 Found 47 hits with Last Name = 'su' and Initial = 'bn'
Found 47 hits with Last Name = 'su' and Initial = 'bn' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057527
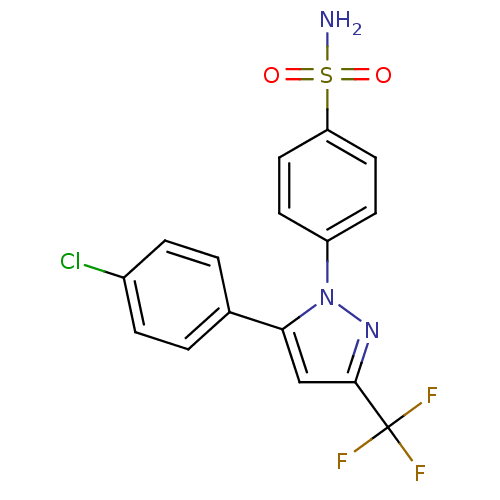
(4-(5-(4-chlorophenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(cc1-c1ccc(Cl)cc1)C(F)(F)F Show InChI InChI=1S/C16H11ClF3N3O2S/c17-11-3-1-10(2-4-11)14-9-15(16(18,19)20)22-23(14)12-5-7-13(8-6-12)26(21,24)25/h1-9H,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50269597
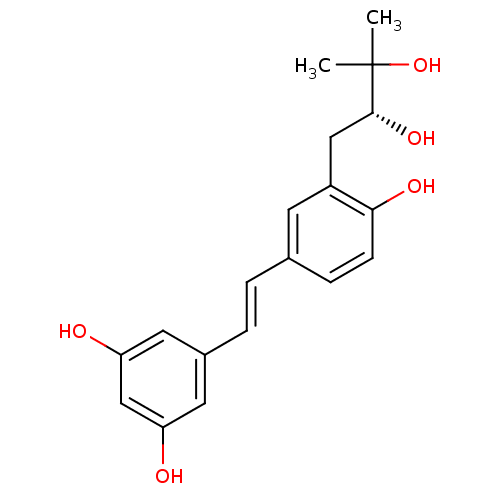
(3-(2,3-dihydroxy-3-methylbutyl)resveratrol | CHEMB...)Show SMILES CC(C)(O)[C@H](O)Cc1cc(\C=C\c2cc(O)cc(O)c2)ccc1O |r| Show InChI InChI=1S/C19H22O5/c1-19(2,24)18(23)10-14-7-12(5-6-17(14)22)3-4-13-8-15(20)11-16(21)9-13/h3-9,11,18,20-24H,10H2,1-2H3/b4-3+/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50250915
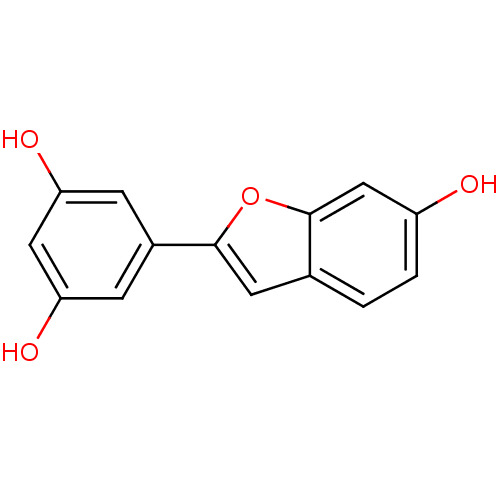
(CHEMBL512578 | moracin M)Show InChI InChI=1S/C14H10O4/c15-10-2-1-8-5-13(18-14(8)7-10)9-3-11(16)6-12(17)4-9/h1-7,15-17H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50269596
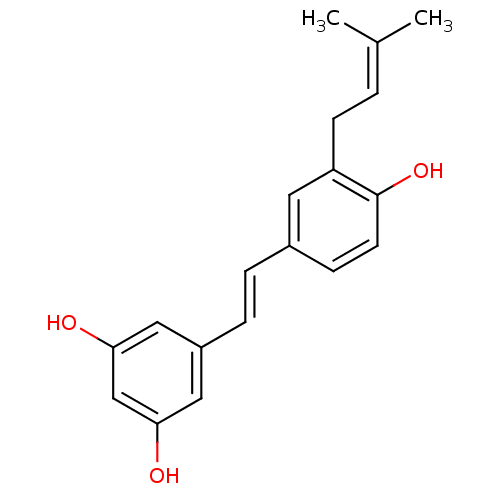
(3-(gamma,gamma-dimethylallyl)resveratrol | CHEMBL4...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1cc(\[#6]=[#6]\c2cc(-[#8])cc(-[#8])c2)ccc1-[#8] Show InChI InChI=1S/C19H20O3/c1-13(2)3-7-16-9-14(6-8-19(16)22)4-5-15-10-17(20)12-18(21)11-15/h3-6,8-12,20-22H,7H2,1-2H3/b5-4+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM23926

((E)-resveratrol | 5-[(E)-2-(4-hydroxyphenyl)etheny...)Show InChI InChI=1S/C14H12O3/c15-12-5-3-10(4-6-12)1-2-11-7-13(16)9-14(17)8-11/h1-9,15-17H/b2-1+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM23926

((E)-resveratrol | 5-[(E)-2-(4-hydroxyphenyl)etheny...)Show InChI InChI=1S/C14H12O3/c15-12-5-3-10(4-6-12)1-2-11-7-13(16)9-14(17)8-11/h1-9,15-17H/b2-1+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50108046
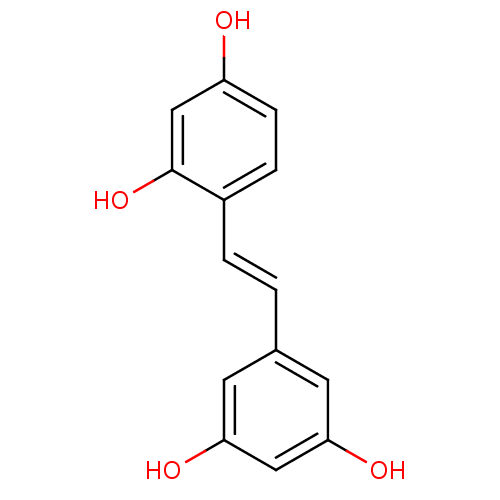
((oxyresveratrol)4-[(E)-2-(3,5-dihydroxyphenyl)viny...)Show InChI InChI=1S/C14H12O4/c15-11-4-3-10(14(18)8-11)2-1-9-5-12(16)7-13(17)6-9/h1-8,15-18H/b2-1+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50269599
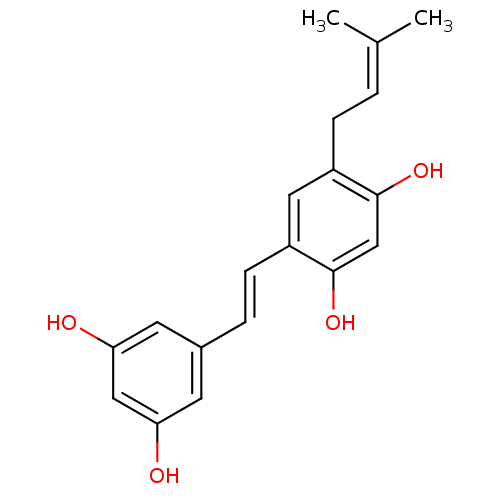
(5-(gamma,gamma-dimethylallyl)-oxyresveratrol | CHE...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1cc(\[#6]=[#6]\c2cc(-[#8])cc(-[#8])c2)c(-[#8])cc1-[#8] Show InChI InChI=1S/C19H20O4/c1-12(2)3-5-14-9-15(19(23)11-18(14)22)6-4-13-7-16(20)10-17(21)8-13/h3-4,6-11,20-23H,5H2,1-2H3/b6-4+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50269598
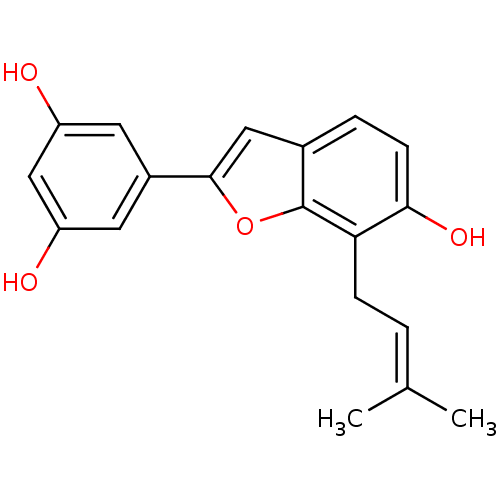
(3-(gamma,gamma-dimethylpropenyl)moracinM | CHEMBL4...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])ccc2cc(oc12)-c1cc(-[#8])cc(-[#8])c1 Show InChI InChI=1S/C19H18O4/c1-11(2)3-5-16-17(22)6-4-12-9-18(23-19(12)16)13-7-14(20)10-15(21)8-13/h3-4,6-10,20-22H,5H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50269605
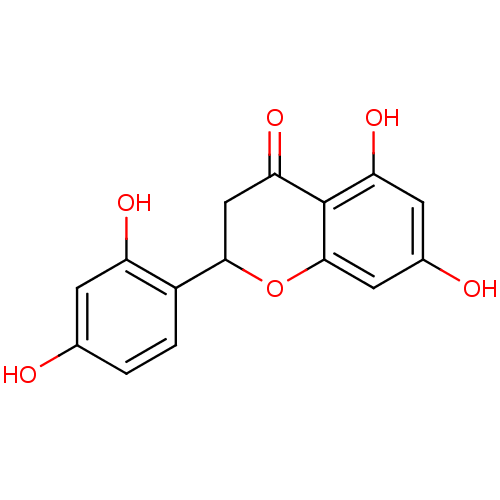
(CHEMBL465194 | steppogenin)Show InChI InChI=1S/C15H12O6/c16-7-1-2-9(10(18)3-7)13-6-12(20)15-11(19)4-8(17)5-14(15)21-13/h1-5,13,16-19H,6H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50240892

((-)-epiafzelechin | (2R,3R)-2-(4-Hydroxy-phenyl)-c...)Show SMILES O[C@@H]1Cc2c(O)cc(O)cc2O[C@@H]1c1ccc(O)cc1 |r| Show InChI InChI=1S/C15H14O5/c16-9-3-1-8(2-4-9)15-13(19)7-11-12(18)5-10(17)6-14(11)20-15/h1-6,13,15-19H,7H2/t13-,15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50269600
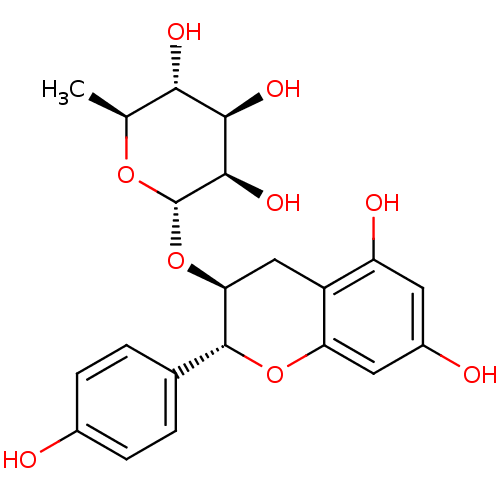
(CHEMBL517149 | afzelechin-3-O-alpha-Lrhamnopyranos...)Show SMILES C[C@@H]1O[C@@H](O[C@H]2Cc3c(O)cc(O)cc3O[C@@H]2c2ccc(O)cc2)[C@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C21H24O9/c1-9-17(25)18(26)19(27)21(28-9)30-16-8-13-14(24)6-12(23)7-15(13)29-20(16)10-2-4-11(22)5-3-10/h2-7,9,16-27H,8H2,1H3/t9-,16-,17-,18+,19+,20+,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50269596

(3-(gamma,gamma-dimethylallyl)resveratrol | CHEMBL4...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1cc(\[#6]=[#6]\c2cc(-[#8])cc(-[#8])c2)ccc1-[#8] Show InChI InChI=1S/C19H20O3/c1-13(2)3-7-16-9-14(6-8-19(16)22)4-5-15-10-17(20)12-18(21)11-15/h3-6,8-12,20-22H,7H2,1-2H3/b5-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50269606
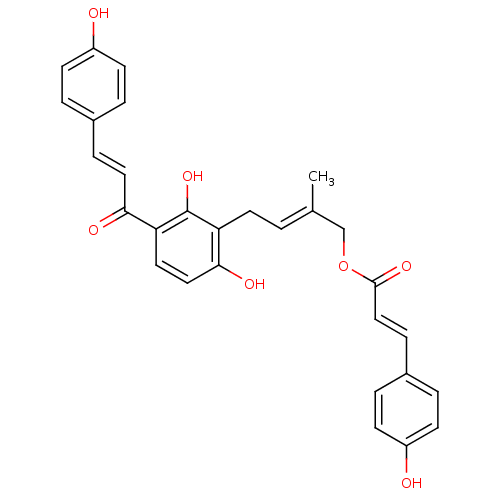
(CHEMBL517334 | isogemichalcone B)Show SMILES C\C(COC(=O)\C=C\c1ccc(O)cc1)=C/Cc1c(O)ccc(C(=O)\C=C\c2ccc(O)cc2)c1O Show InChI InChI=1S/C29H26O7/c1-19(18-36-28(34)17-8-21-5-11-23(31)12-6-21)2-13-24-27(33)16-14-25(29(24)35)26(32)15-7-20-3-9-22(30)10-4-20/h2-12,14-17,30-31,33,35H,13,18H2,1H3/b15-7+,17-8+,19-2+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50269597

(3-(2,3-dihydroxy-3-methylbutyl)resveratrol | CHEMB...)Show SMILES CC(C)(O)[C@H](O)Cc1cc(\C=C\c2cc(O)cc(O)c2)ccc1O |r| Show InChI InChI=1S/C19H22O5/c1-19(2,24)18(23)10-14-7-12(5-6-17(14)22)3-4-13-8-15(20)11-16(21)9-13/h3-9,11,18,20-24H,10H2,1-2H3/b4-3+/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50269277
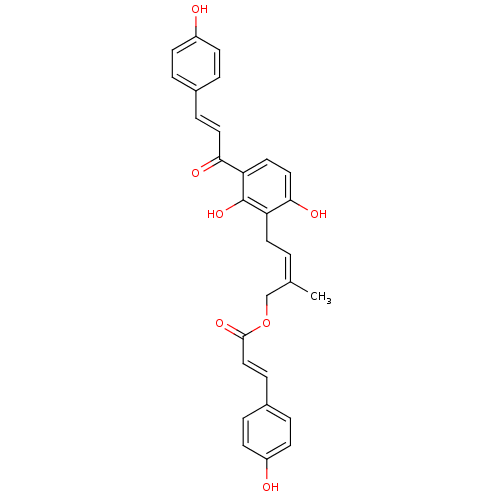
(CHEMBL497716 | gemichalcone B | gemichalcones B)Show SMILES C\C(COC(=O)\C=C\c1ccc(O)cc1)=C\Cc1c(O)ccc(C(=O)\C=C\c2ccc(O)cc2)c1O Show InChI InChI=1S/C29H26O7/c1-19(18-36-28(34)17-8-21-5-11-23(31)12-6-21)2-13-24-27(33)16-14-25(29(24)35)26(32)15-7-20-3-9-22(30)10-4-20/h2-12,14-17,30-31,33,35H,13,18H2,1H3/b15-7+,17-8+,19-2- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50269559
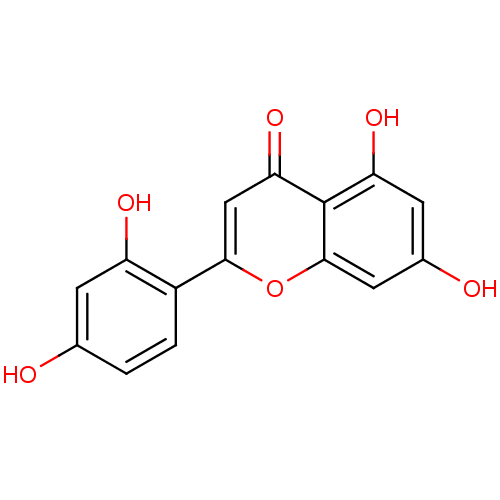
(2-(2,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...)Show InChI InChI=1S/C15H10O6/c16-7-1-2-9(10(18)3-7)13-6-12(20)15-11(19)4-8(17)5-14(15)21-13/h1-6,16-19H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50269606

(CHEMBL517334 | isogemichalcone B)Show SMILES C\C(COC(=O)\C=C\c1ccc(O)cc1)=C/Cc1c(O)ccc(C(=O)\C=C\c2ccc(O)cc2)c1O Show InChI InChI=1S/C29H26O7/c1-19(18-36-28(34)17-8-21-5-11-23(31)12-6-21)2-13-24-27(33)16-14-25(29(24)35)26(32)15-7-20-3-9-22(30)10-4-20/h2-12,14-17,30-31,33,35H,13,18H2,1H3/b15-7+,17-8+,19-2+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50269600

(CHEMBL517149 | afzelechin-3-O-alpha-Lrhamnopyranos...)Show SMILES C[C@@H]1O[C@@H](O[C@H]2Cc3c(O)cc(O)cc3O[C@@H]2c2ccc(O)cc2)[C@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C21H24O9/c1-9-17(25)18(26)19(27)21(28-9)30-16-8-13-14(24)6-12(23)7-15(13)29-20(16)10-2-4-11(22)5-3-10/h2-7,9,16-27H,8H2,1H3/t9-,16-,17-,18+,19+,20+,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50250915

(CHEMBL512578 | moracin M)Show InChI InChI=1S/C14H10O4/c15-10-2-1-8-5-13(18-14(8)7-10)9-3-11(16)6-12(17)4-9/h1-7,15-17H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50057527

(4-(5-(4-chlorophenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(cc1-c1ccc(Cl)cc1)C(F)(F)F Show InChI InChI=1S/C16H11ClF3N3O2S/c17-11-3-1-10(2-4-11)14-9-15(16(18,19)20)22-23(14)12-5-7-13(8-6-12)26(21,24)25/h1-9H,(H2,21,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50269598

(3-(gamma,gamma-dimethylpropenyl)moracinM | CHEMBL4...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])ccc2cc(oc12)-c1cc(-[#8])cc(-[#8])c1 Show InChI InChI=1S/C19H18O4/c1-11(2)3-5-16-17(22)6-4-12-9-18(23-19(12)16)13-7-14(20)10-15(21)8-13/h3-4,6-10,20-22H,5H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50269599

(5-(gamma,gamma-dimethylallyl)-oxyresveratrol | CHE...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1cc(\[#6]=[#6]\c2cc(-[#8])cc(-[#8])c2)c(-[#8])cc1-[#8] Show InChI InChI=1S/C19H20O4/c1-12(2)3-5-14-9-15(19(23)11-18(14)22)6-4-13-7-16(20)10-17(21)8-13/h3-4,6-11,20-23H,5H2,1-2H3/b6-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50269277

(CHEMBL497716 | gemichalcone B | gemichalcones B)Show SMILES C\C(COC(=O)\C=C\c1ccc(O)cc1)=C\Cc1c(O)ccc(C(=O)\C=C\c2ccc(O)cc2)c1O Show InChI InChI=1S/C29H26O7/c1-19(18-36-28(34)17-8-21-5-11-23(31)12-6-21)2-13-24-27(33)16-14-25(29(24)35)26(32)15-7-20-3-9-22(30)10-4-20/h2-12,14-17,30-31,33,35H,13,18H2,1H3/b15-7+,17-8+,19-2- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50269605

(CHEMBL465194 | steppogenin)Show InChI InChI=1S/C15H12O6/c16-7-1-2-9(10(18)3-7)13-6-12(20)15-11(19)4-8(17)5-14(15)21-13/h1-5,13,16-19H,6H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50250912
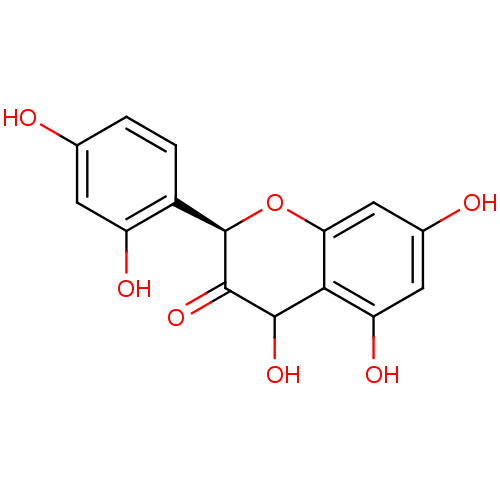
((2R,3R)-5,7,2',4'-tetrahydroxyflavanonol | CHEMBL4...)Show SMILES OC1C(=O)[C@H](Oc2cc(O)cc(O)c12)c1ccc(O)cc1O |r| Show InChI InChI=1S/C15H12O7/c16-6-1-2-8(9(18)3-6)15-14(21)13(20)12-10(19)4-7(17)5-11(12)22-15/h1-5,13,15-20H/t13?,15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50240892

((-)-epiafzelechin | (2R,3R)-2-(4-Hydroxy-phenyl)-c...)Show SMILES O[C@@H]1Cc2c(O)cc(O)cc2O[C@@H]1c1ccc(O)cc1 |r| Show InChI InChI=1S/C15H14O5/c16-9-3-1-8(2-4-9)15-13(19)7-11-12(18)5-10(17)6-14(11)20-15/h1-6,13,15-19H,7H2/t13-,15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50269604
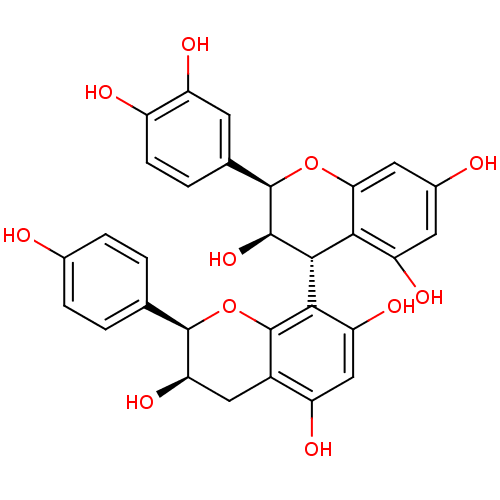
(CHEMBL447023 | epiafzelechin-(4beta->8)-epicatechi...)Show SMILES O[C@@H]1Cc2c(O)cc(O)c([C@@H]3[C@@H](O)[C@H](Oc4cc(O)cc(O)c34)c3ccc(O)c(O)c3)c2O[C@@H]1c1ccc(O)cc1 |r| Show InChI InChI=1S/C30H26O11/c31-14-4-1-12(2-5-14)28-22(38)10-16-18(34)11-21(37)25(30(16)41-28)26-24-20(36)8-15(32)9-23(24)40-29(27(26)39)13-3-6-17(33)19(35)7-13/h1-9,11,22,26-29,31-39H,10H2/t22-,26-,27-,28-,29-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50269559

(2-(2,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...)Show InChI InChI=1S/C15H10O6/c16-7-1-2-9(10(18)3-7)13-6-12(20)15-11(19)4-8(17)5-14(15)21-13/h1-6,16-19H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50250912

((2R,3R)-5,7,2',4'-tetrahydroxyflavanonol | CHEMBL4...)Show SMILES OC1C(=O)[C@H](Oc2cc(O)cc(O)c12)c1ccc(O)cc1O |r| Show InChI InChI=1S/C15H12O7/c16-6-1-2-8(9(18)3-6)15-14(21)13(20)12-10(19)4-7(17)5-11(12)22-15/h1-5,13,15-20H/t13?,15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50269604

(CHEMBL447023 | epiafzelechin-(4beta->8)-epicatechi...)Show SMILES O[C@@H]1Cc2c(O)cc(O)c([C@@H]3[C@@H](O)[C@H](Oc4cc(O)cc(O)c34)c3ccc(O)c(O)c3)c2O[C@@H]1c1ccc(O)cc1 |r| Show InChI InChI=1S/C30H26O11/c31-14-4-1-12(2-5-14)28-22(38)10-16-18(34)11-21(37)25(30(16)41-28)26-24-20(36)8-15(32)9-23(24)40-29(27(26)39)13-3-6-17(33)19(35)7-13/h1-9,11,22,26-29,31-39H,10H2/t22-,26-,27-,28-,29-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50108046

((oxyresveratrol)4-[(E)-2-(3,5-dihydroxyphenyl)viny...)Show InChI InChI=1S/C14H12O4/c15-11-4-3-10(14(18)8-11)2-1-9-5-12(16)7-13(17)6-9/h1-8,15-18H/b2-1+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.09E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50164200
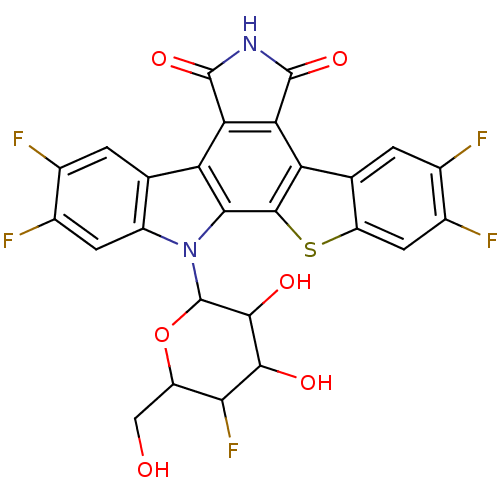
(2,3,9,10-tetrafluoro-13-(5-fluoro-3,4-dihydroxy-6-...)Show SMILES OCC1OC(C(O)C(O)C1F)n1c2cc(F)c(F)cc2c2c3C(=O)NC(=O)c3c3c4cc(F)c(F)cc4sc3c12 Show InChI InChI=1S/C26H15F5N2O6S/c27-8-1-6-12(3-10(8)29)33(26-22(36)21(35)19(31)13(5-34)39-26)20-15(6)17-18(25(38)32-24(17)37)16-7-2-9(28)11(30)4-14(7)40-23(16)20/h1-4,13,19,21-22,26,34-36H,5H2,(H,32,37,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Topoisomerase I activity for single-strand breaks in the DNA substrate |
J Med Chem 48: 2258-61 (2005)
Article DOI: 10.1021/jm049090z
BindingDB Entry DOI: 10.7270/Q20G3JP5 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50164201
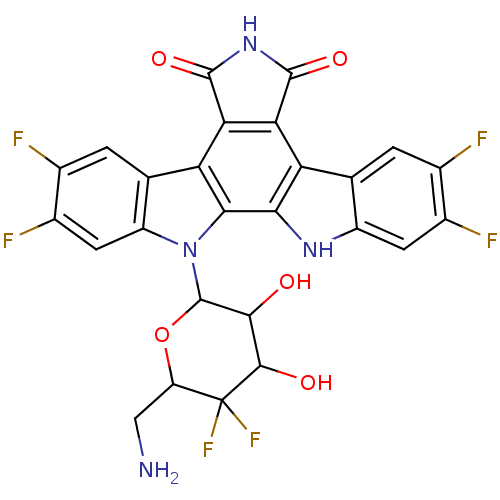
(12-(6-aminomethyl-5,5-difluoro-3,4-dihydroxytetrah...)Show SMILES NCC1OC(C(O)C(O)C1(F)F)n1c2cc(F)c(F)cc2c2c3C(=O)NC(=O)c3c3c4cc(F)c(F)cc4[nH]c3c12 Show InChI InChI=1S/C26H16F6N4O5/c27-8-1-6-12(3-10(8)29)34-19-15(6)17-18(24(40)35-23(17)39)16-7-2-9(28)11(30)4-13(7)36(20(16)19)25-21(37)22(38)26(31,32)14(5-33)41-25/h1-4,14,21-22,25,34,37-38H,5,33H2,(H,35,39,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Topoisomerase I activity for single-strand breaks in the DNA substrate |
J Med Chem 48: 2258-61 (2005)
Article DOI: 10.1021/jm049090z
BindingDB Entry DOI: 10.7270/Q20G3JP5 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50164203
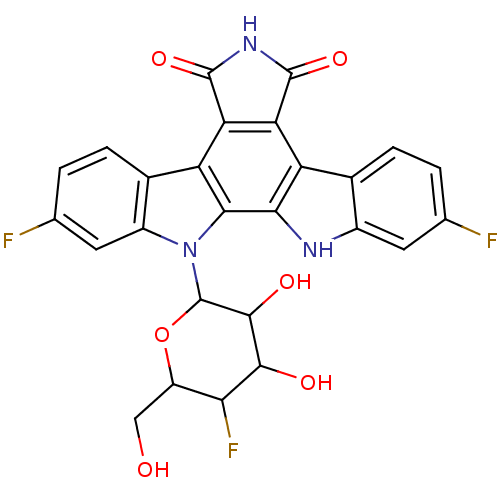
(2,10-difluoro-12-(5-fluoro-3,4-dihydroxy-6-hydroxy...)Show SMILES OCC1OC(C(O)C(O)C1F)n1c2cc(F)ccc2c2c3C(=O)NC(=O)c3c3c4ccc(F)cc4[nH]c3c12 Show InChI InChI=1S/C26H18F3N3O6/c27-8-1-3-10-12(5-8)30-20-15(10)17-18(25(37)31-24(17)36)16-11-4-2-9(28)6-13(11)32(21(16)20)26-23(35)22(34)19(29)14(7-33)38-26/h1-6,14,19,22-23,26,30,33-35H,7H2,(H,31,36,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Topoisomerase I activity for single-strand breaks in the DNA substrate |
J Med Chem 48: 2258-61 (2005)
Article DOI: 10.1021/jm049090z
BindingDB Entry DOI: 10.7270/Q20G3JP5 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50164205

(2,3,9,10-tetrafluoro-12-(5-fluoro-3,4-dihydroxy-6-...)Show SMILES OCC1OC(C(O)C(O)C1F)n1c2cc(F)c(F)cc2c2c3C(=O)NC(=O)c3c3c4cc(F)c(F)cc4[nH]c3c12 Show InChI InChI=1S/C26H16F5N3O6/c27-8-1-6-12(3-10(8)29)32-20-15(6)17-18(25(39)33-24(17)38)16-7-2-9(28)11(30)4-13(7)34(21(16)20)26-23(37)22(36)19(31)14(5-35)40-26/h1-4,14,19,22-23,26,32,35-37H,5H2,(H,33,38,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Topoisomerase I activity for single-strand breaks in the DNA substrate |
J Med Chem 48: 2258-61 (2005)
Article DOI: 10.1021/jm049090z
BindingDB Entry DOI: 10.7270/Q20G3JP5 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50164196
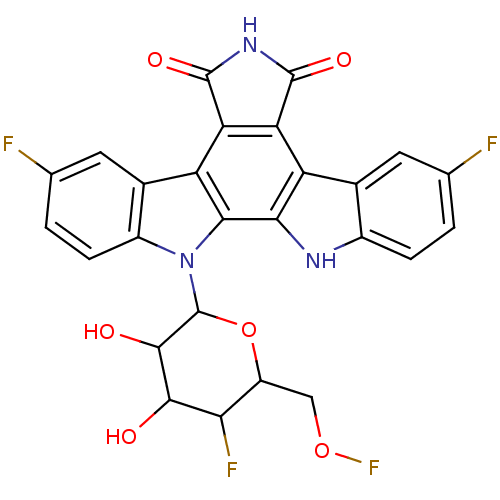
(3,9-difluoro-12-(5-fluoro-3,4-dihydroxy-6-hydroxym...)Show SMILES OC1C(O)C(OC(COF)C1F)n1c2ccc(F)cc2c2c3C(=O)NC(=O)c3c3c4cc(F)ccc4[nH]c3c12 Show InChI InChI=1S/C26H17F4N3O6/c27-8-1-3-12-10(5-8)15-17-18(25(37)32-24(17)36)16-11-6-9(28)2-4-13(11)33(21(16)20(15)31-12)26-23(35)22(34)19(29)14(39-26)7-38-30/h1-6,14,19,22-23,26,31,34-35H,7H2,(H,32,36,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 65.6 | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Topoisomerase I activity for single-strand breaks in the DNA substrate |
J Med Chem 48: 2258-61 (2005)
Article DOI: 10.1021/jm049090z
BindingDB Entry DOI: 10.7270/Q20G3JP5 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50164197

(3,9-difluoro-12-(5-fluoro-3,4-dihydroxy-6-hydroxym...)Show SMILES OCC1OC(C(O)C(O)C1F)n1c2ccc(F)cc2c2c3C(=O)NC(=O)c3c3c4cc(F)ccc4[nH]c3c12 Show InChI InChI=1S/C26H18F3N3O6/c27-8-1-3-12-10(5-8)15-17-18(25(37)31-24(17)36)16-11-6-9(28)2-4-13(11)32(21(16)20(15)30-12)26-23(35)22(34)19(29)14(7-33)38-26/h1-6,14,19,22-23,26,30,33-35H,7H2,(H,31,36,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 17.6 | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Topoisomerase I activity for single-strand breaks in the DNA substrate |
J Med Chem 48: 2258-61 (2005)
Article DOI: 10.1021/jm049090z
BindingDB Entry DOI: 10.7270/Q20G3JP5 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50002738
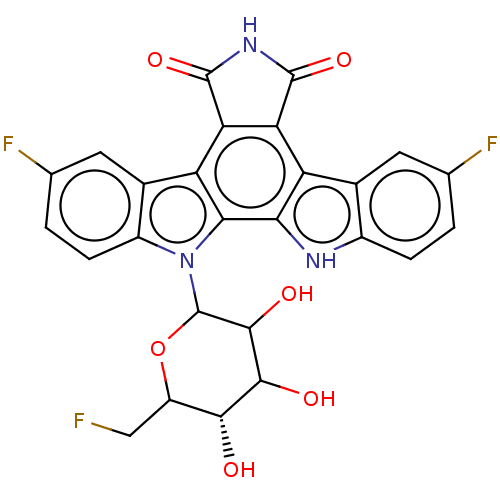
Show SMILES Cn1c(CN2CCNCC2)nc2ccc(NC(=O)COc3ccc(cc3)C(F)(F)F)cc12 Show InChI InChI=1S/C22H24F3N5O2/c1-29-19-12-16(4-7-18(19)28-20(29)13-30-10-8-26-9-11-30)27-21(31)14-32-17-5-2-15(3-6-17)22(23,24)25/h2-7,12,26H,8-11,13-14H2,1H3,(H,27,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 704 | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Topoisomerase I activity for single-strand breaks in the DNA substrate |
J Med Chem 48: 2258-61 (2005)
Article DOI: 10.1021/jm049090z
BindingDB Entry DOI: 10.7270/Q20G3JP5 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50164202

(2,3,9,10-tetrafluoro-13-(6-fluoromethyl-3,4,5-trih...)Show SMILES OC1C(O)C(CF)OC(C1O)n1c2cc(F)c(F)cc2c2c3C(=O)NC(=O)c3c3c4cc(F)c(F)cc4sc3c12 Show InChI InChI=1S/C26H15F5N2O6S/c27-5-13-20(34)21(35)22(36)26(39-13)33-12-3-10(30)8(28)1-6(12)15-17-18(25(38)32-24(17)37)16-7-2-9(29)11(31)4-14(7)40-23(16)19(15)33/h1-4,13,20-22,26,34-36H,5H2,(H,32,37,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 240 | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Topoisomerase I activity for single-strand breaks in the DNA substrate |
J Med Chem 48: 2258-61 (2005)
Article DOI: 10.1021/jm049090z
BindingDB Entry DOI: 10.7270/Q20G3JP5 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50164199
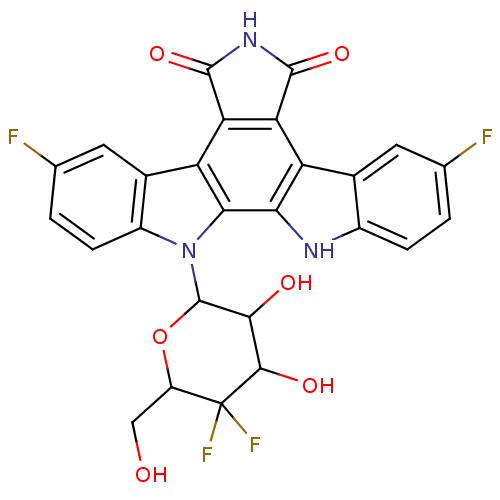
(3,9-difluoro-12-(5-fluoro-3,4,5-trihydroxy-6-hydro...)Show SMILES OCC1OC(C(O)C(O)C1(F)F)n1c2ccc(F)cc2c2c3C(=O)NC(=O)c3c3c4cc(F)ccc4[nH]c3c12 Show InChI InChI=1S/C26H17F4N3O6/c27-8-1-3-12-10(5-8)15-17-18(24(38)32-23(17)37)16-11-6-9(28)2-4-13(11)33(20(16)19(15)31-12)25-21(35)22(36)26(29,30)14(7-34)39-25/h1-6,14,21-22,25,31,34-36H,7H2,(H,32,37,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 12.8 | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Topoisomerase I activity for single-strand breaks in the DNA substrate |
J Med Chem 48: 2258-61 (2005)
Article DOI: 10.1021/jm049090z
BindingDB Entry DOI: 10.7270/Q20G3JP5 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50164198

(3,9-difluoro-12-(6-fluoromethyl-3,4,5-trihydroxyte...)Show SMILES OC1C(O)C(CF)OC(C1O)n1c2ccc(F)cc2c2c3C(=O)NC(=O)c3c3c4cc(F)ccc4[nH]c3c12 Show InChI InChI=1S/C26H18F3N3O6/c27-7-14-21(33)22(34)23(35)26(38-14)32-13-4-2-9(29)6-11(13)16-18-17(24(36)31-25(18)37)15-10-5-8(28)1-3-12(10)30-19(15)20(16)32/h1-6,14,21-23,26,30,33-35H,7H2,(H,31,36,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 76.8 | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Topoisomerase I activity for single-strand breaks in the DNA substrate |
J Med Chem 48: 2258-61 (2005)
Article DOI: 10.1021/jm049090z
BindingDB Entry DOI: 10.7270/Q20G3JP5 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50164204

(13-(5,5-difluoro-3,4-dihydroxy-6-hydroxymethyltetr...)Show SMILES OCC1OC(C(O)C(O)C1(F)F)n1c2cc(F)c(F)cc2c2c3C(=O)NC(=O)c3c3c4cc(F)c(F)cc4sc3c12 Show InChI InChI=1S/C26H14F6N2O6S/c27-8-1-6-12(3-10(8)29)34(25-20(36)22(37)26(31,32)14(5-35)40-25)19-15(6)17-18(24(39)33-23(17)38)16-7-2-9(28)11(30)4-13(7)41-21(16)19/h1-4,14,20,22,25,35-37H,5H2,(H,33,38,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 80 | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Topoisomerase I activity for single-strand breaks in the DNA substrate |
J Med Chem 48: 2258-61 (2005)
Article DOI: 10.1021/jm049090z
BindingDB Entry DOI: 10.7270/Q20G3JP5 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50164207

(2,3,9,10-tetrafluoro-12-(6-fluoromethyl-3,4,5-trih...)Show SMILES OC1C(O)C(CF)OC(C1O)n1c2cc(F)c(F)cc2c2c3C(=O)NC(=O)c3c3c4cc(F)c(F)cc4[nH]c3c12 Show InChI InChI=1S/C26H16F5N3O6/c27-5-14-21(35)22(36)23(37)26(40-14)34-13-4-11(31)9(29)2-7(13)16-18-17(24(38)33-25(18)39)15-6-1-8(28)10(30)3-12(6)32-19(15)20(16)34/h1-4,14,21-23,26,32,35-37H,5H2,(H,33,38,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 480 | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Topoisomerase I activity for single-strand breaks in the DNA substrate |
J Med Chem 48: 2258-61 (2005)
Article DOI: 10.1021/jm049090z
BindingDB Entry DOI: 10.7270/Q20G3JP5 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50164195

(12-(6-aminomethyl-5-fluoro-3,4-dihydroxytetrahydro...)Show SMILES NCC1OC(C(O)C(O)C1F)n1c2ccc(F)cc2c2c3C(=O)NC(=O)c3c3c4cc(F)ccc4[nH]c3c12 Show InChI InChI=1S/C26H19F3N4O5/c27-8-1-3-12-10(5-8)15-17-18(25(37)32-24(17)36)16-11-6-9(28)2-4-13(11)33(21(16)20(15)31-12)26-23(35)22(34)19(29)14(7-30)38-26/h1-6,14,19,22-23,26,31,34-35H,7,30H2,(H,32,36,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 11.2 | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Topoisomerase I activity for single-strand breaks in the DNA substrate |
J Med Chem 48: 2258-61 (2005)
Article DOI: 10.1021/jm049090z
BindingDB Entry DOI: 10.7270/Q20G3JP5 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50142927
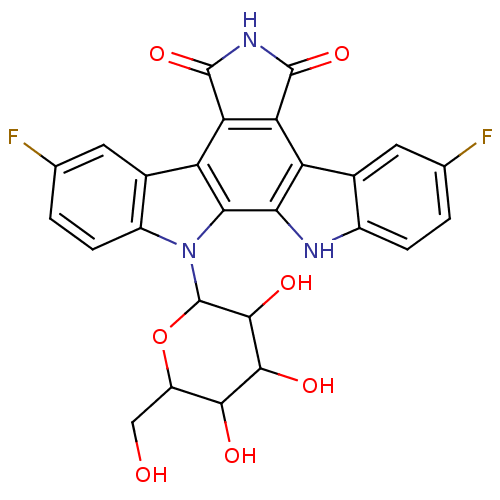
(12-(3,4-dihydroxy-6-hydroxymethyl-5-methoxytetrahy...)Show SMILES OCC1OC(C(O)C(O)C1O)n1c2ccc(F)cc2c2c3C(=O)NC(=O)c3c3c4cc(F)ccc4[nH]c3c12 Show InChI InChI=1S/C26H19F2N3O7/c27-8-1-3-12-10(5-8)15-17-18(25(37)30-24(17)36)16-11-6-9(28)2-4-13(11)31(20(16)19(15)29-12)26-23(35)22(34)21(33)14(7-32)38-26/h1-6,14,21-23,26,29,32-35H,7H2,(H,30,36,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 38.4 | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Topoisomerase I activity for single-strand breaks in the DNA substrate |
J Med Chem 48: 2258-61 (2005)
Article DOI: 10.1021/jm049090z
BindingDB Entry DOI: 10.7270/Q20G3JP5 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50164206

(3,9-difluoro-13-(6-fluoromethyl-3,4,5-trihydroxyte...)Show SMILES OC1C(O)C(CF)OC(C1O)n1c2ccc(F)cc2c2c3C(=O)NC(=O)c3c3c4cc(F)ccc4sc3c12 Show InChI InChI=1S/C26H17F3N2O6S/c27-7-13-20(32)21(33)22(34)26(37-13)31-12-3-1-8(28)5-10(12)15-17-18(25(36)30-24(17)35)16-11-6-9(29)2-4-14(11)38-23(16)19(15)31/h1-6,13,20-22,26,32-34H,7H2,(H,30,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 67.2 | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Topoisomerase I activity for single-strand breaks in the DNA substrate |
J Med Chem 48: 2258-61 (2005)
Article DOI: 10.1021/jm049090z
BindingDB Entry DOI: 10.7270/Q20G3JP5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data