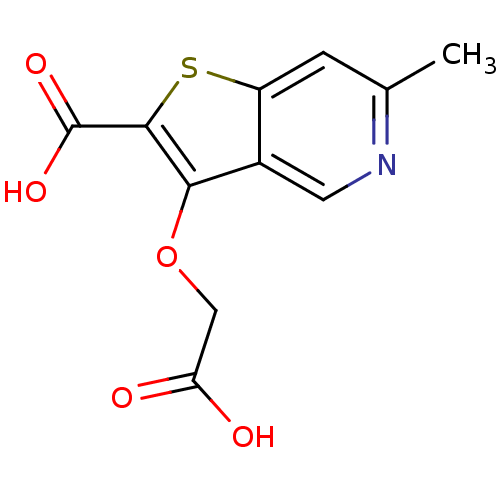 (3-(carboxymethoxy)-6-methylthieno[3,2-c]pyridine-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | >2.50E+6 | >-14.7 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Wyeth Research | Assay Description The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate... | Bioorg Med Chem 14: 2162-77 (2006) Article DOI: 10.1016/j.bmc.2005.11.005 BindingDB Entry DOI: 10.7270/Q289143K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50034758 ((E)-7-(3-{4-[(2S,4S,5R)-5-((Z)-5-Carboxy-pent-2-en...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human microsomal thromboxane synthase. | J Med Chem 38: 1608-28 (1995) BindingDB Entry DOI: 10.7270/Q2319TWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50034762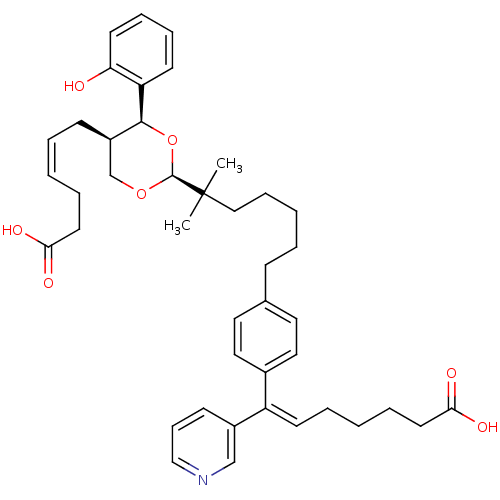 ((E)-7-(4-{6-[(2S,4S,5R)-5-((Z)-5-Carboxy-pent-2-en...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human microsomal thromboxane synthase. | J Med Chem 38: 1608-28 (1995) BindingDB Entry DOI: 10.7270/Q2319TWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50034767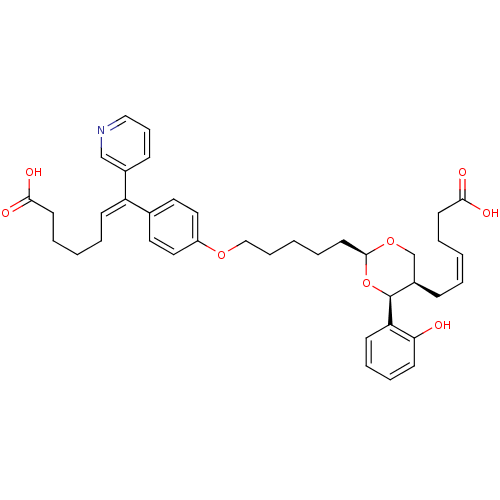 ((E)-7-(4-{5-[(2S,4S,5R)-5-((Z)-5-Carboxy-pent-2-en...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human microsomal thromboxane synthase. | J Med Chem 38: 1608-28 (1995) BindingDB Entry DOI: 10.7270/Q2319TWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50036368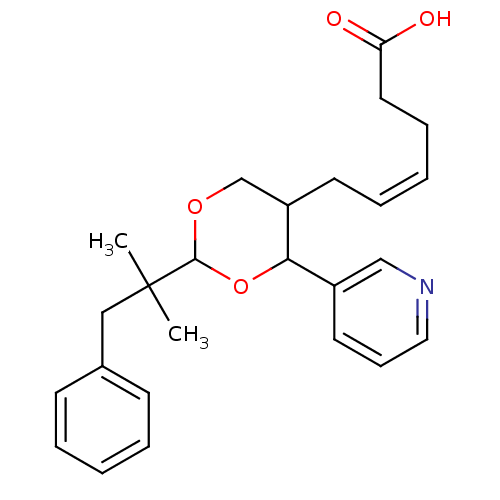 ((Z)-6-[2-(1,1-Dimethyl-2-phenyl-ethyl)-4-pyridin-3...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro Inhibition of thromboxane synthase from human blood platelet microsomes | J Med Chem 38: 686-94 (1995) BindingDB Entry DOI: 10.7270/Q24Q7T2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50025149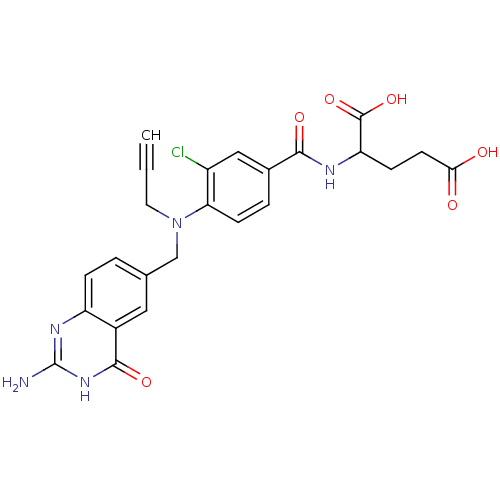 (2-{4-[(2-Amino-4-oxo-3,4-dihydro-quinazolin-6-ylme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of L1210 thymidylate synthase | J Med Chem 29: 468-72 (1986) BindingDB Entry DOI: 10.7270/Q2HH6J3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50025148 (2-{4-[(2-Amino-4-oxo-3,4-dihydro-quinazolin-6-ylme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of L1210 thymidylate synthase with CB3717 as control | J Med Chem 29: 468-72 (1986) BindingDB Entry DOI: 10.7270/Q2HH6J3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50036355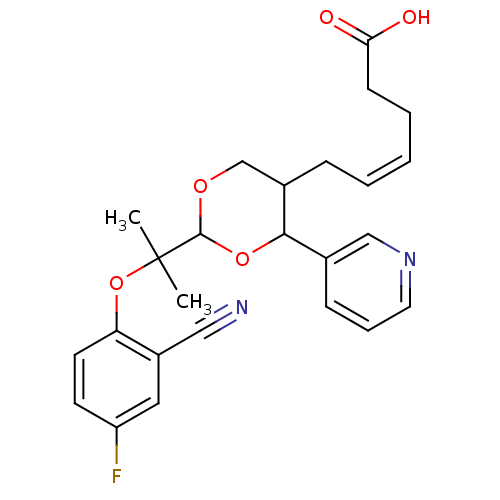 ((Z)-6-{2-[1-(2-Cyano-4-fluoro-phenoxy)-1-methyl-et...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro Inhibition of thromboxane synthase from human blood platelet microsomes | J Med Chem 38: 686-94 (1995) BindingDB Entry DOI: 10.7270/Q24Q7T2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50010960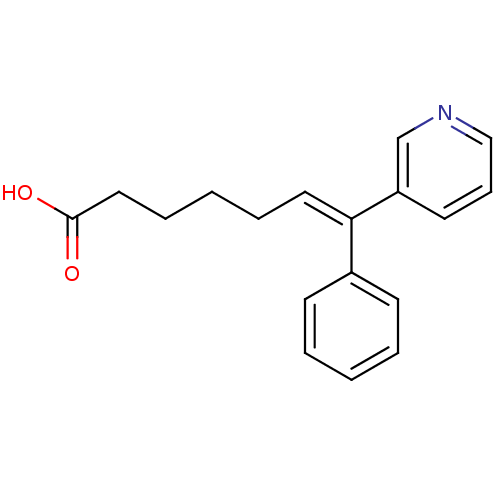 ((E)-7-Phenyl-7-pyridin-3-yl-hept-6-enoic acid | 7-...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro Inhibition of thromboxane synthase from human blood platelet microsomes | J Med Chem 38: 686-94 (1995) BindingDB Entry DOI: 10.7270/Q24Q7T2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50025150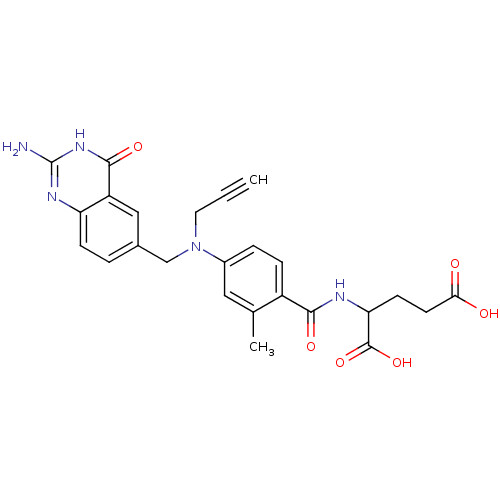 (2-{4-[(2-Amino-4-oxo-3,4-dihydro-quinazolin-6-ylme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of L1210 thymidylate synthase with CB3717 as control | J Med Chem 29: 468-72 (1986) BindingDB Entry DOI: 10.7270/Q2HH6J3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50036354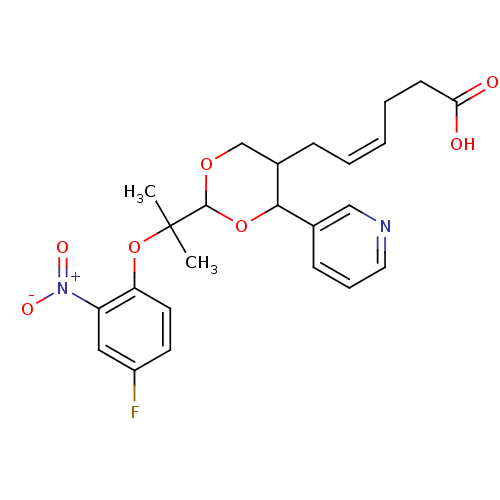 ((Z)-6-{2-[1-(4-Fluoro-2-nitro-phenoxy)-1-methyl-et...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro Inhibition of thromboxane synthase from human blood platelet microsomes | J Med Chem 38: 686-94 (1995) BindingDB Entry DOI: 10.7270/Q24Q7T2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50288601 ((Z)-6-[(2S,4S,5R)-2-(4-Cyano-phenyl)-4-pyridin-3-y...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for thromboxane TXA2 synthase inhibitory activity using human platelet | Bioorg Med Chem Lett 6: 273-278 (1996) Article DOI: 10.1016/0960-894X(96)00004-2 BindingDB Entry DOI: 10.7270/Q2PG1RR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50008294 (2-(4-(((2-amino-4-oxo-1,4-dihydroquinazolin-6-yl)m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against thymidylate synthase | J Med Chem 28: 1468-76 (1985) BindingDB Entry DOI: 10.7270/Q2348JDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50034750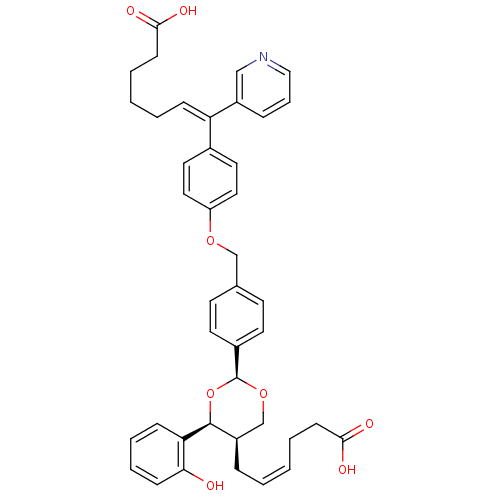 ((E)-7-(4-{4-[(2S,4S,5R)-5-((Z)-5-Carboxy-pent-2-en...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human microsomal thromboxane synthase. | J Med Chem 38: 1608-28 (1995) BindingDB Entry DOI: 10.7270/Q2319TWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50010960 ((E)-7-Phenyl-7-pyridin-3-yl-hept-6-enoic acid | 7-...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human microsomal thromboxane synthase. | J Med Chem 38: 1608-28 (1995) BindingDB Entry DOI: 10.7270/Q2319TWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50036345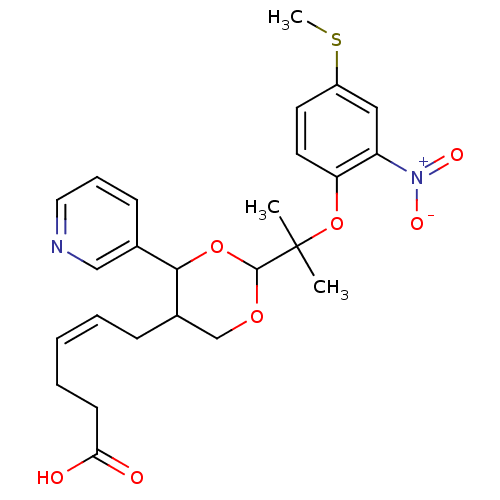 ((Z)-6-{2-[1-Methyl-1-(4-methylsulfanyl-2-nitro-phe...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro Inhibition of thromboxane synthase from human blood platelet microsomes | J Med Chem 38: 686-94 (1995) BindingDB Entry DOI: 10.7270/Q24Q7T2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50036362 ((Z)-6-{2-[1-(4-Bromo-phenoxy)-1-methyl-ethyl]-4-py...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro Inhibition of thromboxane synthase from human blood platelet microsomes | J Med Chem 38: 686-94 (1995) BindingDB Entry DOI: 10.7270/Q24Q7T2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50036362 ((Z)-6-{2-[1-(4-Bromo-phenoxy)-1-methyl-ethyl]-4-py...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro Inhibition of thromboxane synthase from human blood platelet microsomes | J Med Chem 38: 686-94 (1995) BindingDB Entry DOI: 10.7270/Q24Q7T2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 178 total ) | Next | Last >> |