 Found 76 hits with Last Name = 'toth' and Initial = 'lm'
Found 76 hits with Last Name = 'toth' and Initial = 'lm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50186377
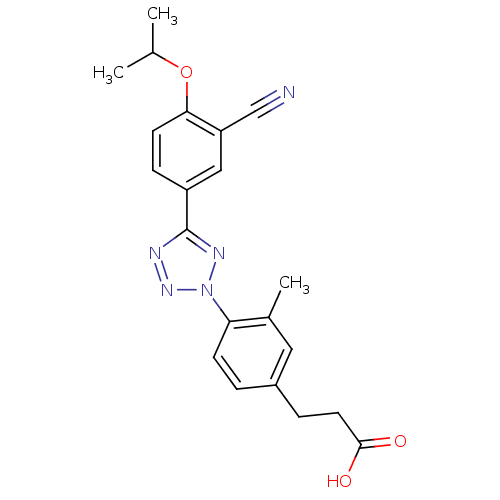
(3-(4-(5-(3-cyano-4-isopropoxyphenyl)-2H-tetrazol-2...)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nnn(n1)-c1ccc(CCC(O)=O)cc1C Show InChI InChI=1S/C21H21N5O3/c1-13(2)29-19-8-6-16(11-17(19)12-22)21-23-25-26(24-21)18-7-4-15(10-14(18)3)5-9-20(27)28/h4,6-8,10-11,13H,5,9H2,1-3H3,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P1 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50186378
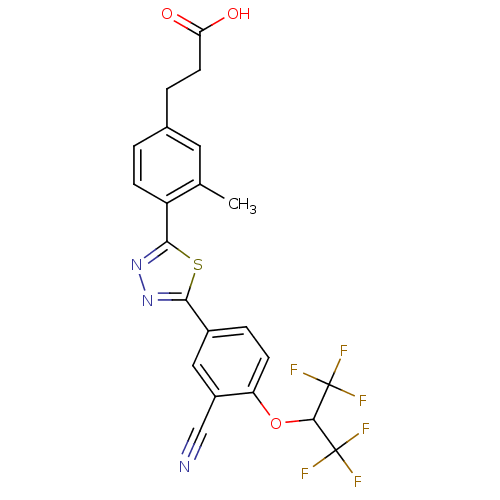
(3-(4-(5-(3-cyano-4-(1,1,1,3,3,3-hexafluoropropan-2...)Show SMILES Cc1cc(CCC(O)=O)ccc1-c1nnc(s1)-c1ccc(OC(C(F)(F)F)C(F)(F)F)c(c1)C#N Show InChI InChI=1S/C22H15F6N3O3S/c1-11-8-12(3-7-17(32)33)2-5-15(11)19-31-30-18(35-19)13-4-6-16(14(9-13)10-29)34-20(21(23,24)25)22(26,27)28/h2,4-6,8-9,20H,3,7H2,1H3,(H,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 38 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P1 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50186379

(3-(4-(5-(3-cyano-4-isopropoxyphenyl)furan-2-yl)-3-...)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1ccc(o1)-c1ccc(CCC(O)=O)cc1C Show InChI InChI=1S/C24H23NO4/c1-15(2)28-22-8-6-18(13-19(22)14-25)21-9-10-23(29-21)20-7-4-17(12-16(20)3)5-11-24(26)27/h4,6-10,12-13,15H,5,11H2,1-3H3,(H,26,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P5 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50186382
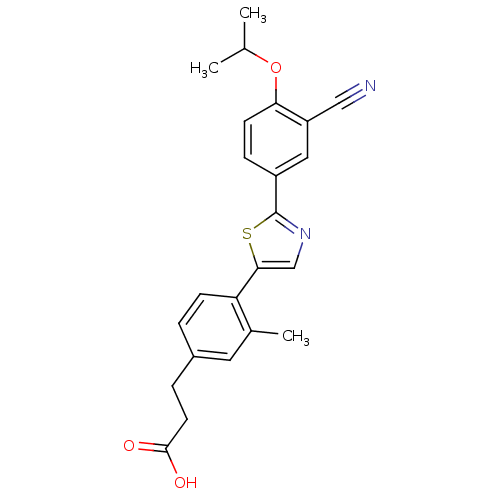
(3-(4-(2-(3-cyano-4-isopropoxyphenyl)thiazol-5-yl)-...)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1ncc(s1)-c1ccc(CCC(O)=O)cc1C Show InChI InChI=1S/C23H22N2O3S/c1-14(2)28-20-8-6-17(11-18(20)12-24)23-25-13-21(29-23)19-7-4-16(10-15(19)3)5-9-22(26)27/h4,6-8,10-11,13-14H,5,9H2,1-3H3,(H,26,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P3 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50186380
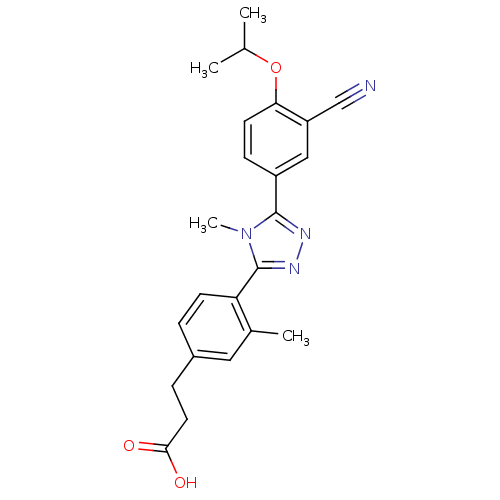
(3-(4-(5-(3-cyano-4-isopropoxyphenyl)-4-methyl-4H-1...)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nnc(-c2ccc(CCC(O)=O)cc2C)n1C Show InChI InChI=1S/C23H24N4O3/c1-14(2)30-20-9-7-17(12-18(20)13-24)22-25-26-23(27(22)4)19-8-5-16(11-15(19)3)6-10-21(28)29/h5,7-9,11-12,14H,6,10H2,1-4H3,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P1 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50186381
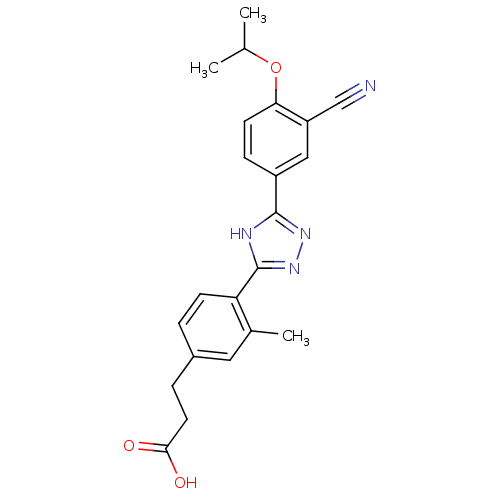
(3-(4-(5-(3-cyano-4-isopropoxyphenyl)-4H-1,2,4-tria...)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nnc([nH]1)-c1ccc(CCC(O)=O)cc1C Show InChI InChI=1S/C22H22N4O3/c1-13(2)29-19-8-6-16(11-17(19)12-23)21-24-22(26-25-21)18-7-4-15(10-14(18)3)5-9-20(27)28/h4,6-8,10-11,13H,5,9H2,1-3H3,(H,27,28)(H,24,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P3 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50186383

(3-(4-(5-(3-cyano-4-(cyanomethoxy)phenyl)-1,3,4-thi...)Show SMILES Cc1cc(CCC(O)=O)ccc1-c1nnc(s1)-c1ccc(OCC#N)c(c1)C#N Show InChI InChI=1S/C21H16N4O3S/c1-13-10-14(3-7-19(26)27)2-5-17(13)21-25-24-20(29-21)15-4-6-18(28-9-8-22)16(11-15)12-23/h2,4-6,10-11H,3,7,9H2,1H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 45 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P1 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50186385
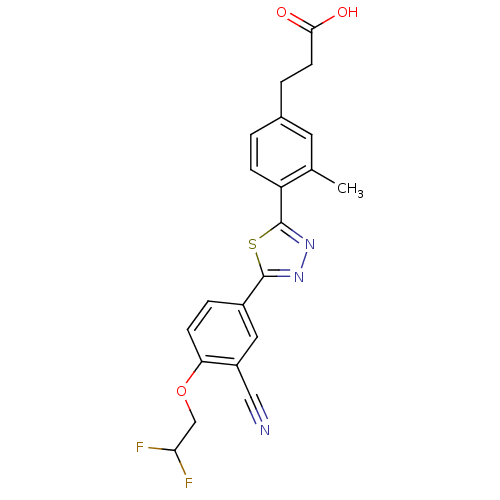
(3-(4-(5-(3-cyano-4-(2,2-difluoroethoxy)phenyl)-1,3...)Show SMILES Cc1cc(CCC(O)=O)ccc1-c1nnc(s1)-c1ccc(OCC(F)F)c(c1)C#N Show InChI InChI=1S/C21H17F2N3O3S/c1-12-8-13(3-7-19(27)28)2-5-16(12)21-26-25-20(30-21)14-4-6-17(15(9-14)10-24)29-11-18(22)23/h2,4-6,8-9,18H,3,7,11H2,1H3,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P1 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50186386
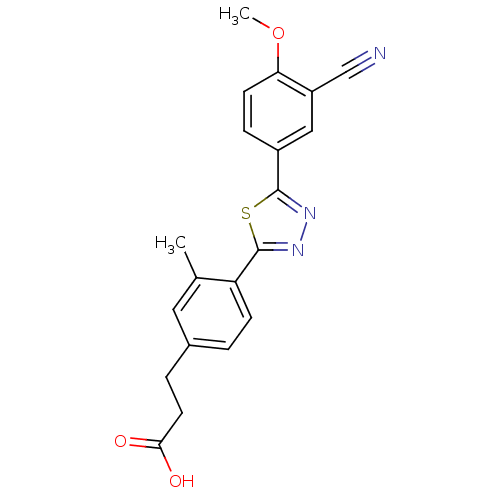
(3-(4-(5-(3-cyano-4-methoxyphenyl)-1,3,4-thiadiazol...)Show SMILES COc1ccc(cc1C#N)-c1nnc(s1)-c1ccc(CCC(O)=O)cc1C Show InChI InChI=1S/C20H17N3O3S/c1-12-9-13(4-8-18(24)25)3-6-16(12)20-23-22-19(27-20)14-5-7-17(26-2)15(10-14)11-21/h3,5-7,9-10H,4,8H2,1-2H3,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 97 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P1 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50186384
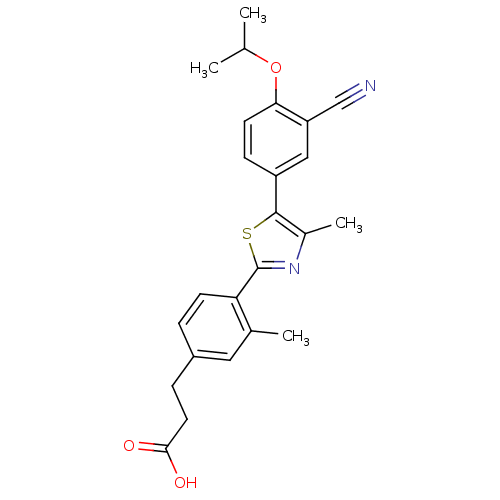
(3-(4-(5-(3-cyano-4-isopropoxyphenyl)-4-methylthiaz...)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1sc(nc1C)-c1ccc(CCC(O)=O)cc1C Show InChI InChI=1S/C24H24N2O3S/c1-14(2)29-21-9-7-18(12-19(21)13-25)23-16(4)26-24(30-23)20-8-5-17(11-15(20)3)6-10-22(27)28/h5,7-9,11-12,14H,6,10H2,1-4H3,(H,27,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P3 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50186379

(3-(4-(5-(3-cyano-4-isopropoxyphenyl)furan-2-yl)-3-...)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1ccc(o1)-c1ccc(CCC(O)=O)cc1C Show InChI InChI=1S/C24H23NO4/c1-15(2)28-22-8-6-18(13-19(22)14-25)21-9-10-23(29-21)20-7-4-17(12-16(20)3)5-11-24(26)27/h4,6-10,12-13,15H,5,11H2,1-3H3,(H,26,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P3 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50186391
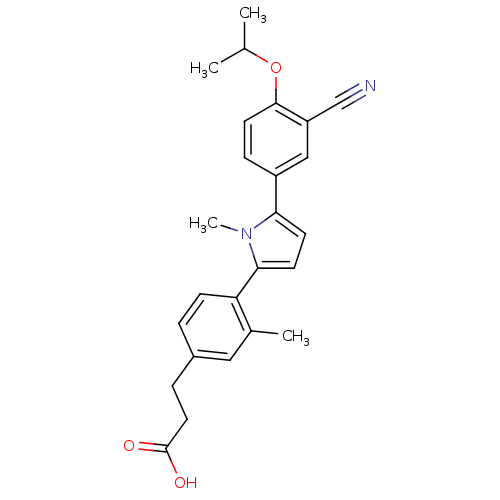
(3-(4-(5-(3-cyano-4-isopropoxyphenyl)-1-methyl-1H-p...)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1ccc(-c2ccc(CCC(O)=O)cc2C)n1C Show InChI InChI=1S/C25H26N2O3/c1-16(2)30-24-11-7-19(14-20(24)15-26)22-9-10-23(27(22)4)21-8-5-18(13-17(21)3)6-12-25(28)29/h5,7-11,13-14,16H,6,12H2,1-4H3,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P1 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50186389
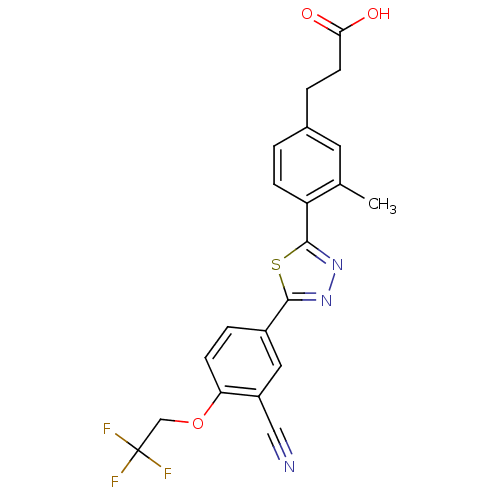
(3-(4-(5-(3-cyano-4-(2,2,2-trifluoroethoxy)phenyl)-...)Show SMILES Cc1cc(CCC(O)=O)ccc1-c1nnc(s1)-c1ccc(OCC(F)(F)F)c(c1)C#N Show InChI InChI=1S/C21H16F3N3O3S/c1-12-8-13(3-7-18(28)29)2-5-16(12)20-27-26-19(31-20)14-4-6-17(15(9-14)10-25)30-11-21(22,23)24/h2,4-6,8-9H,3,7,11H2,1H3,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P1 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50186390

(3-(4-(5-(3-cyano-4-isopropoxyphenyl)thiophen-3-yl)...)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1cc(cs1)-c1ccc(CCC(O)=O)cc1C Show InChI InChI=1S/C24H23NO3S/c1-15(2)28-22-8-6-18(11-19(22)13-25)23-12-20(14-29-23)21-7-4-17(10-16(21)3)5-9-24(26)27/h4,6-8,10-12,14-15H,5,9H2,1-3H3,(H,26,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P5 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50186381

(3-(4-(5-(3-cyano-4-isopropoxyphenyl)-4H-1,2,4-tria...)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nnc([nH]1)-c1ccc(CCC(O)=O)cc1C Show InChI InChI=1S/C22H22N4O3/c1-13(2)29-19-8-6-16(11-17(19)12-23)21-24-22(26-25-21)18-7-4-15(10-14(18)3)5-9-20(27)28/h4,6-8,10-11,13H,5,9H2,1-3H3,(H,27,28)(H,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 380 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P1 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50186388

(3-(4-(5-(3-cyano-4-isopropoxyphenyl)oxazol-2-yl)-3...)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1cnc(o1)-c1ccc(CCC(O)=O)cc1C Show InChI InChI=1S/C23H22N2O4/c1-14(2)28-20-8-6-17(11-18(20)12-24)21-13-25-23(29-21)19-7-4-16(10-15(19)3)5-9-22(26)27/h4,6-8,10-11,13-14H,5,9H2,1-3H3,(H,26,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P5 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50186388

(3-(4-(5-(3-cyano-4-isopropoxyphenyl)oxazol-2-yl)-3...)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1cnc(o1)-c1ccc(CCC(O)=O)cc1C Show InChI InChI=1S/C23H22N2O4/c1-14(2)28-20-8-6-17(11-18(20)12-24)21-13-25-23(29-21)19-7-4-16(10-15(19)3)5-9-22(26)27/h4,6-8,10-11,13-14H,5,9H2,1-3H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P1 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50186387

(3-(4-(5-(3-cyano-4-isobutoxyphenyl)-1,3,4-thiadiaz...)Show SMILES CC(C)COc1ccc(cc1C#N)-c1nnc(s1)-c1ccc(CCC(O)=O)cc1C Show InChI InChI=1S/C23H23N3O3S/c1-14(2)13-29-20-8-6-17(11-18(20)12-24)22-25-26-23(30-22)19-7-4-16(10-15(19)3)5-9-21(27)28/h4,6-8,10-11,14H,5,9,13H2,1-3H3,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P1 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50186392
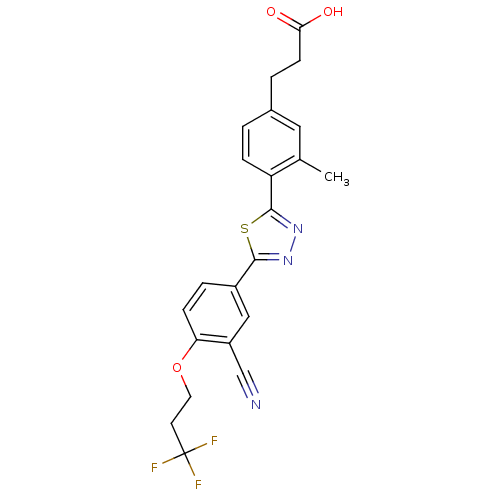
(3-(4-(5-(3-cyano-4-(3,3,3-trifluoropropoxy)phenyl)...)Show SMILES Cc1cc(CCC(O)=O)ccc1-c1nnc(s1)-c1ccc(OCCC(F)(F)F)c(c1)C#N Show InChI InChI=1S/C22H18F3N3O3S/c1-13-10-14(3-7-19(29)30)2-5-17(13)21-28-27-20(32-21)15-4-6-18(16(11-15)12-26)31-9-8-22(23,24)25/h2,4-6,10-11H,3,7-9H2,1H3,(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P1 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM22223
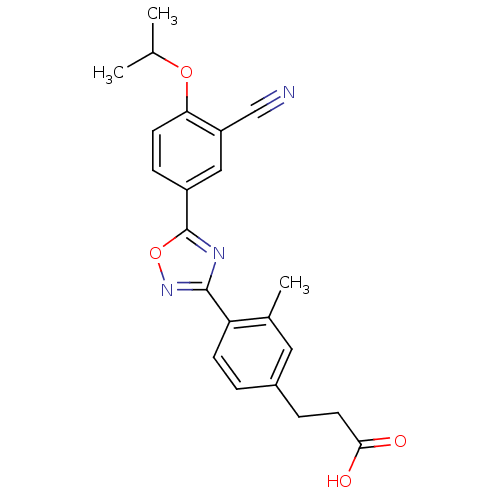
(3-(4-{5-[3-cyano-4-(propan-2-yloxy)phenyl]-1,2,4-o...)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nc(no1)-c1ccc(CCC(O)=O)cc1C Show InChI InChI=1S/C22H21N3O4/c1-13(2)28-19-8-6-16(11-17(19)12-23)22-24-21(25-29-22)18-7-4-15(10-14(18)3)5-9-20(26)27/h4,6-8,10-11,13H,5,9H2,1-3H3,(H,26,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P5 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50186393
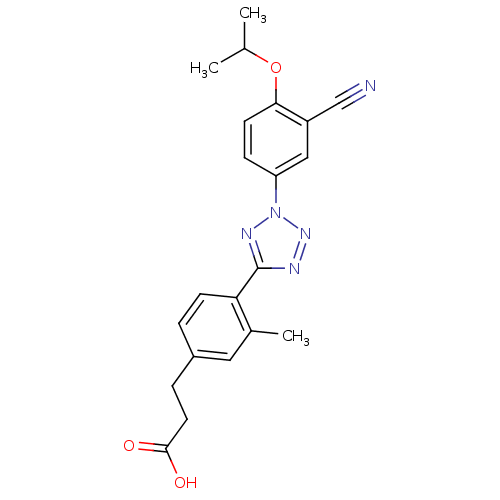
(3-(4-(2-(3-cyano-4-isopropoxyphenyl)-2H-tetrazol-5...)Show SMILES CC(C)Oc1ccc(cc1C#N)-n1nnc(n1)-c1ccc(CCC(O)=O)cc1C Show InChI InChI=1S/C21H21N5O3/c1-13(2)29-19-8-6-17(11-16(19)12-22)26-24-21(23-25-26)18-7-4-15(10-14(18)3)5-9-20(27)28/h4,6-8,10-11,13H,5,9H2,1-3H3,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P1 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50186384

(3-(4-(5-(3-cyano-4-isopropoxyphenyl)-4-methylthiaz...)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1sc(nc1C)-c1ccc(CCC(O)=O)cc1C Show InChI InChI=1S/C24H24N2O3S/c1-14(2)29-21-9-7-18(12-19(21)13-25)23-16(4)26-24(30-23)20-8-5-17(11-15(20)3)6-10-22(27)28/h5,7-9,11-12,14H,6,10H2,1-4H3,(H,27,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P5 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50186396

(3-(4-(4-(3-cyano-4-isopropoxyphenyl)thiophen-2-yl)...)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1csc(c1)-c1ccc(CCC(O)=O)cc1C Show InChI InChI=1S/C24H23NO3S/c1-15(2)28-22-8-6-18(11-19(22)13-25)20-12-23(29-14-20)21-7-4-17(10-16(21)3)5-9-24(26)27/h4,6-8,10-12,14-15H,5,9H2,1-3H3,(H,26,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P5 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50186394
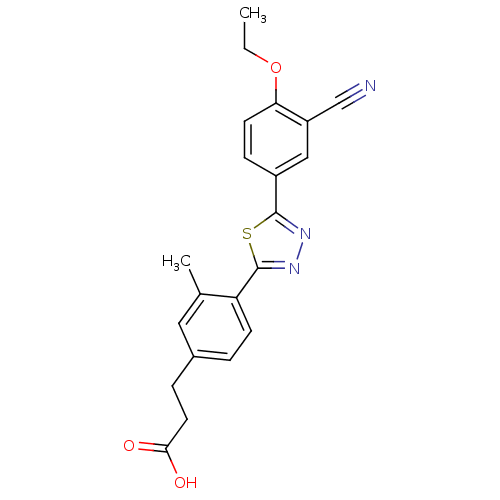
(3-(4-(5-(3-cyano-4-ethoxyphenyl)-1,3,4-thiadiazol-...)Show SMILES CCOc1ccc(cc1C#N)-c1nnc(s1)-c1ccc(CCC(O)=O)cc1C Show InChI InChI=1S/C21H19N3O3S/c1-3-27-18-8-6-15(11-16(18)12-22)20-23-24-21(28-20)17-7-4-14(10-13(17)2)5-9-19(25)26/h4,6-8,10-11H,3,5,9H2,1-2H3,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P1 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50186395
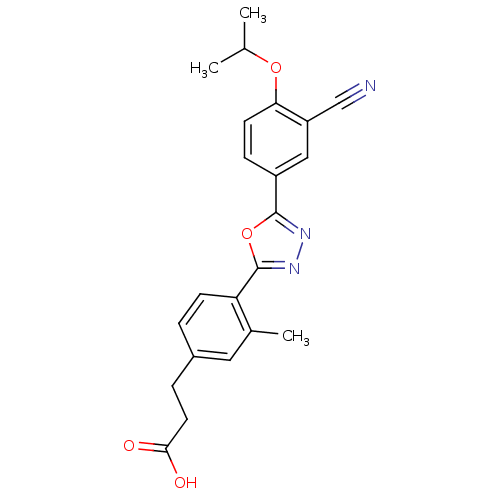
(3-(4-(5-(3-cyano-4-isopropoxyphenyl)-1,3,4-oxadiaz...)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nnc(o1)-c1ccc(CCC(O)=O)cc1C Show InChI InChI=1S/C22H21N3O4/c1-13(2)28-19-8-6-16(11-17(19)12-23)21-24-25-22(29-21)18-7-4-15(10-14(18)3)5-9-20(26)27/h4,6-8,10-11,13H,5,9H2,1-3H3,(H,26,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 460 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P5 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50186377

(3-(4-(5-(3-cyano-4-isopropoxyphenyl)-2H-tetrazol-2...)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nnn(n1)-c1ccc(CCC(O)=O)cc1C Show InChI InChI=1S/C21H21N5O3/c1-13(2)29-19-8-6-16(11-17(19)12-22)21-23-25-26(24-21)18-7-4-15(10-14(18)3)5-9-20(27)28/h4,6-8,10-11,13H,5,9H2,1-3H3,(H,27,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P3 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50186396

(3-(4-(4-(3-cyano-4-isopropoxyphenyl)thiophen-2-yl)...)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1csc(c1)-c1ccc(CCC(O)=O)cc1C Show InChI InChI=1S/C24H23NO3S/c1-15(2)28-22-8-6-18(11-19(22)13-25)20-12-23(29-14-20)21-7-4-17(10-16(21)3)5-9-24(26)27/h4,6-8,10-12,14-15H,5,9H2,1-3H3,(H,26,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P3 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50186398
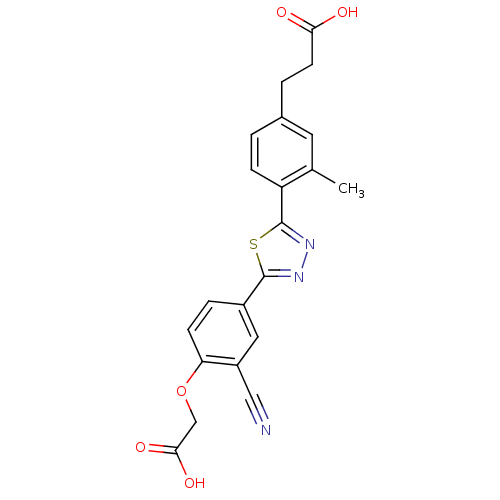
(3-(4-(5-(4-(carboxymethoxy)-3-cyanophenyl)-1,3,4-t...)Show SMILES Cc1cc(CCC(O)=O)ccc1-c1nnc(s1)-c1ccc(OCC(O)=O)c(c1)C#N Show InChI InChI=1S/C21H17N3O5S/c1-12-8-13(3-7-18(25)26)2-5-16(12)21-24-23-20(30-21)14-4-6-17(15(9-14)10-22)29-11-19(27)28/h2,4-6,8-9H,3,7,11H2,1H3,(H,25,26)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 360 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P1 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50186380

(3-(4-(5-(3-cyano-4-isopropoxyphenyl)-4-methyl-4H-1...)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nnc(-c2ccc(CCC(O)=O)cc2C)n1C Show InChI InChI=1S/C23H24N4O3/c1-14(2)30-20-9-7-17(12-18(20)13-24)22-25-26-23(27(22)4)19-8-5-16(11-15(19)3)6-10-21(28)29/h5,7-9,11-12,14H,6,10H2,1-4H3,(H,28,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P3 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50186381

(3-(4-(5-(3-cyano-4-isopropoxyphenyl)-4H-1,2,4-tria...)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nnc([nH]1)-c1ccc(CCC(O)=O)cc1C Show InChI InChI=1S/C22H22N4O3/c1-13(2)29-19-8-6-16(11-17(19)12-23)21-24-22(26-25-21)18-7-4-15(10-14(18)3)5-9-20(27)28/h4,6-8,10-11,13H,5,9H2,1-3H3,(H,27,28)(H,24,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P5 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50186397

(3-(4-(5-(3-cyano-4-isopropoxyphenyl)thiophen-2-yl)...)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1ccc(s1)-c1ccc(CCC(O)=O)cc1C Show InChI InChI=1S/C24H23NO3S/c1-15(2)28-21-8-6-18(13-19(21)14-25)22-9-10-23(29-22)20-7-4-17(12-16(20)3)5-11-24(26)27/h4,6-10,12-13,15H,5,11H2,1-3H3,(H,26,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 220 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P5 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50186382

(3-(4-(2-(3-cyano-4-isopropoxyphenyl)thiazol-5-yl)-...)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1ncc(s1)-c1ccc(CCC(O)=O)cc1C Show InChI InChI=1S/C23H22N2O3S/c1-14(2)28-20-8-6-17(11-18(20)12-24)23-25-13-21(29-23)19-7-4-16(10-15(19)3)5-9-22(26)27/h4,6-8,10-11,13-14H,5,9H2,1-3H3,(H,26,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P5 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50186382

(3-(4-(2-(3-cyano-4-isopropoxyphenyl)thiazol-5-yl)-...)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1ncc(s1)-c1ccc(CCC(O)=O)cc1C Show InChI InChI=1S/C23H22N2O3S/c1-14(2)28-20-8-6-17(11-18(20)12-24)23-25-13-21(29-23)19-7-4-16(10-15(19)3)5-9-22(26)27/h4,6-8,10-11,13-14H,5,9H2,1-3H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P1 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50186391

(3-(4-(5-(3-cyano-4-isopropoxyphenyl)-1-methyl-1H-p...)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1ccc(-c2ccc(CCC(O)=O)cc2C)n1C Show InChI InChI=1S/C25H26N2O3/c1-16(2)30-24-11-7-19(14-20(24)15-26)22-9-10-23(27(22)4)21-8-5-18(13-17(21)3)6-12-25(28)29/h5,7-11,13-14,16H,6,12H2,1-4H3,(H,28,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P3 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50186400

(3-(4-(5-(3-cyano-4-fluorophenyl)-1,3,4-thiadiazol-...)Show SMILES Cc1cc(CCC(O)=O)ccc1-c1nnc(s1)-c1ccc(F)c(c1)C#N Show InChI InChI=1S/C19H14FN3O2S/c1-11-8-12(3-7-17(24)25)2-5-15(11)19-23-22-18(26-19)13-4-6-16(20)14(9-13)10-21/h2,4-6,8-9H,3,7H2,1H3,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 570 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P1 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50186401

(3-(4-(5-(3-cyano-4-isobutylphenyl)-1,3,4-thiadiazo...)Show SMILES CC(C)Cc1ccc(cc1C#N)-c1nnc(s1)-c1ccc(CCC(O)=O)cc1C Show InChI InChI=1S/C23H23N3O2S/c1-14(2)10-17-6-7-18(12-19(17)13-24)22-25-26-23(29-22)20-8-4-16(11-15(20)3)5-9-21(27)28/h4,6-8,11-12,14H,5,9-10H2,1-3H3,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P1 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50186399
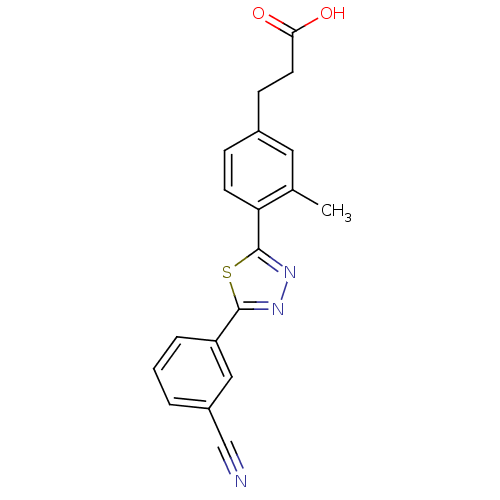
(3-(4-(5-(3-cyanophenyl)-1,3,4-thiadiazol-2-yl)-3-m...)Show SMILES Cc1cc(CCC(O)=O)ccc1-c1nnc(s1)-c1cccc(c1)C#N Show InChI InChI=1S/C19H15N3O2S/c1-12-9-13(6-8-17(23)24)5-7-16(12)19-22-21-18(25-19)15-4-2-3-14(10-15)11-20/h2-5,7,9-10H,6,8H2,1H3,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P1 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50186402

(3-(4-(5-(3-cyano-4-isopropoxyphenyl)thiazol-2-yl)-...)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1cnc(s1)-c1ccc(CCC(O)=O)cc1C Show InChI InChI=1S/C23H22N2O3S/c1-14(2)28-20-8-6-17(11-18(20)12-24)21-13-25-23(29-21)19-7-4-16(10-15(19)3)5-9-22(26)27/h4,6-8,10-11,13-14H,5,9H2,1-3H3,(H,26,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P3 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50186403
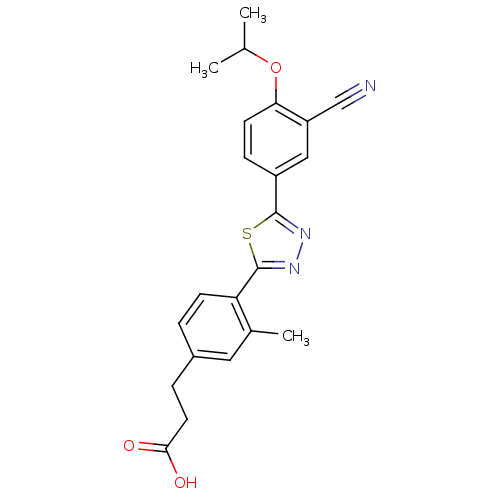
(3-(4-(5-(3-cyano-4-isopropoxyphenyl)-1,3,4-thiadia...)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nnc(s1)-c1ccc(CCC(O)=O)cc1C Show InChI InChI=1S/C22H21N3O3S/c1-13(2)28-19-8-6-16(11-17(19)12-23)21-24-25-22(29-21)18-7-4-15(10-14(18)3)5-9-20(26)27/h4,6-8,10-11,13H,5,9H2,1-3H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P1 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50186404
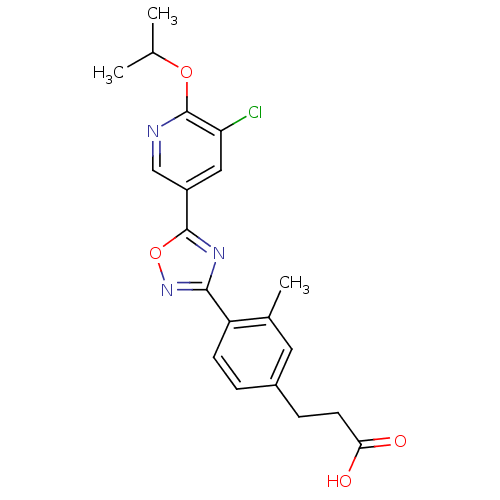
(3-(4-(5-(5-chloro-6-isopropoxypyridin-3-yl)-1,2,4-...)Show SMILES CC(C)Oc1ncc(cc1Cl)-c1nc(no1)-c1ccc(CCC(O)=O)cc1C Show InChI InChI=1S/C20H20ClN3O4/c1-11(2)27-20-16(21)9-14(10-22-20)19-23-18(24-28-19)15-6-4-13(8-12(15)3)5-7-17(25)26/h4,6,8-11H,5,7H2,1-3H3,(H,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 950 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P3 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50186405
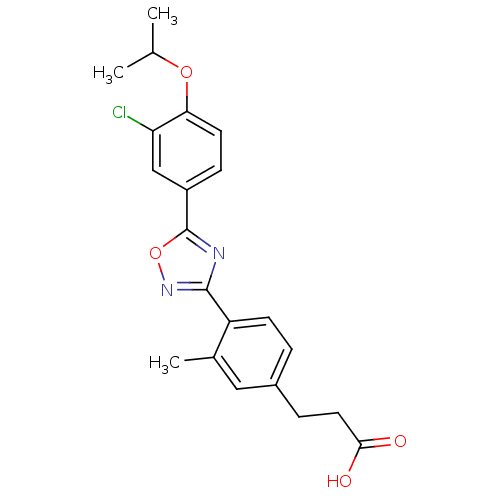
(3-(4-(5-(3-chloro-4-isopropoxyphenyl)-1,2,4-oxadia...)Show SMILES CC(C)Oc1ccc(cc1Cl)-c1nc(no1)-c1ccc(CCC(O)=O)cc1C Show InChI InChI=1S/C21H21ClN2O4/c1-12(2)27-18-8-6-15(11-17(18)22)21-23-20(24-28-21)16-7-4-14(10-13(16)3)5-9-19(25)26/h4,6-8,10-12H,5,9H2,1-3H3,(H,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 73 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P5 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50186406
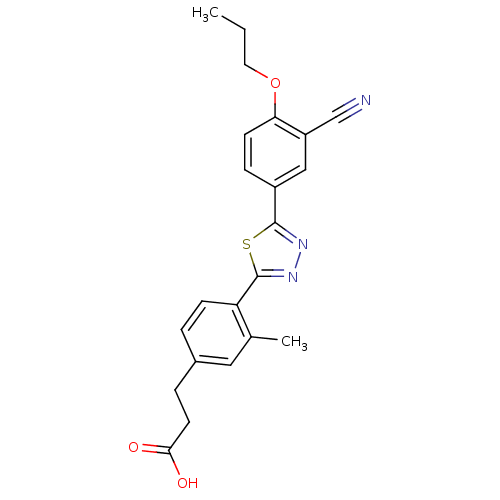
(3-(4-(5-(3-cyano-4-propoxyphenyl)-1,3,4-thiadiazol...)Show SMILES CCCOc1ccc(cc1C#N)-c1nnc(s1)-c1ccc(CCC(O)=O)cc1C Show InChI InChI=1S/C22H21N3O3S/c1-3-10-28-19-8-6-16(12-17(19)13-23)21-24-25-22(29-21)18-7-4-15(11-14(18)2)5-9-20(26)27/h4,6-8,11-12H,3,5,9-10H2,1-2H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P1 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50186407
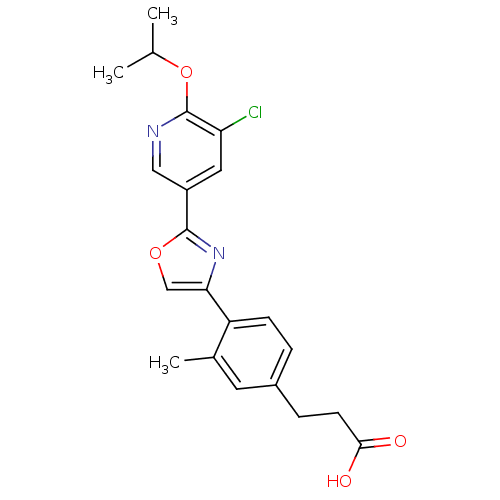
(3-(4-(2-(5-chloro-6-isopropoxypyridin-3-yl)oxazol-...)Show SMILES CC(C)Oc1ncc(cc1Cl)-c1nc(co1)-c1ccc(CCC(O)=O)cc1C Show InChI InChI=1S/C21H21ClN2O4/c1-12(2)28-21-17(22)9-15(10-23-21)20-24-18(11-27-20)16-6-4-14(8-13(16)3)5-7-19(25)26/h4,6,8-12H,5,7H2,1-3H3,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P1 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50186408
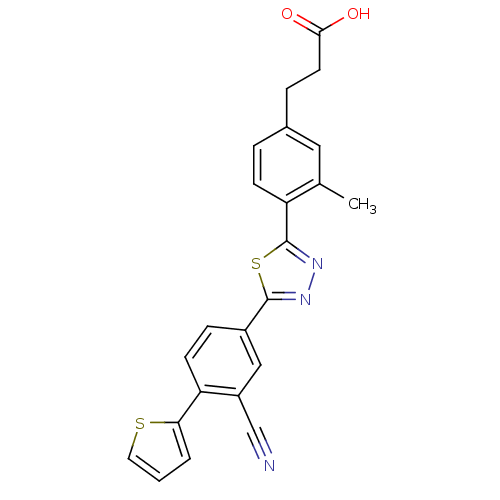
(3-(4-(5-(3-cyano-4-(thiophen-2-yl)phenyl)-1,3,4-th...)Show SMILES Cc1cc(CCC(O)=O)ccc1-c1nnc(s1)-c1ccc(-c2cccs2)c(c1)C#N Show InChI InChI=1S/C23H17N3O2S2/c1-14-11-15(5-9-21(27)28)4-7-18(14)23-26-25-22(30-23)16-6-8-19(17(12-16)13-24)20-3-2-10-29-20/h2-4,6-8,10-12H,5,9H2,1H3,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P1 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50186404

(3-(4-(5-(5-chloro-6-isopropoxypyridin-3-yl)-1,2,4-...)Show SMILES CC(C)Oc1ncc(cc1Cl)-c1nc(no1)-c1ccc(CCC(O)=O)cc1C Show InChI InChI=1S/C20H20ClN3O4/c1-11(2)27-20-16(21)9-14(10-22-20)19-23-18(24-28-19)15-6-4-13(8-12(15)3)5-7-17(25)26/h4,6,8-11H,5,7H2,1-3H3,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P1 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM22223

(3-(4-{5-[3-cyano-4-(propan-2-yloxy)phenyl]-1,2,4-o...)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nc(no1)-c1ccc(CCC(O)=O)cc1C Show InChI InChI=1S/C22H21N3O4/c1-13(2)28-19-8-6-16(11-17(19)12-23)22-24-21(25-29-22)18-7-4-15(10-14(18)3)5-9-20(26)27/h4,6-8,10-11,13H,5,9H2,1-3H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P1 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50186409
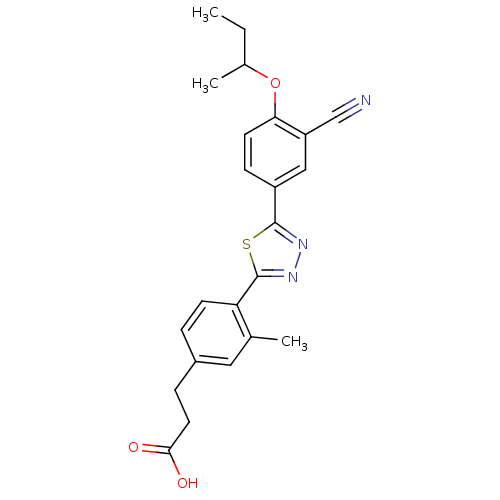
(3-(4-(5-(4-sec-butoxy-3-cyanophenyl)-1,3,4-thiadia...)Show SMILES CCC(C)Oc1ccc(cc1C#N)-c1nnc(s1)-c1ccc(CCC(O)=O)cc1C Show InChI InChI=1S/C23H23N3O3S/c1-4-15(3)29-20-9-7-17(12-18(20)13-24)22-25-26-23(30-22)19-8-5-16(11-14(19)2)6-10-21(27)28/h5,7-9,11-12,15H,4,6,10H2,1-3H3,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P1 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50186410
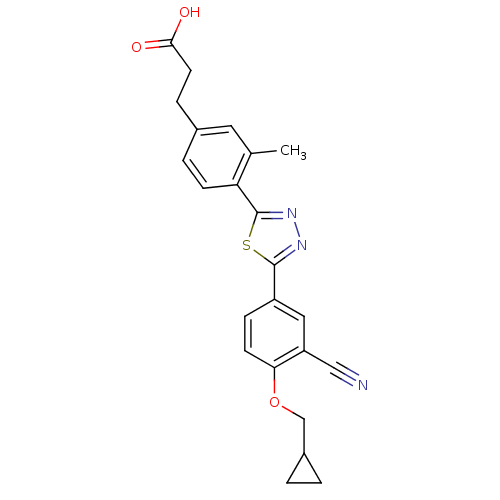
(3-(4-(5-(3-cyano-4-(cyclopropylmethoxy)phenyl)-1,3...)Show SMILES Cc1cc(CCC(O)=O)ccc1-c1nnc(s1)-c1ccc(OCC2CC2)c(c1)C#N Show InChI InChI=1S/C23H21N3O3S/c1-14-10-15(5-9-21(27)28)4-7-19(14)23-26-25-22(30-23)17-6-8-20(18(11-17)12-24)29-13-16-2-3-16/h4,6-8,10-11,16H,2-3,5,9,13H2,1H3,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P1 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50186390

(3-(4-(5-(3-cyano-4-isopropoxyphenyl)thiophen-3-yl)...)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1cc(cs1)-c1ccc(CCC(O)=O)cc1C Show InChI InChI=1S/C24H23NO3S/c1-15(2)28-22-8-6-18(11-19(22)13-25)23-12-20(14-29-23)21-7-4-17(10-16(21)3)5-9-24(26)27/h4,6-8,10-12,14-15H,5,9H2,1-3H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P1 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50186377

(3-(4-(5-(3-cyano-4-isopropoxyphenyl)-2H-tetrazol-2...)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nnn(n1)-c1ccc(CCC(O)=O)cc1C Show InChI InChI=1S/C21H21N5O3/c1-13(2)29-19-8-6-16(11-17(19)12-22)21-23-25-26(24-21)18-7-4-15(10-14(18)3)5-9-20(27)28/h4,6-8,10-11,13H,5,9H2,1-3H3,(H,27,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 150 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at S1P5 receptor assessed as induction of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 16: 3684-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.064
BindingDB Entry DOI: 10.7270/Q2HD7V7J |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data