

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin K (Homo sapiens (Human)) | BDBM50260286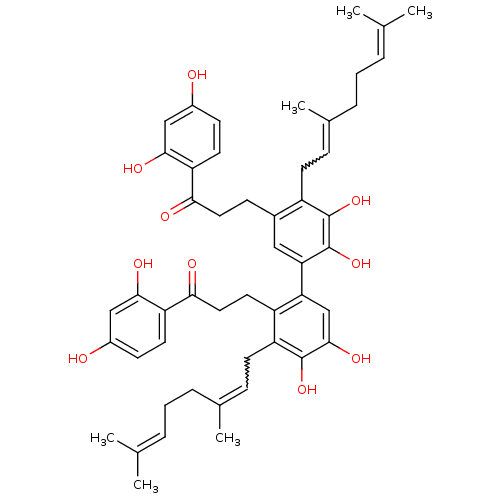 (CHEMBL508746 | cycloaltilisin 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of cathepsin K (unknown origin) by fluorimetric assay | J Nat Prod 65: 624-7 (2002) BindingDB Entry DOI: 10.7270/Q2FT8KRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50029987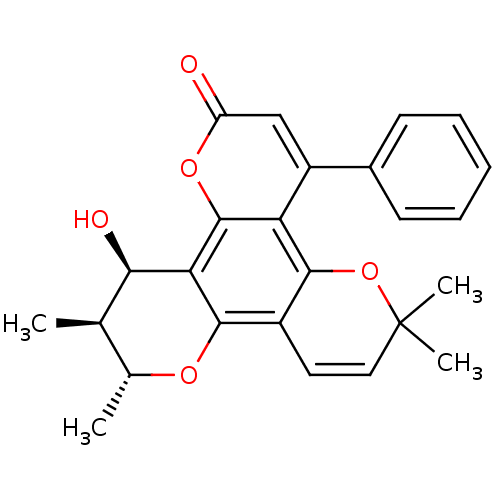 (12-Hydroxy-6,6,10,11-tetramethyl-4-phenyl-11,12-di...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals R& D Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase in scintillation proximity assay | J Med Chem 36: 4131-8 (1994) BindingDB Entry DOI: 10.7270/Q269746K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50029982 (12-Hydroxy-6,6,10,11-tetramethyl-4-phenyl-11,12-di...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals R& D Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase mutant (Tyr-181 to Cys) | J Med Chem 36: 4131-8 (1994) BindingDB Entry DOI: 10.7270/Q269746K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50029982 (12-Hydroxy-6,6,10,11-tetramethyl-4-phenyl-11,12-di...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals R& D Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase mutant (Tyr-181 to Cys) | J Med Chem 36: 4131-8 (1994) BindingDB Entry DOI: 10.7270/Q269746K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50260285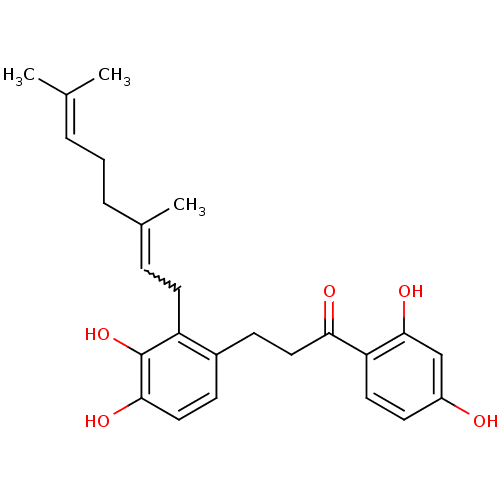 (1-(2,4-dihydroxyphenyl)-3-(2-(3,7-dimethylocta-2,6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of cathepsin K (unknown origin) by fluorimetric assay | J Nat Prod 65: 624-7 (2002) BindingDB Entry DOI: 10.7270/Q2FT8KRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50029982 (12-Hydroxy-6,6,10,11-tetramethyl-4-phenyl-11,12-di...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals R& D Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase mutant (Tyr-181 to Leu) | J Med Chem 36: 4131-8 (1994) BindingDB Entry DOI: 10.7270/Q269746K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50279762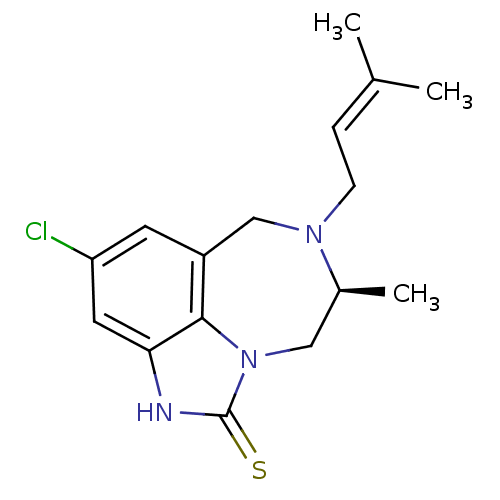 ((5S)-9-chloro-5-methyl-6-(3-methylbut-2-enyl)-4,5,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals R& D Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase in scintillation proximity assay | J Med Chem 36: 4131-8 (1994) BindingDB Entry DOI: 10.7270/Q269746K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50029996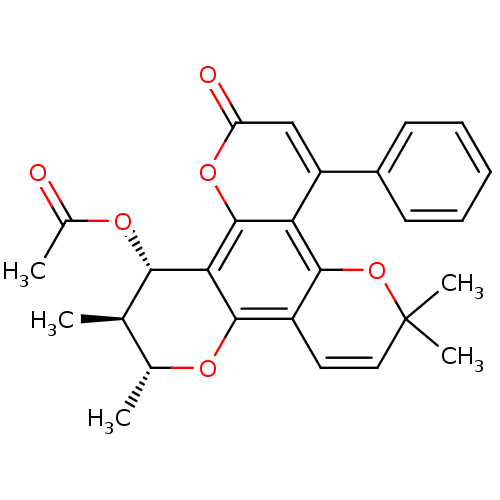 (Acetic acid 2,3,10,10-tetramethyl-6-oxo-8-phenyl-3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals R& D Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase in scintillation proximity assay | J Med Chem 36: 4131-8 (1994) BindingDB Entry DOI: 10.7270/Q269746K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50260287 (CHEMBL494579 | cycloaltilisin 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of cathepsin K (unknown origin) by fluorimetric assay | J Nat Prod 65: 624-7 (2002) BindingDB Entry DOI: 10.7270/Q2FT8KRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50029985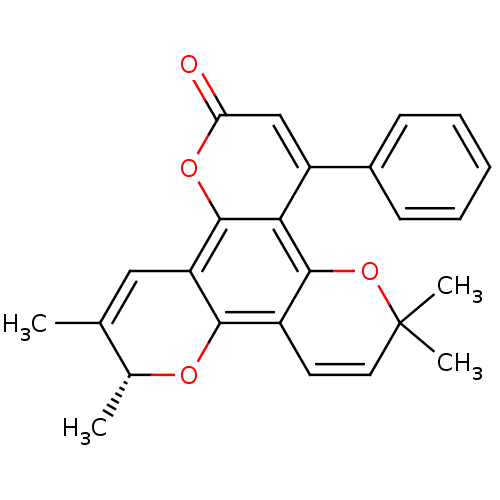 (6,6,10,11-Tetramethyl-4-phenyl-6H,10H-dipyrano[2,3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals R& D Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase in scintillation proximity assay | J Med Chem 36: 4131-8 (1994) BindingDB Entry DOI: 10.7270/Q269746K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50029988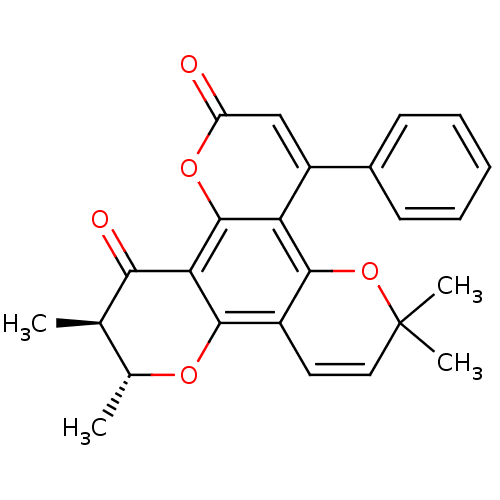 (6,6,10,11-Tetramethyl-4-phenyl-10,11-dihydro-6H-di...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals R& D Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase in scintillation proximity assay | J Med Chem 36: 4131-8 (1994) BindingDB Entry DOI: 10.7270/Q269746K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50029992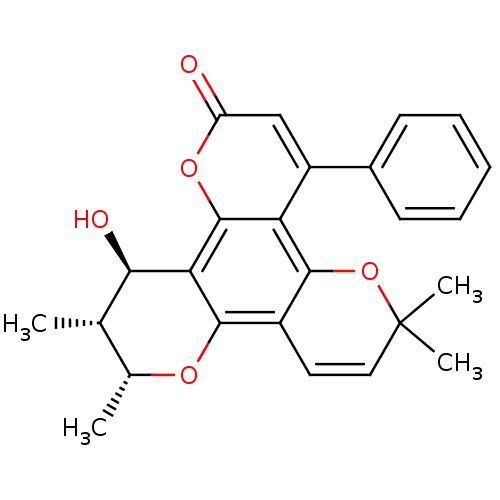 (12-Hydroxy-6,6,10,11-tetramethyl-4-phenyl-11,12-di...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals R& D Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase in scintillation proximity assay | J Med Chem 36: 4131-8 (1994) BindingDB Entry DOI: 10.7270/Q269746K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50024210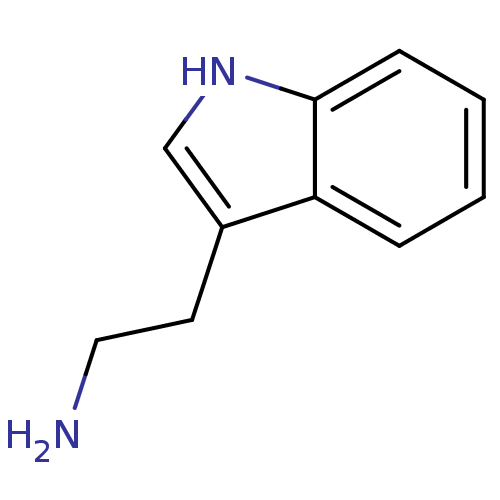 (1H-indole-3-ethanamine | 2-(1H-indol-3-yl)ethanami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of cathepsin K (unknown origin) by fluorimetric assay | J Nat Prod 65: 628-9 (2002) BindingDB Entry DOI: 10.7270/Q2B27V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50260288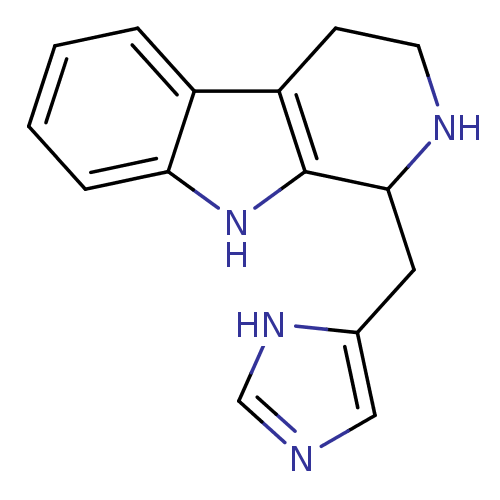 (CHEMBL495256 | Haploscleridamine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of cathepsin K (unknown origin) by fluorimetric assay | J Nat Prod 65: 628-9 (2002) BindingDB Entry DOI: 10.7270/Q2B27V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50029981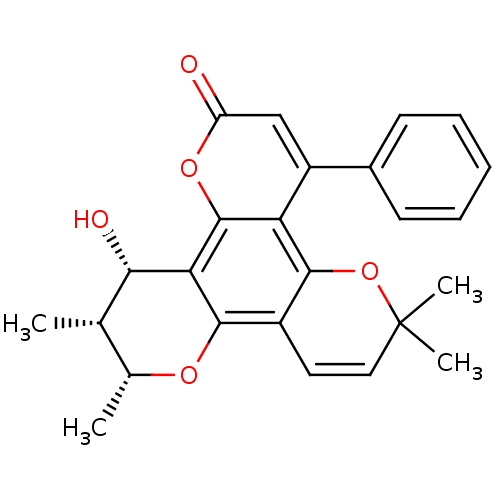 (12-Hydroxy-6,6,10,11-tetramethyl-4-phenyl-11,12-di...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals R& D Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase in scintillation proximity assay | J Med Chem 36: 4131-8 (1994) BindingDB Entry DOI: 10.7270/Q269746K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50029986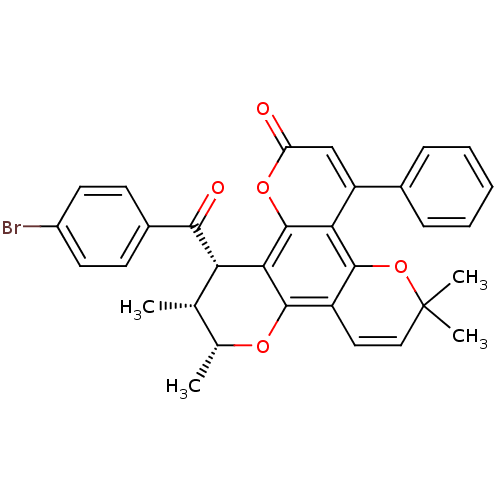 (12-(4-Bromo-benzoyl)-6,6,10,11-tetramethyl-4-pheny...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals R& D Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase in scintillation proximity assay | J Med Chem 36: 4131-8 (1994) BindingDB Entry DOI: 10.7270/Q269746K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50029994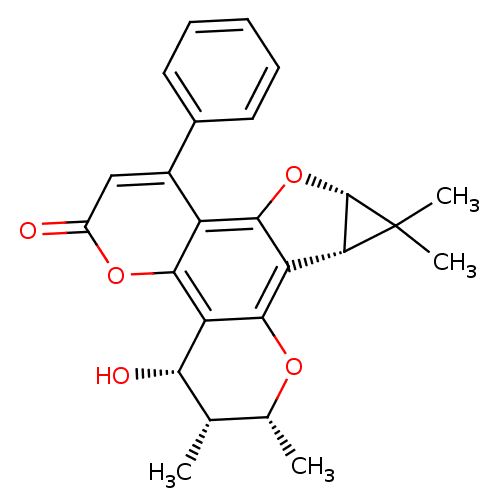 (4-hydroxy-2,3,10,10-tetramethyl-8-phenyl-(2R,3S,4S...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals R& D Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase in scintillation proximity assay | J Med Chem 36: 4131-8 (1994) BindingDB Entry DOI: 10.7270/Q269746K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50029983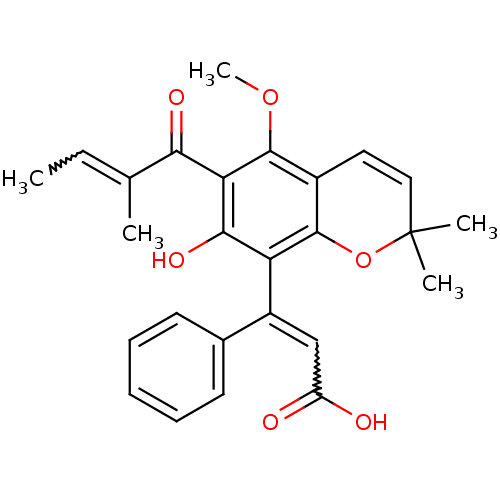 (3-[7-Hydroxy-5-methoxy-2,2-dimethyl-6-(2-methyl-bu...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals R& D Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase in scintillation proximity assay | J Med Chem 36: 4131-8 (1994) BindingDB Entry DOI: 10.7270/Q269746K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50029991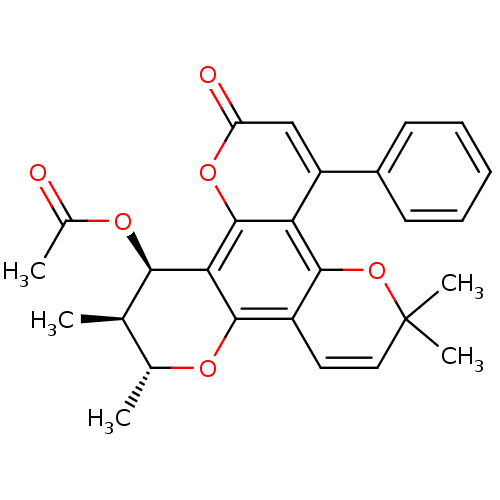 (Acetic acid 2,3,10,10-tetramethyl-6-oxo-8-phenyl-3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals R& D Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase in scintillation proximity assay | J Med Chem 36: 4131-8 (1994) BindingDB Entry DOI: 10.7270/Q269746K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50029984 (4-hydroxy-2,3,10,10-tetramethyl-8-phenyl-(2R,3S,4S...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals R& D Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase in scintillation proximity assay | J Med Chem 36: 4131-8 (1994) BindingDB Entry DOI: 10.7270/Q269746K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50029995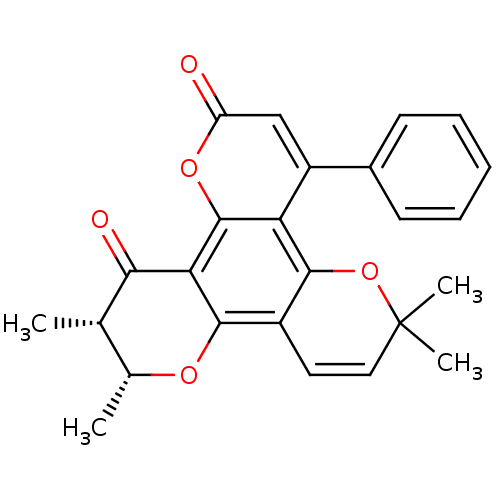 (6,6,10,11-Tetramethyl-4-phenyl-10,11-dihydro-6H-di...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals R& D Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase in scintillation proximity assay | J Med Chem 36: 4131-8 (1994) BindingDB Entry DOI: 10.7270/Q269746K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50029990 (3-(5-Hydroxy-2,3,8,8-tetramethyl-4-oxo-3,4-dihydro...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals R& D Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase in scintillation proximity assay | J Med Chem 36: 4131-8 (1994) BindingDB Entry DOI: 10.7270/Q269746K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50029993 (3-(5-Hydroxy-2,3,8,8-tetramethyl-4-oxo-3,4-dihydro...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals R& D Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase in scintillation proximity assay | J Med Chem 36: 4131-8 (1994) BindingDB Entry DOI: 10.7270/Q269746K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||