

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50367489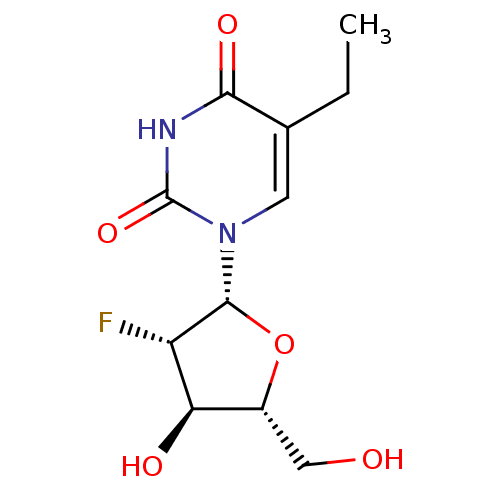 (CHEMBL1269499) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for Kinetic constant for viral thymidine kinase of Herpes simplex virus (HSV) -1 | J Med Chem 30: 867-71 (1987) BindingDB Entry DOI: 10.7270/Q2Z32077 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50367488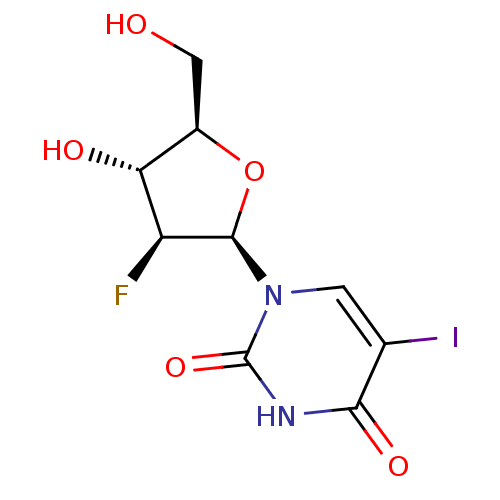 (FIALURIDINE) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for Kinetic constant for viral thymidine kinase of Herpes simplex virus (HSV) -2 | J Med Chem 30: 867-71 (1987) BindingDB Entry DOI: 10.7270/Q2Z32077 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50367487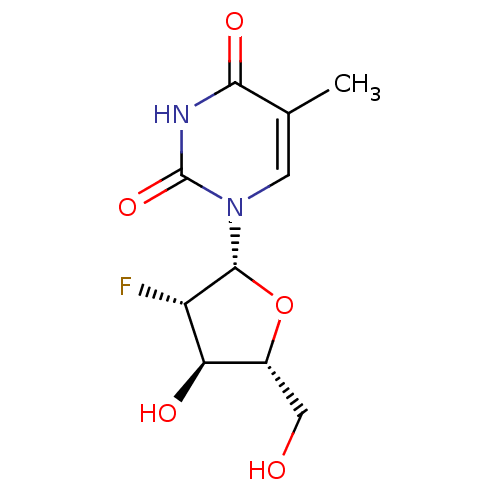 (CHEMBL475717) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for Kinetic constant for viral thymidine kinase of Herpes simplex virus (HSV) -1 | J Med Chem 30: 867-71 (1987) BindingDB Entry DOI: 10.7270/Q2Z32077 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50367489 (CHEMBL1269499) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for Kinetic constant for viral thymidine kinase of Herpes simplex virus (HSV) -2 | J Med Chem 30: 867-71 (1987) BindingDB Entry DOI: 10.7270/Q2Z32077 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50367488 (FIALURIDINE) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | 950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for Kinetic constant for viral thymidine kinase of Herpes simplex virus (HSV) -2 | J Med Chem 30: 867-71 (1987) BindingDB Entry DOI: 10.7270/Q2Z32077 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50367487 (CHEMBL475717) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for Kinetic constant for viral thymidine kinase of Herpes simplex virus (HSV) -1 | J Med Chem 30: 867-71 (1987) BindingDB Entry DOI: 10.7270/Q2Z32077 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50282061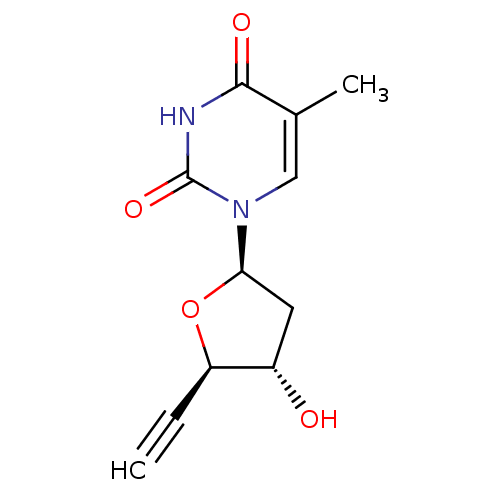 (1-((2R,4S,5R)-5-Ethynyl-4-hydroxy-tetrahydro-furan...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity of the compound against HSV-1 Thymidine kinase | Bioorg Med Chem Lett 3: 1571-1576 (1993) Article DOI: 10.1016/S0960-894X(00)80020-7 BindingDB Entry DOI: 10.7270/Q28P60F3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50282067 (1-((2R,4S,5S)-4-Hydroxy-5-methoxy-tetrahydro-furan...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity of the compound against HSV-1 Thymidine kinase | Bioorg Med Chem Lett 3: 1571-1576 (1993) Article DOI: 10.1016/S0960-894X(00)80020-7 BindingDB Entry DOI: 10.7270/Q28P60F3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50282063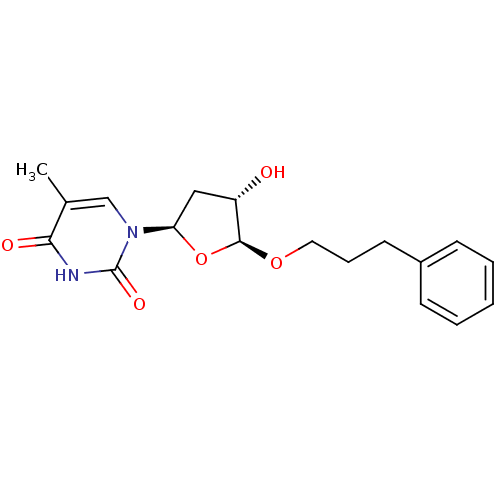 (1-[(2R,4S,5S)-4-Hydroxy-5-(3-phenyl-propoxy)-tetra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity of the compound against HSV-1 Thymidine kinase | Bioorg Med Chem Lett 3: 1571-1576 (1993) Article DOI: 10.1016/S0960-894X(00)80020-7 BindingDB Entry DOI: 10.7270/Q28P60F3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50282072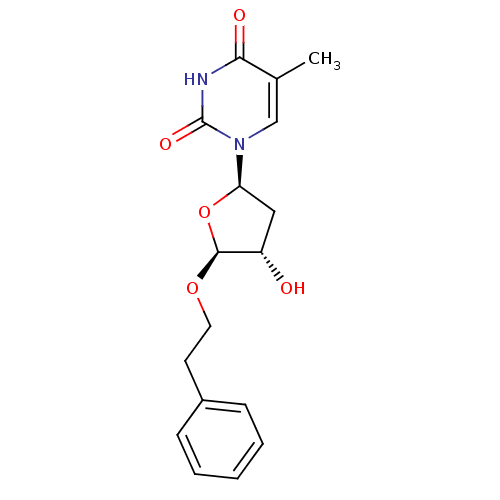 (1-((2R,4S,5S)-4-Hydroxy-5-phenethyloxy-tetrahydro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity of the compound against HSV-1 Thymidine kinase | Bioorg Med Chem Lett 3: 1571-1576 (1993) Article DOI: 10.1016/S0960-894X(00)80020-7 BindingDB Entry DOI: 10.7270/Q28P60F3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50021776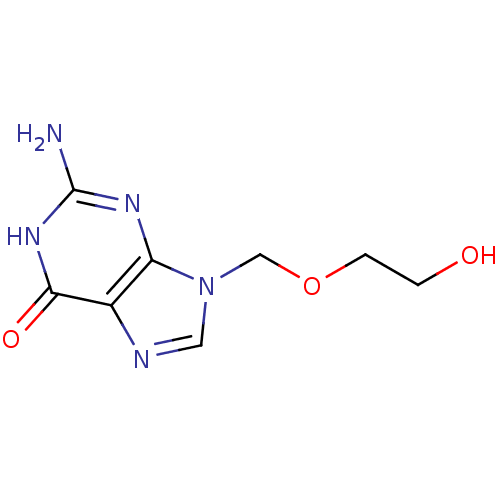 (2-Amino-9-(2-hydroxy-ethoxymethyl)-5,9-dihydro-pur...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for Kinetic constant for viral thymidine kinase of Herpes simplex virus (HSV) -1 | J Med Chem 30: 867-71 (1987) BindingDB Entry DOI: 10.7270/Q2Z32077 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50021776 (2-Amino-9-(2-hydroxy-ethoxymethyl)-5,9-dihydro-pur...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for Kinetic constant for viral thymidine kinase of Herpes simplex virus (HSV) -2 | J Med Chem 30: 867-71 (1987) BindingDB Entry DOI: 10.7270/Q2Z32077 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Mus musculus) | BDBM50468557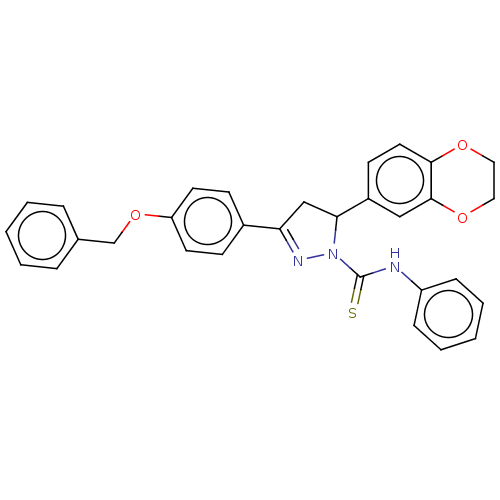 (CHEMBL4294787) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of mouse full length GST-tagged BRAF V600E mutant using recombinant human full length N-terminal His-tagged MEK1 as substrate preincubated... | Eur J Med Chem 155: 725-735 (2018) Article DOI: 10.1016/j.ejmech.2018.06.043 BindingDB Entry DOI: 10.7270/Q2639SF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 5 (Homo sapiens (Human)) | BDBM50022107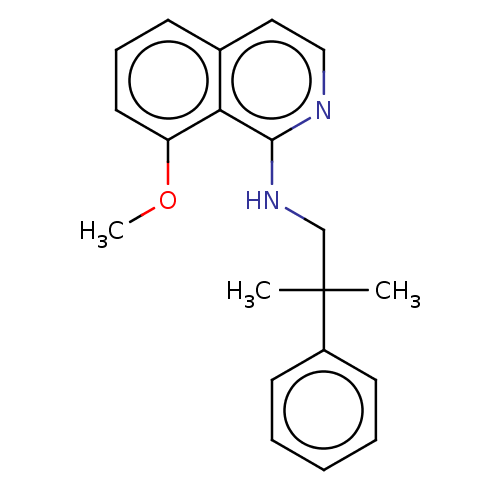 (CHEMBL3298226) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human Kv1.5 expressed in mouse L929 cells assessed as inhibition of current | Bioorg Med Chem Lett 24: 3018-22 (2014) Article DOI: 10.1016/j.bmcl.2014.05.035 BindingDB Entry DOI: 10.7270/Q2HH6MNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 5 (Homo sapiens (Human)) | BDBM50022108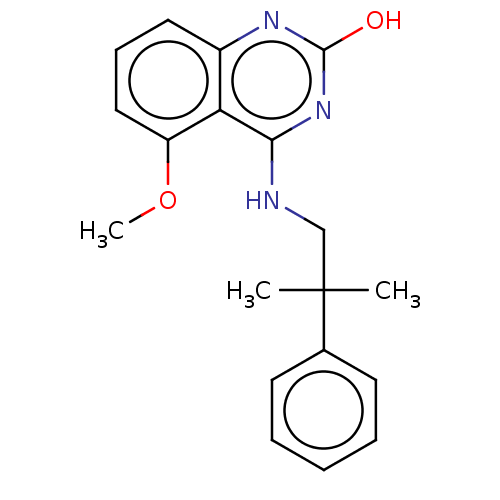 (CHEMBL3298228) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human Kv1.5 expressed in mouse L929 cells assessed as inhibition of current | Bioorg Med Chem Lett 24: 3018-22 (2014) Article DOI: 10.1016/j.bmcl.2014.05.035 BindingDB Entry DOI: 10.7270/Q2HH6MNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 5 (Homo sapiens (Human)) | BDBM50022109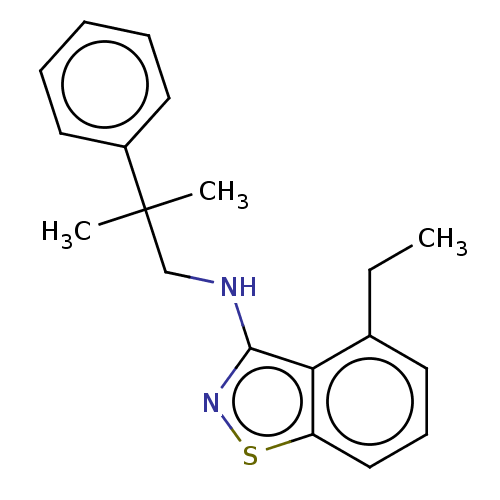 (CHEMBL3298224) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human Kv1.5 expressed in mouse L929 cells assessed as inhibition of current | Bioorg Med Chem Lett 24: 3018-22 (2014) Article DOI: 10.1016/j.bmcl.2014.05.035 BindingDB Entry DOI: 10.7270/Q2HH6MNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Mus musculus) | BDBM50396483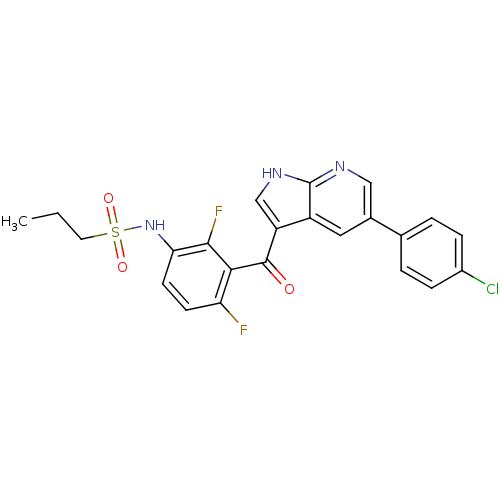 (PLX-4032 | RG 7204 | Ro 5185426 | US10570155, Vemu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of mouse full length GST-tagged BRAF V600E mutant using recombinant human full length N-terminal His-tagged MEK1 as substrate preincubated... | Eur J Med Chem 155: 725-735 (2018) Article DOI: 10.1016/j.ejmech.2018.06.043 BindingDB Entry DOI: 10.7270/Q2639SF8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Potassium voltage-gated channel subfamily A member 5 (Homo sapiens (Human)) | BDBM50430217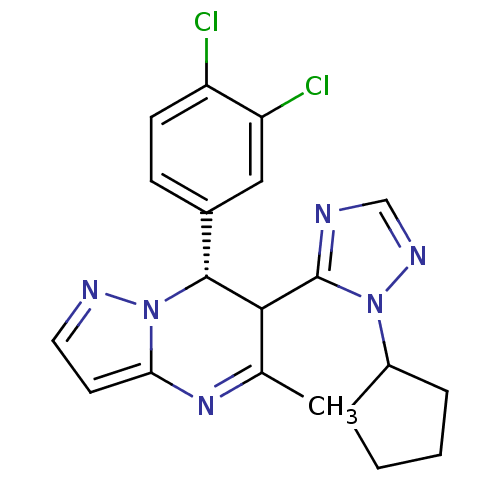 (CHEMBL2331989) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human Kv1.5 expressed in mouse L929 cells assessed as inhibition of delayed rectifier repolarization current by patch clamp as... | Bioorg Med Chem Lett 23: 1743-7 (2013) Article DOI: 10.1016/j.bmcl.2013.01.064 BindingDB Entry DOI: 10.7270/Q2QN685H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 5 (Homo sapiens (Human)) | BDBM50022110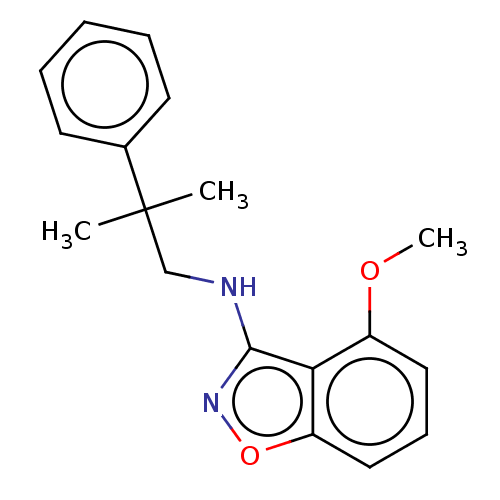 (CHEMBL3298140) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human Kv1.5 expressed in mouse L929 cells assessed as inhibition of current | Bioorg Med Chem Lett 24: 3018-22 (2014) Article DOI: 10.1016/j.bmcl.2014.05.035 BindingDB Entry DOI: 10.7270/Q2HH6MNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 5 (Homo sapiens (Human)) | BDBM50430216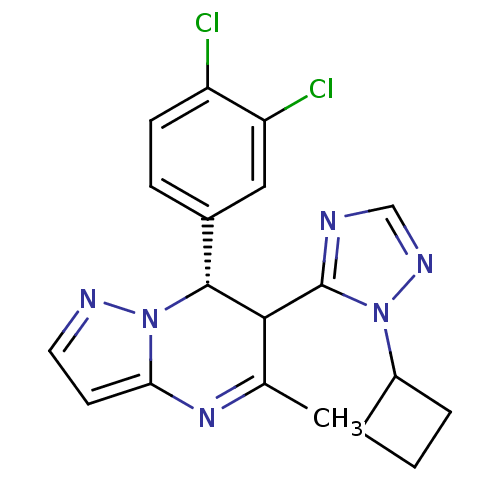 (CHEMBL2331990) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human Kv1.5 expressed in mouse L929 cells assessed as inhibition of delayed rectifier repolarization current by patch clamp as... | Bioorg Med Chem Lett 23: 1743-7 (2013) Article DOI: 10.1016/j.bmcl.2013.01.064 BindingDB Entry DOI: 10.7270/Q2QN685H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Mus musculus) | BDBM50468570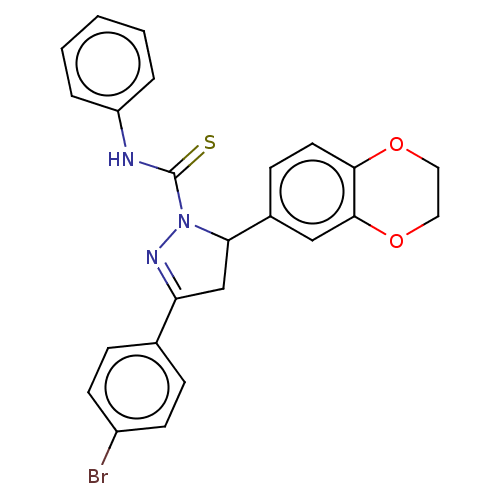 (CHEMBL4290513) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of mouse full length GST-tagged BRAF V600E mutant using recombinant human full length N-terminal His-tagged MEK1 as substrate preincubated... | Eur J Med Chem 155: 725-735 (2018) Article DOI: 10.1016/j.ejmech.2018.06.043 BindingDB Entry DOI: 10.7270/Q2639SF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 5 (Homo sapiens (Human)) | BDBM50430219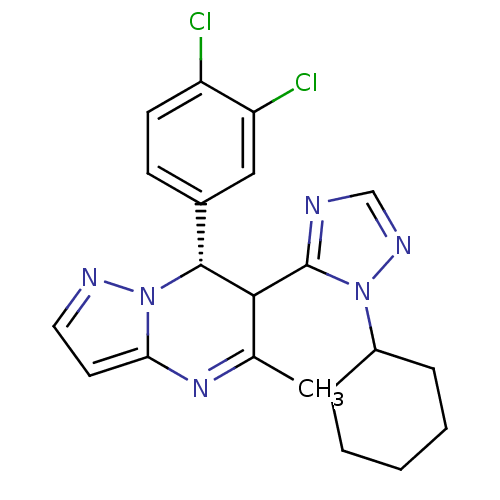 (CHEMBL2331987) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human Kv1.5 expressed in mouse L929 cells assessed as inhibition of delayed rectifier repolarization current by patch clamp as... | Bioorg Med Chem Lett 23: 1743-7 (2013) Article DOI: 10.1016/j.bmcl.2013.01.064 BindingDB Entry DOI: 10.7270/Q2QN685H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Mus musculus) | BDBM50468565 (CHEMBL4277125) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of mouse full length GST-tagged BRAF V600E mutant using recombinant human full length N-terminal His-tagged MEK1 as substrate preincubated... | Eur J Med Chem 155: 725-735 (2018) Article DOI: 10.1016/j.ejmech.2018.06.043 BindingDB Entry DOI: 10.7270/Q2639SF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 5 (Homo sapiens (Human)) | BDBM50022111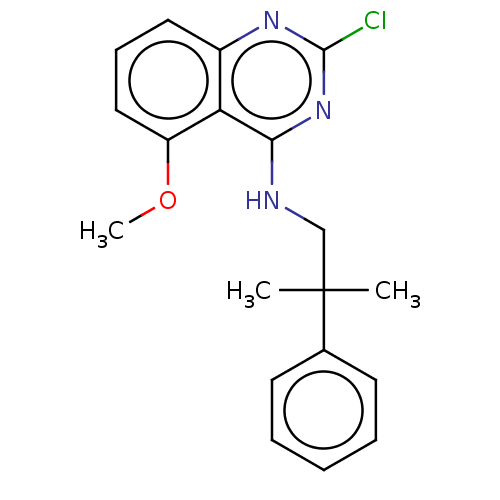 (CHEMBL3298227) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human Kv1.5 expressed in mouse L929 cells assessed as inhibition of current | Bioorg Med Chem Lett 24: 3018-22 (2014) Article DOI: 10.1016/j.bmcl.2014.05.035 BindingDB Entry DOI: 10.7270/Q2HH6MNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 5 (Homo sapiens (Human)) | BDBM50430227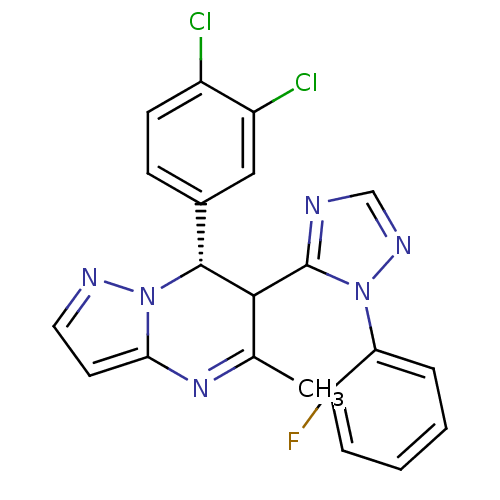 (CHEMBL2332455) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human Kv1.5 expressed in mouse L929 cells assessed as inhibition of delayed rectifier repolarization current by patch clamp as... | Bioorg Med Chem Lett 23: 1743-7 (2013) Article DOI: 10.1016/j.bmcl.2013.01.064 BindingDB Entry DOI: 10.7270/Q2QN685H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 5 (Homo sapiens (Human)) | BDBM50430225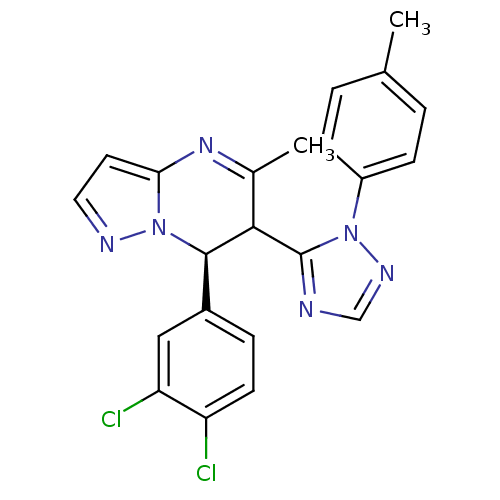 (CHEMBL2332457) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human Kv1.5 expressed in mouse L929 cells assessed as inhibition of delayed rectifier repolarization current by patch clamp as... | Bioorg Med Chem Lett 23: 1743-7 (2013) Article DOI: 10.1016/j.bmcl.2013.01.064 BindingDB Entry DOI: 10.7270/Q2QN685H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 5 (Homo sapiens (Human)) | BDBM50022112 (CHEMBL3298230) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human Kv1.5 expressed in mouse L929 cells assessed as inhibition of current | Bioorg Med Chem Lett 24: 3018-22 (2014) Article DOI: 10.1016/j.bmcl.2014.05.035 BindingDB Entry DOI: 10.7270/Q2HH6MNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 5 (Homo sapiens (Human)) | BDBM50430201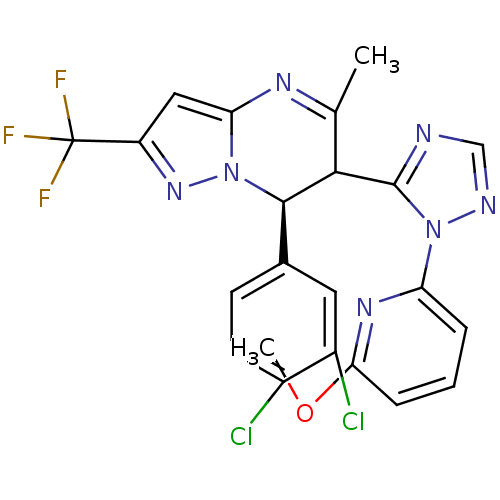 (CHEMBL2332448) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human Kv1.5 expressed in mouse L929 cells assessed as inhibition of delayed rectifier repolarization current by patch clamp as... | Bioorg Med Chem Lett 23: 1743-7 (2013) Article DOI: 10.1016/j.bmcl.2013.01.064 BindingDB Entry DOI: 10.7270/Q2QN685H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 5 (Homo sapiens (Human)) | BDBM50022113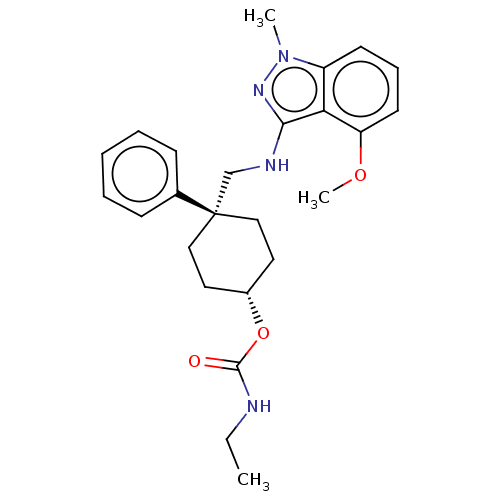 (CHEMBL3298231) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human Kv1.5 expressed in mouse L929 cells assessed as inhibition of current | Bioorg Med Chem Lett 24: 3018-22 (2014) Article DOI: 10.1016/j.bmcl.2014.05.035 BindingDB Entry DOI: 10.7270/Q2HH6MNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 5 (Homo sapiens (Human)) | BDBM50430220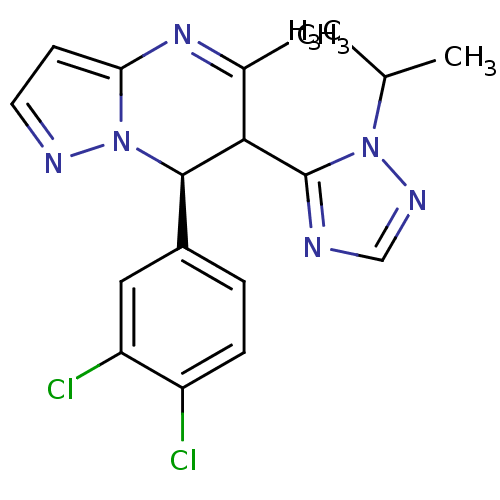 (CHEMBL2331986) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human Kv1.5 expressed in mouse L929 cells assessed as inhibition of delayed rectifier repolarization current by patch clamp as... | Bioorg Med Chem Lett 23: 1743-7 (2013) Article DOI: 10.1016/j.bmcl.2013.01.064 BindingDB Entry DOI: 10.7270/Q2QN685H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 5 (Homo sapiens (Human)) | BDBM50430211 (CHEMBL2331996) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human Kv1.5 expressed in mouse L929 cells assessed as inhibition of delayed rectifier repolarization current by patch clamp as... | Bioorg Med Chem Lett 23: 1743-7 (2013) Article DOI: 10.1016/j.bmcl.2013.01.064 BindingDB Entry DOI: 10.7270/Q2QN685H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 5 (Homo sapiens (Human)) | BDBM50430206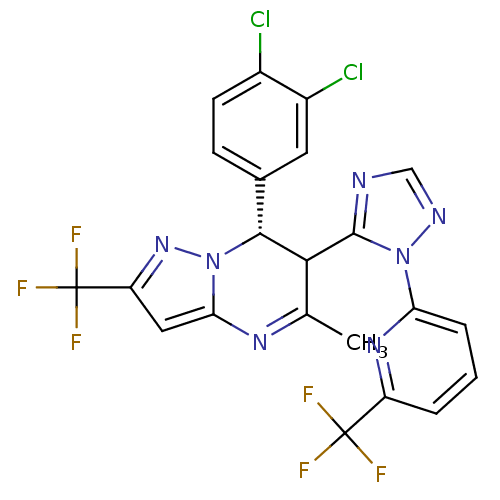 (CHEMBL2332443) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human Kv1.5 expressed in mouse L929 cells assessed as inhibition of delayed rectifier repolarization current by patch clamp as... | Bioorg Med Chem Lett 23: 1743-7 (2013) Article DOI: 10.1016/j.bmcl.2013.01.064 BindingDB Entry DOI: 10.7270/Q2QN685H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 5 (Homo sapiens (Human)) | BDBM50430228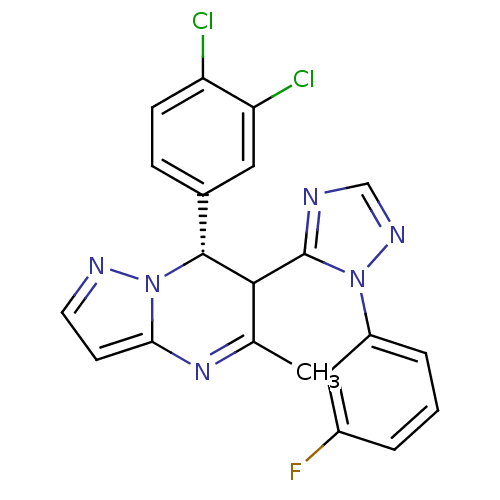 (CHEMBL2332454) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human Kv1.5 expressed in mouse L929 cells assessed as inhibition of delayed rectifier repolarization current by patch clamp as... | Bioorg Med Chem Lett 23: 1743-7 (2013) Article DOI: 10.1016/j.bmcl.2013.01.064 BindingDB Entry DOI: 10.7270/Q2QN685H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily A member 5 (Homo sapiens (Human)) | BDBM50430197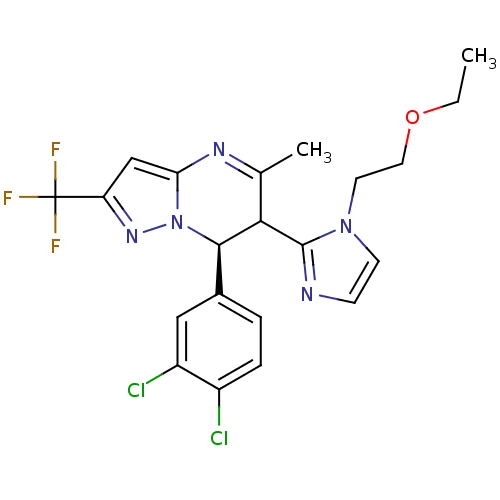 (CHEMBL2332452) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human Kv1.5 expressed in mouse L929 cells assessed as inhibition of delayed rectifier repolarization current by patch clamp as... | Bioorg Med Chem Lett 23: 1743-7 (2013) Article DOI: 10.1016/j.bmcl.2013.01.064 BindingDB Entry DOI: 10.7270/Q2QN685H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 4 (Homo sapiens (Human)) | BDBM461294 (US10774068, Example 1 | US11427560, Example 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
THE SCRIPPS RESEARCH INSTITUTE; UNIVERSITÄT BREMEN US Patent | Assay Description MST1 and MST2 biochemical LanthaScreen Eu Kinase Binding Assay was based on the binding and displacement of kinase tracer to the kinase of interest. ... | US Patent US10774068 (2020) BindingDB Entry DOI: 10.7270/Q2S46W22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 3 (Homo sapiens (Human)) | BDBM461294 (US10774068, Example 1 | US11427560, Example 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
THE SCRIPPS RESEARCH INSTITUTE; UNIVERSITÄT BREMEN US Patent | Assay Description MST1 and MST2 biochemical LanthaScreen Eu Kinase Binding Assay was based on the binding and displacement of kinase tracer to the kinase of interest. ... | US Patent US10774068 (2020) BindingDB Entry DOI: 10.7270/Q2S46W22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 4 (Homo sapiens (Human)) | BDBM461295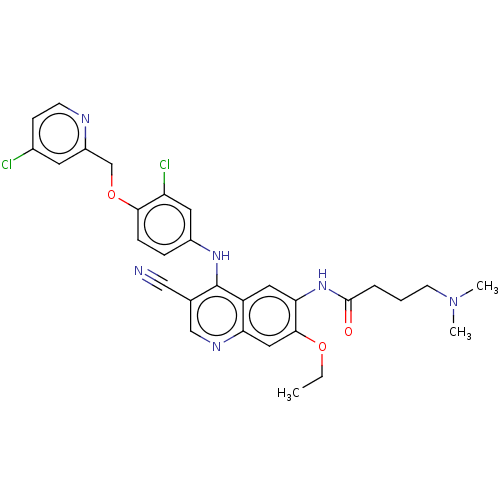 (US10774068, Example 2 | US11427560, Example 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
THE SCRIPPS RESEARCH INSTITUTE; UNIVERSITÄT BREMEN US Patent | Assay Description MST1 and MST2 biochemical LanthaScreen Eu Kinase Binding Assay was based on the binding and displacement of kinase tracer to the kinase of interest. ... | US Patent US10774068 (2020) BindingDB Entry DOI: 10.7270/Q2S46W22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 3 (Homo sapiens (Human)) | BDBM461295 (US10774068, Example 2 | US11427560, Example 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
THE SCRIPPS RESEARCH INSTITUTE; UNIVERSITÄT BREMEN US Patent | Assay Description MST1 and MST2 biochemical LanthaScreen Eu Kinase Binding Assay was based on the binding and displacement of kinase tracer to the kinase of interest. ... | US Patent US10774068 (2020) BindingDB Entry DOI: 10.7270/Q2S46W22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 4 (Homo sapiens (Human)) | BDBM461296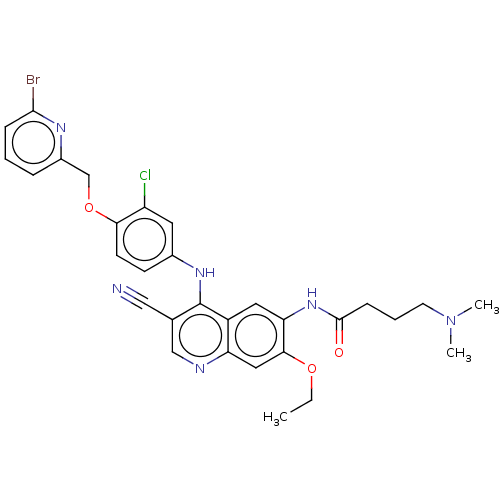 (US10774068, Example 3 | US11427560, Example 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
THE SCRIPPS RESEARCH INSTITUTE; UNIVERSITÄT BREMEN US Patent | Assay Description MST1 and MST2 biochemical LanthaScreen Eu Kinase Binding Assay was based on the binding and displacement of kinase tracer to the kinase of interest. ... | US Patent US10774068 (2020) BindingDB Entry DOI: 10.7270/Q2S46W22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 3 (Homo sapiens (Human)) | BDBM461296 (US10774068, Example 3 | US11427560, Example 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
THE SCRIPPS RESEARCH INSTITUTE; UNIVERSITÄT BREMEN US Patent | Assay Description MST1 and MST2 biochemical LanthaScreen Eu Kinase Binding Assay was based on the binding and displacement of kinase tracer to the kinase of interest. ... | US Patent US10774068 (2020) BindingDB Entry DOI: 10.7270/Q2S46W22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 4 (Homo sapiens (Human)) | BDBM461297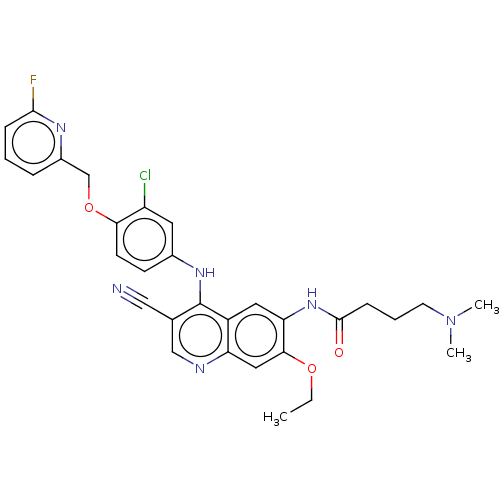 (US10774068, Example 4 | US11427560, Example 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
THE SCRIPPS RESEARCH INSTITUTE; UNIVERSITÄT BREMEN US Patent | Assay Description MST1 and MST2 biochemical LanthaScreen Eu Kinase Binding Assay was based on the binding and displacement of kinase tracer to the kinase of interest. ... | US Patent US10774068 (2020) BindingDB Entry DOI: 10.7270/Q2S46W22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 3 (Homo sapiens (Human)) | BDBM461297 (US10774068, Example 4 | US11427560, Example 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
THE SCRIPPS RESEARCH INSTITUTE; UNIVERSITÄT BREMEN US Patent | Assay Description MST1 and MST2 biochemical LanthaScreen Eu Kinase Binding Assay was based on the binding and displacement of kinase tracer to the kinase of interest. ... | US Patent US10774068 (2020) BindingDB Entry DOI: 10.7270/Q2S46W22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 4 (Homo sapiens (Human)) | BDBM461298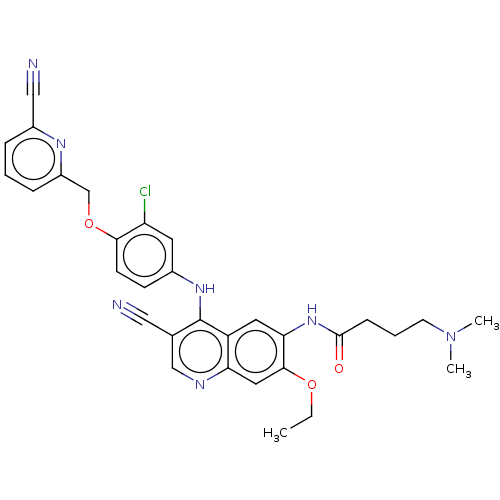 (US10774068, Example 5 | US11427560, Example 5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
THE SCRIPPS RESEARCH INSTITUTE; UNIVERSITÄT BREMEN US Patent | Assay Description MST1 and MST2 biochemical LanthaScreen Eu Kinase Binding Assay was based on the binding and displacement of kinase tracer to the kinase of interest. ... | US Patent US10774068 (2020) BindingDB Entry DOI: 10.7270/Q2S46W22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 4 (Homo sapiens (Human)) | BDBM461300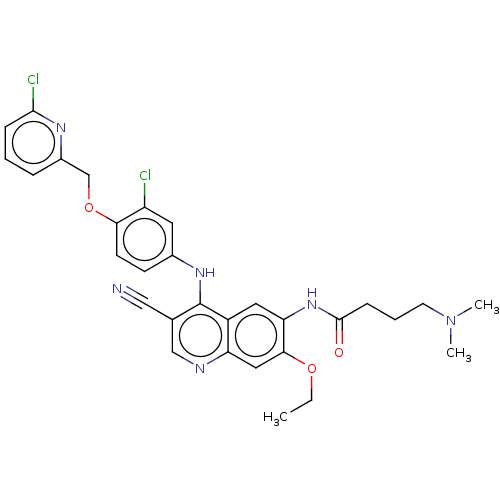 (US10774068, Example 7 | US11427560, Example 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
THE SCRIPPS RESEARCH INSTITUTE; UNIVERSITÄT BREMEN US Patent | Assay Description MST1 and MST2 biochemical LanthaScreen Eu Kinase Binding Assay was based on the binding and displacement of kinase tracer to the kinase of interest. ... | US Patent US10774068 (2020) BindingDB Entry DOI: 10.7270/Q2S46W22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 3 (Homo sapiens (Human)) | BDBM461300 (US10774068, Example 7 | US11427560, Example 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
THE SCRIPPS RESEARCH INSTITUTE; UNIVERSITÄT BREMEN US Patent | Assay Description MST1 and MST2 biochemical LanthaScreen Eu Kinase Binding Assay was based on the binding and displacement of kinase tracer to the kinase of interest. ... | US Patent US10774068 (2020) BindingDB Entry DOI: 10.7270/Q2S46W22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 4 (Homo sapiens (Human)) | BDBM461301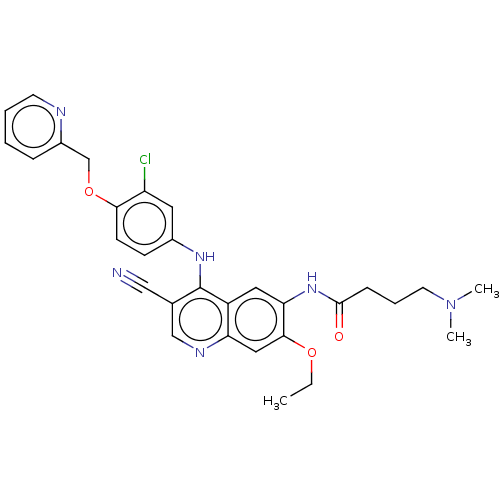 (US10774068, Example 107 | US10774068, Example 8 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
THE SCRIPPS RESEARCH INSTITUTE; UNIVERSITÄT BREMEN US Patent | Assay Description MST1 and MST2 biochemical LanthaScreen Eu Kinase Binding Assay was based on the binding and displacement of kinase tracer to the kinase of interest. ... | US Patent US10774068 (2020) BindingDB Entry DOI: 10.7270/Q2S46W22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 4 (Homo sapiens (Human)) | BDBM461304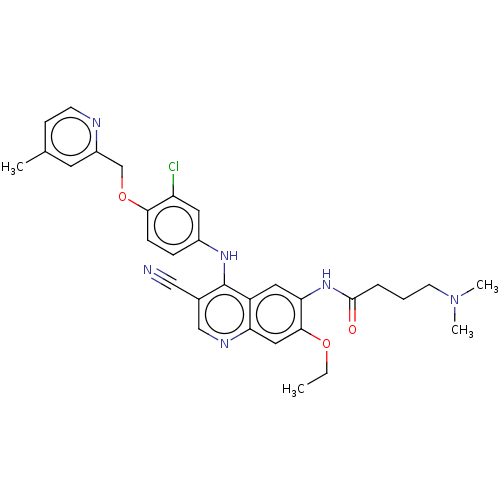 (US10774068, Example 10 | US11427560, Example 10) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
THE SCRIPPS RESEARCH INSTITUTE; UNIVERSITÄT BREMEN US Patent | Assay Description MST1 and MST2 biochemical LanthaScreen Eu Kinase Binding Assay was based on the binding and displacement of kinase tracer to the kinase of interest. ... | US Patent US10774068 (2020) BindingDB Entry DOI: 10.7270/Q2S46W22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 3 (Homo sapiens (Human)) | BDBM461305 (US10774068, Example 11 | US11427560, Example 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
THE SCRIPPS RESEARCH INSTITUTE; UNIVERSITÄT BREMEN US Patent | Assay Description MST1 and MST2 biochemical LanthaScreen Eu Kinase Binding Assay was based on the binding and displacement of kinase tracer to the kinase of interest. ... | US Patent US10774068 (2020) BindingDB Entry DOI: 10.7270/Q2S46W22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 4 (Homo sapiens (Human)) | BDBM461306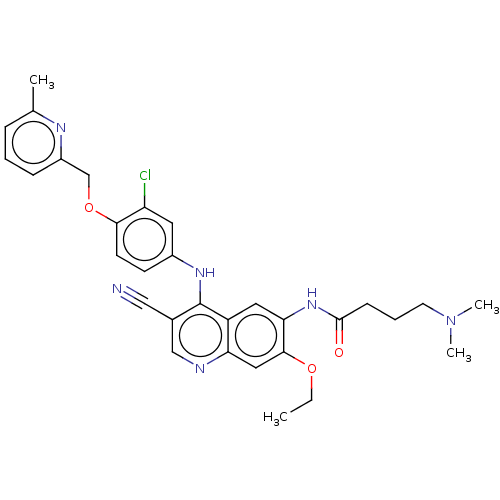 (US10774068, Example 12 | US11427560, Example 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
THE SCRIPPS RESEARCH INSTITUTE; UNIVERSITÄT BREMEN US Patent | Assay Description MST1 and MST2 biochemical LanthaScreen Eu Kinase Binding Assay was based on the binding and displacement of kinase tracer to the kinase of interest. ... | US Patent US10774068 (2020) BindingDB Entry DOI: 10.7270/Q2S46W22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 3 (Homo sapiens (Human)) | BDBM461306 (US10774068, Example 12 | US11427560, Example 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
THE SCRIPPS RESEARCH INSTITUTE; UNIVERSITÄT BREMEN US Patent | Assay Description MST1 and MST2 biochemical LanthaScreen Eu Kinase Binding Assay was based on the binding and displacement of kinase tracer to the kinase of interest. ... | US Patent US10774068 (2020) BindingDB Entry DOI: 10.7270/Q2S46W22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 629 total ) | Next | Last >> |