

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM91581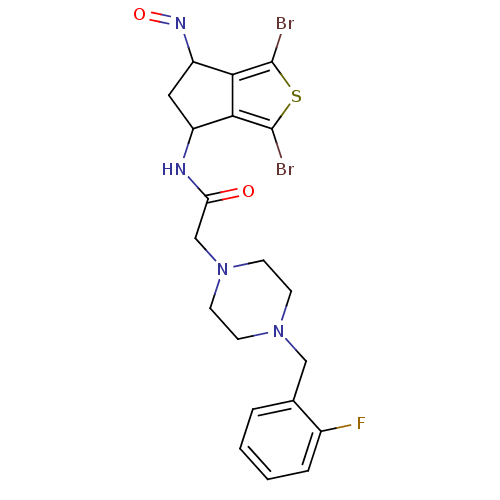 (Thiaindanone, 3) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Centre d'Etudes et de Recherche sur le Médicament de Normandie | Assay Description Inhibitory ability of compounds on AChE activity was evaluated through the use of the spectrometic method of Ellman. Lyophilized electric eel AChE (... | J Enzyme Inhib Med Chem 23: 696-703 (2008) Article DOI: 10.1080/14756360802208053 BindingDB Entry DOI: 10.7270/Q2SX6BT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM91580 (Thiaindanone, 2) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Centre d'Etudes et de Recherche sur le Médicament de Normandie | Assay Description Inhibitory ability of compounds on AChE activity was evaluated through the use of the spectrometic method of Ellman. Lyophilized electric eel AChE (... | J Enzyme Inhib Med Chem 23: 696-703 (2008) Article DOI: 10.1080/14756360802208053 BindingDB Entry DOI: 10.7270/Q2SX6BT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM91582 (Thiaindanone, 11) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Centre d'Etudes et de Recherche sur le Médicament de Normandie | Assay Description Inhibitory ability of compounds on AChE activity was evaluated through the use of the spectrometic method of Ellman. Lyophilized electric eel AChE (... | J Enzyme Inhib Med Chem 23: 696-703 (2008) Article DOI: 10.1080/14756360802208053 BindingDB Entry DOI: 10.7270/Q2SX6BT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM91587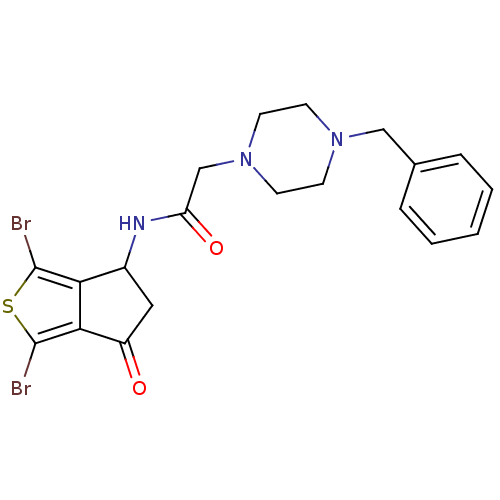 (Thiaindanone, 1) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Centre d'Etudes et de Recherche sur le Médicament de Normandie | Assay Description Inhibitory ability of compounds on AChE activity was evaluated through the use of the spectrometic method of Ellman. Lyophilized electric eel AChE (... | J Enzyme Inhib Med Chem 23: 696-703 (2008) Article DOI: 10.1080/14756360802208053 BindingDB Entry DOI: 10.7270/Q2SX6BT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50209245 (CHEMBL247378) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
UPRES-EA 3915 Curated by ChEMBL | Assay Description Inhibition of rat neuronal NOS | Bioorg Med Chem Lett 17: 3177-80 (2007) Article DOI: 10.1016/j.bmcl.2007.03.024 BindingDB Entry DOI: 10.7270/Q2Z60NR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM91584 (Thiaindanone, 13) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Centre d'Etudes et de Recherche sur le Médicament de Normandie | Assay Description Inhibitory ability of compounds on AChE activity was evaluated through the use of the spectrometic method of Ellman. Lyophilized electric eel AChE (... | J Enzyme Inhib Med Chem 23: 696-703 (2008) Article DOI: 10.1080/14756360802208053 BindingDB Entry DOI: 10.7270/Q2SX6BT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM91585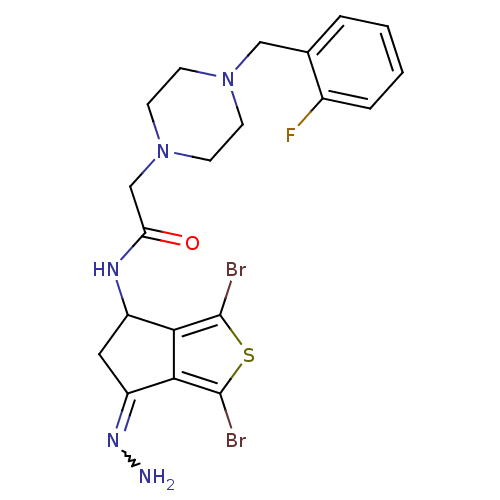 (Thiaindanone, 14) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Centre d'Etudes et de Recherche sur le Médicament de Normandie | Assay Description Inhibitory ability of compounds on AChE activity was evaluated through the use of the spectrometic method of Ellman. Lyophilized electric eel AChE (... | J Enzyme Inhib Med Chem 23: 696-703 (2008) Article DOI: 10.1080/14756360802208053 BindingDB Entry DOI: 10.7270/Q2SX6BT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM91583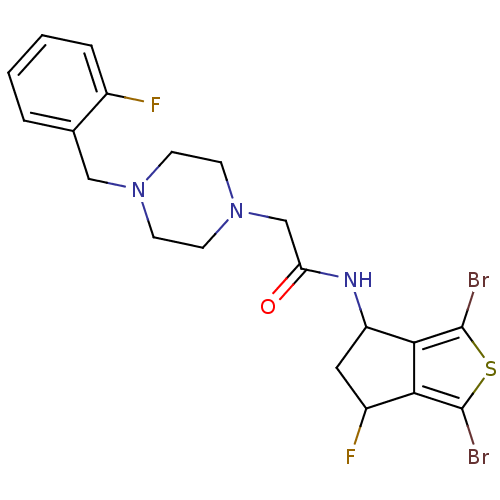 (Thiaindanone, 12) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Centre d'Etudes et de Recherche sur le Médicament de Normandie | Assay Description Inhibitory ability of compounds on AChE activity was evaluated through the use of the spectrometic method of Ellman. Lyophilized electric eel AChE (... | J Enzyme Inhib Med Chem 23: 696-703 (2008) Article DOI: 10.1080/14756360802208053 BindingDB Entry DOI: 10.7270/Q2SX6BT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50209241 (4-bromo-1H-indazole | CHEMBL246393) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UPRES-EA 3915 Curated by ChEMBL | Assay Description Inhibition of rat neuronal NOS | Bioorg Med Chem Lett 17: 3177-80 (2007) Article DOI: 10.1016/j.bmcl.2007.03.024 BindingDB Entry DOI: 10.7270/Q2Z60NR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50209244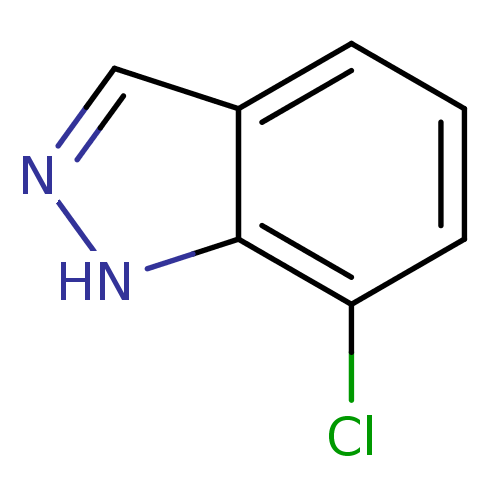 (7-chloro-1H-indazole | CHEMBL247367) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UPRES-EA 3915 Curated by ChEMBL | Assay Description Inhibition of rat neuronal NOS | Bioorg Med Chem Lett 17: 3177-80 (2007) Article DOI: 10.1016/j.bmcl.2007.03.024 BindingDB Entry DOI: 10.7270/Q2Z60NR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50209237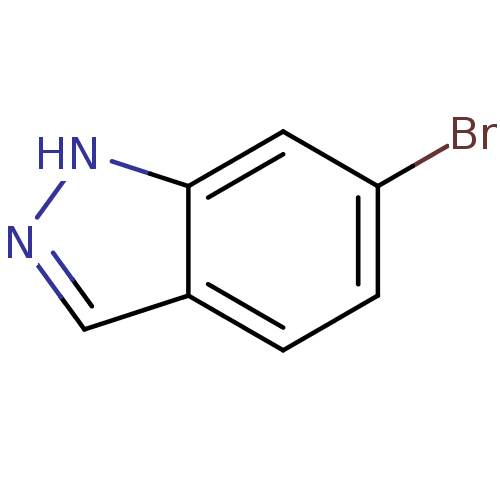 (6-bromo-1H-indazole | CHEMBL247365) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UPRES-EA 3915 Curated by ChEMBL | Assay Description Inhibition of rat neuronal NOS | Bioorg Med Chem Lett 17: 3177-80 (2007) Article DOI: 10.1016/j.bmcl.2007.03.024 BindingDB Entry DOI: 10.7270/Q2Z60NR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM91590 (Thiaindanone, 19) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Centre d'Etudes et de Recherche sur le Médicament de Normandie | Assay Description Inhibitory ability of compounds on AChE activity was evaluated through the use of the spectrometic method of Ellman. Lyophilized electric eel AChE (... | J Enzyme Inhib Med Chem 23: 696-703 (2008) Article DOI: 10.1080/14756360802208053 BindingDB Entry DOI: 10.7270/Q2SX6BT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM91588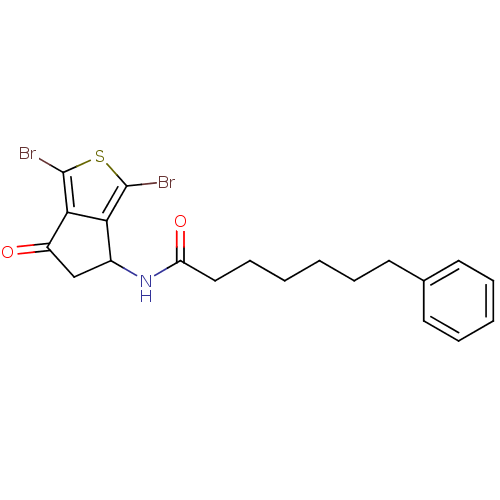 (Thiaindanone, 16) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Centre d'Etudes et de Recherche sur le Médicament de Normandie | Assay Description Inhibitory ability of compounds on AChE activity was evaluated through the use of the spectrometic method of Ellman. Lyophilized electric eel AChE (... | J Enzyme Inhib Med Chem 23: 696-703 (2008) Article DOI: 10.1080/14756360802208053 BindingDB Entry DOI: 10.7270/Q2SX6BT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM91586 (Thiaindanone, 15) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Centre d'Etudes et de Recherche sur le Médicament de Normandie | Assay Description Inhibitory ability of compounds on AChE activity was evaluated through the use of the spectrometic method of Ellman. Lyophilized electric eel AChE (... | J Enzyme Inhib Med Chem 23: 696-703 (2008) Article DOI: 10.1080/14756360802208053 BindingDB Entry DOI: 10.7270/Q2SX6BT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM91589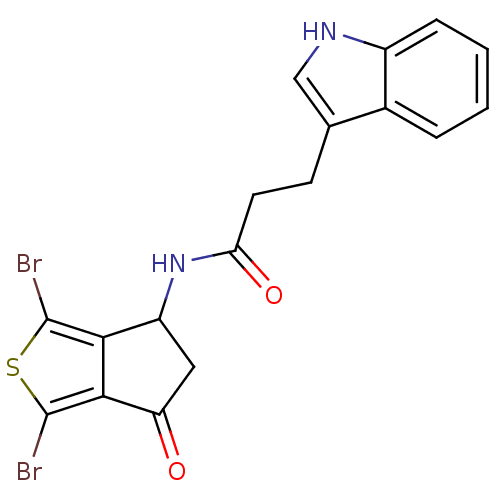 (Thiaindanone, 17) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Centre d'Etudes et de Recherche sur le Médicament de Normandie | Assay Description Inhibitory ability of compounds on AChE activity was evaluated through the use of the spectrometic method of Ellman. Lyophilized electric eel AChE (... | J Enzyme Inhib Med Chem 23: 696-703 (2008) Article DOI: 10.1080/14756360802208053 BindingDB Entry DOI: 10.7270/Q2SX6BT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50390019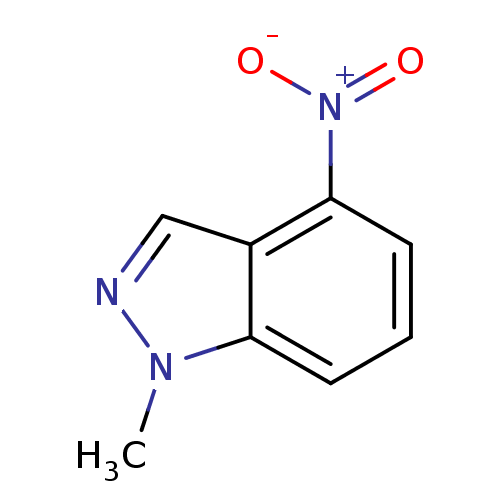 (CHEMBL2071543) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Caen Basse-Normandie Curated by ChEMBL | Assay Description Inhibition of nNOS in rat cerebellum homogenates assessed as reduction in conversion of L-[3H]arginine to L-[3H]citrulline | Bioorg Med Chem 20: 5296-304 (2012) Article DOI: 10.1016/j.bmc.2012.06.025 BindingDB Entry DOI: 10.7270/Q26W9C46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50209239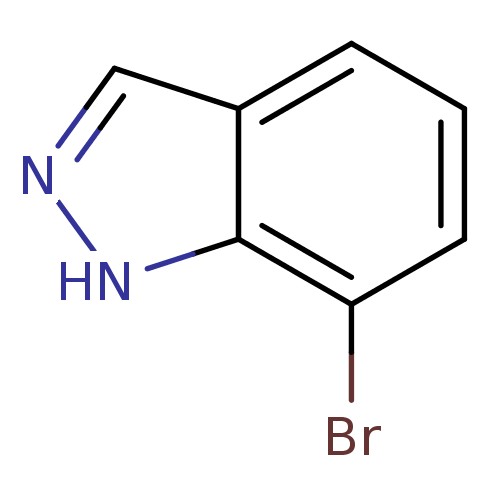 (7-bromo-1H-indazole | CHEMBL439566) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
UPRES-EA 3915 Curated by ChEMBL | Assay Description Inhibition of rat neuronal NOS | Bioorg Med Chem Lett 17: 3177-80 (2007) Article DOI: 10.1016/j.bmcl.2007.03.024 BindingDB Entry DOI: 10.7270/Q2Z60NR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50209235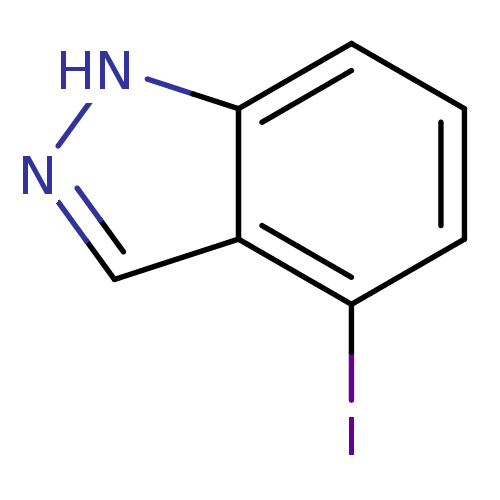 (4-iodo-1H-indazole | CHEMBL246534) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
UPRES-EA 3915 Curated by ChEMBL | Assay Description Inhibition of rat neuronal NOS | Bioorg Med Chem Lett 17: 3177-80 (2007) Article DOI: 10.1016/j.bmcl.2007.03.024 BindingDB Entry DOI: 10.7270/Q2Z60NR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50209236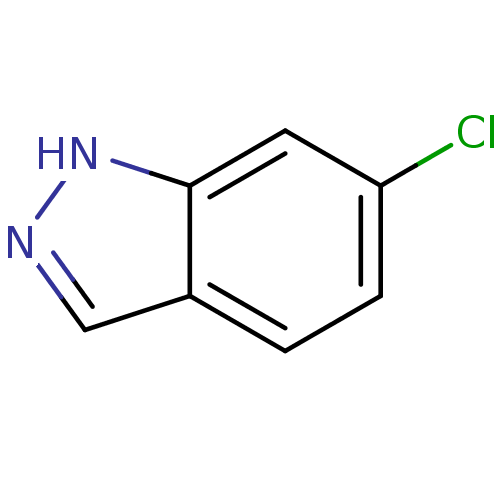 (6-Chloro-1H-indazole | CHEMBL392184) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
UPRES-EA 3915 Curated by ChEMBL | Assay Description Inhibition of rat neuronal NOS | Bioorg Med Chem Lett 17: 3177-80 (2007) Article DOI: 10.1016/j.bmcl.2007.03.024 BindingDB Entry DOI: 10.7270/Q2Z60NR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50390023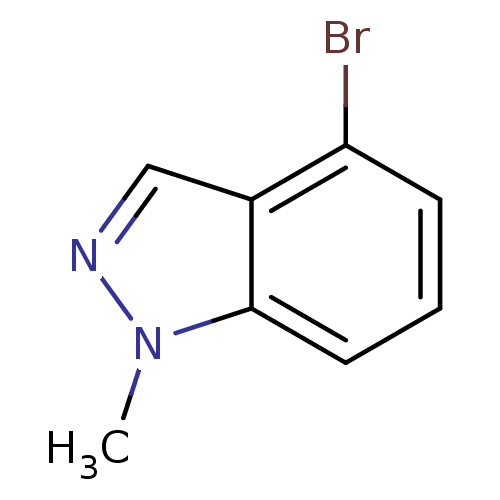 (CHEMBL2071547) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Caen Basse-Normandie Curated by ChEMBL | Assay Description Inhibition of nNOS in rat cerebellum homogenates assessed as reduction in conversion of L-[3H]arginine to L-[3H]citrulline | Bioorg Med Chem 20: 5296-304 (2012) Article DOI: 10.1016/j.bmc.2012.06.025 BindingDB Entry DOI: 10.7270/Q26W9C46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50209243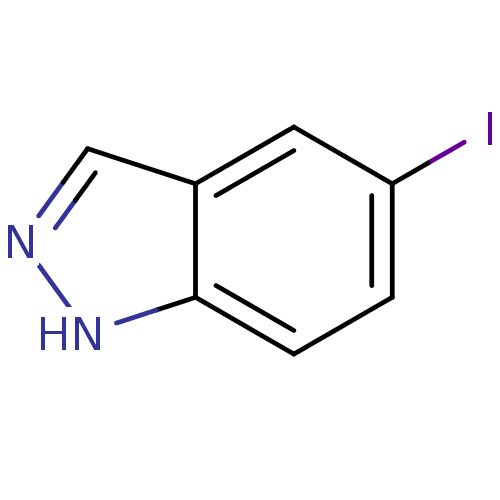 (5-iodo-1H-indazole | CHEMBL391348) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
UPRES-EA 3915 Curated by ChEMBL | Assay Description Inhibition of rat neuronal NOS | Bioorg Med Chem Lett 17: 3177-80 (2007) Article DOI: 10.1016/j.bmcl.2007.03.024 BindingDB Entry DOI: 10.7270/Q2Z60NR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50390027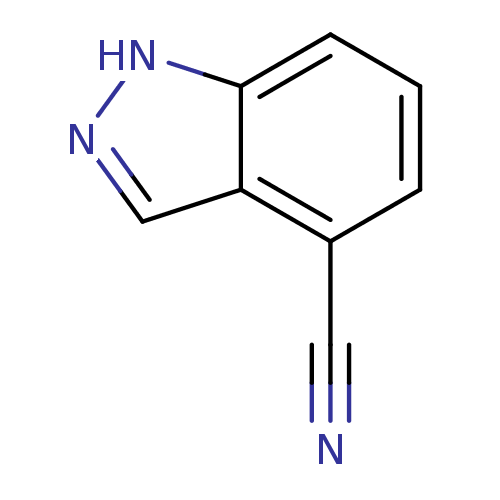 (CHEMBL2071551) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Caen Basse-Normandie Curated by ChEMBL | Assay Description Inhibition of nNOS in rat cerebellum homogenates assessed as reduction in conversion of L-[3H]arginine to L-[3H]citrulline | Bioorg Med Chem 20: 5296-304 (2012) Article DOI: 10.1016/j.bmc.2012.06.025 BindingDB Entry DOI: 10.7270/Q26W9C46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50209234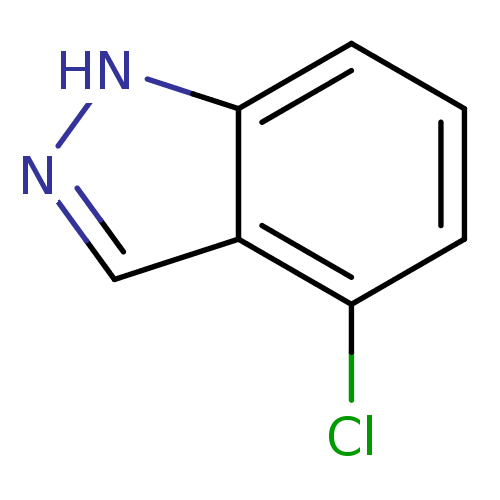 (4-chloro-1H-indazole | CHEMBL246533) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
UPRES-EA 3915 Curated by ChEMBL | Assay Description Inhibition of rat neuronal NOS | Bioorg Med Chem Lett 17: 3177-80 (2007) Article DOI: 10.1016/j.bmcl.2007.03.024 BindingDB Entry DOI: 10.7270/Q2Z60NR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50390021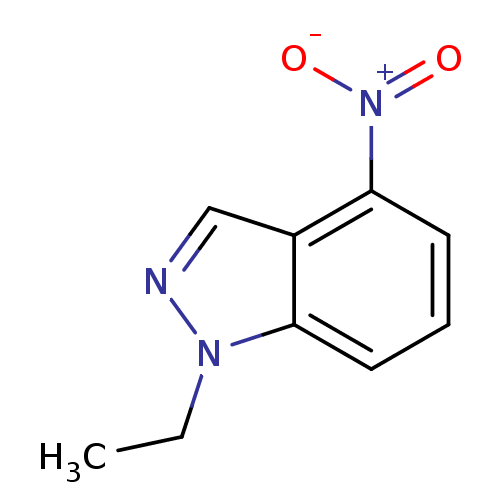 (CHEMBL2071545) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Caen Basse-Normandie Curated by ChEMBL | Assay Description Inhibition of nNOS in rat cerebellum homogenates assessed as reduction in conversion of L-[3H]arginine to L-[3H]citrulline | Bioorg Med Chem 20: 5296-304 (2012) Article DOI: 10.1016/j.bmc.2012.06.025 BindingDB Entry DOI: 10.7270/Q26W9C46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50209242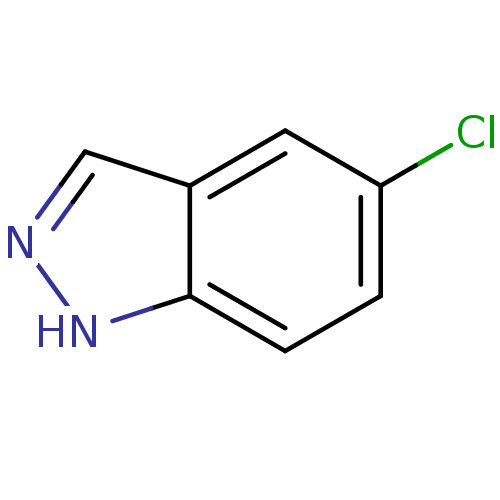 (5-chloro-1H-indazole | CHEMBL246746) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
UPRES-EA 3915 Curated by ChEMBL | Assay Description Inhibition of rat neuronal NOS | Bioorg Med Chem Lett 17: 3177-80 (2007) Article DOI: 10.1016/j.bmcl.2007.03.024 BindingDB Entry DOI: 10.7270/Q2Z60NR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50099398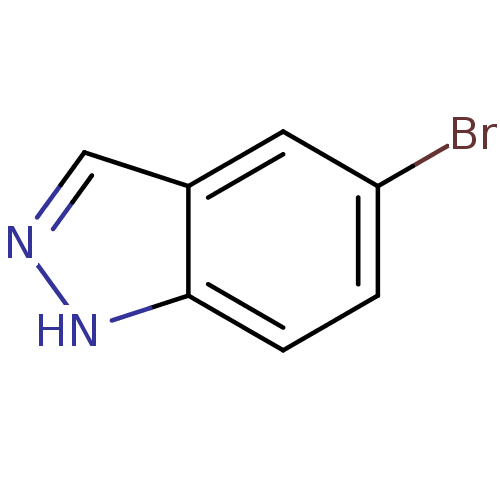 (5-Bromo-1H-indazole | CHEMBL16425 | cid_761929) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
UPRES-EA 3915 Curated by ChEMBL | Assay Description Inhibition of rat neuronal NOS | Bioorg Med Chem Lett 17: 3177-80 (2007) Article DOI: 10.1016/j.bmcl.2007.03.024 BindingDB Entry DOI: 10.7270/Q2Z60NR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50390025 (CHEMBL2071549) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Caen Basse-Normandie Curated by ChEMBL | Assay Description Inhibition of nNOS in rat cerebellum homogenates assessed as reduction in conversion of L-[3H]arginine to L-[3H]citrulline | Bioorg Med Chem 20: 5296-304 (2012) Article DOI: 10.1016/j.bmc.2012.06.025 BindingDB Entry DOI: 10.7270/Q26W9C46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50390026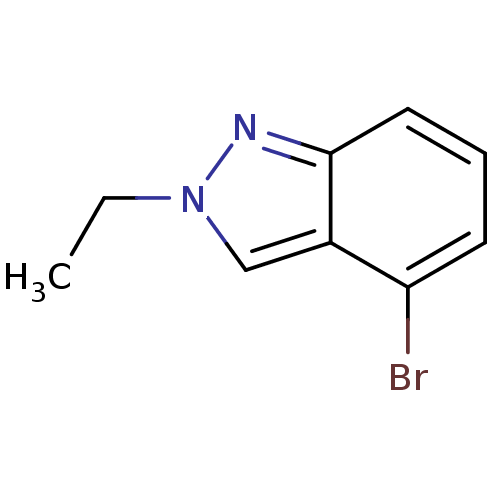 (CHEMBL2071550) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Caen Basse-Normandie Curated by ChEMBL | Assay Description Inhibition of nNOS in rat cerebellum homogenates assessed as reduction in conversion of L-[3H]arginine to L-[3H]citrulline | Bioorg Med Chem 20: 5296-304 (2012) Article DOI: 10.1016/j.bmc.2012.06.025 BindingDB Entry DOI: 10.7270/Q26W9C46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50390024 (CHEMBL2071548) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Caen Basse-Normandie Curated by ChEMBL | Assay Description Inhibition of nNOS in rat cerebellum homogenates assessed as reduction in conversion of L-[3H]arginine to L-[3H]citrulline | Bioorg Med Chem 20: 5296-304 (2012) Article DOI: 10.1016/j.bmc.2012.06.025 BindingDB Entry DOI: 10.7270/Q26W9C46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50390022 (CHEMBL2071546) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Caen Basse-Normandie Curated by ChEMBL | Assay Description Inhibition of nNOS in rat cerebellum homogenates assessed as reduction in conversion of L-[3H]arginine to L-[3H]citrulline | Bioorg Med Chem 20: 5296-304 (2012) Article DOI: 10.1016/j.bmc.2012.06.025 BindingDB Entry DOI: 10.7270/Q26W9C46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50390020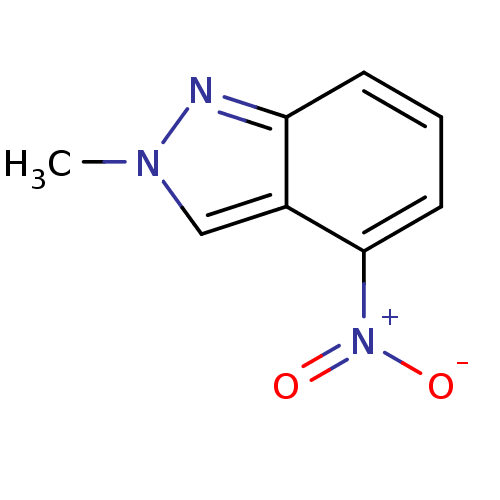 (CHEMBL2071544) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Caen Basse-Normandie Curated by ChEMBL | Assay Description Inhibition of nNOS in rat cerebellum homogenates assessed as reduction in conversion of L-[3H]arginine to L-[3H]citrulline | Bioorg Med Chem 20: 5296-304 (2012) Article DOI: 10.1016/j.bmc.2012.06.025 BindingDB Entry DOI: 10.7270/Q26W9C46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50209238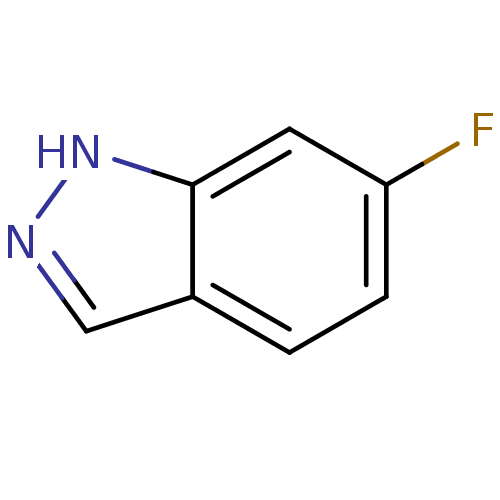 (6-fluoro-1H-indazole | CHEMBL247366) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
UPRES-EA 3915 Curated by ChEMBL | Assay Description Inhibition of rat neuronal NOS | Bioorg Med Chem Lett 17: 3177-80 (2007) Article DOI: 10.1016/j.bmcl.2007.03.024 BindingDB Entry DOI: 10.7270/Q2Z60NR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50099405 (5-Fluoro-1H-indazole | CHEMBL16076) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
UPRES-EA 3915 Curated by ChEMBL | Assay Description Inhibition of rat neuronal NOS | Bioorg Med Chem Lett 17: 3177-80 (2007) Article DOI: 10.1016/j.bmcl.2007.03.024 BindingDB Entry DOI: 10.7270/Q2Z60NR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50209240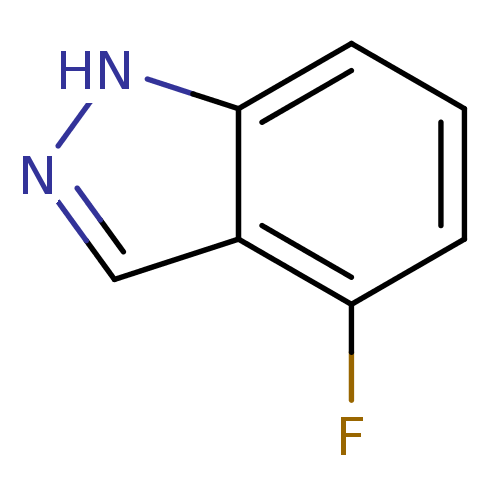 (4-fluoro-1H-indazole | CHEMBL392263) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
UPRES-EA 3915 Curated by ChEMBL | Assay Description Inhibition of rat neuronal NOS | Bioorg Med Chem Lett 17: 3177-80 (2007) Article DOI: 10.1016/j.bmcl.2007.03.024 BindingDB Entry DOI: 10.7270/Q2Z60NR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||