

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50099006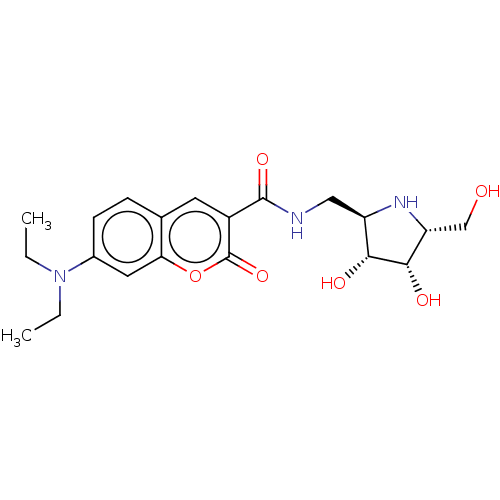 (7-Diethylamino-2-oxo-2H-chromene-3-carboxylic acid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität Graz Curated by ChEMBL | Assay Description Tested for inhibitory activity of the compound against Beta-glucosidase from Agrobacterium species | Bioorg Med Chem Lett 11: 1063-4 (2001) BindingDB Entry DOI: 10.7270/Q2222T12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50100428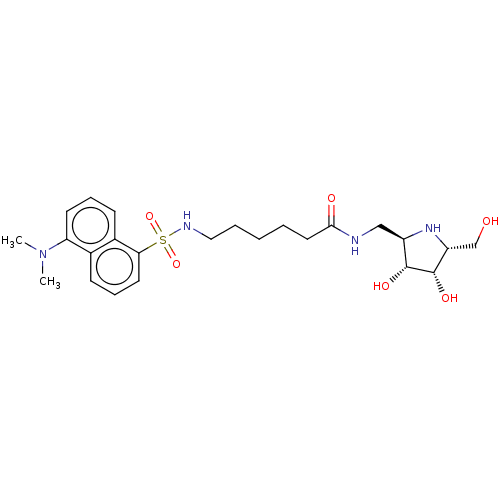 (6-(5-Dimethylamino-naphthalene-1-sulfonylamino)-he...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie der Technischen Universität Graz Curated by ChEMBL | Assay Description Inhibitory activity against Agrobacterium sp. Beta-glucosidase employing fluorescence spectrometric method | Bioorg Med Chem Lett 11: 1339-42 (2001) BindingDB Entry DOI: 10.7270/Q2JQ1097 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50100426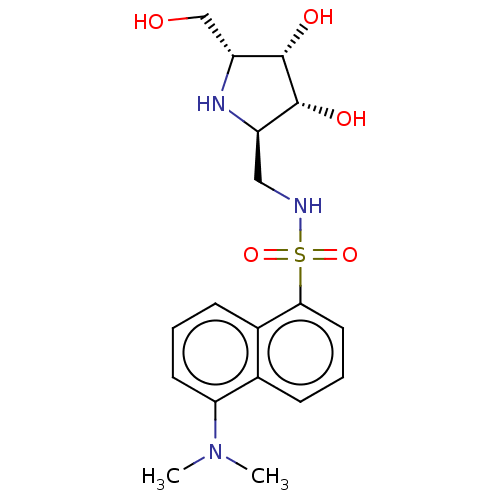 (5-Dimethylamino-naphthalene-1-sulfonic acid ((3S,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie der Technischen Universität Graz Curated by ChEMBL | Assay Description Inhibitory activity against Agrobacterium sp. Beta-glucosidase employing fluorescence spectrometric method | Bioorg Med Chem Lett 11: 1339-42 (2001) BindingDB Entry DOI: 10.7270/Q2JQ1097 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucosidase (Agrobacterium tumefaciens) | BDBM50182801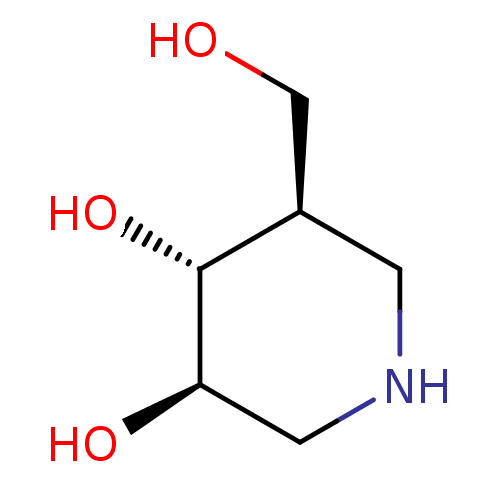 ((3R,4R,5R)-5-(Hydroxymethyl)piperidine-3,4-diol, 8...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität Graz Curated by ChEMBL | Assay Description Inhibition of Agrobacterium sp. beta-glucosidase | Bioorg Med Chem Lett 16: 2067-70 (2006) Article DOI: 10.1016/j.bmcl.2006.01.095 BindingDB Entry DOI: 10.7270/Q26Q1WV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Homo sapiens (Human)) | BDBM50065258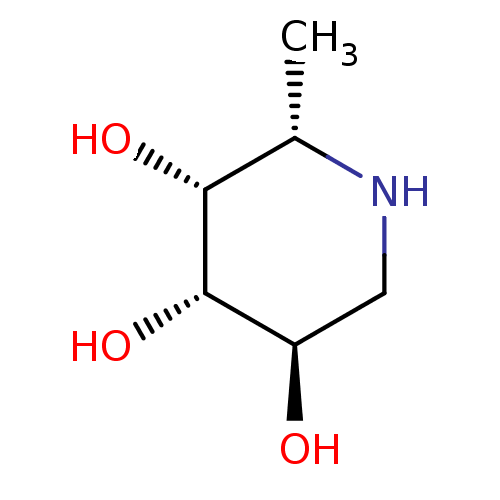 ((2S,3R,4S,5R)-2-Methyl-piperidine-3,4,5-triol | (2...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität Graz Curated by ChEMBL | Assay Description Inhibition of human liver alpha-L-fucosidase | Bioorg Med Chem Lett 16: 2067-70 (2006) Article DOI: 10.1016/j.bmcl.2006.01.095 BindingDB Entry DOI: 10.7270/Q26Q1WV2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Alpha-galactosidase (Coffea arabica (Coffee beans)) | BDBM50163440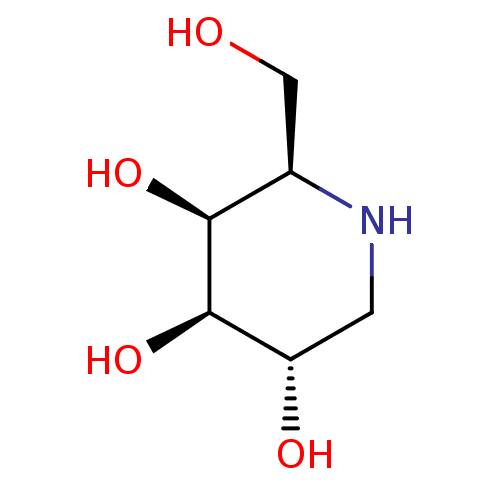 ((2R,3S,4R,5S)-2-(hydroxymethyl)piperidine-3,4,5-tr...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Graz Curated by ChEMBL | Assay Description Inhibition of alpha-galactosidase green coffee beans | Bioorg Med Chem Lett 20: 4077-9 (2010) Article DOI: 10.1016/j.bmcl.2010.05.084 BindingDB Entry DOI: 10.7270/Q24Q7VZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50099009 ((3S,4S,5R)-2-Aminomethyl-5-hydroxymethyl-pyrrolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie der Technischen Universität Graz Curated by ChEMBL | Assay Description Inhibitory activity against Agrobacterium sp. Beta-glucosidase employing fluorescence spectrometric method | Bioorg Med Chem Lett 11: 1339-42 (2001) BindingDB Entry DOI: 10.7270/Q2JQ1097 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50099009 ((3S,4S,5R)-2-Aminomethyl-5-hydroxymethyl-pyrrolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität Graz Curated by ChEMBL | Assay Description Tested for inhibitory activity of the compound against Beta-glucosidase from Agrobacterium species | Bioorg Med Chem Lett 11: 1063-4 (2001) BindingDB Entry DOI: 10.7270/Q2222T12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50099007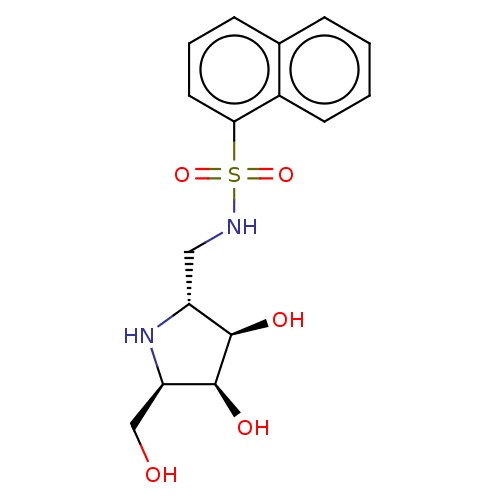 (CHEMBL3350089 | Naphthalene-1-sulfonic acid ((R)-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität Graz Curated by ChEMBL | Assay Description Tested for inhibitory activity of the compound against Beta-glucosidase from Agrobacterium species | Bioorg Med Chem Lett 11: 1063-4 (2001) BindingDB Entry DOI: 10.7270/Q2222T12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50099005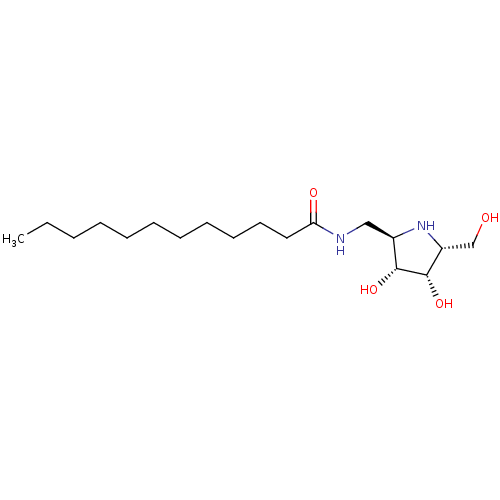 (CHEMBL3350087 | Dodecanoic acid ((3S,4S,5R)-3,4-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität Graz Curated by ChEMBL | Assay Description Tested for inhibitory activity of the compound against Beta-glucosidase from Agrobacterium species | Bioorg Med Chem Lett 11: 1063-4 (2001) BindingDB Entry DOI: 10.7270/Q2222T12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucosidase (Agrobacterium tumefaciens) | BDBM50182793 (CHEMBL208677 | N-(2-(1-(dimethylamino)naphthalene-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität Graz Curated by ChEMBL | Assay Description Inhibition of Agrobacterium sp. beta-glucosidase | Bioorg Med Chem Lett 16: 2067-70 (2006) Article DOI: 10.1016/j.bmcl.2006.01.095 BindingDB Entry DOI: 10.7270/Q26Q1WV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50099004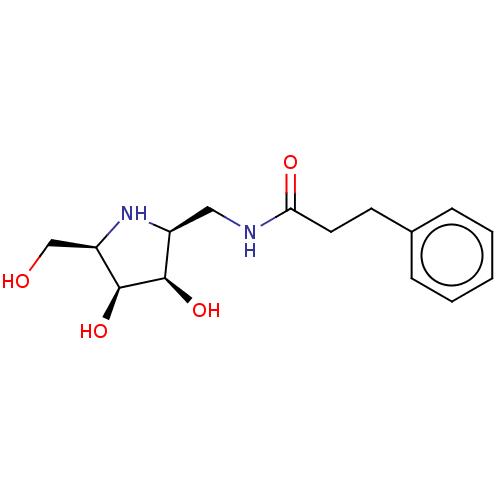 (CHEMBL3350088 | N-((3S,4S,5R)-3,4-Dihydroxy-5-hydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität Graz Curated by ChEMBL | Assay Description Tested for inhibitory activity of the compound against Beta-glucosidase from Agrobacterium species | Bioorg Med Chem Lett 11: 1063-4 (2001) BindingDB Entry DOI: 10.7270/Q2222T12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50241865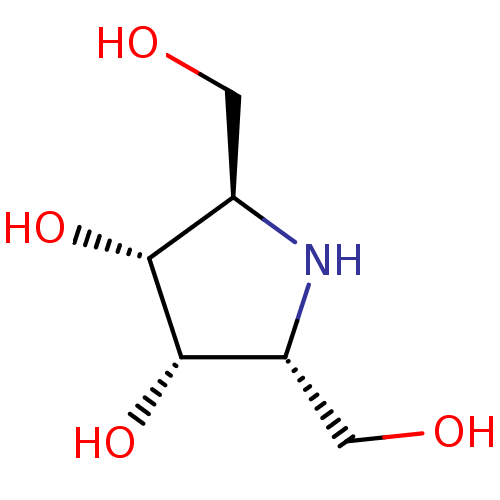 (2,5-dideoxy-2,5-imino-D-altritol | 2R,5R-dihydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie der Technischen Universität Graz Curated by ChEMBL | Assay Description Inhibitory activity against Agrobacterium sp. Beta-glucosidase employing fluorescence spectrometric method | Bioorg Med Chem Lett 11: 1339-42 (2001) BindingDB Entry DOI: 10.7270/Q2JQ1097 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50099008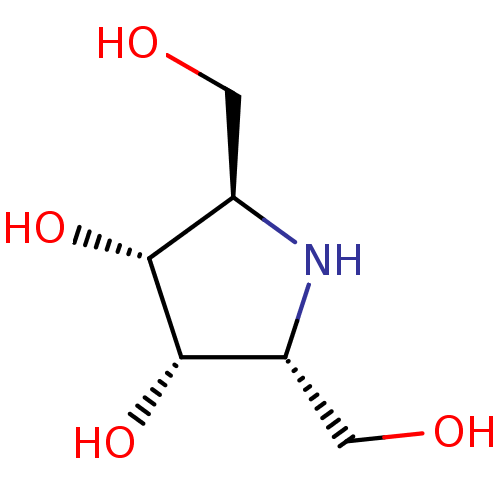 ((2R,3S,4S)-2,5-Bis-hydroxymethyl-pyrrolidine-3,4-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität Graz Curated by ChEMBL | Assay Description Tested for inhibitory activity of the compound against Beta-glucosidase from Agrobacterium species | Bioorg Med Chem Lett 11: 1063-4 (2001) BindingDB Entry DOI: 10.7270/Q2222T12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Escherichia coli (Enterobacteria)) | BDBM50182795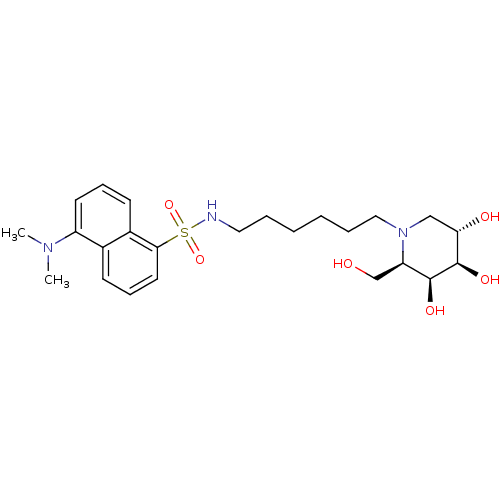 (5-(dimethylamino)-N-(6-((2R,3S,4R,5S)-3,4,5-trihyd...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of Escherichia coli beta-galactosidase assessed as inhibition of nitrophenolate release by spectrophotometry | Bioorg Med Chem Lett 21: 6872-5 (2011) Article DOI: 10.1016/j.bmcl.2011.09.012 BindingDB Entry DOI: 10.7270/Q24B31RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Escherichia coli (Enterobacteria)) | BDBM50356096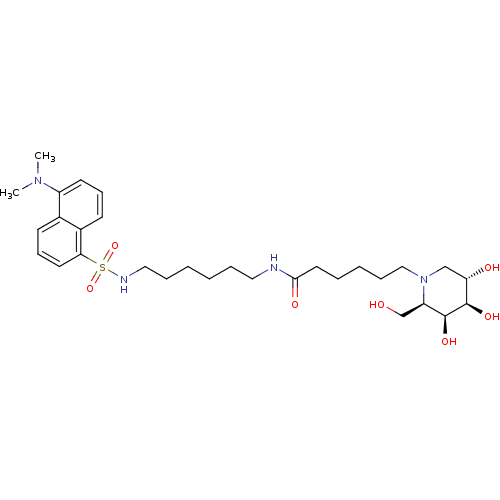 (CHEMBL1911831) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of Escherichia coli beta-galactosidase assessed as inhibition of nitrophenolate release by spectrophotometry | Bioorg Med Chem Lett 21: 6872-5 (2011) Article DOI: 10.1016/j.bmcl.2011.09.012 BindingDB Entry DOI: 10.7270/Q24B31RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Homo sapiens (Human)) | BDBM50182795 (5-(dimethylamino)-N-(6-((2R,3S,4R,5S)-3,4,5-trihyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human lysosomal beta-galactosidase assessed as inhibition of hydrolyzed 4-methylumbelliferone production after 30 mins by luminescence ... | Bioorg Med Chem Lett 21: 6872-5 (2011) Article DOI: 10.1016/j.bmcl.2011.09.012 BindingDB Entry DOI: 10.7270/Q24B31RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucosidase (Agrobacterium tumefaciens) | BDBM50182796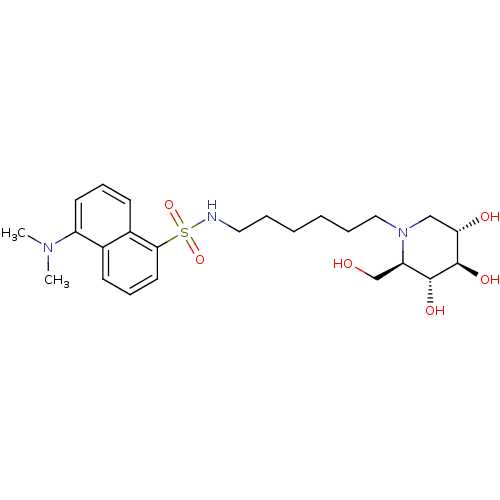 (5-(dimethylamino)-N-(6-((2R,3R,4R,5S)-3,4,5-trihyd...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität Graz Curated by ChEMBL | Assay Description Inhibition of Agrobacterium sp. beta-glucosidase | Bioorg Med Chem Lett 16: 2067-70 (2006) Article DOI: 10.1016/j.bmcl.2006.01.095 BindingDB Entry DOI: 10.7270/Q26Q1WV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Homo sapiens (Human)) | BDBM50182797 (5-(dimethylamino)-N-(6-((2S,3R,4S,5R)-3,4,5-trihyd...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität Graz Curated by ChEMBL | Assay Description Inhibition of human liver alpha-L-fucosidase | Bioorg Med Chem Lett 16: 2067-70 (2006) Article DOI: 10.1016/j.bmcl.2006.01.095 BindingDB Entry DOI: 10.7270/Q26Q1WV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50100427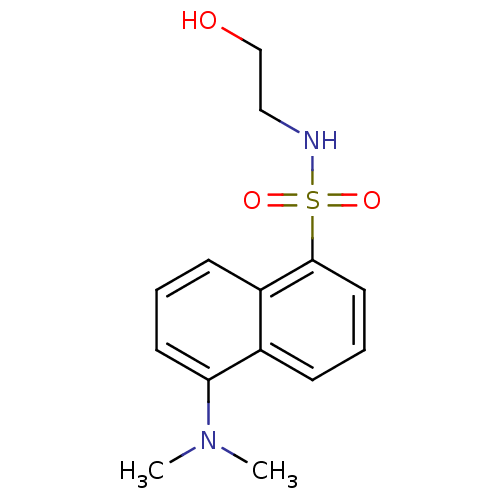 (5-Dimethylamino-naphthalene-1-sulfonic acid (2-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie der Technischen Universität Graz Curated by ChEMBL | Assay Description Inhibitory activity against Agrobacterium sp. Beta-glucosidase employing fluorescence spectrometric method | Bioorg Med Chem Lett 11: 1339-42 (2001) BindingDB Entry DOI: 10.7270/Q2JQ1097 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50099003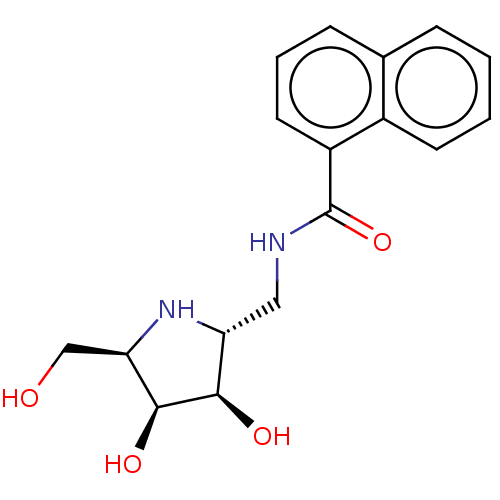 (CHEMBL3350093 | Naphthalene-1-carboxylic acid ((3S...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität Graz Curated by ChEMBL | Assay Description Tested for inhibitory activity of the compound against Beta-glucosidase from Agrobacterium species | Bioorg Med Chem Lett 11: 1063-4 (2001) BindingDB Entry DOI: 10.7270/Q2222T12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Homo sapiens (Human)) | BDBM50246569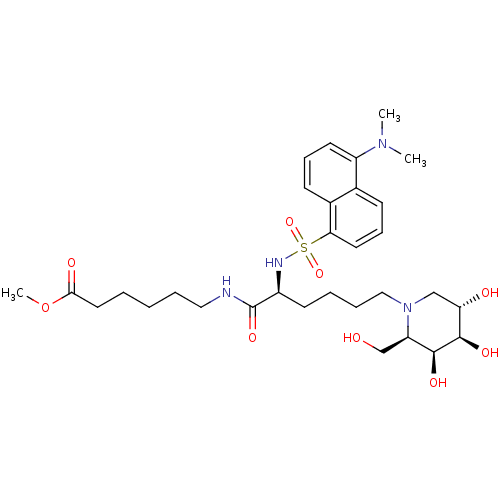 (CHEMBL505422 | Methyl 6-[N2-dansyl-N6-(1,5-dideoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human lysosomal beta-galactosidase assessed as inhibition of hydrolyzed 4-methylumbelliferone production after 30 mins by luminescence ... | Bioorg Med Chem Lett 21: 6872-5 (2011) Article DOI: 10.1016/j.bmcl.2011.09.012 BindingDB Entry DOI: 10.7270/Q24B31RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Homo sapiens (Human)) | BDBM50356097 (CHEMBL461161) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human lysosomal beta-galactosidase assessed as inhibition of hydrolyzed 4-methylumbelliferone production after 30 mins by luminescence ... | Bioorg Med Chem Lett 21: 6872-5 (2011) Article DOI: 10.1016/j.bmcl.2011.09.012 BindingDB Entry DOI: 10.7270/Q24B31RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Homo sapiens (Human)) | BDBM50356096 (CHEMBL1911831) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human lysosomal beta-galactosidase assessed as inhibition of hydrolyzed 4-methylumbelliferone production after 30 mins by luminescence ... | Bioorg Med Chem Lett 21: 6872-5 (2011) Article DOI: 10.1016/j.bmcl.2011.09.012 BindingDB Entry DOI: 10.7270/Q24B31RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucosidase (Agrobacterium sp. ATCC 21400) | BDBM50371431 (CHEMBL402127) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Graz Curated by ChEMBL | Assay Description Inhibition of Agrobacterium sp. beta-glucosidase | Bioorg Med Chem Lett 18: 1922-5 (2008) Article DOI: 10.1016/j.bmcl.2008.01.124 BindingDB Entry DOI: 10.7270/Q2Q52QF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Rhizobium meliloti) | BDBM50182795 (5-(dimethylamino)-N-(6-((2R,3S,4R,5S)-3,4,5-trihyd...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität Graz Curated by ChEMBL | Assay Description Inhibition of Agrobacterium sp. beta-galactosidase | Bioorg Med Chem Lett 16: 2067-70 (2006) Article DOI: 10.1016/j.bmcl.2006.01.095 BindingDB Entry DOI: 10.7270/Q26Q1WV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucosidase (Agrobacterium tumefaciens) | BDBM50182794 (4-(5-(4-(dimethylamino)phenyl)oxazol-2-yl)-N-(6-((...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität Graz Curated by ChEMBL | Assay Description Inhibition of Agrobacterium sp. beta-glucosidase | Bioorg Med Chem Lett 16: 2067-70 (2006) Article DOI: 10.1016/j.bmcl.2006.01.095 BindingDB Entry DOI: 10.7270/Q26Q1WV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-galactosidase (Coffea arabica (Coffee beans)) | BDBM50321618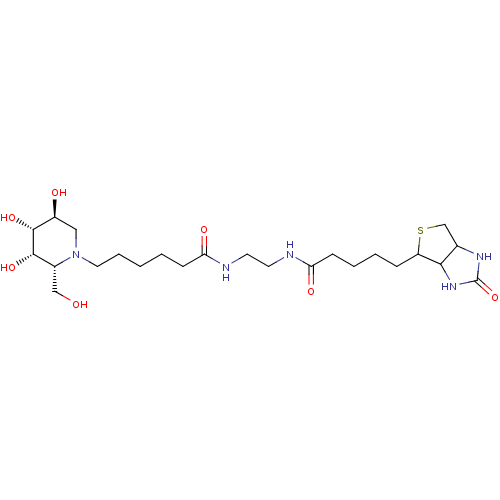 (CHEMBL1172470 | N-(N'-Biotinylaminoethyl)-aminocar...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Graz Curated by ChEMBL | Assay Description Inhibition of alpha-galactosidase green coffee beans | Bioorg Med Chem Lett 20: 4077-9 (2010) Article DOI: 10.1016/j.bmcl.2010.05.084 BindingDB Entry DOI: 10.7270/Q24Q7VZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-galactosidase (Coffea arabica (Coffee beans)) | BDBM50182795 (5-(dimethylamino)-N-(6-((2R,3S,4R,5S)-3,4,5-trihyd...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of green coffee bean alpha-galactosidase assessed as inhibition of nitrophenolate release by spectrophotometry | Bioorg Med Chem Lett 21: 6872-5 (2011) Article DOI: 10.1016/j.bmcl.2011.09.012 BindingDB Entry DOI: 10.7270/Q24B31RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-N-acetylhexosaminidase (Streptomyces coelicolor) | BDBM50182803 (CHEMBL205036 | N-((3S,4R,5R,6R)-1-(6-(1-(dimethyla...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität Graz Curated by ChEMBL | Assay Description Inhibition of Streptomyces plicatus beta-D-hexosaminidase | Bioorg Med Chem Lett 16: 2067-70 (2006) Article DOI: 10.1016/j.bmcl.2006.01.095 BindingDB Entry DOI: 10.7270/Q26Q1WV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-galactosidase (Coffea arabica (Coffee beans)) | BDBM50356096 (CHEMBL1911831) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of green coffee bean alpha-galactosidase assessed as inhibition of nitrophenolate release by spectrophotometry | Bioorg Med Chem Lett 21: 6872-5 (2011) Article DOI: 10.1016/j.bmcl.2011.09.012 BindingDB Entry DOI: 10.7270/Q24B31RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Bos taurus) | BDBM50329782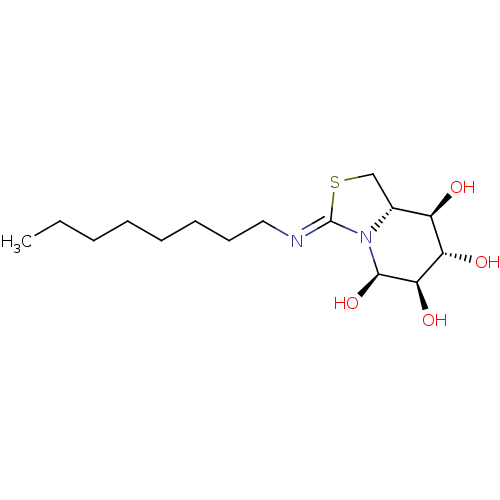 ((5R,6R,7S,8R,8aS)-3-(octylimino)hexahydro-1H-thiaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-glucosidase after 10 to 30 mins | Bioorg Med Chem 18: 7439-45 (2010) Article DOI: 10.1016/j.bmc.2010.09.003 BindingDB Entry DOI: 10.7270/Q2JS9QPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucosidase (Agrobacterium sp. ATCC 21400) | BDBM50371433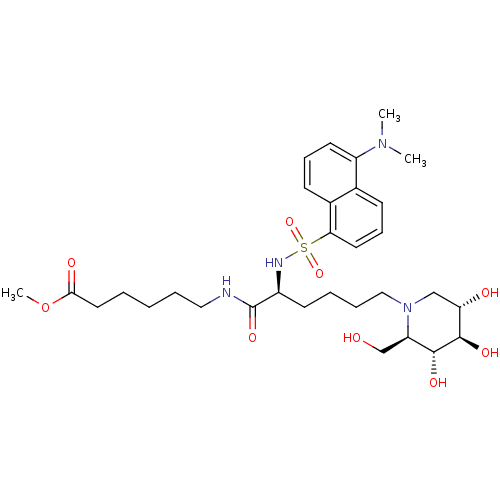 (CHEMBL402128) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Graz Curated by ChEMBL | Assay Description Inhibition of Agrobacterium sp. beta-glucosidase | Bioorg Med Chem Lett 18: 1922-5 (2008) Article DOI: 10.1016/j.bmcl.2008.01.124 BindingDB Entry DOI: 10.7270/Q2Q52QF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-xylosidase (Thermoanaerobacter saccharolyticum) | BDBM50182802 (5-(dimethylamino)-N-(6-((3R,4r,5S)-3,4,5-trihydrox...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität Graz Curated by ChEMBL | Assay Description Inhibition of Thermoanaerobacterium saccharolyticum beta-xylosidase | Bioorg Med Chem Lett 16: 2067-70 (2006) Article DOI: 10.1016/j.bmcl.2006.01.095 BindingDB Entry DOI: 10.7270/Q26Q1WV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucosidase (Agrobacterium tumefaciens) | BDBM50182799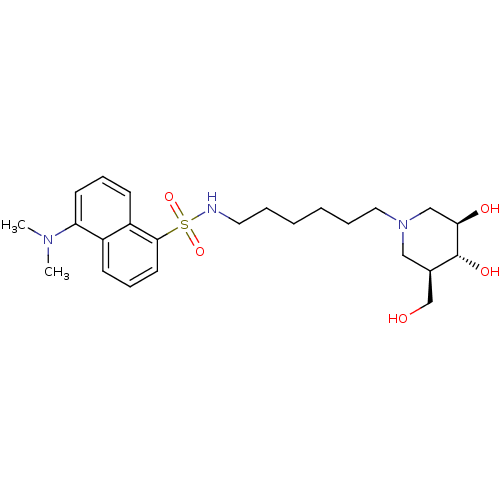 (CHEMBL205592 | N-(6-((3R,4R,5R)-3,4-dihydroxy-5-(h...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität Graz Curated by ChEMBL | Assay Description Inhibition of Agrobacterium sp. beta-glucosidase | Bioorg Med Chem Lett 16: 2067-70 (2006) Article DOI: 10.1016/j.bmcl.2006.01.095 BindingDB Entry DOI: 10.7270/Q26Q1WV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucosidase (Agrobacterium tumefaciens) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität Graz Curated by ChEMBL | Assay Description Inhibition of Agrobacterium sp. beta-glucosidase | Bioorg Med Chem Lett 16: 2067-70 (2006) Article DOI: 10.1016/j.bmcl.2006.01.095 BindingDB Entry DOI: 10.7270/Q26Q1WV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Rhizobium meliloti) | BDBM50163440 ((2R,3S,4R,5S)-2-(hydroxymethyl)piperidine-3,4,5-tr...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität Graz Curated by ChEMBL | Assay Description Inhibition of Agrobacterium sp. beta-galactosidase | Bioorg Med Chem Lett 16: 2067-70 (2006) Article DOI: 10.1016/j.bmcl.2006.01.095 BindingDB Entry DOI: 10.7270/Q26Q1WV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| alpha-1,2-Mannosidase (Glycine max) | BDBM50065259 ((2R,3R,4R,5R)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität Graz Curated by ChEMBL | Assay Description Inhibition of jack beans alpha-mannosidase | Bioorg Med Chem Lett 16: 2067-70 (2006) Article DOI: 10.1016/j.bmcl.2006.01.095 BindingDB Entry DOI: 10.7270/Q26Q1WV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Bos taurus) | BDBM50329779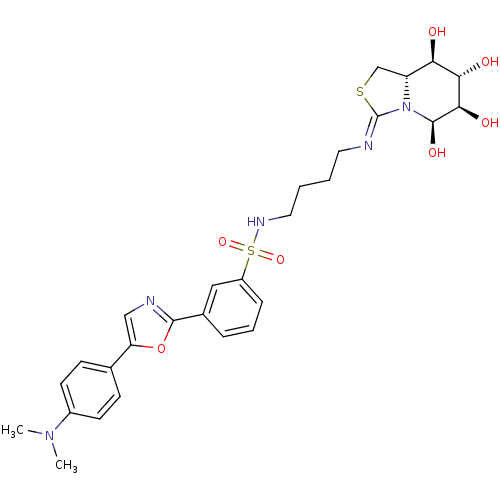 (5-N,6-S-[N'-(4-Dapoxylsulfonylamino)butyliminometh...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-glucosidase after 10 to 30 mins | Bioorg Med Chem 18: 7439-45 (2010) Article DOI: 10.1016/j.bmc.2010.09.003 BindingDB Entry DOI: 10.7270/Q2JS9QPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Bos taurus) | BDBM50329783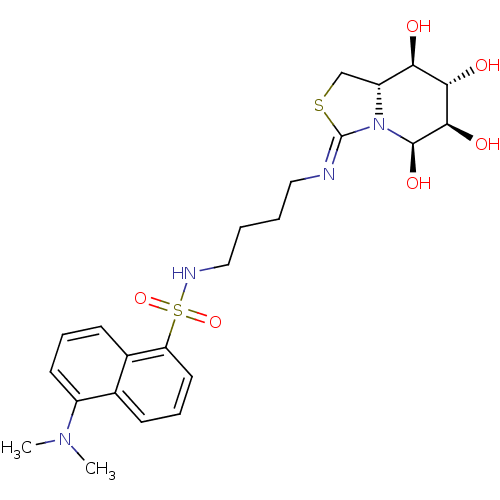 (5-N,6-S-[N'-(4-Dansylamino)butyliminomethylidene]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-glucosidase after 10 to 30 mins | Bioorg Med Chem 18: 7439-45 (2010) Article DOI: 10.1016/j.bmc.2010.09.003 BindingDB Entry DOI: 10.7270/Q2JS9QPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Bos taurus) | BDBM50329781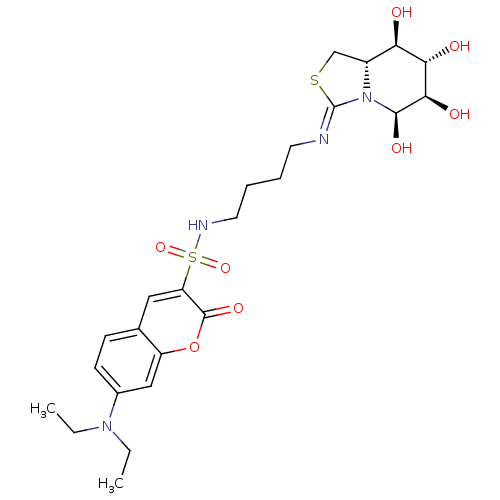 (5-N,6-S-[N'-[4-(7'-Diethylaminocoumarin-30-ylcarbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-glucosidase after 10 to 30 mins | Bioorg Med Chem 18: 7439-45 (2010) Article DOI: 10.1016/j.bmc.2010.09.003 BindingDB Entry DOI: 10.7270/Q2JS9QPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Bos taurus) | BDBM50329780 (5-N,6-S-[N'-[4-(6-Dansylaminohexanamido)butyl]imin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-glucosidase after 10 to 30 mins | Bioorg Med Chem 18: 7439-45 (2010) Article DOI: 10.1016/j.bmc.2010.09.003 BindingDB Entry DOI: 10.7270/Q2JS9QPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| alpha-1,2-Mannosidase (Glycine max) | BDBM50182794 (4-(5-(4-(dimethylamino)phenyl)oxazol-2-yl)-N-(6-((...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität Graz Curated by ChEMBL | Assay Description Inhibition of jack beans alpha-mannosidase | Bioorg Med Chem Lett 16: 2067-70 (2006) Article DOI: 10.1016/j.bmcl.2006.01.095 BindingDB Entry DOI: 10.7270/Q26Q1WV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| alpha-1,2-Mannosidase (Glycine max) | BDBM50182800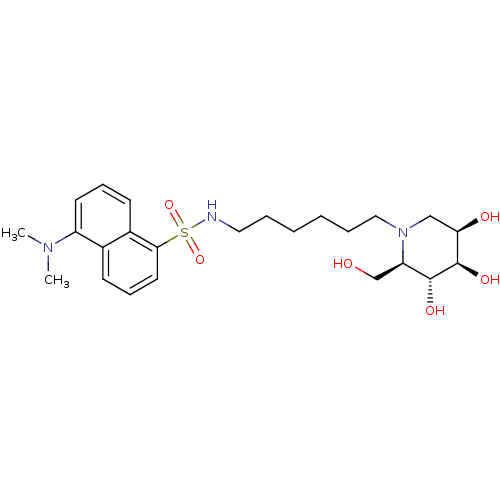 (5-(dimethylamino)-N-(6-((2R,3R,4R,5R)-3,4,5-trihyd...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität Graz Curated by ChEMBL | Assay Description Inhibition of jack beans alpha-mannosidase | Bioorg Med Chem Lett 16: 2067-70 (2006) Article DOI: 10.1016/j.bmcl.2006.01.095 BindingDB Entry DOI: 10.7270/Q26Q1WV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-N-acetylhexosaminidase (Streptomyces coelicolor) | BDBM50182804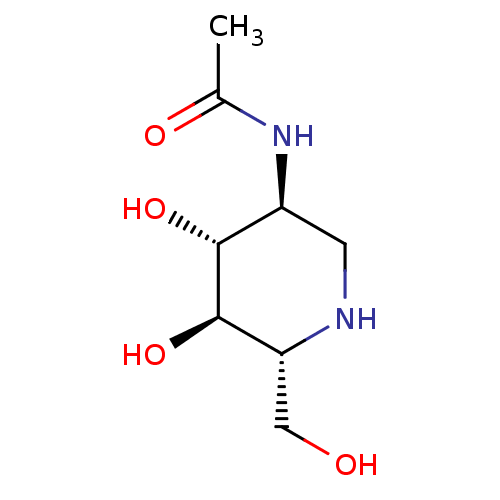 (2-ACETAMIDO-1,2-DIDEOXYNOJIRMYCIN | CHEMBL382689 |...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität Graz Curated by ChEMBL | Assay Description Inhibition of Streptomyces plicatus beta-D-hexosaminidase | Bioorg Med Chem Lett 16: 2067-70 (2006) Article DOI: 10.1016/j.bmcl.2006.01.095 BindingDB Entry DOI: 10.7270/Q26Q1WV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-xylosidase (Thermoanaerobacter saccharolyticum) | BDBM50182798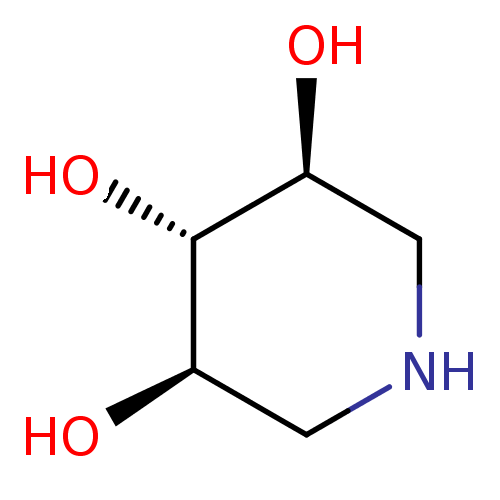 ((3R,4r,5S)-piperidine-3,4,5-triol | 1,5-Dideoxy-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.06E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität Graz Curated by ChEMBL | Assay Description Inhibition of Thermoanaerobacterium saccharolyticum beta-xylosidase | Bioorg Med Chem Lett 16: 2067-70 (2006) Article DOI: 10.1016/j.bmcl.2006.01.095 BindingDB Entry DOI: 10.7270/Q26Q1WV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oligo-1,6-glucosidase IMA1 (Saccharomyces cerevisiae S288c (Baker's yeast)) | BDBM50329783 (5-N,6-S-[N'-(4-Dansylamino)butyliminomethylidene]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.46E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of yeast alpha-glucosidase after 10 to 30 mins | Bioorg Med Chem 18: 7439-45 (2010) Article DOI: 10.1016/j.bmc.2010.09.003 BindingDB Entry DOI: 10.7270/Q2JS9QPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oligo-1,6-glucosidase IMA1 (Saccharomyces cerevisiae S288c (Baker's yeast)) | BDBM50329781 (5-N,6-S-[N'-[4-(7'-Diethylaminocoumarin-30-ylcarbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.13E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of yeast alpha-glucosidase after 10 to 30 mins | Bioorg Med Chem 18: 7439-45 (2010) Article DOI: 10.1016/j.bmc.2010.09.003 BindingDB Entry DOI: 10.7270/Q2JS9QPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oligo-1,6-glucosidase IMA1 (Saccharomyces cerevisiae S288c (Baker's yeast)) | BDBM50329780 (5-N,6-S-[N'-[4-(6-Dansylaminohexanamido)butyl]imin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.51E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of yeast alpha-glucosidase after 10 to 30 mins | Bioorg Med Chem 18: 7439-45 (2010) Article DOI: 10.1016/j.bmc.2010.09.003 BindingDB Entry DOI: 10.7270/Q2JS9QPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oligo-1,6-glucosidase IMA1 (Saccharomyces cerevisiae S288c (Baker's yeast)) | BDBM50329779 (5-N,6-S-[N'-(4-Dapoxylsulfonylamino)butyliminometh...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 7.14E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of yeast alpha-glucosidase after 10 to 30 mins | Bioorg Med Chem 18: 7439-45 (2010) Article DOI: 10.1016/j.bmc.2010.09.003 BindingDB Entry DOI: 10.7270/Q2JS9QPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 52 total ) | Next | Last >> |