

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50318133 ((14S)-16-amino-10,14-dicyclohexyl-2-oxa-10,15,17,2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 20: 3158-60 (2010) Article DOI: 10.1016/j.bmcl.2010.03.097 BindingDB Entry DOI: 10.7270/Q2C53M1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50318123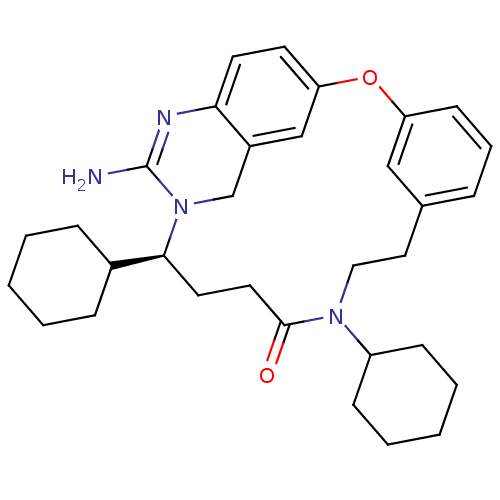 ((14S)-16-amino-10,14-dicyclohexyl-2-oxa-10,15,17-t...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 20: 3158-60 (2010) Article DOI: 10.1016/j.bmcl.2010.03.097 BindingDB Entry DOI: 10.7270/Q2C53M1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50318131 ((14S)-16-amino-10,14-dicyclohexyl-20-fluoro-2-oxa-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 20: 3158-60 (2010) Article DOI: 10.1016/j.bmcl.2010.03.097 BindingDB Entry DOI: 10.7270/Q2C53M1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50318125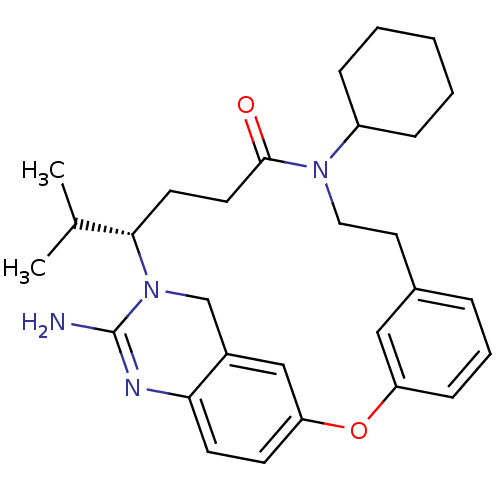 ((14S)-16-amino-10-cyclohexyl-14-(propan-2-yl)-2-ox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 20: 3158-60 (2010) Article DOI: 10.1016/j.bmcl.2010.03.097 BindingDB Entry DOI: 10.7270/Q2C53M1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50318132 ((14S)-16-amino-10,14-dicyclohexyl-20-methoxy-2-oxa...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 20: 3158-60 (2010) Article DOI: 10.1016/j.bmcl.2010.03.097 BindingDB Entry DOI: 10.7270/Q2C53M1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50318124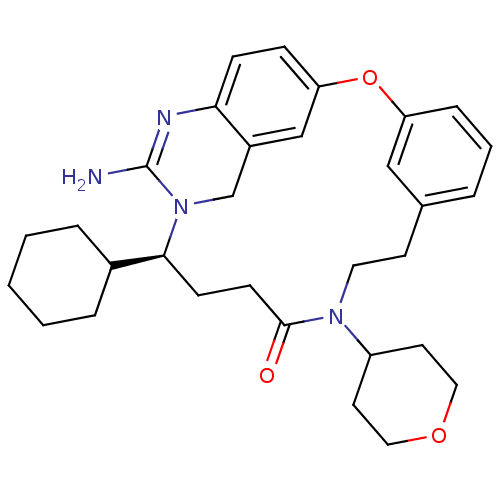 ((14S)-16-amino-14-cyclohexyl-10-(oxan-4-yl)-2-oxa-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 20: 3158-60 (2010) Article DOI: 10.1016/j.bmcl.2010.03.097 BindingDB Entry DOI: 10.7270/Q2C53M1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50318129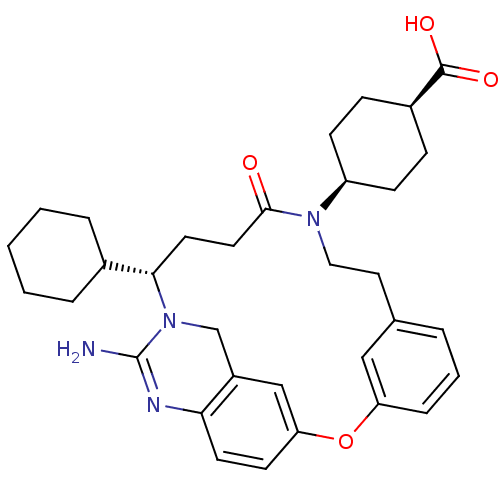 (4-[(14S)-16-amino-14-cyclohexyl-11-oxo-2-oxa-10,15...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 20: 3158-60 (2010) Article DOI: 10.1016/j.bmcl.2010.03.097 BindingDB Entry DOI: 10.7270/Q2C53M1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50318128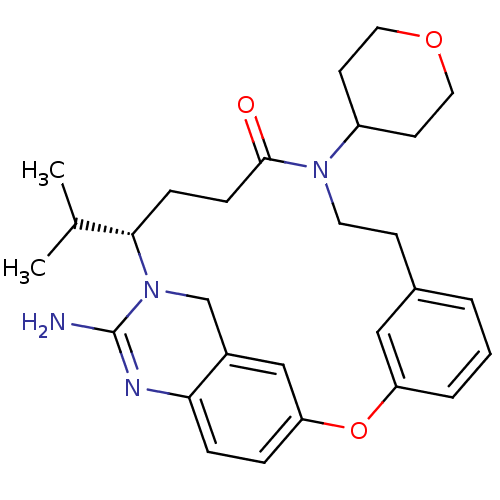 ((14S)-16-amino-10-(oxan-4-yl)-14-(propan-2-yl)-2-o...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 20: 3158-60 (2010) Article DOI: 10.1016/j.bmcl.2010.03.097 BindingDB Entry DOI: 10.7270/Q2C53M1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50318127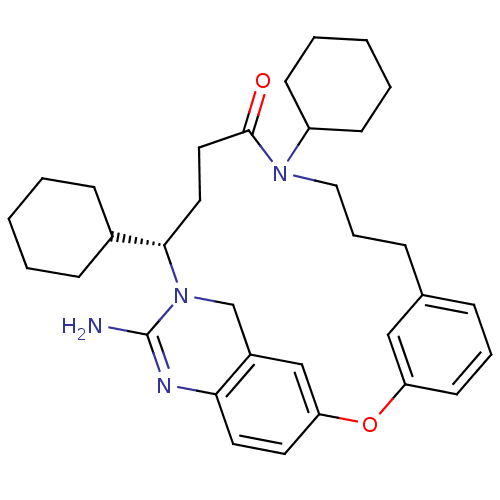 ((15S)-17-amino-11,15-dicyclohexyl-2-oxa-11,16,18-t...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 20: 3158-60 (2010) Article DOI: 10.1016/j.bmcl.2010.03.097 BindingDB Entry DOI: 10.7270/Q2C53M1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50318126 ((15R)-17-amino-11,15-dicyclohexyl-2-oxa-11,16,18-t...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 186 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 20: 3158-60 (2010) Article DOI: 10.1016/j.bmcl.2010.03.097 BindingDB Entry DOI: 10.7270/Q2C53M1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50318130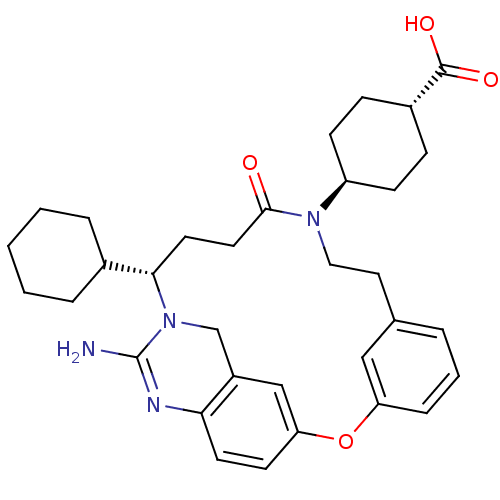 (4-[(14S)-16-amino-14-cyclohexyl-11-oxo-2-oxa-10,15...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 20: 3158-60 (2010) Article DOI: 10.1016/j.bmcl.2010.03.097 BindingDB Entry DOI: 10.7270/Q2C53M1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50235018 (CHEMBL4072070) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Lexicon Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human HA-tagged SGLT2 expressed in HEK293 cells assessed as decrease in [14C]-AMG uptake measured after 1 to 2 hrs by scintillation cou... | J Med Chem 60: 710-721 (2017) Article DOI: 10.1021/acs.jmedchem.6b01541 BindingDB Entry DOI: 10.7270/Q2C24ZQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50179206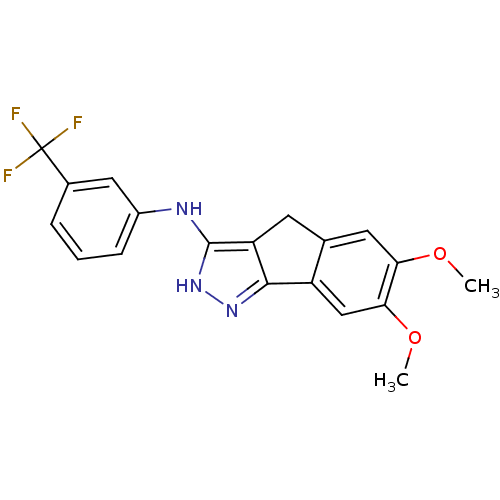 (3-trifluoromethyl-N-(6,7-dimethoxy-2,4-dihydroinde...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antiproliferative activity against PDGF-BB stimulated HCASMC | J Med Chem 48: 8163-73 (2005) Article DOI: 10.1021/jm050680m BindingDB Entry DOI: 10.7270/Q2W095HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM176693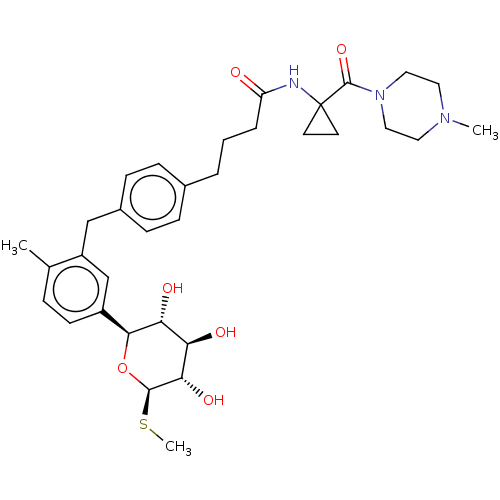 (US9688710, 116 4-(4-(2-methyl-5-((2S,3R,4R,5S,6R)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lexicon Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human HA-tagged SGLT1 expressed in HEK293 cells assessed as decrease in [14C]-AMG uptake measured after 1 to 2 hrs by scintillation cou... | J Med Chem 60: 710-721 (2017) Article DOI: 10.1021/acs.jmedchem.6b01541 BindingDB Entry DOI: 10.7270/Q2C24ZQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM176709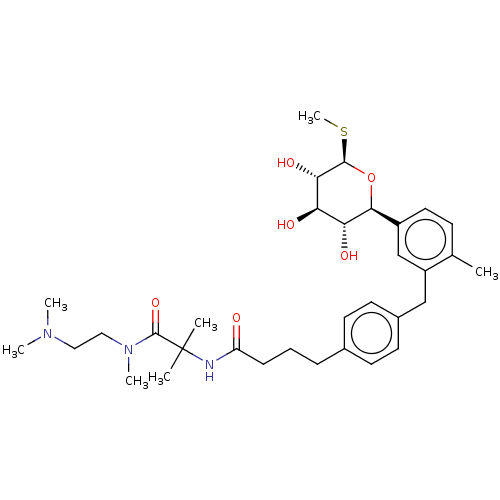 (US9688710, 132 N-(1-((2-(dimethylamino)ethyl)(meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lexicon Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human HA-tagged SGLT2 expressed in HEK293 cells assessed as decrease in [14C]-AMG uptake measured after 1 to 2 hrs by scintillation cou... | J Med Chem 60: 710-721 (2017) Article DOI: 10.1021/acs.jmedchem.6b01541 BindingDB Entry DOI: 10.7270/Q2C24ZQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM176724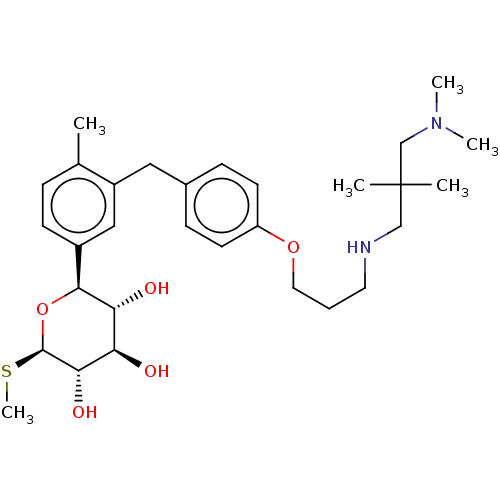 (US9688710, 146 (3R,4R,5S,6R)-2-(3-(4-(3-((3-(dimet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lexicon Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human HA-tagged SGLT2 expressed in HEK293 cells assessed as decrease in [14C]-AMG uptake measured after 1 to 2 hrs by scintillation cou... | J Med Chem 60: 710-721 (2017) Article DOI: 10.1021/acs.jmedchem.6b01541 BindingDB Entry DOI: 10.7270/Q2C24ZQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM176697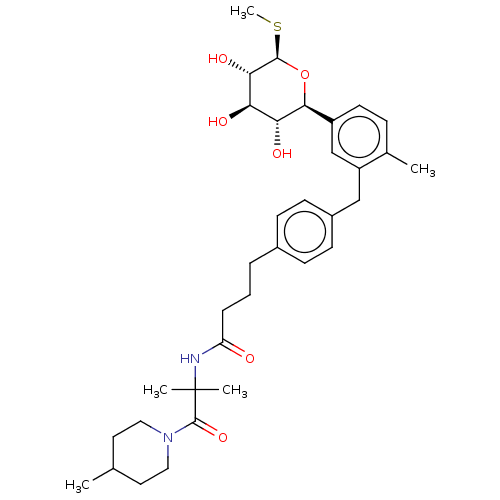 (US9688710, 120 N-(2-methyl-1-(4-methylpiperidin-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lexicon Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human HA-tagged SGLT1 expressed in HEK293 cells assessed as decrease in [14C]-AMG uptake measured after 1 to 2 hrs by scintillation cou... | J Med Chem 60: 710-721 (2017) Article DOI: 10.1021/acs.jmedchem.6b01541 BindingDB Entry DOI: 10.7270/Q2C24ZQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM176709 (US9688710, 132 N-(1-((2-(dimethylamino)ethyl)(meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Lexicon Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human HA-tagged SGLT1 expressed in HEK293 cells assessed as decrease in [14C]-AMG uptake measured after 1 to 2 hrs by scintillation cou... | J Med Chem 60: 710-721 (2017) Article DOI: 10.1021/acs.jmedchem.6b01541 BindingDB Entry DOI: 10.7270/Q2C24ZQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50235017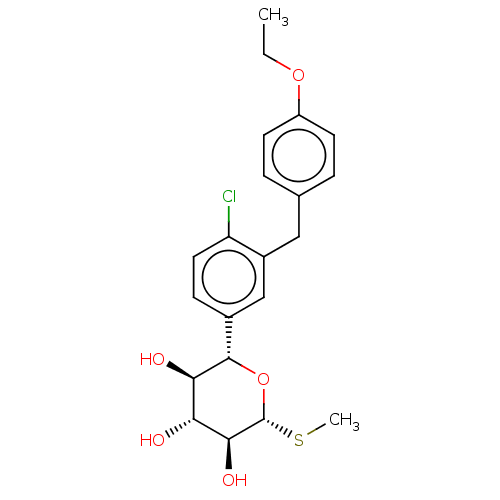 (LP-802034 | LX-4211 | Sotagliflozin) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Lexicon Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human HA-tagged SGLT2 expressed in HEK293 cells assessed as decrease in [14C]-AMG uptake measured after 1 to 2 hrs by scintillation cou... | J Med Chem 60: 710-721 (2017) Article DOI: 10.1021/acs.jmedchem.6b01541 BindingDB Entry DOI: 10.7270/Q2C24ZQ7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM176414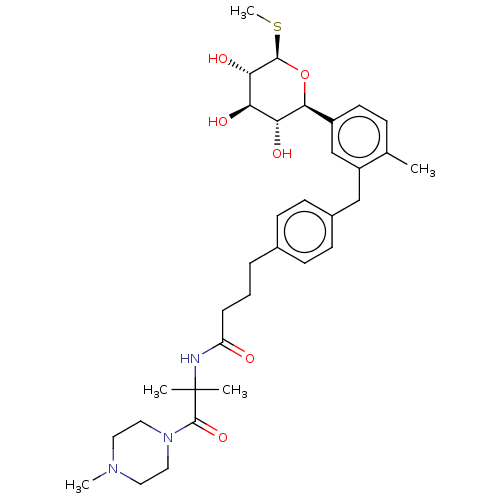 (US9688710, 113 N-(2-methyl-1-(4-methylpiperazin-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Lexicon Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human HA-tagged SGLT1 expressed in HEK293 cells assessed as decrease in [14C]-AMG uptake measured after 1 to 2 hrs by scintillation cou... | J Med Chem 60: 710-721 (2017) Article DOI: 10.1021/acs.jmedchem.6b01541 BindingDB Entry DOI: 10.7270/Q2C24ZQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM176414 (US9688710, 113 N-(2-methyl-1-(4-methylpiperazin-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Lexicon Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human HA-tagged SGLT2 expressed in HEK293 cells assessed as decrease in [14C]-AMG uptake measured after 1 to 2 hrs by scintillation cou... | J Med Chem 60: 710-721 (2017) Article DOI: 10.1021/acs.jmedchem.6b01541 BindingDB Entry DOI: 10.7270/Q2C24ZQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM176724 (US9688710, 146 (3R,4R,5S,6R)-2-(3-(4-(3-((3-(dimet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lexicon Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human HA-tagged SGLT1 expressed in HEK293 cells assessed as decrease in [14C]-AMG uptake measured after 1 to 2 hrs by scintillation cou... | J Med Chem 60: 710-721 (2017) Article DOI: 10.1021/acs.jmedchem.6b01541 BindingDB Entry DOI: 10.7270/Q2C24ZQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM176714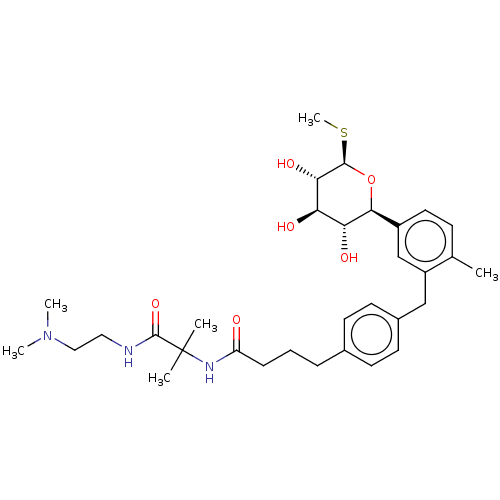 (US9688710, 136 N-(1-((2-(dimethylamino)ethyl)amino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lexicon Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human HA-tagged SGLT1 expressed in HEK293 cells assessed as decrease in [14C]-AMG uptake measured after 1 to 2 hrs by scintillation cou... | J Med Chem 60: 710-721 (2017) Article DOI: 10.1021/acs.jmedchem.6b01541 BindingDB Entry DOI: 10.7270/Q2C24ZQ7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM176544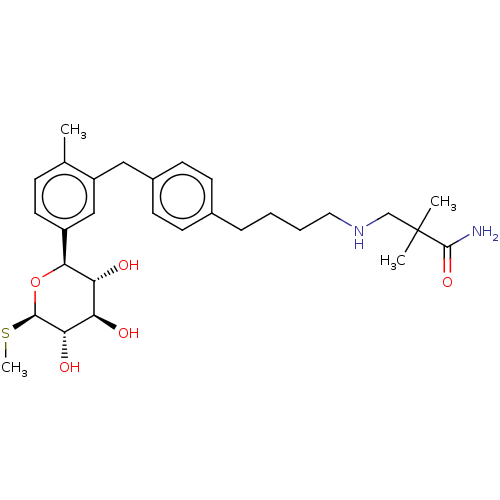 (US9688710, 104 2,2-dimethyl-3-((4-(4-(2-methyl-5-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lexicon Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human HA-tagged SGLT1 expressed in HEK293 cells assessed as decrease in [14C]-AMG uptake measured after 1 to 2 hrs by scintillation cou... | J Med Chem 60: 710-721 (2017) Article DOI: 10.1021/acs.jmedchem.6b01541 BindingDB Entry DOI: 10.7270/Q2C24ZQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50235018 (CHEMBL4072070) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Lexicon Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human HA-tagged SGLT1 expressed in HEK293 cells assessed as decrease in [14C]-AMG uptake measured after 1 to 2 hrs by scintillation cou... | J Med Chem 60: 710-721 (2017) Article DOI: 10.1021/acs.jmedchem.6b01541 BindingDB Entry DOI: 10.7270/Q2C24ZQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM176701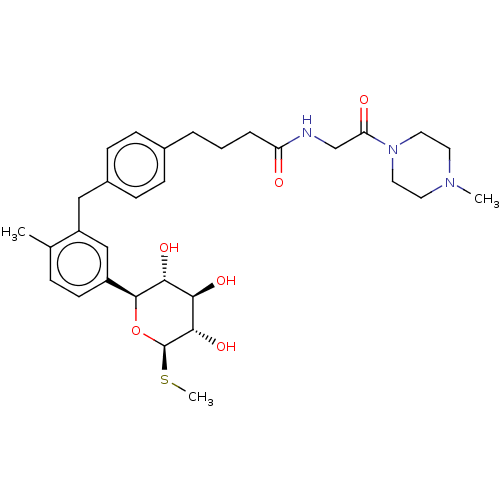 (US9688710, 124 4-(4-(2-methyl-5-((2S,3R,4R,5S,6R)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lexicon Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human HA-tagged SGLT2 expressed in HEK293 cells assessed as decrease in [14C]-AMG uptake measured after 1 to 2 hrs by scintillation cou... | J Med Chem 60: 710-721 (2017) Article DOI: 10.1021/acs.jmedchem.6b01541 BindingDB Entry DOI: 10.7270/Q2C24ZQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM176712 (US9688710, 135 (3R,4R,5S,6R)-2-(3-(4-(3-((1-hydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lexicon Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human HA-tagged SGLT2 expressed in HEK293 cells assessed as decrease in [14C]-AMG uptake measured after 1 to 2 hrs by scintillation cou... | J Med Chem 60: 710-721 (2017) Article DOI: 10.1021/acs.jmedchem.6b01541 BindingDB Entry DOI: 10.7270/Q2C24ZQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM176693 (US9688710, 116 4-(4-(2-methyl-5-((2S,3R,4R,5S,6R)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lexicon Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human HA-tagged SGLT2 expressed in HEK293 cells assessed as decrease in [14C]-AMG uptake measured after 1 to 2 hrs by scintillation cou... | J Med Chem 60: 710-721 (2017) Article DOI: 10.1021/acs.jmedchem.6b01541 BindingDB Entry DOI: 10.7270/Q2C24ZQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM176697 (US9688710, 120 N-(2-methyl-1-(4-methylpiperidin-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lexicon Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human HA-tagged SGLT2 expressed in HEK293 cells assessed as decrease in [14C]-AMG uptake measured after 1 to 2 hrs by scintillation cou... | J Med Chem 60: 710-721 (2017) Article DOI: 10.1021/acs.jmedchem.6b01541 BindingDB Entry DOI: 10.7270/Q2C24ZQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM176714 (US9688710, 136 N-(1-((2-(dimethylamino)ethyl)amino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Lexicon Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human HA-tagged SGLT2 expressed in HEK293 cells assessed as decrease in [14C]-AMG uptake measured after 1 to 2 hrs by scintillation cou... | J Med Chem 60: 710-721 (2017) Article DOI: 10.1021/acs.jmedchem.6b01541 BindingDB Entry DOI: 10.7270/Q2C24ZQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM176725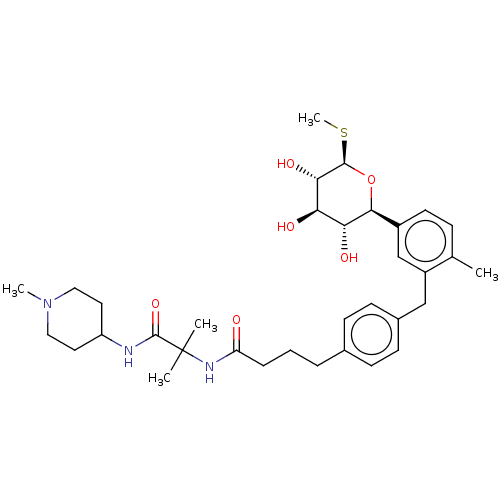 (US9688710, 147 N-(2-methyl-1-((1-methylpiperidin-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Lexicon Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human HA-tagged SGLT1 expressed in HEK293 cells assessed as decrease in [14C]-AMG uptake measured after 1 to 2 hrs by scintillation cou... | J Med Chem 60: 710-721 (2017) Article DOI: 10.1021/acs.jmedchem.6b01541 BindingDB Entry DOI: 10.7270/Q2C24ZQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM176446 (US9688710, 45 N-(2-methyl-1-(4-methylpiperazin-1-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Lexicon Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human HA-tagged SGLT1 expressed in HEK293 cells assessed as decrease in [14C]-AMG uptake measured after 1 to 2 hrs by scintillation cou... | J Med Chem 60: 710-721 (2017) Article DOI: 10.1021/acs.jmedchem.6b01541 BindingDB Entry DOI: 10.7270/Q2C24ZQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50179212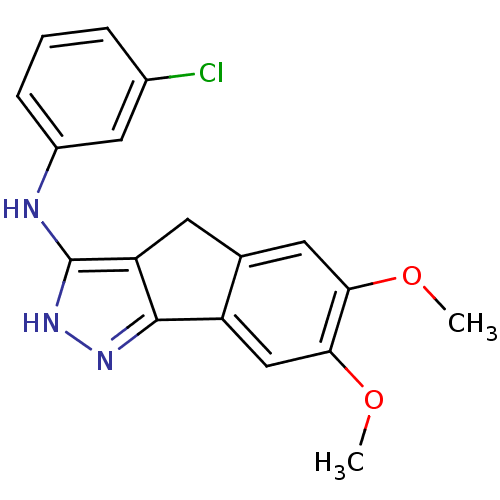 (3-chloro-N-(6,7-dimethoxy-2,4-dihydroindeno[1,2-c]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against PDGFRbeta kinase | J Med Chem 48: 8163-73 (2005) Article DOI: 10.1021/jm050680m BindingDB Entry DOI: 10.7270/Q2W095HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50179207 (3-fluoro-N-(6,7-dimethoxy-2,4-dihydroindeno[1,2-c]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antiproliferative activity against PDGF-BB stimulated HCASMC | J Med Chem 48: 8163-73 (2005) Article DOI: 10.1021/jm050680m BindingDB Entry DOI: 10.7270/Q2W095HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| LIM domain kinase 2 (Homo sapiens (Human)) | BDBM50043510 (CHEMBL3355507) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lexicon Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human full-length LIMK2 expressed in Sf9 cells assessed as incorporation of [33P] from ATP into biotinylated-cofilin substrate in prese... | ACS Med Chem Lett 6: 53-7 (2015) Article DOI: 10.1021/ml500242y BindingDB Entry DOI: 10.7270/Q2SQ9207 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM176706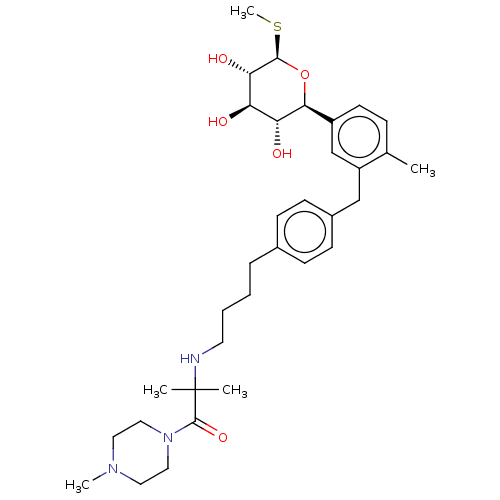 (US9688710, 129 2-methyl-2-((4-(4-(2-methyl-5-((2S,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lexicon Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human HA-tagged SGLT2 expressed in HEK293 cells assessed as decrease in [14C]-AMG uptake measured after 1 to 2 hrs by scintillation cou... | J Med Chem 60: 710-721 (2017) Article DOI: 10.1021/acs.jmedchem.6b01541 BindingDB Entry DOI: 10.7270/Q2C24ZQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM176712 (US9688710, 135 (3R,4R,5S,6R)-2-(3-(4-(3-((1-hydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lexicon Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human HA-tagged SGLT1 expressed in HEK293 cells assessed as decrease in [14C]-AMG uptake measured after 1 to 2 hrs by scintillation cou... | J Med Chem 60: 710-721 (2017) Article DOI: 10.1021/acs.jmedchem.6b01541 BindingDB Entry DOI: 10.7270/Q2C24ZQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM176704 (US9688710, 127 N-(2,2-dimethyl-3-(4-methylpiperazi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Lexicon Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human HA-tagged SGLT2 expressed in HEK293 cells assessed as decrease in [14C]-AMG uptake measured after 1 to 2 hrs by scintillation cou... | J Med Chem 60: 710-721 (2017) Article DOI: 10.1021/acs.jmedchem.6b01541 BindingDB Entry DOI: 10.7270/Q2C24ZQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM176544 (US9688710, 104 2,2-dimethyl-3-((4-(4-(2-methyl-5-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lexicon Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human HA-tagged SGLT2 expressed in HEK293 cells assessed as decrease in [14C]-AMG uptake measured after 1 to 2 hrs by scintillation cou... | J Med Chem 60: 710-721 (2017) Article DOI: 10.1021/acs.jmedchem.6b01541 BindingDB Entry DOI: 10.7270/Q2C24ZQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM176727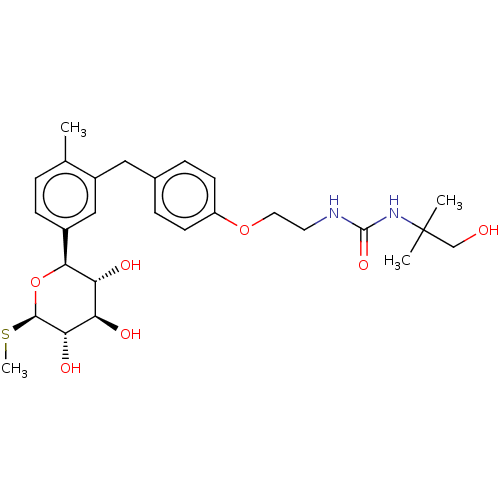 (US9688710, 149 1-(1-hydroxy-2-methylpropan-2-yl)-3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Lexicon Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human HA-tagged SGLT2 expressed in HEK293 cells assessed as decrease in [14C]-AMG uptake measured after 1 to 2 hrs by scintillation cou... | J Med Chem 60: 710-721 (2017) Article DOI: 10.1021/acs.jmedchem.6b01541 BindingDB Entry DOI: 10.7270/Q2C24ZQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM176446 (US9688710, 45 N-(2-methyl-1-(4-methylpiperazin-1-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Lexicon Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human HA-tagged SGLT2 expressed in HEK293 cells assessed as decrease in [14C]-AMG uptake measured after 1 to 2 hrs by scintillation cou... | J Med Chem 60: 710-721 (2017) Article DOI: 10.1021/acs.jmedchem.6b01541 BindingDB Entry DOI: 10.7270/Q2C24ZQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM176725 (US9688710, 147 N-(2-methyl-1-((1-methylpiperidin-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Lexicon Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human HA-tagged SGLT2 expressed in HEK293 cells assessed as decrease in [14C]-AMG uptake measured after 1 to 2 hrs by scintillation cou... | J Med Chem 60: 710-721 (2017) Article DOI: 10.1021/acs.jmedchem.6b01541 BindingDB Entry DOI: 10.7270/Q2C24ZQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50179207 (3-fluoro-N-(6,7-dimethoxy-2,4-dihydroindeno[1,2-c]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against PDGFRbeta kinase | J Med Chem 48: 8163-73 (2005) Article DOI: 10.1021/jm050680m BindingDB Entry DOI: 10.7270/Q2W095HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM176403 (US9688710, 2 4-(4-(2-chloro-5-((2S,3R,4R,5S,6R)-3,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lexicon Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human HA-tagged SGLT2 expressed in HEK293 cells assessed as decrease in [14C]-AMG uptake measured after 1 to 2 hrs by scintillation cou... | J Med Chem 60: 710-721 (2017) Article DOI: 10.1021/acs.jmedchem.6b01541 BindingDB Entry DOI: 10.7270/Q2C24ZQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50179211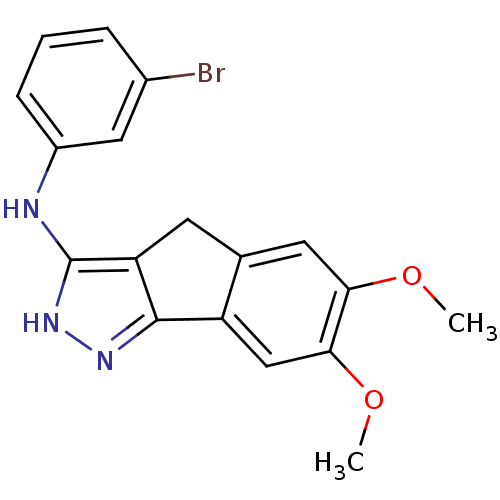 (3-bromo-N-(6,7-dimethoxy-2,4-dihydroindeno[1,2-c]p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against PDGFRbeta kinase | J Med Chem 48: 8163-73 (2005) Article DOI: 10.1021/jm050680m BindingDB Entry DOI: 10.7270/Q2W095HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM176438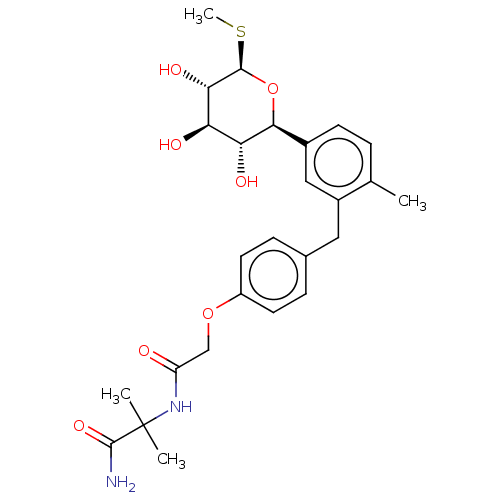 (US9688710, 37 2-methyl-2-(2-(4-(2-methyl-5-((2S,3R...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lexicon Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human HA-tagged SGLT2 expressed in HEK293 cells assessed as decrease in [14C]-AMG uptake measured after 1 to 2 hrs by scintillation cou... | J Med Chem 60: 710-721 (2017) Article DOI: 10.1021/acs.jmedchem.6b01541 BindingDB Entry DOI: 10.7270/Q2C24ZQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM176704 (US9688710, 127 N-(2,2-dimethyl-3-(4-methylpiperazi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Lexicon Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human HA-tagged SGLT1 expressed in HEK293 cells assessed as decrease in [14C]-AMG uptake measured after 1 to 2 hrs by scintillation cou... | J Med Chem 60: 710-721 (2017) Article DOI: 10.1021/acs.jmedchem.6b01541 BindingDB Entry DOI: 10.7270/Q2C24ZQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50318133 ((14S)-16-amino-10,14-dicyclohexyl-2-oxa-10,15,17,2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of BACE1 assessed as amyloid beta (1-40) production | Bioorg Med Chem Lett 20: 3158-60 (2010) Article DOI: 10.1016/j.bmcl.2010.03.097 BindingDB Entry DOI: 10.7270/Q2C53M1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM176701 (US9688710, 124 4-(4-(2-methyl-5-((2S,3R,4R,5S,6R)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Lexicon Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human HA-tagged SGLT1 expressed in HEK293 cells assessed as decrease in [14C]-AMG uptake measured after 1 to 2 hrs by scintillation cou... | J Med Chem 60: 710-721 (2017) Article DOI: 10.1021/acs.jmedchem.6b01541 BindingDB Entry DOI: 10.7270/Q2C24ZQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50179209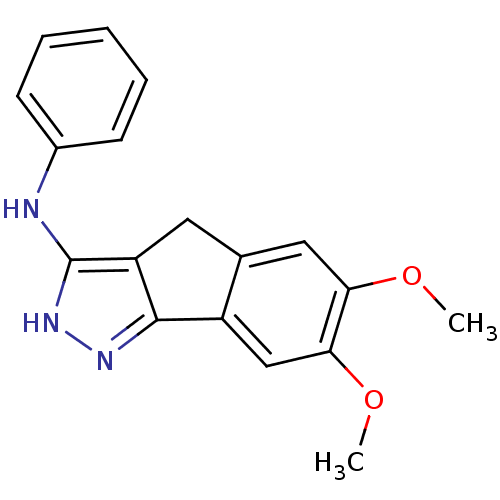 ((6,7-dimethoxy-2,4-dihydroindeno[1,2-c]pyrazol-3-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against PDGFRbeta kinase | J Med Chem 48: 8163-73 (2005) Article DOI: 10.1021/jm050680m BindingDB Entry DOI: 10.7270/Q2W095HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 185 total ) | Next | Last >> |