 Found 57 hits with Last Name = 'terashima' and Initial = 's'
Found 57 hits with Last Name = 'terashima' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50076089

((3S,3aR,4R,4aS,8aR,9aS)-3-Methyl-4-[2-((R)-1-methy...)Show SMILES C[C@@H]1OC(=O)[C@H]2C[C@H]3CCCC[C@@H]3[C@@H](\C=C\[C@H]3CCC[C@H](C)N3C)[C@@H]12 Show InChI InChI=1S/C22H35NO2/c1-14-7-6-9-17(23(14)3)11-12-19-18-10-5-4-8-16(18)13-20-21(19)15(2)25-22(20)24/h11-12,14-21H,4-10,13H2,1-3H3/b12-11+/t14-,15-,16+,17+,18-,19+,20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity against the muscarinic M2 (brainstem) subtype was evaluated using [3H]quinuclidinyl benzilate |
Bioorg Med Chem Lett 12: 2871-3 (2002)
BindingDB Entry DOI: 10.7270/Q2QC04PJ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50076089

((3S,3aR,4R,4aS,8aR,9aS)-3-Methyl-4-[2-((R)-1-methy...)Show SMILES C[C@@H]1OC(=O)[C@H]2C[C@H]3CCCC[C@@H]3[C@@H](\C=C\[C@H]3CCC[C@H](C)N3C)[C@@H]12 Show InChI InChI=1S/C22H35NO2/c1-14-7-6-9-17(23(14)3)11-12-19-18-10-5-4-8-16(18)13-20-21(19)15(2)25-22(20)24/h11-12,14-21H,4-10,13H2,1-3H3/b12-11+/t14-,15-,16+,17+,18-,19+,20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for binding activity against M2 muscarinic receptor in homogenates of the brainstem of rat using [3H]quinuclidinyl b... |
Bioorg Med Chem Lett 12: 3271-3 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8N6H |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50076089

((3S,3aR,4R,4aS,8aR,9aS)-3-Methyl-4-[2-((R)-1-methy...)Show SMILES C[C@@H]1OC(=O)[C@H]2C[C@H]3CCCC[C@@H]3[C@@H](\C=C\[C@H]3CCC[C@H](C)N3C)[C@@H]12 Show InChI InChI=1S/C22H35NO2/c1-14-7-6-9-17(23(14)3)11-12-19-18-10-5-4-8-16(18)13-20-21(19)15(2)25-22(20)24/h11-12,14-21H,4-10,13H2,1-3H3/b12-11+/t14-,15-,16+,17+,18-,19+,20-,21+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity against the muscarinic M1 (cortex) subtype was evaluated using [3H]pirenzepine |
Bioorg Med Chem Lett 12: 2871-3 (2002)
BindingDB Entry DOI: 10.7270/Q2QC04PJ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50076089

((3S,3aR,4R,4aS,8aR,9aS)-3-Methyl-4-[2-((R)-1-methy...)Show SMILES C[C@@H]1OC(=O)[C@H]2C[C@H]3CCCC[C@@H]3[C@@H](\C=C\[C@H]3CCC[C@H](C)N3C)[C@@H]12 Show InChI InChI=1S/C22H35NO2/c1-14-7-6-9-17(23(14)3)11-12-19-18-10-5-4-8-16(18)13-20-21(19)15(2)25-22(20)24/h11-12,14-21H,4-10,13H2,1-3H3/b12-11+/t14-,15-,16+,17+,18-,19+,20-,21+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for binding activity against M1 muscarinic receptor in homogenates of the cerebral cortex of rat using [3H]pirenzepi... |
Bioorg Med Chem Lett 12: 3271-3 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8N6H |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50403996
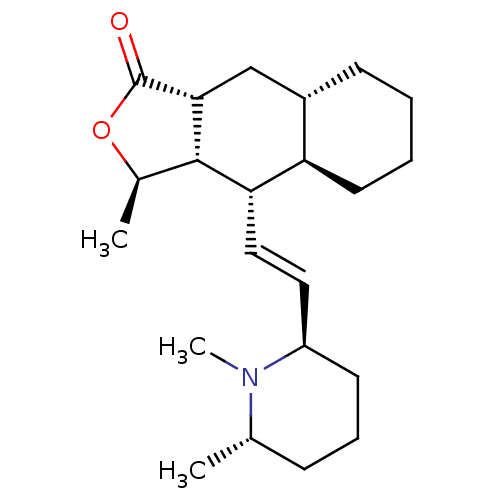
(CHEMBL97765)Show SMILES C[C@H]1OC(=O)[C@@H]2C[C@@H]3CCCC[C@H]3[C@H](\C=C\[C@H]3CCC[C@H](C)N3C)[C@H]12 Show InChI InChI=1S/C22H35NO2/c1-14-7-6-9-17(23(14)3)11-12-19-18-10-5-4-8-16(18)13-20-21(19)15(2)25-22(20)24/h11-12,14-21H,4-10,13H2,1-3H3/b12-11+/t14-,15+,16-,17+,18+,19-,20+,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity against the muscarinic M2 (brainstem) subtype was evaluated using [3H]quinuclidinyl benzilate |
Bioorg Med Chem Lett 12: 2871-3 (2002)
BindingDB Entry DOI: 10.7270/Q2QC04PJ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50403998
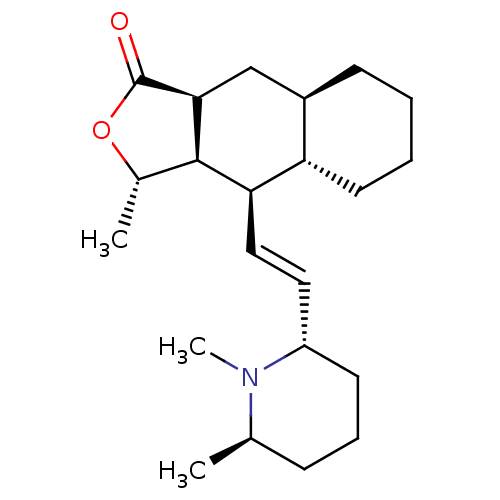
(CHEMBL318849)Show SMILES C[C@@H]1OC(=O)[C@H]2C[C@H]3CCCC[C@@H]3[C@@H](\C=C\[C@@H]3CCC[C@@H](C)N3C)[C@@H]12 Show InChI InChI=1S/C22H35NO2/c1-14-7-6-9-17(23(14)3)11-12-19-18-10-5-4-8-16(18)13-20-21(19)15(2)25-22(20)24/h11-12,14-21H,4-10,13H2,1-3H3/b12-11+/t14-,15+,16-,17+,18+,19-,20+,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity against the muscarinic M2 (brainstem) subtype was evaluated using [3H]quinuclidinyl benzilate |
Bioorg Med Chem Lett 12: 2871-3 (2002)
BindingDB Entry DOI: 10.7270/Q2QC04PJ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50403998

(CHEMBL318849)Show SMILES C[C@@H]1OC(=O)[C@H]2C[C@H]3CCCC[C@@H]3[C@@H](\C=C\[C@@H]3CCC[C@@H](C)N3C)[C@@H]12 Show InChI InChI=1S/C22H35NO2/c1-14-7-6-9-17(23(14)3)11-12-19-18-10-5-4-8-16(18)13-20-21(19)15(2)25-22(20)24/h11-12,14-21H,4-10,13H2,1-3H3/b12-11+/t14-,15+,16-,17+,18+,19-,20+,21-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity against the muscarinic M1 (cortex) subtype was evaluated using [3H]pirenzepine |
Bioorg Med Chem Lett 12: 2871-3 (2002)
BindingDB Entry DOI: 10.7270/Q2QC04PJ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50403996

(CHEMBL97765)Show SMILES C[C@H]1OC(=O)[C@@H]2C[C@@H]3CCCC[C@H]3[C@H](\C=C\[C@H]3CCC[C@H](C)N3C)[C@H]12 Show InChI InChI=1S/C22H35NO2/c1-14-7-6-9-17(23(14)3)11-12-19-18-10-5-4-8-16(18)13-20-21(19)15(2)25-22(20)24/h11-12,14-21H,4-10,13H2,1-3H3/b12-11+/t14-,15+,16-,17+,18+,19-,20+,21-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity against the muscarinic M1 (cortex) subtype was evaluated using [3H]pirenzepine |
Bioorg Med Chem Lett 12: 2871-3 (2002)
BindingDB Entry DOI: 10.7270/Q2QC04PJ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50404003
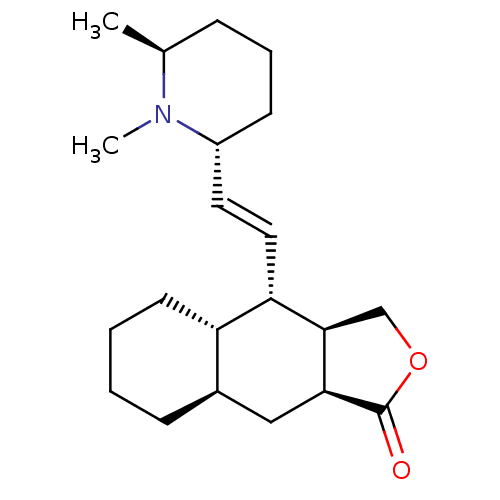
(CHEMBL324725)Show SMILES C[C@H]1CCC[C@H](\C=C\[C@@H]2[C@H]3COC(=O)[C@H]3C[C@H]3CCCC[C@H]23)N1C Show InChI InChI=1S/C21H33NO2/c1-14-6-5-8-16(22(14)2)10-11-18-17-9-4-3-7-15(17)12-19-20(18)13-24-21(19)23/h10-11,14-20H,3-9,12-13H2,1-2H3/b11-10+/t14-,15+,16+,17-,18-,19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for binding activity against M2 muscarinic receptor in homogenates of the brainstem of rat using [3H]quinuclidinyl b... |
Bioorg Med Chem Lett 12: 3271-3 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8N6H |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50404003

(CHEMBL324725)Show SMILES C[C@H]1CCC[C@H](\C=C\[C@@H]2[C@H]3COC(=O)[C@H]3C[C@H]3CCCC[C@H]23)N1C Show InChI InChI=1S/C21H33NO2/c1-14-6-5-8-16(22(14)2)10-11-18-17-9-4-3-7-15(17)12-19-20(18)13-24-21(19)23/h10-11,14-20H,3-9,12-13H2,1-2H3/b11-10+/t14-,15+,16+,17-,18-,19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for binding activity against M2 muscarinic receptor in homogenates of the brainstem of rat using [3H]quinuclidinyl b... |
Bioorg Med Chem Lett 12: 3271-3 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8N6H |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50404003

(CHEMBL324725)Show SMILES C[C@H]1CCC[C@H](\C=C\[C@@H]2[C@H]3COC(=O)[C@H]3C[C@H]3CCCC[C@H]23)N1C Show InChI InChI=1S/C21H33NO2/c1-14-6-5-8-16(22(14)2)10-11-18-17-9-4-3-7-15(17)12-19-20(18)13-24-21(19)23/h10-11,14-20H,3-9,12-13H2,1-2H3/b11-10+/t14-,15+,16+,17-,18-,19-,20+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for binding activity against M1 muscarinic receptor in homogenates of the cerebral cortex of rat using [3H]pirenzepi... |
Bioorg Med Chem Lett 12: 3271-3 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8N6H |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50404003

(CHEMBL324725)Show SMILES C[C@H]1CCC[C@H](\C=C\[C@@H]2[C@H]3COC(=O)[C@H]3C[C@H]3CCCC[C@H]23)N1C Show InChI InChI=1S/C21H33NO2/c1-14-6-5-8-16(22(14)2)10-11-18-17-9-4-3-7-15(17)12-19-20(18)13-24-21(19)23/h10-11,14-20H,3-9,12-13H2,1-2H3/b11-10+/t14-,15+,16+,17-,18-,19-,20+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for binding activity against M1 muscarinic receptor in homogenates of the cerebral cortex of rat using [3H]pirenzepi... |
Bioorg Med Chem Lett 12: 3271-3 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8N6H |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50404004
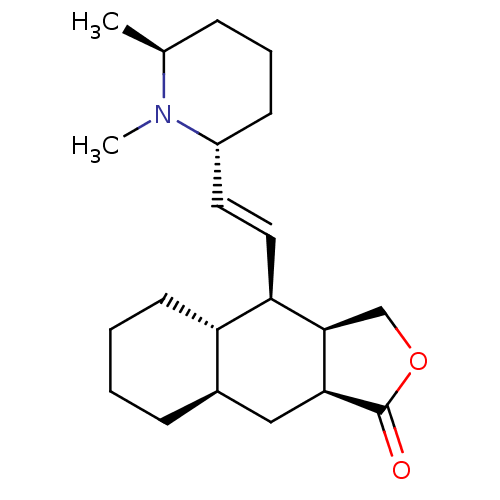
(CHEMBL111224)Show SMILES C[C@H]1CCC[C@H](\C=C\[C@H]2[C@H]3COC(=O)[C@H]3C[C@H]3CCCC[C@H]23)N1C Show InChI InChI=1S/C21H33NO2/c1-14-6-5-8-16(22(14)2)10-11-18-17-9-4-3-7-15(17)12-19-20(18)13-24-21(19)23/h10-11,14-20H,3-9,12-13H2,1-2H3/b11-10+/t14-,15+,16+,17-,18+,19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for binding activity against M2 muscarinic receptor in homogenates of the brainstem of rat using [3H]quinuclidinyl b... |
Bioorg Med Chem Lett 12: 3271-3 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8N6H |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50404004

(CHEMBL111224)Show SMILES C[C@H]1CCC[C@H](\C=C\[C@H]2[C@H]3COC(=O)[C@H]3C[C@H]3CCCC[C@H]23)N1C Show InChI InChI=1S/C21H33NO2/c1-14-6-5-8-16(22(14)2)10-11-18-17-9-4-3-7-15(17)12-19-20(18)13-24-21(19)23/h10-11,14-20H,3-9,12-13H2,1-2H3/b11-10+/t14-,15+,16+,17-,18+,19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for binding activity against M2 muscarinic receptor in homogenates of the brainstem of rat using [3H]quinuclidinyl b... |
Bioorg Med Chem Lett 12: 3271-3 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8N6H |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50403997
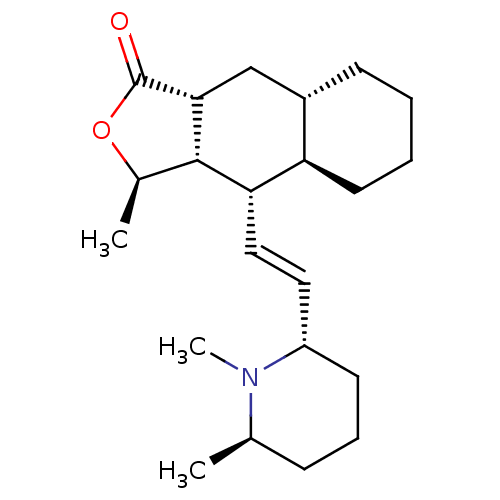
(CHEMBL99618)Show SMILES C[C@H]1OC(=O)[C@@H]2C[C@@H]3CCCC[C@H]3[C@H](\C=C\[C@@H]3CCC[C@@H](C)N3C)[C@H]12 Show InChI InChI=1S/C22H35NO2/c1-14-7-6-9-17(23(14)3)11-12-19-18-10-5-4-8-16(18)13-20-21(19)15(2)25-22(20)24/h11-12,14-21H,4-10,13H2,1-3H3/b12-11+/t14-,15-,16+,17+,18-,19+,20-,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity against the muscarinic M2 (brainstem) subtype was evaluated using [3H]quinuclidinyl benzilate |
Bioorg Med Chem Lett 12: 2871-3 (2002)
BindingDB Entry DOI: 10.7270/Q2QC04PJ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50404004

(CHEMBL111224)Show SMILES C[C@H]1CCC[C@H](\C=C\[C@H]2[C@H]3COC(=O)[C@H]3C[C@H]3CCCC[C@H]23)N1C Show InChI InChI=1S/C21H33NO2/c1-14-6-5-8-16(22(14)2)10-11-18-17-9-4-3-7-15(17)12-19-20(18)13-24-21(19)23/h10-11,14-20H,3-9,12-13H2,1-2H3/b11-10+/t14-,15+,16+,17-,18+,19-,20+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 999 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for binding activity against M1 muscarinic receptor in homogenates of the cerebral cortex of rat using [3H]pirenzepi... |
Bioorg Med Chem Lett 12: 3271-3 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8N6H |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50403997

(CHEMBL99618)Show SMILES C[C@H]1OC(=O)[C@@H]2C[C@@H]3CCCC[C@H]3[C@H](\C=C\[C@@H]3CCC[C@@H](C)N3C)[C@H]12 Show InChI InChI=1S/C22H35NO2/c1-14-7-6-9-17(23(14)3)11-12-19-18-10-5-4-8-16(18)13-20-21(19)15(2)25-22(20)24/h11-12,14-21H,4-10,13H2,1-3H3/b12-11+/t14-,15-,16+,17+,18-,19+,20-,21+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity against the muscarinic M1 (cortex) subtype was evaluated using [3H]pirenzepine |
Bioorg Med Chem Lett 12: 2871-3 (2002)
BindingDB Entry DOI: 10.7270/Q2QC04PJ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50404004

(CHEMBL111224)Show SMILES C[C@H]1CCC[C@H](\C=C\[C@H]2[C@H]3COC(=O)[C@H]3C[C@H]3CCCC[C@H]23)N1C Show InChI InChI=1S/C21H33NO2/c1-14-6-5-8-16(22(14)2)10-11-18-17-9-4-3-7-15(17)12-19-20(18)13-24-21(19)23/h10-11,14-20H,3-9,12-13H2,1-2H3/b11-10+/t14-,15+,16+,17-,18+,19-,20+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for binding activity against M1 muscarinic receptor in homogenates of the cerebral cortex of rat using [3H]pirenzepi... |
Bioorg Med Chem Lett 12: 3271-3 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8N6H |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50342601

(CHEMBL1255901 | Huperzine A)Show SMILES C\C=C1/[C@@H]2Cc3[nH]c(=O)ccc3[C@]1(N)CC(C)=C2 |r,c:18,TLB:1:2:11.5.4:17.14.15| Show InChI InChI=1S/C15H18N2O/c1-3-11-10-6-9(2)8-15(11,16)12-4-5-14(18)17-13(12)7-10/h3-6,10H,7-8,16H2,1-2H3,(H,17,18)/b11-3+/t10-,15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Acetylcholinesterase (AChE). |
Bioorg Med Chem Lett 6: 1927-1930 (1996)
Article DOI: 10.1016/0960-894X(96)00337-X
BindingDB Entry DOI: 10.7270/Q2P84CD1 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50287625
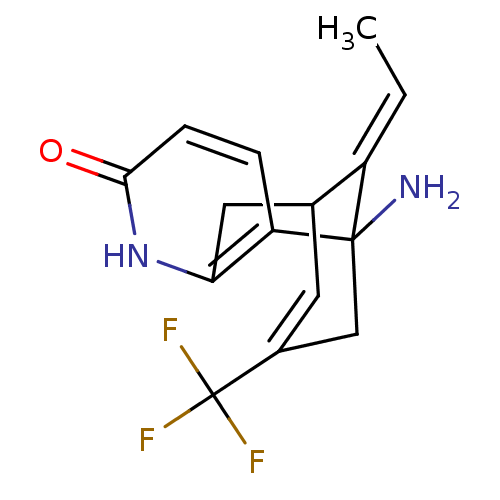
(1-Amino-13-eth-(E)-ylidene-11-trifluoromethyl-6-az...)Show SMILES C\C=C1/C2Cc3[nH]c(=O)ccc3C1(N)CC(=C2)C(F)(F)F |c:17,TLB:10:11:2:15.14.16,THB:6:5:2:15.14.16| Show InChI InChI=1S/C15H15F3N2O/c1-2-10-8-5-9(15(16,17)18)7-14(10,19)11-3-4-13(21)20-12(11)6-8/h2-5,8H,6-7,19H2,1H3,(H,20,21)/b10-2+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Acetylcholinesterase (AChE). |
Bioorg Med Chem Lett 6: 1927-1930 (1996)
Article DOI: 10.1016/0960-894X(96)00337-X
BindingDB Entry DOI: 10.7270/Q2P84CD1 |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50301400
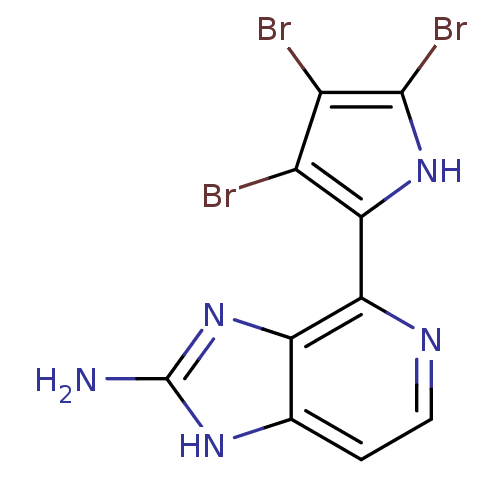
(4-(3,4,5-tribromo-1H-pyrrol-2-yl)-1H-imidazo[4,5-c...)Show InChI InChI=1S/C10H6Br3N5/c11-4-5(12)9(13)17-7(4)8-6-3(1-2-15-8)16-10(14)18-6/h1-2,17H,(H3,14,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human MMP12 catalytic domain |
Bioorg Med Chem Lett 19: 5461-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.099
BindingDB Entry DOI: 10.7270/Q2HX1CRC |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50287624
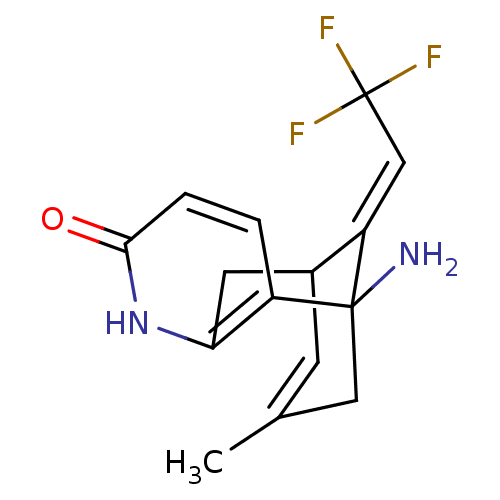
(1-Amino-13-eth-(E)-ylidene-11-trifluoromethyl-6-az...)Show SMILES CC1=CC2Cc3[nH]c(=O)ccc3C(N)(C1)\C2=C\C(F)(F)F |t:1,TLB:6:5:15:2.14.1,THB:10:11:15:2.14.1| Show InChI InChI=1S/C15H15F3N2O/c1-8-4-9-5-12-10(2-3-13(21)20-12)14(19,6-8)11(9)7-15(16,17)18/h2-4,7,9H,5-6,19H2,1H3,(H,20,21)/b11-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Acetylcholinesterase (AChE). |
Bioorg Med Chem Lett 6: 1927-1930 (1996)
Article DOI: 10.1016/0960-894X(96)00337-X
BindingDB Entry DOI: 10.7270/Q2P84CD1 |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50301402
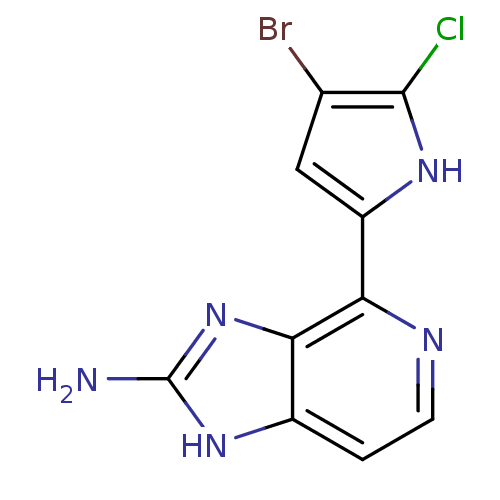
(4-(4-bromo-5-chloro-1H-pyrrol-2-yl)-1H-imidazo[4,5...)Show InChI InChI=1S/C10H7BrClN5/c11-4-3-6(15-9(4)12)7-8-5(1-2-14-7)16-10(13)17-8/h1-3,15H,(H3,13,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human MMP12 catalytic domain |
Bioorg Med Chem Lett 19: 5461-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.099
BindingDB Entry DOI: 10.7270/Q2HX1CRC |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50301414
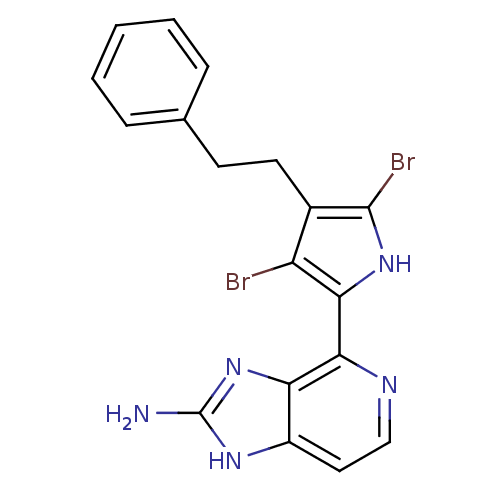
(4-(3,5-dibromo-4-phenethyl-1H-pyrrol-2-yl)-1H-imid...)Show SMILES Nc1nc2c(nccc2[nH]1)-c1[nH]c(Br)c(CCc2ccccc2)c1Br Show InChI InChI=1S/C18H15Br2N5/c19-13-11(7-6-10-4-2-1-3-5-10)17(20)24-15(13)16-14-12(8-9-22-16)23-18(21)25-14/h1-5,8-9,24H,6-7H2,(H3,21,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human MMP12 catalytic domain |
Bioorg Med Chem Lett 19: 5461-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.099
BindingDB Entry DOI: 10.7270/Q2HX1CRC |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50287623

(1-Amino-13-[2,2,2-trifluoro-eth-(E)-ylidene]-11-tr...)Show SMILES NC12CC(=CC(Cc3[nH]c(=O)ccc13)\C2=C/C(F)(F)F)C(F)(F)F |c:3,TLB:8:7:14:3.2.4,THB:12:13:14:3.2.4| Show InChI InChI=1S/C15H12F6N2O/c16-14(17,18)6-10-7-3-8(15(19,20)21)5-13(10,22)9-1-2-12(24)23-11(9)4-7/h1-3,6-7H,4-5,22H2,(H,23,24)/b10-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Acetylcholinesterase (AChE). |
Bioorg Med Chem Lett 6: 1927-1930 (1996)
Article DOI: 10.1016/0960-894X(96)00337-X
BindingDB Entry DOI: 10.7270/Q2P84CD1 |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50215926
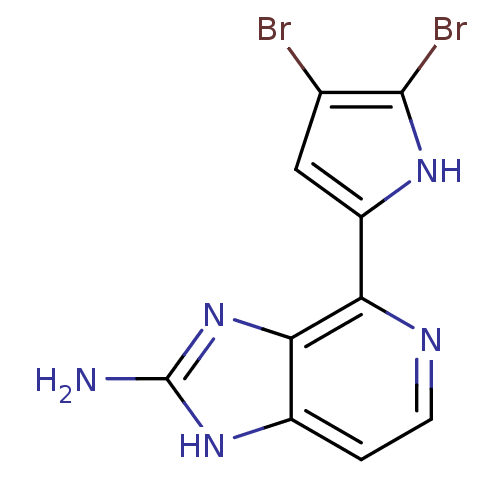
(4-(4,5-dibromo-1H-pyrrol-2-yl)-1H-imidazo[4,5-c]py...)Show InChI InChI=1S/C10H7Br2N5/c11-4-3-6(15-9(4)12)7-8-5(1-2-14-7)16-10(13)17-8/h1-3,15H,(H3,13,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human MMP12 catalytic domain |
Bioorg Med Chem Lett 19: 5461-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.099
BindingDB Entry DOI: 10.7270/Q2HX1CRC |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50215926

(4-(4,5-dibromo-1H-pyrrol-2-yl)-1H-imidazo[4,5-c]py...)Show InChI InChI=1S/C10H7Br2N5/c11-4-3-6(15-9(4)12)7-8-5(1-2-14-7)16-10(13)17-8/h1-3,15H,(H3,13,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem Lett 17: 4495-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.005
BindingDB Entry DOI: 10.7270/Q2MW2GVX |
More data for this
Ligand-Target Pair | |
Acidic mammalian chitinase
(Homo sapiens (Human)) | BDBM50173286
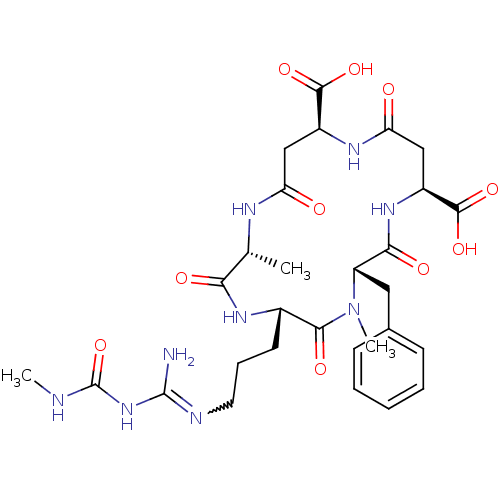
(5-[3-[amino-(methylcarbamoylamino)methylidene]amin...)Show SMILES CNC(=O)NC(N)=NCCC[C@@H]1NC(=O)[C@@H](C)NC(=O)C[C@H](NC(=O)C[C@H](NC(=O)[C@H](Cc2ccccc2)N(C)C1=O)C(O)=O)C(O)=O |r,w:7.7| Show InChI InChI=1S/C29H41N9O10/c1-15-23(41)35-17(10-7-11-32-28(30)37-29(48)31-2)25(43)38(3)20(12-16-8-5-4-6-9-16)24(42)36-19(27(46)47)14-22(40)34-18(26(44)45)13-21(39)33-15/h4-6,8-9,15,17-20H,7,10-14H2,1-3H3,(H,33,39)(H,34,40)(H,35,41)(H,36,42)(H,44,45)(H,46,47)(H4,30,31,32,37,48)/t15-,17+,18+,19+,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of human acidic mammalian chitinase |
Bioorg Med Chem 17: 6270-8 (2009)
Article DOI: 10.1016/j.bmc.2009.07.045
BindingDB Entry DOI: 10.7270/Q2TB17TJ |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50301409
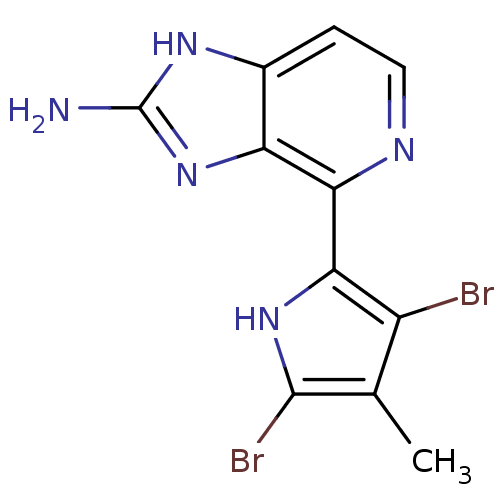
(4-(3,5-dibromo-4-methyl-1H-pyrrol-2-yl)-1H-imidazo...)Show InChI InChI=1S/C11H9Br2N5/c1-4-6(12)8(17-10(4)13)9-7-5(2-3-15-9)16-11(14)18-7/h2-3,17H,1H3,(H3,14,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human MMP12 catalytic domain |
Bioorg Med Chem Lett 19: 5461-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.099
BindingDB Entry DOI: 10.7270/Q2HX1CRC |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50301401
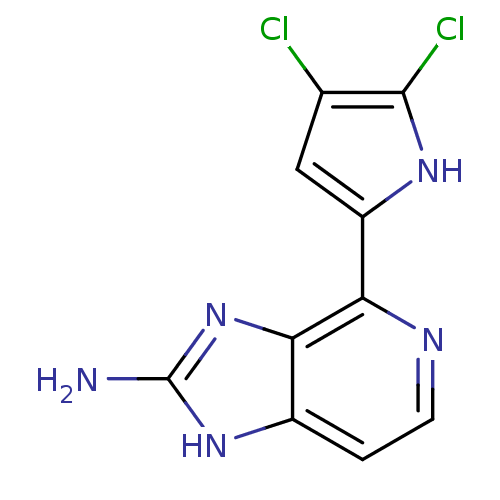
(4-(4,5-dichloro-1H-pyrrol-2-yl)-1H-imidazo[4,5-c]p...)Show InChI InChI=1S/C10H7Cl2N5/c11-4-3-6(15-9(4)12)7-8-5(1-2-14-7)16-10(13)17-8/h1-3,15H,(H3,13,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human MMP12 catalytic domain |
Bioorg Med Chem Lett 19: 5461-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.099
BindingDB Entry DOI: 10.7270/Q2HX1CRC |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50301413
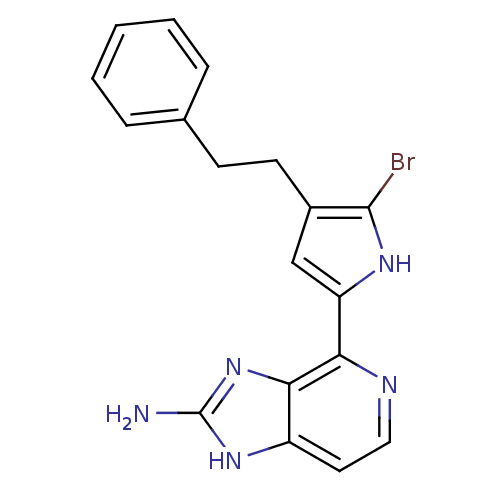
(4-(5-bromo-4-phenethyl-1H-pyrrol-2-yl)-1H-imidazo[...)Show SMILES Nc1nc2c(nccc2[nH]1)-c1cc(CCc2ccccc2)c(Br)[nH]1 Show InChI InChI=1S/C18H16BrN5/c19-17-12(7-6-11-4-2-1-3-5-11)10-14(22-17)15-16-13(8-9-21-15)23-18(20)24-16/h1-5,8-10,22H,6-7H2,(H3,20,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human MMP12 catalytic domain |
Bioorg Med Chem Lett 19: 5461-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.099
BindingDB Entry DOI: 10.7270/Q2HX1CRC |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50215933

(4-(4,5-dibromo-1H-pyrrol-2-yl)-N-methyl-1H-imidazo...)Show InChI InChI=1S/C11H9Br2N5/c1-14-11-17-6-2-3-15-8(9(6)18-11)7-4-5(12)10(13)16-7/h2-4,16H,1H3,(H2,14,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem Lett 17: 4495-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.005
BindingDB Entry DOI: 10.7270/Q2MW2GVX |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50301410

(4-(5-bromo-4-phenyl-1H-pyrrol-2-yl)-1H-imidazo[4,5...)Show SMILES Nc1nc2c(nccc2[nH]1)-c1cc(c(Br)[nH]1)-c1ccccc1 Show InChI InChI=1S/C16H12BrN5/c17-15-10(9-4-2-1-3-5-9)8-12(20-15)13-14-11(6-7-19-13)21-16(18)22-14/h1-8,20H,(H3,18,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human MMP12 catalytic domain |
Bioorg Med Chem Lett 19: 5461-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.099
BindingDB Entry DOI: 10.7270/Q2HX1CRC |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50215931

(4-(4,5-dibromo-1H-pyrrol-2-yl)-1H-imidazo[4,5-c]py...)Show InChI InChI=1S/C10H6Br2N4/c11-5-3-7(16-10(5)12)9-8-6(1-2-13-9)14-4-15-8/h1-4,16H,(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem Lett 17: 4495-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.005
BindingDB Entry DOI: 10.7270/Q2MW2GVX |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50215934

(4-(4,5-dibromo-1H-pyrrol-2-yl)-1-methyl-1H-imidazo...)Show InChI InChI=1S/C11H9Br2N5/c1-18-7-2-3-15-8(9(7)17-11(18)14)6-4-5(12)10(13)16-6/h2-4,16H,1H3,(H2,14,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem Lett 17: 4495-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.005
BindingDB Entry DOI: 10.7270/Q2MW2GVX |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50301407

(4-(5-(biphenyl-4-yl)-4-bromo-1H-pyrrol-2-yl)-1H-im...)Show SMILES Nc1nc2c(nccc2[nH]1)-c1cc(Br)c([nH]1)-c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C22H16BrN5/c23-16-12-18(20-21-17(10-11-25-20)27-22(24)28-21)26-19(16)15-8-6-14(7-9-15)13-4-2-1-3-5-13/h1-12,26H,(H3,24,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human MMP12 catalytic domain |
Bioorg Med Chem Lett 19: 5461-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.099
BindingDB Entry DOI: 10.7270/Q2HX1CRC |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50301406
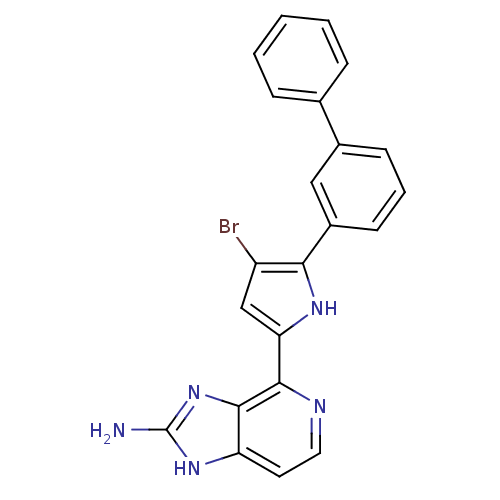
(4-(5-(biphenyl-3-yl)-4-bromo-1H-pyrrol-2-yl)-1H-im...)Show SMILES Nc1nc2c(nccc2[nH]1)-c1cc(Br)c([nH]1)-c1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C22H16BrN5/c23-16-12-18(20-21-17(9-10-25-20)27-22(24)28-21)26-19(16)15-8-4-7-14(11-15)13-5-2-1-3-6-13/h1-12,26H,(H3,24,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human MMP12 catalytic domain |
Bioorg Med Chem Lett 19: 5461-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.099
BindingDB Entry DOI: 10.7270/Q2HX1CRC |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50301405
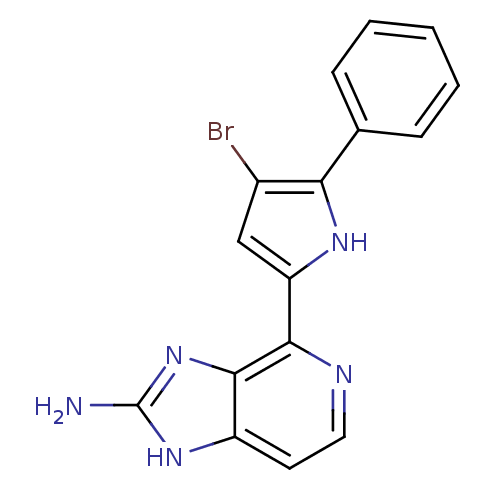
(4-(4-bromo-5-phenyl-1H-pyrrol-2-yl)-1H-imidazo[4,5...)Show SMILES Nc1nc2c(nccc2[nH]1)-c1cc(Br)c([nH]1)-c1ccccc1 Show InChI InChI=1S/C16H12BrN5/c17-10-8-12(20-13(10)9-4-2-1-3-5-9)14-15-11(6-7-19-14)21-16(18)22-15/h1-8,20H,(H3,18,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human MMP12 catalytic domain |
Bioorg Med Chem Lett 19: 5461-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.099
BindingDB Entry DOI: 10.7270/Q2HX1CRC |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50215932

(4-(4,5-dibromo-1-methyl-1H-pyrrol-2-yl)-1H-imidazo...)Show InChI InChI=1S/C11H9Br2N5/c1-18-7(4-5(12)10(18)13)9-8-6(2-3-15-9)16-11(14)17-8/h2-4H,1H3,(H3,14,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem Lett 17: 4495-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.005
BindingDB Entry DOI: 10.7270/Q2MW2GVX |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
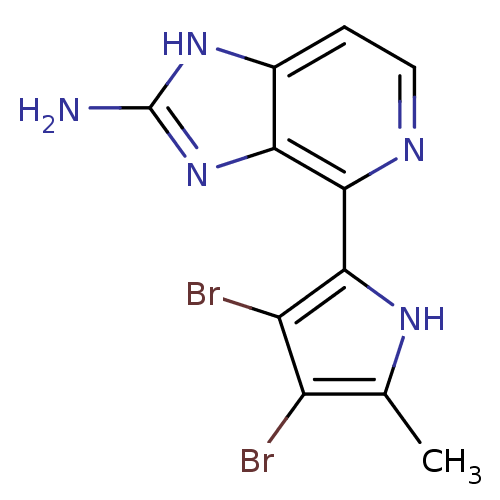
(Homo sapiens (Human)) | BDBM50301404
(4-(3,4-dibromo-5-methyl-1H-pyrrol-2-yl)-1H-imidazo...)Show InChI InChI=1S/C11H9Br2N5/c1-4-6(12)7(13)9(16-4)10-8-5(2-3-15-10)17-11(14)18-8/h2-3,16H,1H3,(H3,14,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human MMP12 catalytic domain |
Bioorg Med Chem Lett 19: 5461-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.099
BindingDB Entry DOI: 10.7270/Q2HX1CRC |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50215929

(4-(4,5-dibromo-1H-pyrrol-2-yl)-N,1-dimethyl-1H-imi...)Show InChI InChI=1S/C12H11Br2N5/c1-15-12-18-10-8(19(12)2)3-4-16-9(10)7-5-6(13)11(14)17-7/h3-5,17H,1-2H3,(H,15,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem Lett 17: 4495-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.005
BindingDB Entry DOI: 10.7270/Q2MW2GVX |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50215930

(4-(5-bromo-1H-pyrrol-2-yl)-1H-imidazo[4,5-c]pyridi...)Show InChI InChI=1S/C10H8BrN5/c11-7-2-1-5(14-7)8-9-6(3-4-13-8)15-10(12)16-9/h1-4,14H,(H3,12,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem Lett 17: 4495-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.005
BindingDB Entry DOI: 10.7270/Q2MW2GVX |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50215928
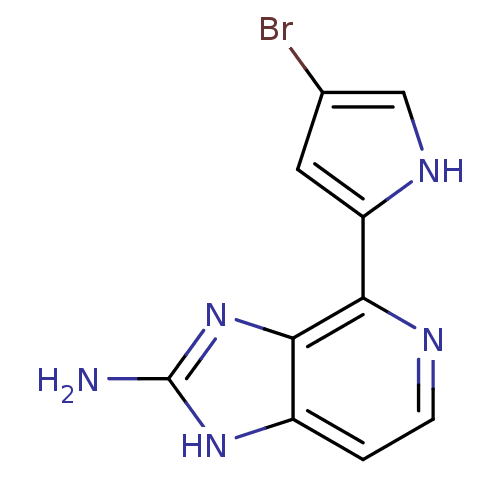
(4-(4-bromo-1H-pyrrol-2-yl)-1H-imidazo[4,5-c]pyridi...)Show InChI InChI=1S/C10H8BrN5/c11-5-3-7(14-4-5)8-9-6(1-2-13-8)15-10(12)16-9/h1-4,14H,(H3,12,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem Lett 17: 4495-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.005
BindingDB Entry DOI: 10.7270/Q2MW2GVX |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
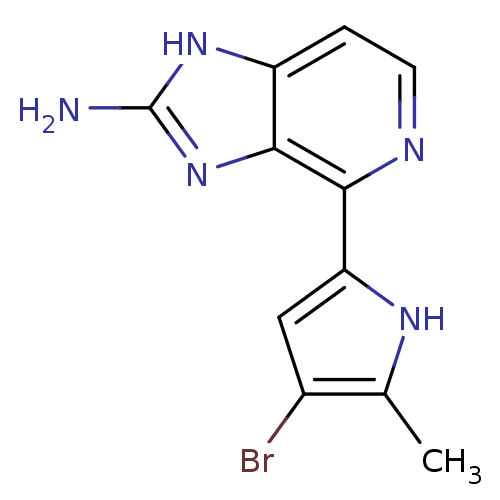
(Homo sapiens (Human)) | BDBM50301403
(4-(4-bromo-5-methyl-1H-pyrrol-2-yl)-1H-imidazo[4,5...)Show InChI InChI=1S/C11H10BrN5/c1-5-6(12)4-8(15-5)9-10-7(2-3-14-9)16-11(13)17-10/h2-4,15H,1H3,(H3,13,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human MMP12 catalytic domain |
Bioorg Med Chem Lett 19: 5461-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.099
BindingDB Entry DOI: 10.7270/Q2HX1CRC |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50215927
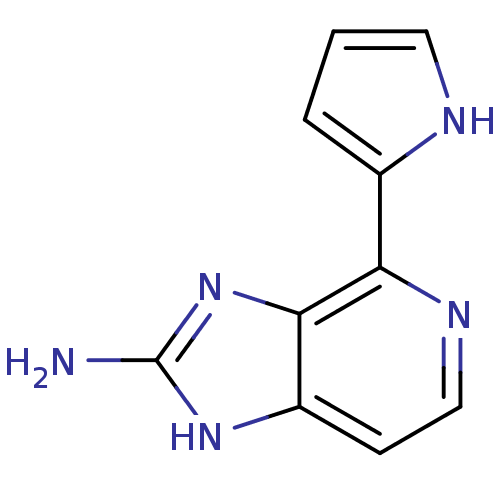
(4-(1H-pyrrol-2-yl)-1H-imidazo[4,5-c]pyridin-2-amin...)Show InChI InChI=1S/C10H9N5/c11-10-14-7-3-5-13-8(9(7)15-10)6-2-1-4-12-6/h1-5,12H,(H3,11,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem Lett 17: 4495-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.005
BindingDB Entry DOI: 10.7270/Q2MW2GVX |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50215925

(4-(4,5-dibromo-1H-pyrrol-2-yl)-N,N-dimethyl-1H-imi...)Show InChI InChI=1S/C12H11Br2N5/c1-19(2)12-17-7-3-4-15-9(10(7)18-12)8-5-6(13)11(14)16-8/h3-5,16H,1-2H3,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem Lett 17: 4495-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.005
BindingDB Entry DOI: 10.7270/Q2MW2GVX |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50215928

(4-(4-bromo-1H-pyrrol-2-yl)-1H-imidazo[4,5-c]pyridi...)Show InChI InChI=1S/C10H8BrN5/c11-5-3-7(14-4-5)8-9-6(1-2-13-8)15-10(12)16-9/h1-4,14H,(H3,12,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human MMP12 catalytic domain |
Bioorg Med Chem Lett 19: 5461-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.099
BindingDB Entry DOI: 10.7270/Q2HX1CRC |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50215930

(4-(5-bromo-1H-pyrrol-2-yl)-1H-imidazo[4,5-c]pyridi...)Show InChI InChI=1S/C10H8BrN5/c11-7-2-1-5(14-7)8-9-6(3-4-13-8)15-10(12)16-9/h1-4,14H,(H3,12,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human MMP12 catalytic domain |
Bioorg Med Chem Lett 19: 5461-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.099
BindingDB Entry DOI: 10.7270/Q2HX1CRC |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50301420
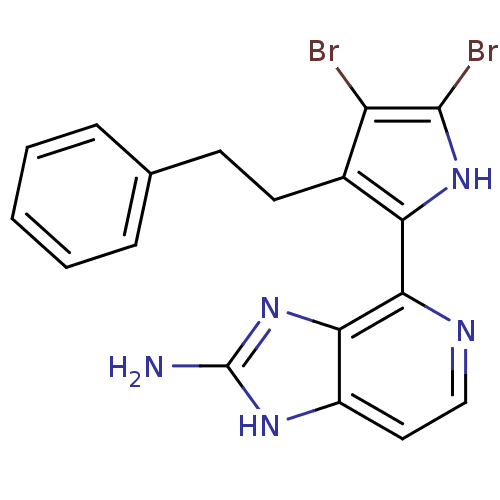
(4-(4,5-dibromo-3-phenethyl-1H-pyrrol-2-yl)-1H-imid...)Show SMILES Nc1nc2c(nccc2[nH]1)-c1[nH]c(Br)c(Br)c1CCc1ccccc1 Show InChI InChI=1S/C18H15Br2N5/c19-13-11(7-6-10-4-2-1-3-5-10)14(24-17(13)20)16-15-12(8-9-22-16)23-18(21)25-15/h1-5,8-9,24H,6-7H2,(H3,21,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human MMP12 catalytic domain |
Bioorg Med Chem Lett 19: 5461-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.099
BindingDB Entry DOI: 10.7270/Q2HX1CRC |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50301419
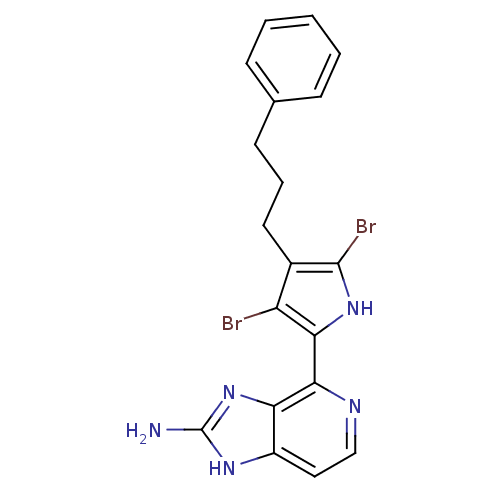
(4-(3,5-dibromo-4-(3-phenylpropyl)-1H-pyrrol-2-yl)-...)Show SMILES Nc1nc2c(nccc2[nH]1)-c1[nH]c(Br)c(CCCc2ccccc2)c1Br Show InChI InChI=1S/C19H17Br2N5/c20-14-12(8-4-7-11-5-2-1-3-6-11)18(21)25-16(14)17-15-13(9-10-23-17)24-19(22)26-15/h1-3,5-6,9-10,25H,4,7-8H2,(H3,22,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human MMP12 catalytic domain |
Bioorg Med Chem Lett 19: 5461-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.099
BindingDB Entry DOI: 10.7270/Q2HX1CRC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data