 Found 195 hits with Last Name = 'suleman' and Initial = 'a'
Found 195 hits with Last Name = 'suleman' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50116590
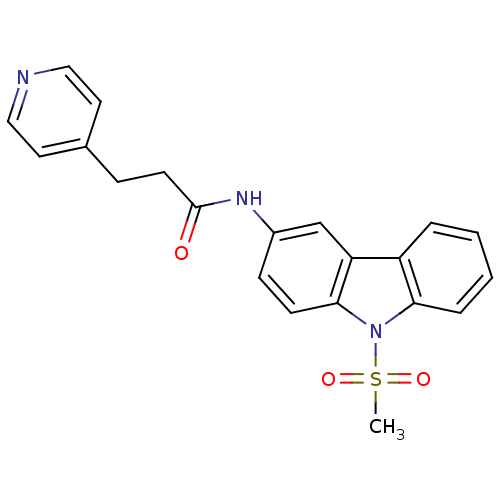
(CHEMBL325486 | N-(9-Methanesulfonyl-9H-carbazol-3-...)Show SMILES CS(=O)(=O)n1c2ccccc2c2cc(NC(=O)CCc3ccncc3)ccc12 Show InChI InChI=1S/C21H19N3O3S/c1-28(26,27)24-19-5-3-2-4-17(19)18-14-16(7-8-20(18)24)23-21(25)9-6-15-10-12-22-13-11-15/h2-5,7-8,10-14H,6,9H2,1H3,(H,23,25) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) |
J Med Chem 45: 3509-23 (2002)
BindingDB Entry DOI: 10.7270/Q27S7N3K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50116610
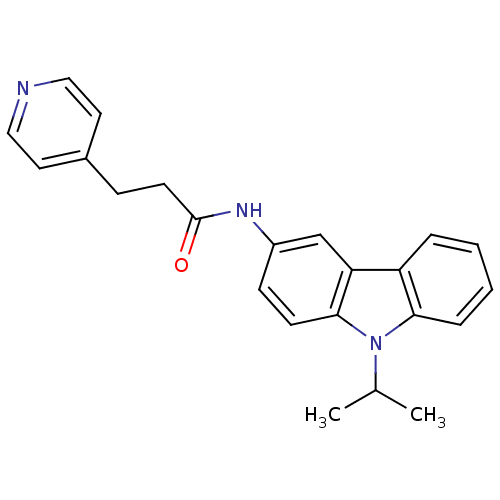
(CHEMBL119743 | N-(9-Isopropyl-9H-carbazol-3-yl)-3-...)Show InChI InChI=1S/C23H23N3O/c1-16(2)26-21-6-4-3-5-19(21)20-15-18(8-9-22(20)26)25-23(27)10-7-17-11-13-24-14-12-17/h3-6,8-9,11-16H,7,10H2,1-2H3,(H,25,27) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) |
J Med Chem 45: 3509-23 (2002)
BindingDB Entry DOI: 10.7270/Q27S7N3K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50116619
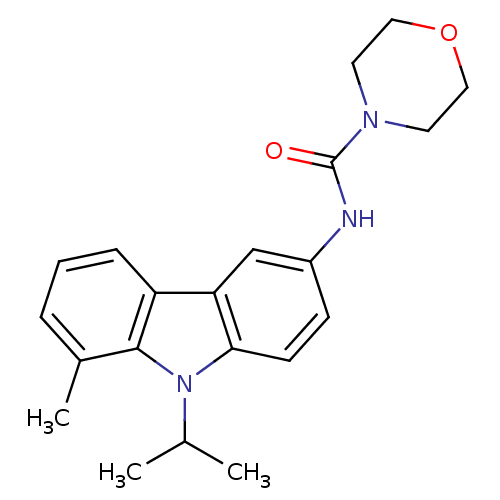
(CHEMBL117563 | Morpholine-4-carboxylic acid (9-iso...)Show SMILES CC(C)n1c2ccc(NC(=O)N3CCOCC3)cc2c2cccc(C)c12 Show InChI InChI=1S/C21H25N3O2/c1-14(2)24-19-8-7-16(22-21(25)23-9-11-26-12-10-23)13-18(19)17-6-4-5-15(3)20(17)24/h4-8,13-14H,9-12H2,1-3H3,(H,22,25) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) |
J Med Chem 45: 3509-23 (2002)
BindingDB Entry DOI: 10.7270/Q27S7N3K |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50371358
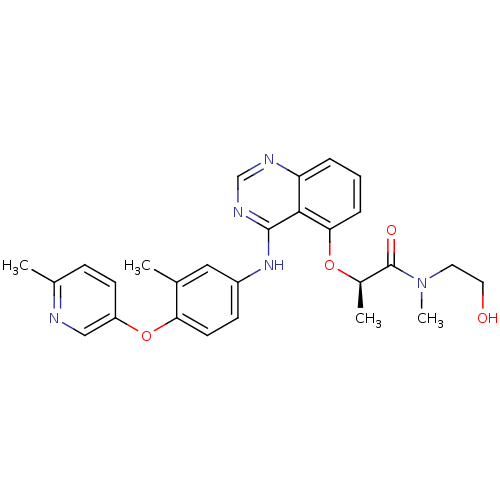
(CHEMBL257478)Show SMILES C[C@@H](Oc1cccc2ncnc(Nc3ccc(Oc4ccc(C)nc4)c(C)c3)c12)C(=O)N(C)CCO Show InChI InChI=1S/C27H29N5O4/c1-17-14-20(9-11-23(17)36-21-10-8-18(2)28-15-21)31-26-25-22(29-16-30-26)6-5-7-24(25)35-19(3)27(34)32(4)12-13-33/h5-11,14-16,19,33H,12-13H2,1-4H3,(H,29,30,31)/t19-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of ebrB2 |
Bioorg Med Chem Lett 18: 674-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.052
BindingDB Entry DOI: 10.7270/Q2736RSH |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50222489

((R)-N-(2-(4-(3-chloro-4-(pyridin-2-ylmethoxy)pheny...)Show SMILES C[C@H](CN(C)C(=O)CO)Oc1cccc2ncnc(Nc3ccc(OCc4ccccn4)c(Cl)c3)c12 Show InChI InChI=1S/C26H26ClN5O4/c1-17(13-32(2)24(34)14-33)36-23-8-5-7-21-25(23)26(30-16-29-21)31-18-9-10-22(20(27)12-18)35-15-19-6-3-4-11-28-19/h3-12,16-17,33H,13-15H2,1-2H3,(H,29,30,31)/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 17: 6326-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.073
BindingDB Entry DOI: 10.7270/Q2CF9PTZ |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50222495

((S)-1-(2-((4-(3-chloro-4-(pyridin-2-ylmethoxy)phen...)Show SMILES OCC(=O)N1CCC[C@H]1COc1cccc2ncnc(Nc3ccc(OCc4ccccn4)c(Cl)c3)c12 Show InChI InChI=1S/C27H26ClN5O4/c28-21-13-18(9-10-23(21)36-15-19-5-1-2-11-29-19)32-27-26-22(30-17-31-27)7-3-8-24(26)37-16-20-6-4-12-33(20)25(35)14-34/h1-3,5,7-11,13,17,20,34H,4,6,12,14-16H2,(H,30,31,32)/t20-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 kinase |
Bioorg Med Chem Lett 17: 6326-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.073
BindingDB Entry DOI: 10.7270/Q2CF9PTZ |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50116602
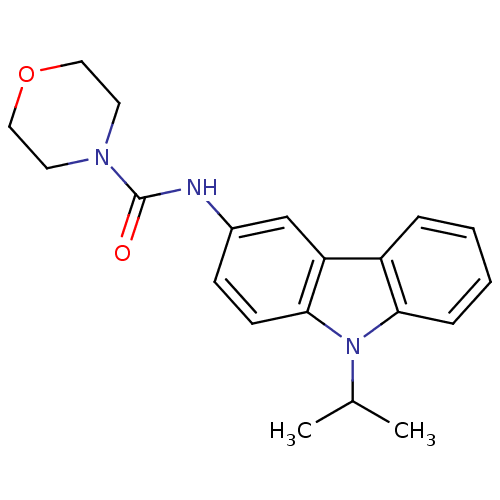
(CHEMBL419951 | Morpholine-4-carboxylic acid (9-iso...)Show InChI InChI=1S/C20H23N3O2/c1-14(2)23-18-6-4-3-5-16(18)17-13-15(7-8-19(17)23)21-20(24)22-9-11-25-12-10-22/h3-8,13-14H,9-12H2,1-2H3,(H,21,24) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) |
J Med Chem 45: 3509-23 (2002)
BindingDB Entry DOI: 10.7270/Q27S7N3K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50116600
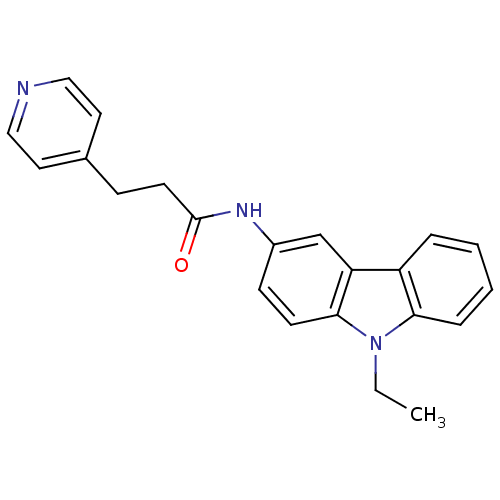
(CHEMBL325475 | N-(9-Ethyl-9H-carbazol-3-yl)-3-pyri...)Show InChI InChI=1S/C22H21N3O/c1-2-25-20-6-4-3-5-18(20)19-15-17(8-9-21(19)25)24-22(26)10-7-16-11-13-23-14-12-16/h3-6,8-9,11-15H,2,7,10H2,1H3,(H,24,26) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) |
J Med Chem 45: 3509-23 (2002)
BindingDB Entry DOI: 10.7270/Q27S7N3K |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50222493

((R)-N-(1-(4-(3-chloro-4-(pyridin-2-ylmethoxy)pheny...)Show SMILES C[C@H](COc1cccc2ncnc(Nc3ccc(OCc4ccccn4)c(Cl)c3)c12)N(C)C(=O)CO Show InChI InChI=1S/C26H26ClN5O4/c1-17(32(2)24(34)13-33)14-35-23-8-5-7-21-25(23)26(30-16-29-21)31-18-9-10-22(20(27)12-18)36-15-19-6-3-4-11-28-19/h3-12,16-17,33H,13-15H2,1-2H3,(H,29,30,31)/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 17: 6326-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.073
BindingDB Entry DOI: 10.7270/Q2CF9PTZ |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50116617
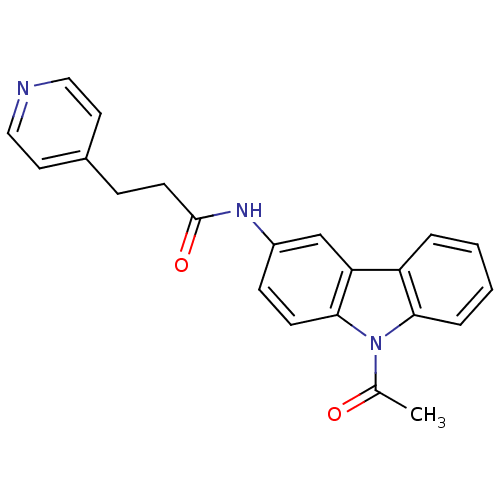
(CHEMBL116210 | N-(9-Acetyl-9H-carbazol-3-yl)-3-pyr...)Show SMILES CC(=O)n1c2ccccc2c2cc(NC(=O)CCc3ccncc3)ccc12 Show InChI InChI=1S/C22H19N3O2/c1-15(26)25-20-5-3-2-4-18(20)19-14-17(7-8-21(19)25)24-22(27)9-6-16-10-12-23-13-11-16/h2-5,7-8,10-14H,6,9H2,1H3,(H,24,27) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) |
J Med Chem 45: 3509-23 (2002)
BindingDB Entry DOI: 10.7270/Q27S7N3K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50116605
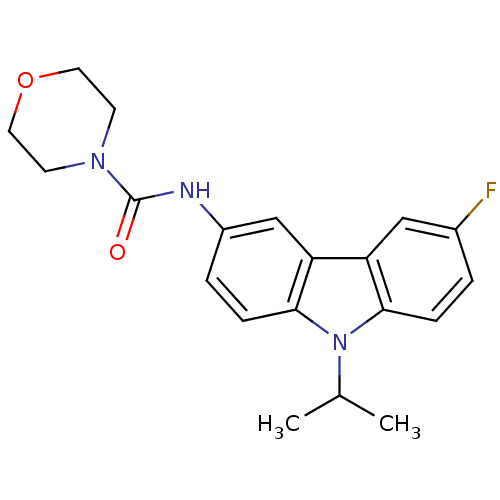
(CHEMBL432628 | Morpholine-4-carboxylic acid (6-flu...)Show SMILES CC(C)n1c2ccc(F)cc2c2cc(NC(=O)N3CCOCC3)ccc12 Show InChI InChI=1S/C20H22FN3O2/c1-13(2)24-18-5-3-14(21)11-16(18)17-12-15(4-6-19(17)24)22-20(25)23-7-9-26-10-8-23/h3-6,11-13H,7-10H2,1-2H3,(H,22,25) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) |
J Med Chem 45: 3509-23 (2002)
BindingDB Entry DOI: 10.7270/Q27S7N3K |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50427322
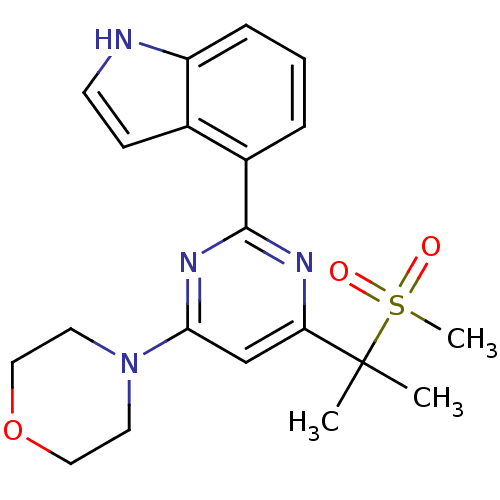
(CHEMBL2325703)Show SMILES CC(C)(c1cc(nc(n1)-c1cccc2[nH]ccc12)N1CCOCC1)S(C)(=O)=O Show InChI InChI=1S/C20H24N4O3S/c1-20(2,28(3,25)26)17-13-18(24-9-11-27-12-10-24)23-19(22-17)15-5-4-6-16-14(15)7-8-21-16/h4-8,13,21H,9-12H2,1-3H3 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATR-mediated CHK1 phosphorylation at serine 345 in human HT29 cells after 1 hr in presence of 4-nitroquinoline 1-oxide |
J Med Chem 56: 2125-38 (2013)
Article DOI: 10.1021/jm301859s
BindingDB Entry DOI: 10.7270/Q2VH5Q5C |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50116592
(CHEMBL325226 | Morpholine-4-carboxylic acid (9-iso...)Show SMILES CC(C)n1c2ccccc2c2c(C)c(NC(=O)N3CCOCC3)ccc12 Show InChI InChI=1S/C21H25N3O2/c1-14(2)24-18-7-5-4-6-16(18)20-15(3)17(8-9-19(20)24)22-21(25)23-10-12-26-13-11-23/h4-9,14H,10-13H2,1-3H3,(H,22,25) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) |
J Med Chem 45: 3509-23 (2002)
BindingDB Entry DOI: 10.7270/Q27S7N3K |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50222491

(CHEMBL249928 | N-(2-(4-(3-chloro-4-(pyridin-2-ylme...)Show SMILES CN(CCOc1cccc2ncnc(Nc3ccc(OCc4ccccn4)c(Cl)c3)c12)C(=O)CO Show InChI InChI=1S/C25H24ClN5O4/c1-31(23(33)14-32)11-12-34-22-7-4-6-20-24(22)25(29-16-28-20)30-17-8-9-21(19(26)13-17)35-15-18-5-2-3-10-27-18/h2-10,13,16,32H,11-12,14-15H2,1H3,(H,28,29,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 kinase |
Bioorg Med Chem Lett 17: 6326-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.073
BindingDB Entry DOI: 10.7270/Q2CF9PTZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50427320

(CHEMBL2325705)Show SMILES CS(=O)(=O)C1(CCOCC1)c1cc(nc(n1)-c1cccc2[nH]ccc12)N1CCOCC1 Show InChI InChI=1S/C22H26N4O4S/c1-31(27,28)22(6-11-29-12-7-22)19-15-20(26-9-13-30-14-10-26)25-21(24-19)17-3-2-4-18-16(17)5-8-23-18/h2-5,8,15,23H,6-7,9-14H2,1H3 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATR-mediated CHK1 phosphorylation at serine 345 in human HT29 cells after 1 hr in presence of 4-nitroquinoline 1-oxide |
J Med Chem 56: 2125-38 (2013)
Article DOI: 10.1021/jm301859s
BindingDB Entry DOI: 10.7270/Q2VH5Q5C |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50427326
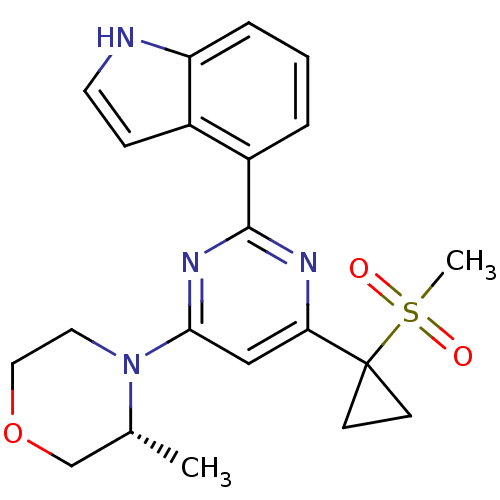
(CHEMBL2325697)Show SMILES C[C@@H]1COCCN1c1cc(nc(n1)-c1cccc2[nH]ccc12)C1(CC1)S(C)(=O)=O |r| Show InChI InChI=1S/C21H24N4O3S/c1-14-13-28-11-10-25(14)19-12-18(21(7-8-21)29(2,26)27)23-20(24-19)16-4-3-5-17-15(16)6-9-22-17/h3-6,9,12,14,22H,7-8,10-11,13H2,1-2H3/t14-/m1/s1 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATR in human HeLa cell nuclear extracts using glutathione S-transferase-p53N66 and ATP as substrate incubated for 10 mins prior to ATP ... |
J Med Chem 56: 2125-38 (2013)
Article DOI: 10.1021/jm301859s
BindingDB Entry DOI: 10.7270/Q2VH5Q5C |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50427314

(CHEMBL2325711)Show SMILES O=S(=O)(c1ccccc1)C1(CC1)c1cc(nc(n1)-c1cccc2[nH]ccc12)N1CCOCC1 Show InChI InChI=1S/C25H24N4O3S/c30-33(31,18-5-2-1-3-6-18)25(10-11-25)22-17-23(29-13-15-32-16-14-29)28-24(27-22)20-7-4-8-21-19(20)9-12-26-21/h1-9,12,17,26H,10-11,13-16H2 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATR-mediated CHK1 phosphorylation at serine 345 in human HT29 cells after 1 hr in presence of 4-nitroquinoline 1-oxide |
J Med Chem 56: 2125-38 (2013)
Article DOI: 10.1021/jm301859s
BindingDB Entry DOI: 10.7270/Q2VH5Q5C |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50116592

(CHEMBL325226 | Morpholine-4-carboxylic acid (9-iso...)Show SMILES CC(C)n1c2ccccc2c2c(C)c(NC(=O)N3CCOCC3)ccc12 Show InChI InChI=1S/C21H25N3O2/c1-14(2)24-18-7-5-4-6-16(18)20-15(3)17(8-9-19(20)24)22-21(25)23-10-12-26-13-11-23/h4-9,14H,10-13H2,1-3H3,(H,22,25) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Compound was evaluated for functional antagonism of Neuropeptide Y receptor Y5 activity in cellular Ca flux |
J Med Chem 45: 3509-23 (2002)
BindingDB Entry DOI: 10.7270/Q27S7N3K |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50427327

(CHEMBL2325699)Show SMILES CS(=O)(=O)C1(CC1)c1cc(nc(n1)-c1cccc2[nH]ccc12)N1CC2CCC(C1)O2 |TLB:9:22:29:25.26| Show InChI InChI=1S/C22H24N4O3S/c1-30(27,28)22(8-9-22)19-11-20(26-12-14-5-6-15(13-26)29-14)25-21(24-19)17-3-2-4-18-16(17)7-10-23-18/h2-4,7,10-11,14-15,23H,5-6,8-9,12-13H2,1H3 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATR-mediated CHK1 phosphorylation at serine 345 in human HT29 cells after 1 hr in presence of 4-nitroquinoline 1-oxide |
J Med Chem 56: 2125-38 (2013)
Article DOI: 10.1021/jm301859s
BindingDB Entry DOI: 10.7270/Q2VH5Q5C |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Rat 6B) | BDBM50116600

(CHEMBL325475 | N-(9-Ethyl-9H-carbazol-3-yl)-3-pyri...)Show InChI InChI=1S/C22H21N3O/c1-2-25-20-6-4-3-5-18(20)19-15-17(8-9-21(19)25)24-22(26)10-7-16-11-13-23-14-12-16/h3-6,8-9,11-15H,2,7,10H2,1H3,(H,24,26) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity against rat Neuropeptide Y receptor Y5 |
J Med Chem 45: 3509-23 (2002)
BindingDB Entry DOI: 10.7270/Q27S7N3K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50116597
(CHEMBL119247 | Morpholine-4-carboxylic acid (9-eth...)Show InChI InChI=1S/C19H21N3O2/c1-2-22-17-6-4-3-5-15(17)16-13-14(7-8-18(16)22)20-19(23)21-9-11-24-12-10-21/h3-8,13H,2,9-12H2,1H3,(H,20,23) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) |
J Med Chem 45: 3509-23 (2002)
BindingDB Entry DOI: 10.7270/Q27S7N3K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50116608
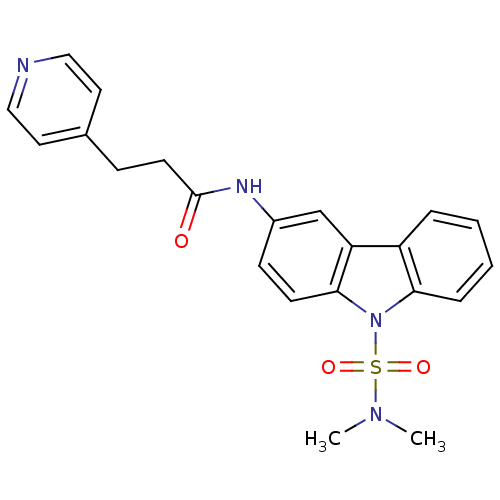
(CHEMBL117922 | N-(9-Dimethylsulfamoyl-9H-carbazol-...)Show SMILES CN(C)S(=O)(=O)n1c2ccccc2c2cc(NC(=O)CCc3ccncc3)ccc12 Show InChI InChI=1S/C22H22N4O3S/c1-25(2)30(28,29)26-20-6-4-3-5-18(20)19-15-17(8-9-21(19)26)24-22(27)10-7-16-11-13-23-14-12-16/h3-6,8-9,11-15H,7,10H2,1-2H3,(H,24,27) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) |
J Med Chem 45: 3509-23 (2002)
BindingDB Entry DOI: 10.7270/Q27S7N3K |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50427305
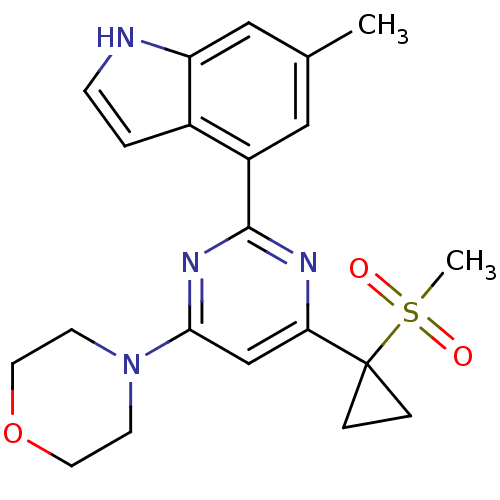
(CHEMBL2325720)Show SMILES Cc1cc(-c2nc(cc(n2)C2(CC2)S(C)(=O)=O)N2CCOCC2)c2cc[nH]c2c1 Show InChI InChI=1S/C21H24N4O3S/c1-14-11-16(15-3-6-22-17(15)12-14)20-23-18(21(4-5-21)29(2,26)27)13-19(24-20)25-7-9-28-10-8-25/h3,6,11-13,22H,4-5,7-10H2,1-2H3 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATR in human HeLa cell nuclear extracts using glutathione S-transferase-p53N66 and ATP as substrate incubated for 10 mins prior to ATP ... |
J Med Chem 56: 2125-38 (2013)
Article DOI: 10.1021/jm301859s
BindingDB Entry DOI: 10.7270/Q2VH5Q5C |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50427321
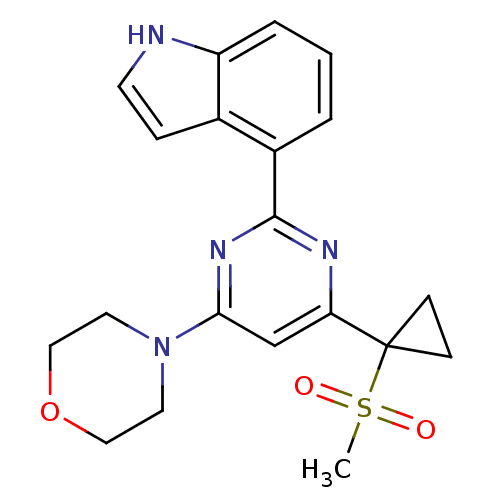
(CHEMBL2325704)Show SMILES CS(=O)(=O)C1(CC1)c1cc(nc(n1)-c1cccc2[nH]ccc12)N1CCOCC1 Show InChI InChI=1S/C20H22N4O3S/c1-28(25,26)20(6-7-20)17-13-18(24-9-11-27-12-10-24)23-19(22-17)15-3-2-4-16-14(15)5-8-21-16/h2-5,8,13,21H,6-7,9-12H2,1H3 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATR-mediated CHK1 phosphorylation at serine 345 in human HT29 cells after 1 hr in presence of 4-nitroquinoline 1-oxide |
J Med Chem 56: 2125-38 (2013)
Article DOI: 10.1021/jm301859s
BindingDB Entry DOI: 10.7270/Q2VH5Q5C |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50427319
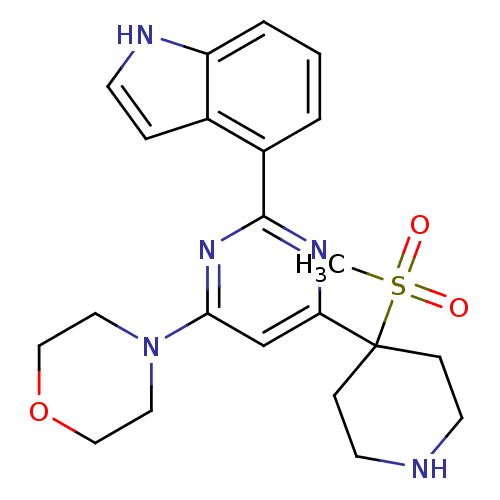
(CHEMBL2325706)Show SMILES CS(=O)(=O)C1(CCNCC1)c1cc(nc(n1)-c1cccc2[nH]ccc12)N1CCOCC1 Show InChI InChI=1S/C22H27N5O3S/c1-31(28,29)22(6-9-23-10-7-22)19-15-20(27-11-13-30-14-12-27)26-21(25-19)17-3-2-4-18-16(17)5-8-24-18/h2-5,8,15,23-24H,6-7,9-14H2,1H3 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATR-mediated CHK1 phosphorylation at serine 345 in human HT29 cells after 1 hr in presence of 4-nitroquinoline 1-oxide |
J Med Chem 56: 2125-38 (2013)
Article DOI: 10.1021/jm301859s
BindingDB Entry DOI: 10.7270/Q2VH5Q5C |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50222493

((R)-N-(1-(4-(3-chloro-4-(pyridin-2-ylmethoxy)pheny...)Show SMILES C[C@H](COc1cccc2ncnc(Nc3ccc(OCc4ccccn4)c(Cl)c3)c12)N(C)C(=O)CO Show InChI InChI=1S/C26H26ClN5O4/c1-17(32(2)24(34)13-33)14-35-23-8-5-7-21-25(23)26(30-16-29-21)31-18-9-10-22(20(27)12-18)36-15-19-6-3-4-11-28-19/h3-12,16-17,33H,13-15H2,1-2H3,(H,29,30,31)/t17-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Erb2 autophosphorylation by cellular clone 24 assay |
Bioorg Med Chem Lett 17: 6326-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.073
BindingDB Entry DOI: 10.7270/Q2CF9PTZ |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50222489

((R)-N-(2-(4-(3-chloro-4-(pyridin-2-ylmethoxy)pheny...)Show SMILES C[C@H](CN(C)C(=O)CO)Oc1cccc2ncnc(Nc3ccc(OCc4ccccn4)c(Cl)c3)c12 Show InChI InChI=1S/C26H26ClN5O4/c1-17(13-32(2)24(34)14-33)36-23-8-5-7-21-25(23)26(30-16-29-21)31-18-9-10-22(20(27)12-18)35-15-19-6-3-4-11-28-19/h3-12,16-17,33H,13-15H2,1-2H3,(H,29,30,31)/t17-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Erb2 autophosphorylation by cellular clone 24 assay |
Bioorg Med Chem Lett 17: 6326-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.073
BindingDB Entry DOI: 10.7270/Q2CF9PTZ |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50116600

(CHEMBL325475 | N-(9-Ethyl-9H-carbazol-3-yl)-3-pyri...)Show InChI InChI=1S/C22H21N3O/c1-2-25-20-6-4-3-5-18(20)19-15-17(8-9-21(19)25)24-22(26)10-7-16-11-13-23-14-12-16/h3-6,8-9,11-15H,2,7,10H2,1H3,(H,24,26) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for human Neuropeptide Y receptor Y5 functional antagonism (reporter gene assay) |
J Med Chem 45: 3509-23 (2002)
BindingDB Entry DOI: 10.7270/Q27S7N3K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50116607
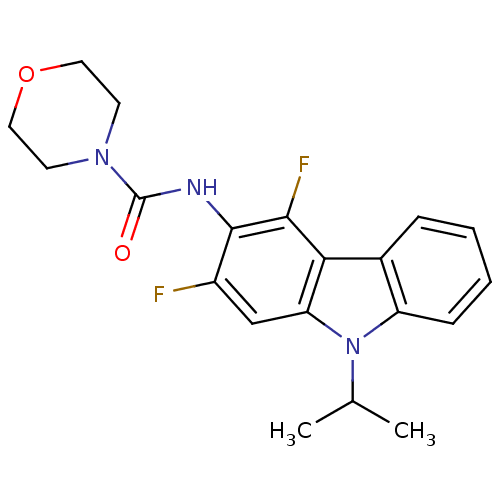
(CHEMBL325639 | Morpholine-4-carboxylic acid (2,4-d...)Show SMILES CC(C)n1c2ccccc2c2c(F)c(NC(=O)N3CCOCC3)c(F)cc12 Show InChI InChI=1S/C20H21F2N3O2/c1-12(2)25-15-6-4-3-5-13(15)17-16(25)11-14(21)19(18(17)22)23-20(26)24-7-9-27-10-8-24/h3-6,11-12H,7-10H2,1-2H3,(H,23,26) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) |
J Med Chem 45: 3509-23 (2002)
BindingDB Entry DOI: 10.7270/Q27S7N3K |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50427317
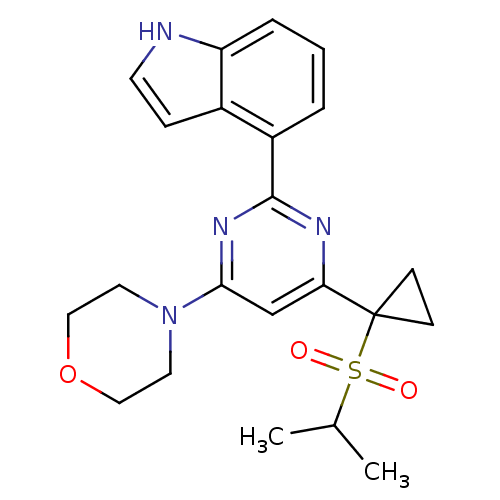
(CHEMBL2325708)Show SMILES CC(C)S(=O)(=O)C1(CC1)c1cc(nc(n1)-c1cccc2[nH]ccc12)N1CCOCC1 Show InChI InChI=1S/C22H26N4O3S/c1-15(2)30(27,28)22(7-8-22)19-14-20(26-10-12-29-13-11-26)25-21(24-19)17-4-3-5-18-16(17)6-9-23-18/h3-6,9,14-15,23H,7-8,10-13H2,1-2H3 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATR-mediated CHK1 phosphorylation at serine 345 in human HT29 cells after 1 hr in presence of 4-nitroquinoline 1-oxide |
J Med Chem 56: 2125-38 (2013)
Article DOI: 10.1021/jm301859s
BindingDB Entry DOI: 10.7270/Q2VH5Q5C |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50116603
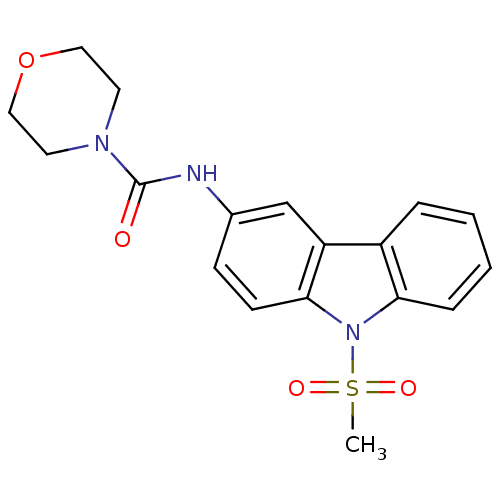
(CHEMBL443809 | Morpholine-4-carboxylic acid (9-met...)Show SMILES CS(=O)(=O)n1c2ccccc2c2cc(NC(=O)N3CCOCC3)ccc12 Show InChI InChI=1S/C18H19N3O4S/c1-26(23,24)21-16-5-3-2-4-14(16)15-12-13(6-7-17(15)21)19-18(22)20-8-10-25-11-9-20/h2-7,12H,8-11H2,1H3,(H,19,22) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) |
J Med Chem 45: 3509-23 (2002)
BindingDB Entry DOI: 10.7270/Q27S7N3K |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50427297
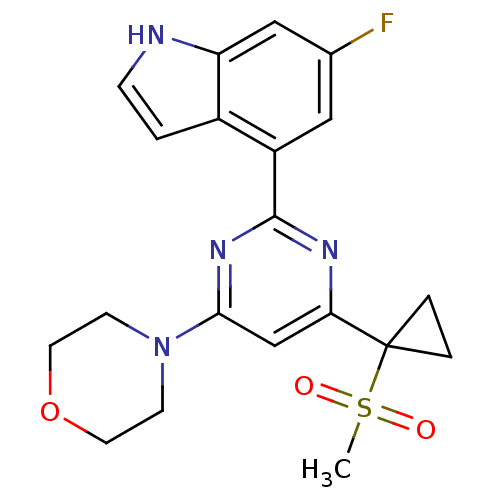
(CHEMBL2325439)Show SMILES CS(=O)(=O)C1(CC1)c1cc(nc(n1)-c1cc(F)cc2[nH]ccc12)N1CCOCC1 Show InChI InChI=1S/C20H21FN4O3S/c1-29(26,27)20(3-4-20)17-12-18(25-6-8-28-9-7-25)24-19(23-17)15-10-13(21)11-16-14(15)2-5-22-16/h2,5,10-12,22H,3-4,6-9H2,1H3 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATR-mediated CHK1 phosphorylation at serine 345 in human HT29 cells after 1 hr in presence of 4-nitroquinoline 1-oxide |
J Med Chem 56: 2125-38 (2013)
Article DOI: 10.1021/jm301859s
BindingDB Entry DOI: 10.7270/Q2VH5Q5C |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50427296
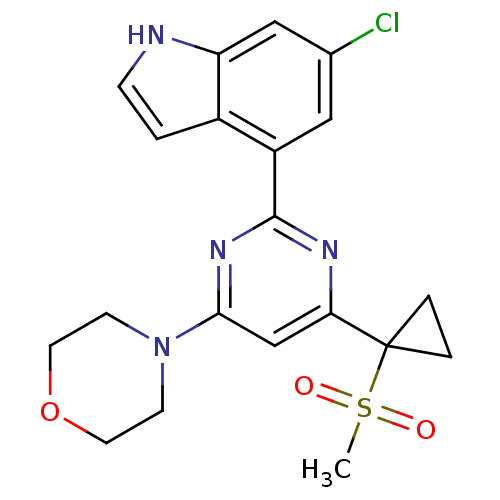
(CHEMBL2325440)Show SMILES CS(=O)(=O)C1(CC1)c1cc(nc(n1)-c1cc(Cl)cc2[nH]ccc12)N1CCOCC1 Show InChI InChI=1S/C20H21ClN4O3S/c1-29(26,27)20(3-4-20)17-12-18(25-6-8-28-9-7-25)24-19(23-17)15-10-13(21)11-16-14(15)2-5-22-16/h2,5,10-12,22H,3-4,6-9H2,1H3 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATR-mediated CHK1 phosphorylation at serine 345 in human HT29 cells after 1 hr in presence of 4-nitroquinoline 1-oxide |
J Med Chem 56: 2125-38 (2013)
Article DOI: 10.1021/jm301859s
BindingDB Entry DOI: 10.7270/Q2VH5Q5C |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50371359
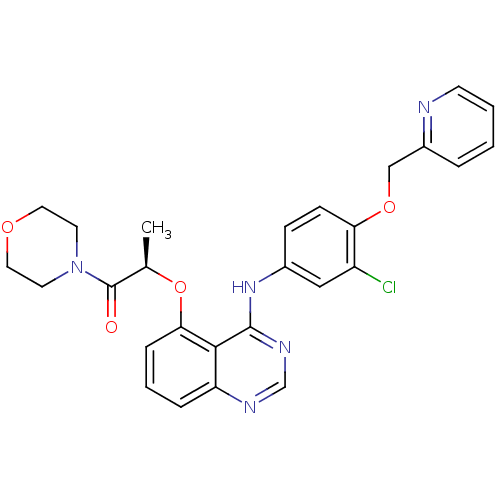
(CHEMBL403774)Show SMILES C[C@@H](Oc1cccc2ncnc(Nc3ccc(OCc4ccccn4)c(Cl)c3)c12)C(=O)N1CCOCC1 Show InChI InChI=1S/C27H26ClN5O4/c1-18(27(34)33-11-13-35-14-12-33)37-24-7-4-6-22-25(24)26(31-17-30-22)32-19-8-9-23(21(28)15-19)36-16-20-5-2-3-10-29-20/h2-10,15,17-18H,11-14,16H2,1H3,(H,30,31,32)/t18-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of ebrB2 expressed in human MCF7 cells by autophosphorylation assay |
Bioorg Med Chem Lett 18: 674-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.052
BindingDB Entry DOI: 10.7270/Q2736RSH |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50222492
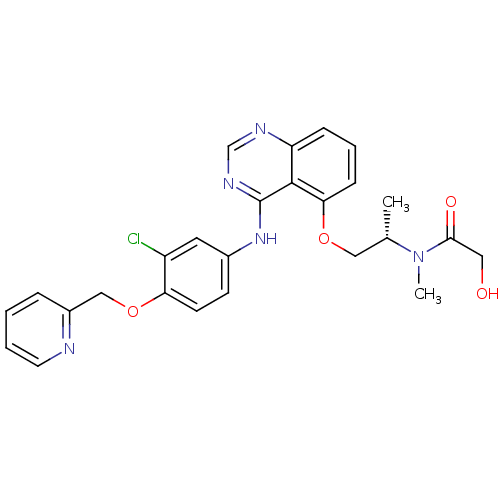
((S)-N-(1-(4-(3-chloro-4-(pyridin-2-ylmethoxy)pheny...)Show SMILES C[C@@H](COc1cccc2ncnc(Nc3ccc(OCc4ccccn4)c(Cl)c3)c12)N(C)C(=O)CO Show InChI InChI=1S/C26H26ClN5O4/c1-17(32(2)24(34)13-33)14-35-23-8-5-7-21-25(23)26(30-16-29-21)31-18-9-10-22(20(27)12-18)36-15-19-6-3-4-11-28-19/h3-12,16-17,33H,13-15H2,1-2H3,(H,29,30,31)/t17-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 kinase |
Bioorg Med Chem Lett 17: 6326-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.073
BindingDB Entry DOI: 10.7270/Q2CF9PTZ |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50222494
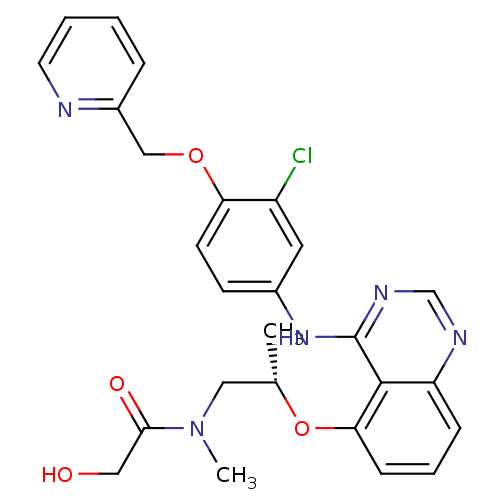
((S)-N-(2-(4-(3-chloro-4-(pyridin-2-ylmethoxy)pheny...)Show SMILES C[C@@H](CN(C)C(=O)CO)Oc1cccc2ncnc(Nc3ccc(OCc4ccccn4)c(Cl)c3)c12 Show InChI InChI=1S/C26H26ClN5O4/c1-17(13-32(2)24(34)14-33)36-23-8-5-7-21-25(23)26(30-16-29-21)31-18-9-10-22(20(27)12-18)35-15-19-6-3-4-11-28-19/h3-12,16-17,33H,13-15H2,1-2H3,(H,29,30,31)/t17-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 kinase |
Bioorg Med Chem Lett 17: 6326-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.073
BindingDB Entry DOI: 10.7270/Q2CF9PTZ |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50371373
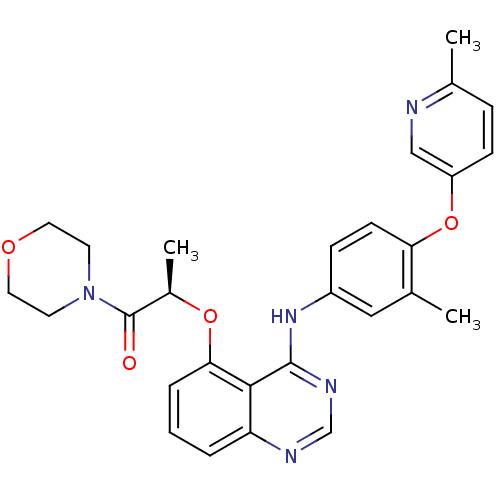
(CHEMBL402507)Show SMILES C[C@@H](Oc1cccc2ncnc(Nc3ccc(Oc4ccc(C)nc4)c(C)c3)c12)C(=O)N1CCOCC1 Show InChI InChI=1S/C28H29N5O4/c1-18-15-21(8-10-24(18)37-22-9-7-19(2)29-16-22)32-27-26-23(30-17-31-27)5-4-6-25(26)36-20(3)28(34)33-11-13-35-14-12-33/h4-10,15-17,20H,11-14H2,1-3H3,(H,30,31,32)/t20-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of ebrB2 expressed in human MCF7 cells by autophosphorylation assay |
Bioorg Med Chem Lett 18: 674-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.052
BindingDB Entry DOI: 10.7270/Q2736RSH |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50371364
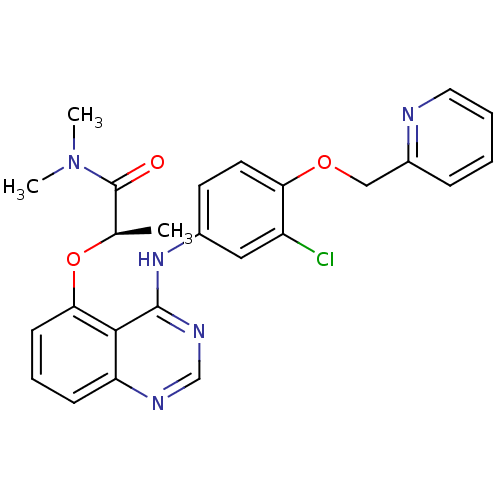
(CHEMBL428777)Show SMILES C[C@@H](Oc1cccc2ncnc(Nc3ccc(OCc4ccccn4)c(Cl)c3)c12)C(=O)N(C)C Show InChI InChI=1S/C25H24ClN5O3/c1-16(25(32)31(2)3)34-22-9-6-8-20-23(22)24(29-15-28-20)30-17-10-11-21(19(26)13-17)33-14-18-7-4-5-12-27-18/h4-13,15-16H,14H2,1-3H3,(H,28,29,30)/t16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 18: 674-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.052
BindingDB Entry DOI: 10.7270/Q2736RSH |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50371371
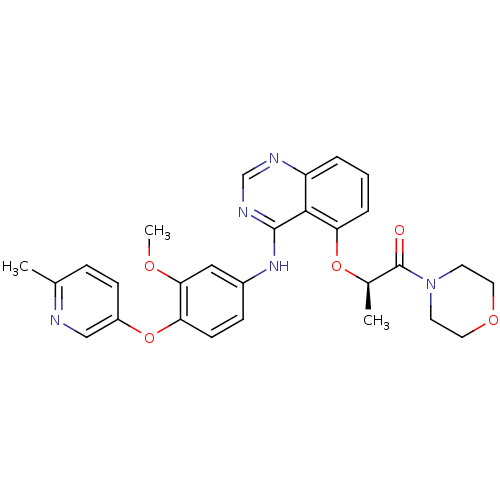
(CHEMBL411799)Show SMILES COc1cc(Nc2ncnc3cccc(O[C@H](C)C(=O)N4CCOCC4)c23)ccc1Oc1ccc(C)nc1 Show InChI InChI=1S/C28H29N5O5/c1-18-7-9-21(16-29-18)38-23-10-8-20(15-25(23)35-3)32-27-26-22(30-17-31-27)5-4-6-24(26)37-19(2)28(34)33-11-13-36-14-12-33/h4-10,15-17,19H,11-14H2,1-3H3,(H,30,31,32)/t19-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of ebrB2 expressed in human MCF7 cells by autophosphorylation assay |
Bioorg Med Chem Lett 18: 674-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.052
BindingDB Entry DOI: 10.7270/Q2736RSH |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50229343
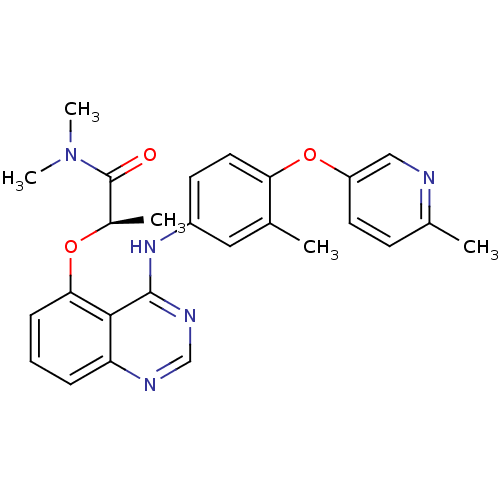
((R)-N,N-dimethyl-2-(4-(3-methyl-4-(6-methylpyridin...)Show SMILES C[C@@H](Oc1cccc2ncnc(Nc3ccc(Oc4ccc(C)nc4)c(C)c3)c12)C(=O)N(C)C Show InChI InChI=1S/C26H27N5O3/c1-16-13-19(10-12-22(16)34-20-11-9-17(2)27-14-20)30-25-24-21(28-15-29-25)7-6-8-23(24)33-18(3)26(32)31(4)5/h6-15,18H,1-5H3,(H,28,29,30)/t18-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of ebrB2 expressed in human MCF7 cells by autophosphorylation assay |
Bioorg Med Chem Lett 18: 674-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.052
BindingDB Entry DOI: 10.7270/Q2736RSH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50427295
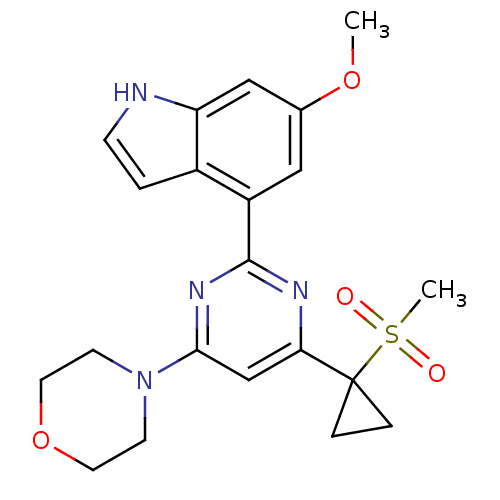
(CHEMBL2325695)Show SMILES COc1cc(-c2nc(cc(n2)C2(CC2)S(C)(=O)=O)N2CCOCC2)c2cc[nH]c2c1 Show InChI InChI=1S/C21H24N4O4S/c1-28-14-11-16(15-3-6-22-17(15)12-14)20-23-18(21(4-5-21)30(2,26)27)13-19(24-20)25-7-9-29-10-8-25/h3,6,11-13,22H,4-5,7-10H2,1-2H3 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATR in human HeLa cell nuclear extracts using glutathione S-transferase-p53N66 and ATP as substrate incubated for 10 mins prior to ATP ... |
J Med Chem 56: 2125-38 (2013)
Article DOI: 10.1021/jm301859s
BindingDB Entry DOI: 10.7270/Q2VH5Q5C |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50371372
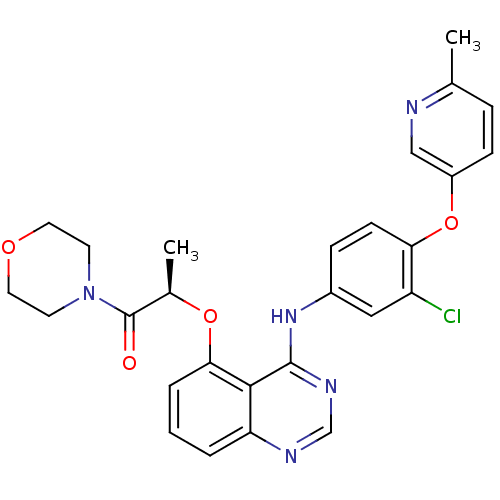
(CHEMBL402798)Show SMILES C[C@@H](Oc1cccc2ncnc(Nc3ccc(Oc4ccc(C)nc4)c(Cl)c3)c12)C(=O)N1CCOCC1 Show InChI InChI=1S/C27H26ClN5O4/c1-17-6-8-20(15-29-17)37-23-9-7-19(14-21(23)28)32-26-25-22(30-16-31-26)4-3-5-24(25)36-18(2)27(34)33-10-12-35-13-11-33/h3-9,14-16,18H,10-13H2,1-2H3,(H,30,31,32)/t18-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of ebrB2 expressed in human MCF7 cells by autophosphorylation assay |
Bioorg Med Chem Lett 18: 674-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.052
BindingDB Entry DOI: 10.7270/Q2736RSH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50427307
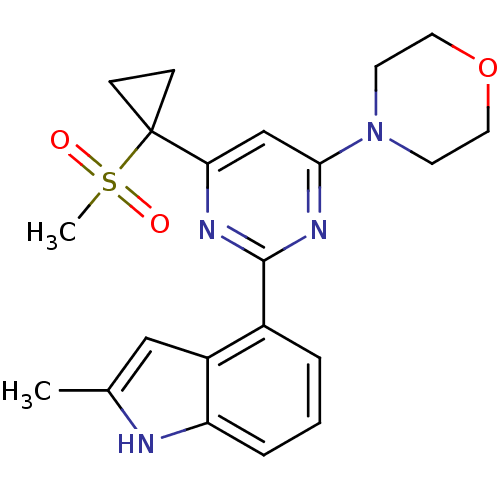
(CHEMBL2325718)Show SMILES Cc1cc2c(cccc2[nH]1)-c1nc(cc(n1)C1(CC1)S(C)(=O)=O)N1CCOCC1 Show InChI InChI=1S/C21H24N4O3S/c1-14-12-16-15(4-3-5-17(16)22-14)20-23-18(21(6-7-21)29(2,26)27)13-19(24-20)25-8-10-28-11-9-25/h3-5,12-13,22H,6-11H2,1-2H3 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATR-mediated CHK1 phosphorylation at serine 345 in human HT29 cells after 1 hr in presence of 4-nitroquinoline 1-oxide |
J Med Chem 56: 2125-38 (2013)
Article DOI: 10.1021/jm301859s
BindingDB Entry DOI: 10.7270/Q2VH5Q5C |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50371366
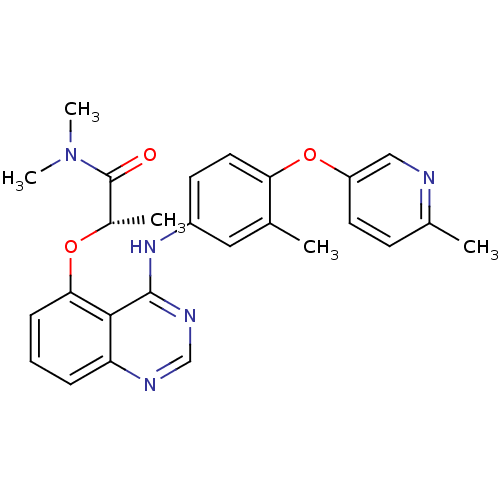
(CHEMBL429760)Show SMILES C[C@H](Oc1cccc2ncnc(Nc3ccc(Oc4ccc(C)nc4)c(C)c3)c12)C(=O)N(C)C Show InChI InChI=1S/C26H27N5O3/c1-16-13-19(10-12-22(16)34-20-11-9-17(2)27-14-20)30-25-24-21(28-15-29-25)7-6-8-23(24)33-18(3)26(32)31(4)5/h6-15,18H,1-5H3,(H,28,29,30)/t18-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of ebrB2 expressed in human MCF7 cells by autophosphorylation assay |
Bioorg Med Chem Lett 18: 674-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.052
BindingDB Entry DOI: 10.7270/Q2736RSH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50384254

(CHEMBL2030442)Show SMILES CS(=O)(=O)Cc1cc(nc(n1)-c1cccc2[nH]ccc12)N1CCOCC1 Show InChI InChI=1S/C18H20N4O3S/c1-26(23,24)12-13-11-17(22-7-9-25-10-8-22)21-18(20-13)15-3-2-4-16-14(15)5-6-19-16/h2-6,11,19H,7-10,12H2,1H3 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATR-mediated CHK1 phosphorylation at serine 345 in human HT29 cells after 1 hr in presence of 4-nitroquinoline 1-oxide |
J Med Chem 56: 2125-38 (2013)
Article DOI: 10.1021/jm301859s
BindingDB Entry DOI: 10.7270/Q2VH5Q5C |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50371368
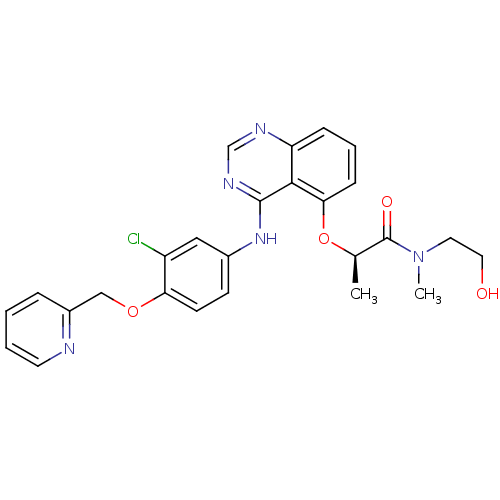
(CHEMBL258313)Show SMILES C[C@@H](Oc1cccc2ncnc(Nc3ccc(OCc4ccccn4)c(Cl)c3)c12)C(=O)N(C)CCO Show InChI InChI=1S/C26H26ClN5O4/c1-17(26(34)32(2)12-13-33)36-23-8-5-7-21-24(23)25(30-16-29-21)31-18-9-10-22(20(27)14-18)35-15-19-6-3-4-11-28-19/h3-11,14,16-17,33H,12-13,15H2,1-2H3,(H,29,30,31)/t17-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of ebrB2 expressed in human MCF7 cells by autophosphorylation assay |
Bioorg Med Chem Lett 18: 674-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.052
BindingDB Entry DOI: 10.7270/Q2736RSH |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50116612
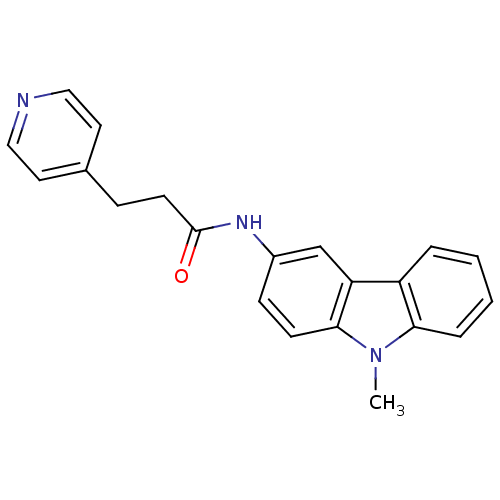
(CHEMBL118973 | N-(9-Methyl-9H-carbazol-3-yl)-3-pyr...)Show InChI InChI=1S/C21H19N3O/c1-24-19-5-3-2-4-17(19)18-14-16(7-8-20(18)24)23-21(25)9-6-15-10-12-22-13-11-15/h2-5,7-8,10-14H,6,9H2,1H3,(H,23,25) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) |
J Med Chem 45: 3509-23 (2002)
BindingDB Entry DOI: 10.7270/Q27S7N3K |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50427316
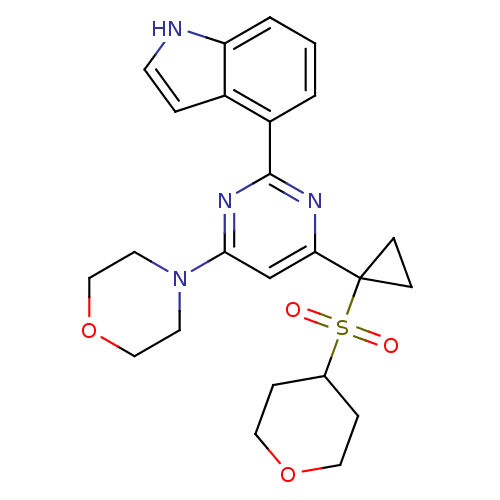
(CHEMBL2325709)Show SMILES O=S(=O)(C1CCOCC1)C1(CC1)c1cc(nc(n1)-c1cccc2[nH]ccc12)N1CCOCC1 Show InChI InChI=1S/C24H28N4O4S/c29-33(30,17-5-12-31-13-6-17)24(7-8-24)21-16-22(28-10-14-32-15-11-28)27-23(26-21)19-2-1-3-20-18(19)4-9-25-20/h1-4,9,16-17,25H,5-8,10-15H2 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATR-mediated CHK1 phosphorylation at serine 345 in human HT29 cells after 1 hr in presence of 4-nitroquinoline 1-oxide |
J Med Chem 56: 2125-38 (2013)
Article DOI: 10.1021/jm301859s
BindingDB Entry DOI: 10.7270/Q2VH5Q5C |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50427313
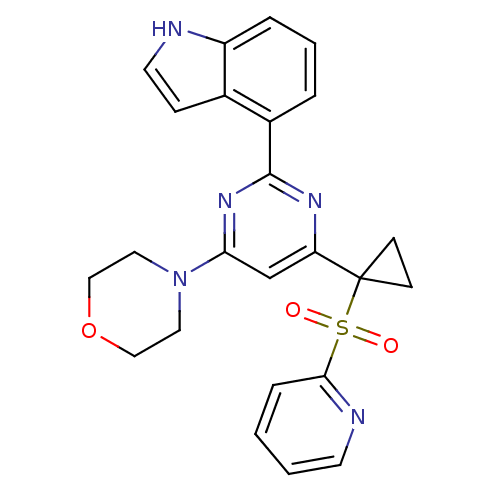
(CHEMBL2325712)Show SMILES O=S(=O)(c1ccccn1)C1(CC1)c1cc(nc(n1)-c1cccc2[nH]ccc12)N1CCOCC1 Show InChI InChI=1S/C24H23N5O3S/c30-33(31,22-6-1-2-10-26-22)24(8-9-24)20-16-21(29-12-14-32-15-13-29)28-23(27-20)18-4-3-5-19-17(18)7-11-25-19/h1-7,10-11,16,25H,8-9,12-15H2 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATR-mediated CHK1 phosphorylation at serine 345 in human HT29 cells after 1 hr in presence of 4-nitroquinoline 1-oxide |
J Med Chem 56: 2125-38 (2013)
Article DOI: 10.1021/jm301859s
BindingDB Entry DOI: 10.7270/Q2VH5Q5C |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50427329
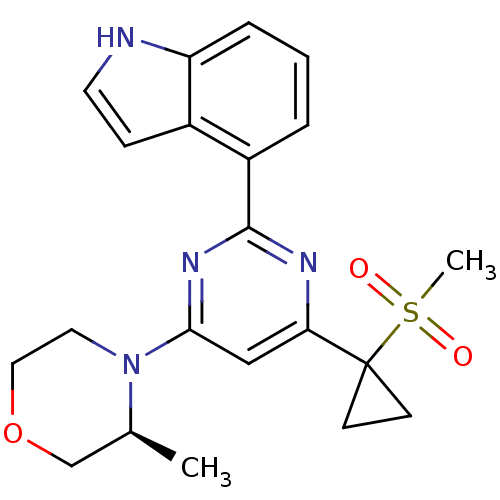
(CHEMBL2325696)Show SMILES C[C@H]1COCCN1c1cc(nc(n1)-c1cccc2[nH]ccc12)C1(CC1)S(C)(=O)=O |r| Show InChI InChI=1S/C21H24N4O3S/c1-14-13-28-11-10-25(14)19-12-18(21(7-8-21)29(2,26)27)23-20(24-19)16-4-3-5-17-15(16)6-9-22-17/h3-6,9,12,14,22H,7-8,10-11,13H2,1-2H3/t14-/m0/s1 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ATR in human HeLa cell nuclear extracts using glutathione S-transferase-p53N66 and ATP as substrate incubated for 10 mins prior to ATP ... |
J Med Chem 56: 2125-38 (2013)
Article DOI: 10.1021/jm301859s
BindingDB Entry DOI: 10.7270/Q2VH5Q5C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data