 Found 296 hits with Last Name = 'toth' and Initial = 'm'
Found 296 hits with Last Name = 'toth' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM82122

(Vinylsulfone, 7)Show InChI InChI=1S/C15H14O3S2/c16-20(17,12-4-11-19)15-9-7-14(8-10-15)18-13-5-2-1-3-6-13/h1-10,12,19H,11H2/b12-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
| Assay Description
Inhibition assay using matrix metalloproteinase (MMP-2). |
Chem Biol Drug Des 74: 527-34 (2009)
Checked by Author
Article DOI: 10.1111/j.1747-0285.2009.00881.x
BindingDB Entry DOI: 10.7270/Q20P0XJV |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50010495

(Acetyl-Ser-Leu-Asn-Phe-[CH(OH)CH2N]Pro-Ile-Val-OMe...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1CC(O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](C(C)C)C(=O)OC Show InChI InChI=1S/C42H68N8O11/c1-9-25(6)36(41(59)48-35(24(4)5)42(60)61-8)49-40(58)32-16-13-17-50(32)21-33(53)28(19-27-14-11-10-12-15-27)45-38(56)30(20-34(43)54)47-37(55)29(18-23(2)3)46-39(57)31(22-51)44-26(7)52/h10-12,14-15,23-25,28-33,35-36,51,53H,9,13,16-22H2,1-8H3,(H2,43,54)(H,44,52)(H,45,56)(H,46,57)(H,47,55)(H,48,59)(H,49,58)/t25-,28-,29-,30-,31-,32-,33?,35-,36-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
In vitro binding affinity of the compound against HIV protease was measured |
J Med Chem 34: 1222-5 (1991)
BindingDB Entry DOI: 10.7270/Q2057DWM |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50014015
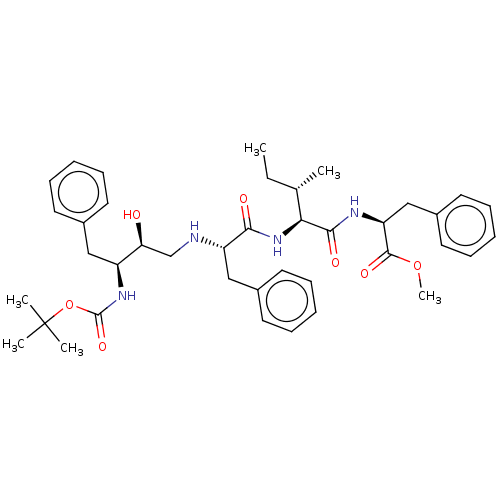
(2-{2-[2-(3-tert-Butoxycarbonylamino-2-hydroxy-4-ph...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccccc1)NC[C@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)N[C@@H](Cc1ccccc1)C(=O)OC |r| Show InChI InChI=1S/C40H54N4O7/c1-7-27(2)35(37(47)42-33(38(48)50-6)25-30-21-15-10-16-22-30)44-36(46)32(24-29-19-13-9-14-20-29)41-26-34(45)31(23-28-17-11-8-12-18-28)43-39(49)51-40(3,4)5/h8-22,27,31-35,41,45H,7,23-26H2,1-6H3,(H,42,47)(H,43,49)(H,44,46) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | 6.4 | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Inhibition of HIV protease at pH 6.4, 37 degree C |
J Med Chem 33: 1285-8 (1990)
BindingDB Entry DOI: 10.7270/Q2SB44QS |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50010497
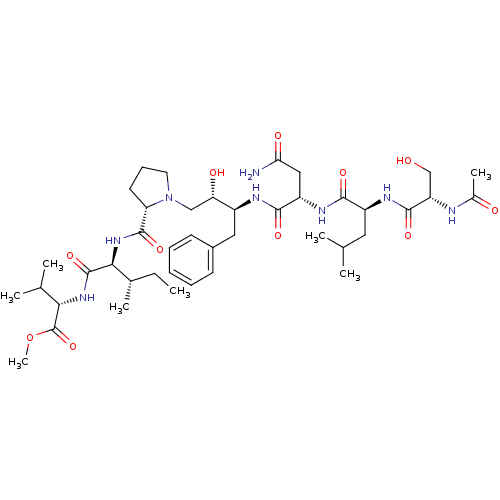
(Acetyl-Ser-Leu-Asn-Phe-[S]-[CH(OH)CH2N]Pro-Ile-Val...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](C(C)C)C(=O)OC Show InChI InChI=1S/C42H68N8O11/c1-9-25(6)36(41(59)48-35(24(4)5)42(60)61-8)49-40(58)32-16-13-17-50(32)21-33(53)28(19-27-14-11-10-12-15-27)45-38(56)30(20-34(43)54)47-37(55)29(18-23(2)3)46-39(57)31(22-51)44-26(7)52/h10-12,14-15,23-25,28-33,35-36,51,53H,9,13,16-22H2,1-8H3,(H2,43,54)(H,44,52)(H,45,56)(H,46,57)(H,47,55)(H,48,59)(H,49,58)/t25-,28-,29-,30-,31-,32-,33-,35-,36-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | 6.4 | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Binding affinity against Cysteinyl leukotriene D4 receptor from guinea pig trachea using [3H]LTD4 |
J Med Chem 33: 1285-8 (1990)
BindingDB Entry DOI: 10.7270/Q2SB44QS |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50335495
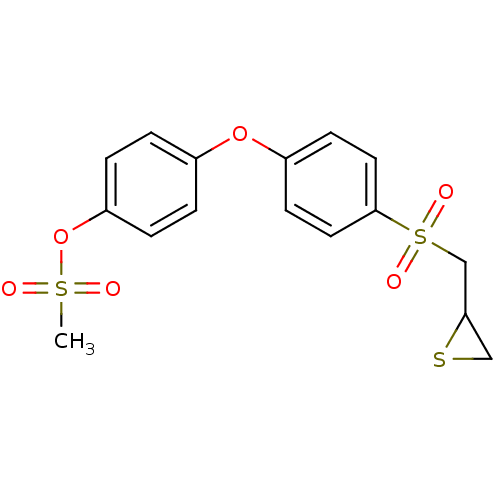
(4-(4-(thiiran-2-ylmethylsulfonyl)phenoxy)phenyl me...)Show SMILES CS(=O)(=O)Oc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C16H16O6S3/c1-24(17,18)22-14-4-2-12(3-5-14)21-13-6-8-16(9-7-13)25(19,20)11-15-10-23-15/h2-9,15H,10-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP9 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50335495

(4-(4-(thiiran-2-ylmethylsulfonyl)phenoxy)phenyl me...)Show SMILES CS(=O)(=O)Oc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C16H16O6S3/c1-24(17,18)22-14-4-2-12(3-5-14)21-13-6-8-16(9-7-13)25(19,20)11-15-10-23-15/h2-9,15H,10-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP9 catalytic domain by substrate hydrolysis based fluorescence spectrophotometry |
ACS Med Chem Lett 2: 177-181 (2011)
Article DOI: 10.1021/ml100254e
BindingDB Entry DOI: 10.7270/Q2N29X6G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50388914
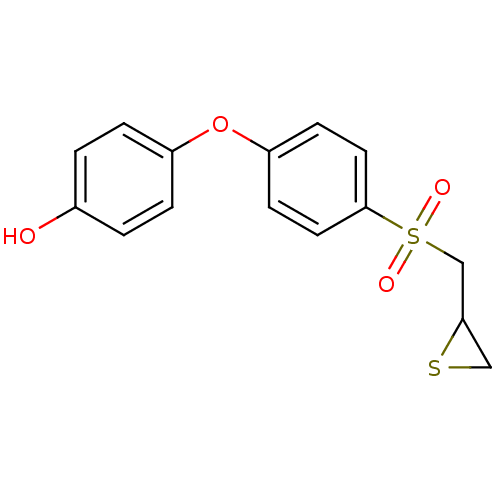
(CHEMBL2063274 | US10357546, p-OH SB-3CT)Show InChI InChI=1S/C15H14O4S2/c16-11-1-3-12(4-2-11)19-13-5-7-15(8-6-13)21(17,18)10-14-9-20-14/h1-8,14,16H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP2 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50014024
(2-(2-{[1-(2-{2-[2-(2-Acetylamino-3-hydroxy-propion...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](C(C)C)C(=O)OC |r| Show InChI InChI=1S/C39H70N8O11/c1-11-23(8)33(38(56)45-32(22(6)7)39(57)58-10)46-37(55)29-13-12-14-47(29)18-30(50)25(15-20(2)3)42-35(53)27(17-31(40)51)44-34(52)26(16-21(4)5)43-36(54)28(19-48)41-24(9)49/h20-23,25-30,32-33,48,50H,11-19H2,1-10H3,(H2,40,51)(H,41,49)(H,42,53)(H,43,54)(H,44,52)(H,45,56)(H,46,55)/t23-,25-,26-,27-,28-,29-,30-,32-,33-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | 6.4 | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV Protease was measured at pH 6.4 and 37 degrees C |
J Med Chem 33: 1285-8 (1990)
BindingDB Entry DOI: 10.7270/Q2SB44QS |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50264809

(2-((4-phenoxyphenylsulfonyl)methyl)thiirane | CHEM...)Show InChI InChI=1S/C15H14O3S2/c16-20(17,11-14-10-19-14)15-8-6-13(7-9-15)18-12-4-2-1-3-5-12/h1-9,14H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP2 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50264809

(2-((4-phenoxyphenylsulfonyl)methyl)thiirane | CHEM...)Show InChI InChI=1S/C15H14O3S2/c16-20(17,11-14-10-19-14)15-8-6-13(7-9-15)18-12-4-2-1-3-5-12/h1-9,14H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 by substrate hydrolysis based fluorescence spectrophotometry |
ACS Med Chem Lett 2: 177-181 (2011)
Article DOI: 10.1021/ml100254e
BindingDB Entry DOI: 10.7270/Q2N29X6G |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50014020
(2-[2-(2-{3-[2-(2-Amino-propionylamino)-propionylam...)Show SMILES COC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CNC[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)[C@H](C)N)C(C)C)C(C)C |r| Show InChI InChI=1S/C29H48N6O7/c1-16(2)24(28(40)35-25(17(3)4)29(41)42-7)34-23(37)15-31-14-22(36)21(13-20-11-9-8-10-12-20)33-27(39)19(6)32-26(38)18(5)30/h8-12,16-19,21-22,24-25,31,36H,13-15,30H2,1-7H3,(H,32,38)(H,33,39)(H,34,37)(H,35,40)/t18-,19-,21-,22-,24-,25-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | 6.4 | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Inhibition of HIV protease at pH 6.4, 37 degree C |
J Med Chem 33: 1285-8 (1990)
BindingDB Entry DOI: 10.7270/Q2SB44QS |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50010497

(Acetyl-Ser-Leu-Asn-Phe-[S]-[CH(OH)CH2N]Pro-Ile-Val...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](C(C)C)C(=O)OC Show InChI InChI=1S/C42H68N8O11/c1-9-25(6)36(41(59)48-35(24(4)5)42(60)61-8)49-40(58)32-16-13-17-50(32)21-33(53)28(19-27-14-11-10-12-15-27)45-38(56)30(20-34(43)54)47-37(55)29(18-23(2)3)46-39(57)31(22-51)44-26(7)52/h10-12,14-15,23-25,28-33,35-36,51,53H,9,13,16-22H2,1-8H3,(H2,43,54)(H,44,52)(H,45,56)(H,46,57)(H,47,55)(H,48,59)(H,49,58)/t25-,28-,29-,30-,31-,32-,33-,35-,36-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
In vitro concentration of the compound required to inhibit 50% activity of HIV protease was measured (exp 2) |
J Med Chem 34: 1222-5 (1991)
BindingDB Entry DOI: 10.7270/Q2057DWM |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50010497

(Acetyl-Ser-Leu-Asn-Phe-[S]-[CH(OH)CH2N]Pro-Ile-Val...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](C(C)C)C(=O)OC Show InChI InChI=1S/C42H68N8O11/c1-9-25(6)36(41(59)48-35(24(4)5)42(60)61-8)49-40(58)32-16-13-17-50(32)21-33(53)28(19-27-14-11-10-12-15-27)45-38(56)30(20-34(43)54)47-37(55)29(18-23(2)3)46-39(57)31(22-51)44-26(7)52/h10-12,14-15,23-25,28-33,35-36,51,53H,9,13,16-22H2,1-8H3,(H2,43,54)(H,44,52)(H,45,56)(H,46,57)(H,47,55)(H,48,59)(H,49,58)/t25-,28-,29-,30-,31-,32-,33-,35-,36-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
In vitro concentration of the compound required to inhibit 50% activity of HIV protease was measured |
J Med Chem 34: 1222-5 (1991)
BindingDB Entry DOI: 10.7270/Q2057DWM |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50421882
(CHEMBL2311136)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H]1CCCN1C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(C)=O)C(=O)N[C@@H](C(C)C)C(=O)OC |r| Show InChI InChI=1S/C39H63N7O9/c1-9-24(6)34(38(53)44-33(23(4)5)39(54)55-8)45-37(52)30-16-13-17-46(30)21-31(48)27(19-26-14-11-10-12-15-26)42-36(51)29(20-32(40)49)43-35(50)28(18-22(2)3)41-25(7)47/h10-12,14-15,22-24,27-31,33-34,48H,9,13,16-21H2,1-8H3,(H2,40,49)(H,41,47)(H,42,51)(H,43,50)(H,44,53)(H,45,52)/t24-,27+,28+,29+,30+,31-,33+,34+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | 6.4 | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Inhibition of HIV protease at pH 6.4, 37 degree C |
J Med Chem 33: 1285-8 (1990)
BindingDB Entry DOI: 10.7270/Q2SB44QS |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50014019

(2-{2-[(1-{2-[2-(2-Acetylamino-4-methyl-pentanoylam...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(C)=O)C(=O)N[C@@H](C(C)C)C(=O)OC |r| Show InChI InChI=1S/C36H65N7O9/c1-11-22(8)31(35(50)41-30(21(6)7)36(51)52-10)42-34(49)27-13-12-14-43(27)18-28(45)24(15-19(2)3)39-33(48)26(17-29(37)46)40-32(47)25(16-20(4)5)38-23(9)44/h19-22,24-28,30-31,45H,11-18H2,1-10H3,(H2,37,46)(H,38,44)(H,39,48)(H,40,47)(H,41,50)(H,42,49) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | 6.4 | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Inhibition of HIV protease at pH 6.4, 37 degree C |
J Med Chem 33: 1285-8 (1990)
BindingDB Entry DOI: 10.7270/Q2SB44QS |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50335495

(4-(4-(thiiran-2-ylmethylsulfonyl)phenoxy)phenyl me...)Show SMILES CS(=O)(=O)Oc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C16H16O6S3/c1-24(17,18)22-14-4-2-12(3-5-14)21-13-6-8-16(9-7-13)25(19,20)11-15-10-23-15/h2-9,15H,10-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 by substrate hydrolysis based fluorescence spectrophotometry |
ACS Med Chem Lett 2: 177-181 (2011)
Article DOI: 10.1021/ml100254e
BindingDB Entry DOI: 10.7270/Q2N29X6G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50335495

(4-(4-(thiiran-2-ylmethylsulfonyl)phenoxy)phenyl me...)Show SMILES CS(=O)(=O)Oc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C16H16O6S3/c1-24(17,18)22-14-4-2-12(3-5-14)21-13-6-8-16(9-7-13)25(19,20)11-15-10-23-15/h2-9,15H,10-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP2 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50335498
(4-(4-((Thiiran-2-ylmethyl)sulfonyl)phenoxy)phenyl ...)Show SMILES CCCS(=O)(=O)Oc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C18H20O6S3/c1-2-11-27(21,22)24-16-5-3-14(4-6-16)23-15-7-9-18(10-8-15)26(19,20)13-17-12-25-17/h3-10,17H,2,11-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 by substrate hydrolysis based fluorescence spectrophotometry |
ACS Med Chem Lett 2: 177-181 (2011)
Article DOI: 10.1021/ml100254e
BindingDB Entry DOI: 10.7270/Q2N29X6G |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50264809

(2-((4-phenoxyphenylsulfonyl)methyl)thiirane | CHEM...)Show InChI InChI=1S/C15H14O3S2/c16-20(17,11-14-10-19-14)15-8-6-13(7-9-15)18-12-4-2-1-3-5-12/h1-9,14H,10-11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP14 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50388911
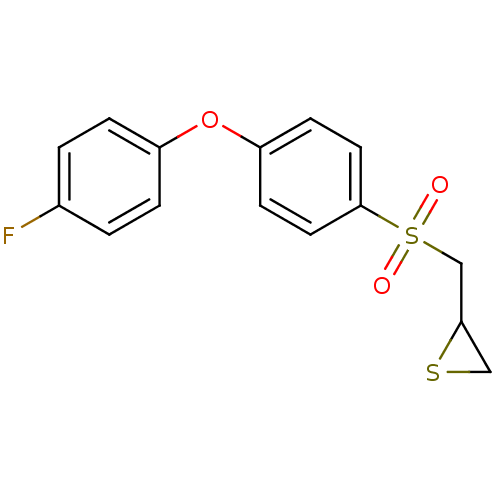
(CHEMBL2063270)Show InChI InChI=1S/C15H13FO3S2/c16-11-1-3-12(4-2-11)19-13-5-7-15(8-6-13)21(17,18)10-14-9-20-14/h1-8,14H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP9 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50388911

(CHEMBL2063270)Show InChI InChI=1S/C15H13FO3S2/c16-11-1-3-12(4-2-11)19-13-5-7-15(8-6-13)21(17,18)10-14-9-20-14/h1-8,14H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP2 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50335499

(4-(4-((Thiiran-2-ylmethyl)sulfonyl)phenoxy)phenyl ...)Show SMILES CC(C)CS(=O)(=O)Oc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C19H22O6S3/c1-14(2)12-28(22,23)25-17-5-3-15(4-6-17)24-16-7-9-19(10-8-16)27(20,21)13-18-11-26-18/h3-10,14,18H,11-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 by substrate hydrolysis based fluorescence spectrophotometry |
ACS Med Chem Lett 2: 177-181 (2011)
Article DOI: 10.1021/ml100254e
BindingDB Entry DOI: 10.7270/Q2N29X6G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50264809

(2-((4-phenoxyphenylsulfonyl)methyl)thiirane | CHEM...)Show InChI InChI=1S/C15H14O3S2/c16-20(17,11-14-10-19-14)15-8-6-13(7-9-15)18-12-4-2-1-3-5-12/h1-9,14H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
| Assay Description
Inhibition assay using matrix metalloproteinase (MMP-2). |
Chem Biol Drug Des 74: 527-34 (2009)
Checked by Author
Article DOI: 10.1111/j.1747-0285.2009.00881.x
BindingDB Entry DOI: 10.7270/Q20P0XJV |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50335497
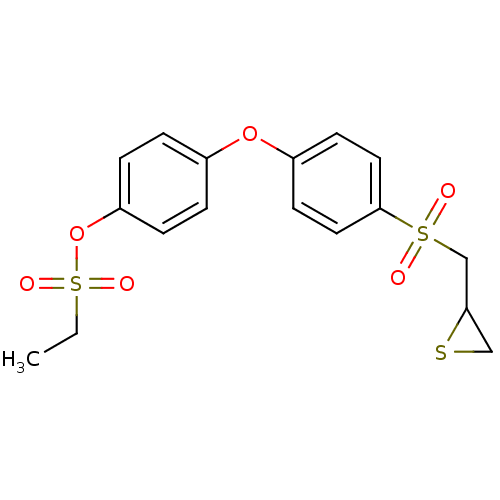
(4-(4-(Thiiranylmethylsulfonyl)phenoxy)-phenylethan...)Show SMILES CCS(=O)(=O)Oc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C17H18O6S3/c1-2-26(20,21)23-15-5-3-13(4-6-15)22-14-7-9-17(10-8-14)25(18,19)12-16-11-24-16/h3-10,16H,2,11-12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 by substrate hydrolysis based fluorescence spectrophotometry |
ACS Med Chem Lett 2: 177-181 (2011)
Article DOI: 10.1021/ml100254e
BindingDB Entry DOI: 10.7270/Q2N29X6G |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50014014
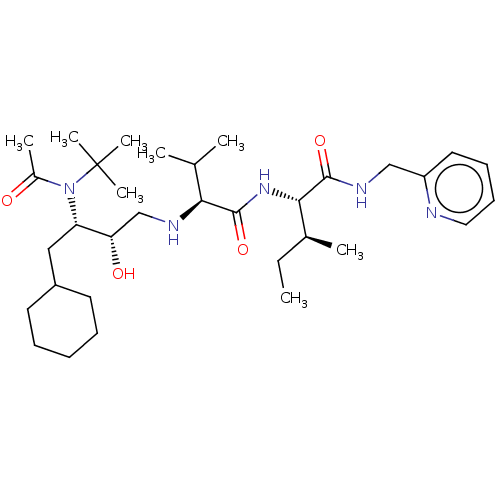
(2-{2-[3-(Acetyl-tert-butyl-amino)-4-cyclohexyl-2-h...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC[C@H](O)[C@H](CC1CCCCC1)N(C(C)=O)C(C)(C)C)C(C)C)C(=O)NCc1ccccn1 |r| Show InChI InChI=1S/C33H57N5O4/c1-9-23(4)30(31(41)36-20-26-17-13-14-18-34-26)37-32(42)29(22(2)3)35-21-28(40)27(19-25-15-11-10-12-16-25)38(24(5)39)33(6,7)8/h13-14,17-18,22-23,25,27-30,35,40H,9-12,15-16,19-21H2,1-8H3,(H,36,41)(H,37,42) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | 6.4 | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Inhibition of HIV protease at pH 6.4, 37 degree C |
J Med Chem 33: 1285-8 (1990)
BindingDB Entry DOI: 10.7270/Q2SB44QS |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50388912
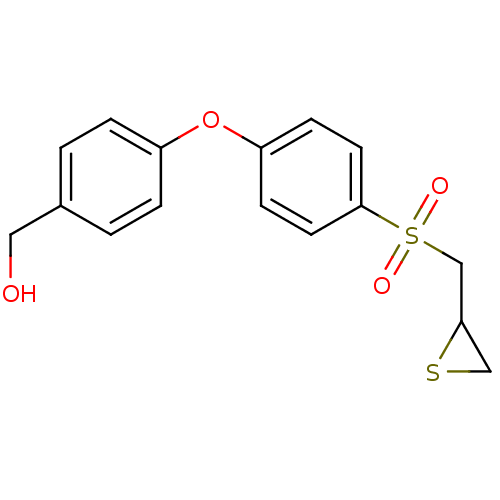
(CHEMBL2063273)Show InChI InChI=1S/C16H16O4S2/c17-9-12-1-3-13(4-2-12)20-14-5-7-16(8-6-14)22(18,19)11-15-10-21-15/h1-8,15,17H,9-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP2 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50388914

(CHEMBL2063274 | US10357546, p-OH SB-3CT)Show InChI InChI=1S/C15H14O4S2/c16-11-1-3-12(4-2-11)19-13-5-7-15(8-6-13)21(17,18)10-14-9-20-14/h1-8,14,16H,9-10H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP14 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50335500
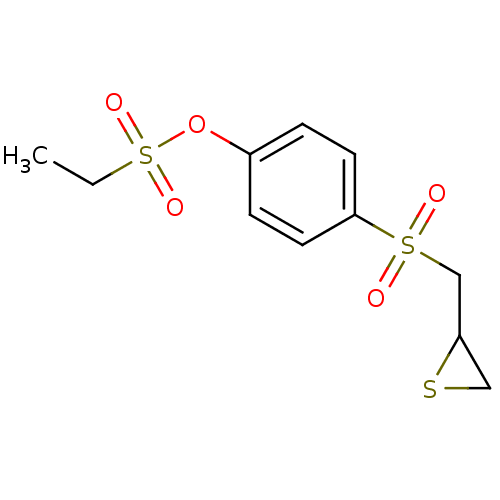
(4-(Thiiran-2-ylmethylsulfonyl)phenyl ethanesulfona...)Show InChI InChI=1S/C11H14O5S3/c1-2-19(14,15)16-9-3-5-11(6-4-9)18(12,13)8-10-7-17-10/h3-6,10H,2,7-8H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 by substrate hydrolysis based fluorescence spectrophotometry |
ACS Med Chem Lett 2: 177-181 (2011)
Article DOI: 10.1021/ml100254e
BindingDB Entry DOI: 10.7270/Q2N29X6G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50388917
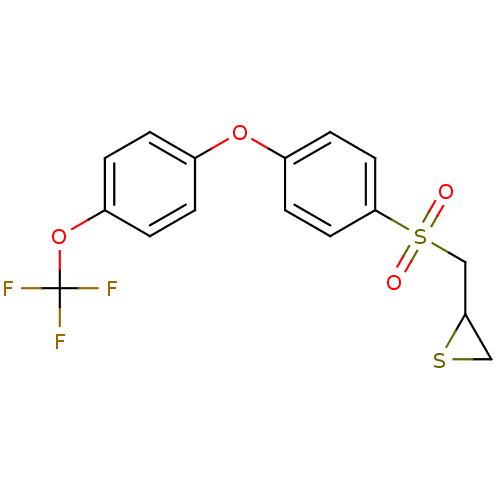
(CHEMBL2063277)Show SMILES FC(F)(F)Oc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C16H13F3O4S2/c17-16(18,19)23-13-3-1-11(2-4-13)22-12-5-7-15(8-6-12)25(20,21)10-14-9-24-14/h1-8,14H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP2 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50264809

(2-((4-phenoxyphenylsulfonyl)methyl)thiirane | CHEM...)Show InChI InChI=1S/C15H14O3S2/c16-20(17,11-14-10-19-14)15-8-6-13(7-9-15)18-12-4-2-1-3-5-12/h1-9,14H,10-11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP14 catalytic domain by substrate hydrolysis based fluorescence spectrophotometry |
ACS Med Chem Lett 2: 177-181 (2011)
Article DOI: 10.1021/ml100254e
BindingDB Entry DOI: 10.7270/Q2N29X6G |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50388916
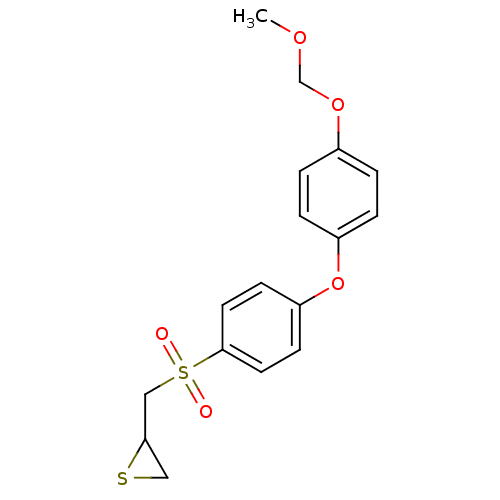
(CHEMBL2063276)Show InChI InChI=1S/C17H18O5S2/c1-20-12-21-13-2-4-14(5-3-13)22-15-6-8-17(9-7-15)24(18,19)11-16-10-23-16/h2-9,16H,10-12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP14 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50335495

(4-(4-(thiiran-2-ylmethylsulfonyl)phenoxy)phenyl me...)Show SMILES CS(=O)(=O)Oc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C16H16O6S3/c1-24(17,18)22-14-4-2-12(3-5-14)21-13-6-8-16(9-7-13)25(19,20)11-15-10-23-15/h2-9,15H,10-11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP14 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50335495

(4-(4-(thiiran-2-ylmethylsulfonyl)phenoxy)phenyl me...)Show SMILES CS(=O)(=O)Oc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C16H16O6S3/c1-24(17,18)22-14-4-2-12(3-5-14)21-13-6-8-16(9-7-13)25(19,20)11-15-10-23-15/h2-9,15H,10-11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP14 catalytic domain by substrate hydrolysis based fluorescence spectrophotometry |
ACS Med Chem Lett 2: 177-181 (2011)
Article DOI: 10.1021/ml100254e
BindingDB Entry DOI: 10.7270/Q2N29X6G |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50388914

(CHEMBL2063274 | US10357546, p-OH SB-3CT)Show InChI InChI=1S/C15H14O4S2/c16-11-1-3-12(4-2-11)19-13-5-7-15(8-6-13)21(17,18)10-14-9-20-14/h1-8,14,16H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP9 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50388916

(CHEMBL2063276)Show InChI InChI=1S/C17H18O5S2/c1-20-12-21-13-2-4-14(5-3-13)22-15-6-8-17(9-7-15)24(18,19)11-16-10-23-16/h2-9,16H,10-12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP9 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50388916

(CHEMBL2063276)Show InChI InChI=1S/C17H18O5S2/c1-20-12-21-13-2-4-14(5-3-13)22-15-6-8-17(9-7-15)24(18,19)11-16-10-23-16/h2-9,16H,10-12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP2 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50388910
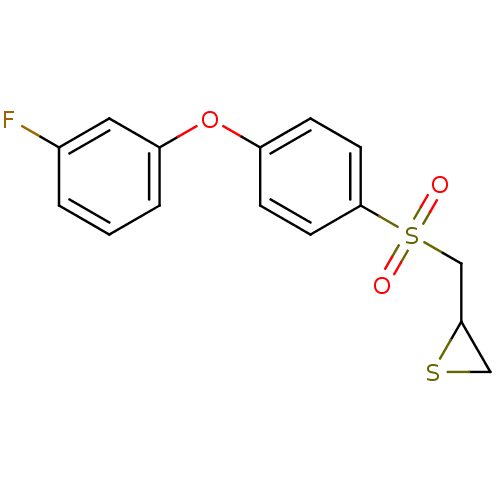
(CHEMBL2063271)Show InChI InChI=1S/C15H13FO3S2/c16-11-2-1-3-13(8-11)19-12-4-6-15(7-5-12)21(17,18)10-14-9-20-14/h1-8,14H,9-10H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP14 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50388912

(CHEMBL2063273)Show InChI InChI=1S/C16H16O4S2/c17-9-12-1-3-13(4-2-12)20-14-5-7-16(8-6-14)22(18,19)11-15-10-21-15/h1-8,15,17H,9-11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 215 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP14 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50335498

(4-(4-((Thiiran-2-ylmethyl)sulfonyl)phenoxy)phenyl ...)Show SMILES CCCS(=O)(=O)Oc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C18H20O6S3/c1-2-11-27(21,22)24-16-5-3-14(4-6-16)23-15-7-9-18(10-8-15)26(19,20)13-17-12-25-17/h3-10,17H,2,11-13H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP14 catalytic domain by substrate hydrolysis based fluorescence spectrophotometry |
ACS Med Chem Lett 2: 177-181 (2011)
Article DOI: 10.1021/ml100254e
BindingDB Entry DOI: 10.7270/Q2N29X6G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50335502

(4-(Thiiran-2-ylmethylsulfonyl)phenyl propane-2-sul...)Show InChI InChI=1S/C13H18O5S3/c1-10(2)8-21(16,17)18-11-3-5-13(6-4-11)20(14,15)9-12-7-19-12/h3-6,10,12H,7-9H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 by substrate hydrolysis based fluorescence spectrophotometry |
ACS Med Chem Lett 2: 177-181 (2011)
Article DOI: 10.1021/ml100254e
BindingDB Entry DOI: 10.7270/Q2N29X6G |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50388915
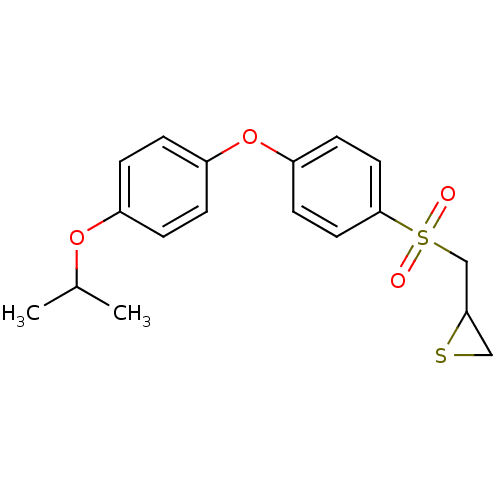
(CHEMBL2063275)Show InChI InChI=1S/C18H20O4S2/c1-13(2)21-14-3-5-15(6-4-14)22-16-7-9-18(10-8-16)24(19,20)12-17-11-23-17/h3-10,13,17H,11-12H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP14 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50335501
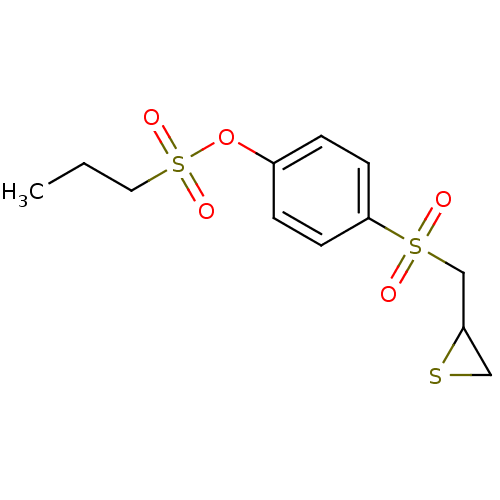
(4-(Thiiran-2-ylmethylsulfonyl)phenyl propane-1-sul...)Show InChI InChI=1S/C12H16O5S3/c1-2-7-20(15,16)17-10-3-5-12(6-4-10)19(13,14)9-11-8-18-11/h3-6,11H,2,7-9H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 by substrate hydrolysis based fluorescence spectrophotometry |
ACS Med Chem Lett 2: 177-181 (2011)
Article DOI: 10.1021/ml100254e
BindingDB Entry DOI: 10.7270/Q2N29X6G |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50388913
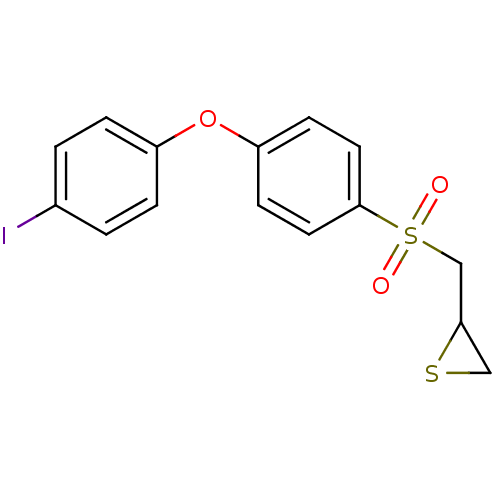
(CHEMBL2063272)Show InChI InChI=1S/C15H13IO3S2/c16-11-1-3-12(4-2-11)19-13-5-7-15(8-6-13)21(17,18)10-14-9-20-14/h1-8,14H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP9 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50388915

(CHEMBL2063275)Show InChI InChI=1S/C18H20O4S2/c1-13(2)21-14-3-5-15(6-4-14)22-16-7-9-18(10-8-16)24(19,20)12-17-11-23-17/h3-10,13,17H,11-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP2 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50264809

(2-((4-phenoxyphenylsulfonyl)methyl)thiirane | CHEM...)Show InChI InChI=1S/C15H14O3S2/c16-20(17,11-14-10-19-14)15-8-6-13(7-9-15)18-12-4-2-1-3-5-12/h1-9,14H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
| Assay Description
Inhibition assay using matrix metalloproteinase (MMP-2). |
Chem Biol Drug Des 74: 527-34 (2009)
Checked by Author
Article DOI: 10.1111/j.1747-0285.2009.00881.x
BindingDB Entry DOI: 10.7270/Q20P0XJV |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50335497

(4-(4-(Thiiranylmethylsulfonyl)phenoxy)-phenylethan...)Show SMILES CCS(=O)(=O)Oc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C17H18O6S3/c1-2-26(20,21)23-15-5-3-13(4-6-15)22-14-7-9-17(10-8-14)25(18,19)12-16-11-24-16/h3-10,16H,2,11-12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP9 catalytic domain by substrate hydrolysis based fluorescence spectrophotometry |
ACS Med Chem Lett 2: 177-181 (2011)
Article DOI: 10.1021/ml100254e
BindingDB Entry DOI: 10.7270/Q2N29X6G |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50335497

(4-(4-(Thiiranylmethylsulfonyl)phenoxy)-phenylethan...)Show SMILES CCS(=O)(=O)Oc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C17H18O6S3/c1-2-26(20,21)23-15-5-3-13(4-6-15)22-14-7-9-17(10-8-14)25(18,19)12-16-11-24-16/h3-10,16H,2,11-12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP14 catalytic domain by substrate hydrolysis based fluorescence spectrophotometry |
ACS Med Chem Lett 2: 177-181 (2011)
Article DOI: 10.1021/ml100254e
BindingDB Entry DOI: 10.7270/Q2N29X6G |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50335499

(4-(4-((Thiiran-2-ylmethyl)sulfonyl)phenoxy)phenyl ...)Show SMILES CC(C)CS(=O)(=O)Oc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C19H22O6S3/c1-14(2)12-28(22,23)25-17-5-3-15(4-6-17)24-16-7-9-19(10-8-16)27(20,21)13-18-11-26-18/h3-10,14,18H,11-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP9 catalytic domain by substrate hydrolysis based fluorescence spectrophotometry |
ACS Med Chem Lett 2: 177-181 (2011)
Article DOI: 10.1021/ml100254e
BindingDB Entry DOI: 10.7270/Q2N29X6G |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50388912

(CHEMBL2063273)Show InChI InChI=1S/C16H16O4S2/c17-9-12-1-3-13(4-2-12)20-14-5-7-16(8-6-14)22(18,19)11-15-10-21-15/h1-8,15,17H,9-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP9 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50335496
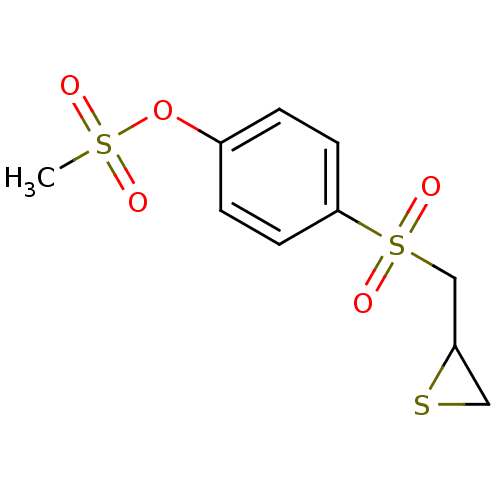
(4-(Thiiran-2-ylmethylsulfonyl)phenyl methanesulfon...)Show InChI InChI=1S/C10H12O5S3/c1-17(11,12)15-8-2-4-10(5-3-8)18(13,14)7-9-6-16-9/h2-5,9H,6-7H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 by substrate hydrolysis based fluorescence spectrophotometry |
ACS Med Chem Lett 2: 177-181 (2011)
Article DOI: 10.1021/ml100254e
BindingDB Entry DOI: 10.7270/Q2N29X6G |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data