 Found 1506 hits with Last Name = 'tu' and Initial = 'y'
Found 1506 hits with Last Name = 'tu' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50056101
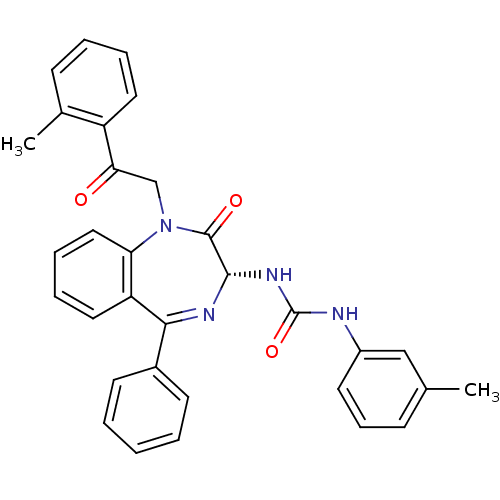
(1-[(R)-2-Oxo-1-(2-oxo-2-o-tolyl-ethyl)-5-phenyl-2,...)Show SMILES Cc1cccc(NC(=O)N[C@@H]2N=C(c3ccccc3)c3ccccc3N(CC(=O)c3ccccc3C)C2=O)c1 |t:11| Show InChI InChI=1S/C32H28N4O3/c1-21-11-10-15-24(19-21)33-32(39)35-30-31(38)36(20-28(37)25-16-7-6-12-22(25)2)27-18-9-8-17-26(27)29(34-30)23-13-4-3-5-14-23/h3-19,30H,20H2,1-2H3,(H2,33,35,39)/t30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 269: 725-31 (1994)
BindingDB Entry DOI: 10.7270/Q2WD3Z2F |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 269: 725-31 (1994)
BindingDB Entry DOI: 10.7270/Q2WD3Z2F |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50056101

(1-[(R)-2-Oxo-1-(2-oxo-2-o-tolyl-ethyl)-5-phenyl-2,...)Show SMILES Cc1cccc(NC(=O)N[C@@H]2N=C(c3ccccc3)c3ccccc3N(CC(=O)c3ccccc3C)C2=O)c1 |t:11| Show InChI InChI=1S/C32H28N4O3/c1-21-11-10-15-24(19-21)33-32(39)35-30-31(38)36(20-28(37)25-16-7-6-12-22(25)2)27-18-9-8-17-26(27)29(34-30)23-13-4-3-5-14-23/h3-19,30H,20H2,1-2H3,(H2,33,35,39)/t30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 269: 725-31 (1994)
BindingDB Entry DOI: 10.7270/Q2WD3Z2F |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50253105

(6-Chloro-N-cyclopropyl-7-(1-((S)-3-methylmorpholin...)Show SMILES CNc1noc2c(c(Cl)ccc12)-c1ccc2c(nncc2c1)N1CCOC[C@@H]1C |r| Show InChI InChI=1S/C21H20ClN5O2/c1-12-11-28-8-7-27(12)21-15-4-3-13(9-14(15)10-24-25-21)18-17(22)6-5-16-19(18)29-26-20(16)23-2/h3-6,9-10,12H,7-8,11H2,1-2H3,(H,23,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged p38alphaMAPK-induced ATF2 phosphorylation |
J Med Chem 51: 6280-92 (2008)
Article DOI: 10.1021/jm8005405
BindingDB Entry DOI: 10.7270/Q218369B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50253148

(CHEMBL522689 | N-(S)-sec-Butyl-6-(6-methyl-3-(meth...)Show SMILES CC[C@H](C)Nc1nncc2cc(ccc12)-c1c(C)ccc2c(NC)noc12 |r| Show InChI InChI=1S/C21H23N5O/c1-5-13(3)24-21-16-9-7-14(10-15(16)11-23-25-21)18-12(2)6-8-17-19(18)27-26-20(17)22-4/h6-11,13H,5H2,1-4H3,(H,22,26)(H,24,25)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged p38alphaMAPK-induced ATF2 phosphorylation |
J Med Chem 51: 6280-92 (2008)
Article DOI: 10.1021/jm8005405
BindingDB Entry DOI: 10.7270/Q218369B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50252776
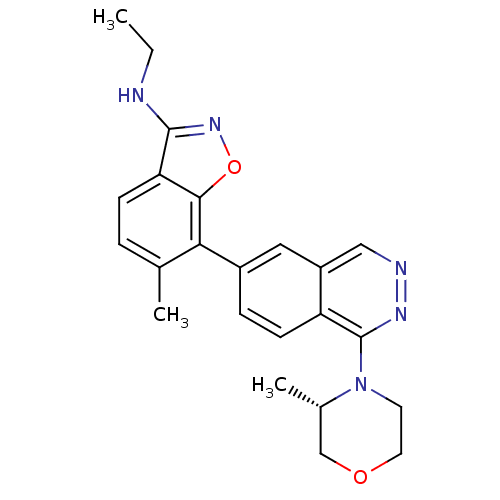
(CHEMBL495493 | N-Ethyl-6-methyl-7-(1-((S)-3-methyl...)Show SMILES CCNc1noc2c(c(C)ccc12)-c1ccc2c(nncc2c1)N1CCOC[C@@H]1C |r| Show InChI InChI=1S/C23H25N5O2/c1-4-24-22-19-7-5-14(2)20(21(19)30-27-22)16-6-8-18-17(11-16)12-25-26-23(18)28-9-10-29-13-15(28)3/h5-8,11-12,15H,4,9-10,13H2,1-3H3,(H,24,27)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged p38alphaMAPK-induced ATF2 phosphorylation |
J Med Chem 51: 6280-92 (2008)
Article DOI: 10.1021/jm8005405
BindingDB Entry DOI: 10.7270/Q218369B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50253102
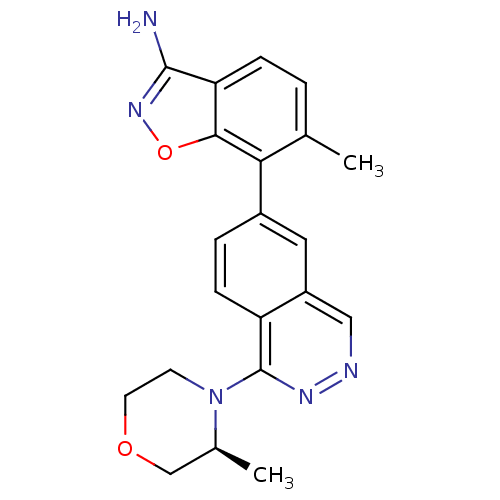
(6-Methyl-7-(1-((S)-3-methylmorpholino)phthalazin-6...)Show SMILES C[C@H]1COCCN1c1nncc2cc(ccc12)-c1c(C)ccc2c(N)noc12 |r| Show InChI InChI=1S/C21H21N5O2/c1-12-3-5-17-19(28-25-20(17)22)18(12)14-4-6-16-15(9-14)10-23-24-21(16)26-7-8-27-11-13(26)2/h3-6,9-10,13H,7-8,11H2,1-2H3,(H2,22,25)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged p38alphaMAPK-induced ATF2 phosphorylation |
J Med Chem 51: 6280-92 (2008)
Article DOI: 10.1021/jm8005405
BindingDB Entry DOI: 10.7270/Q218369B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50253150

(CHEMBL493496 | N,6-Dimethyl-7-(1-((R)-3-methylmorp...)Show SMILES CNc1noc2c(c(C)ccc12)-c1ccc2c(nncc2c1)N1CCOC[C@H]1C |r| Show InChI InChI=1S/C22H23N5O2/c1-13-4-6-18-20(29-26-21(18)23-3)19(13)15-5-7-17-16(10-15)11-24-25-22(17)27-8-9-28-12-14(27)2/h4-7,10-11,14H,8-9,12H2,1-3H3,(H,23,26)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged p38alphaMAPK-induced ATF2 phosphorylation |
J Med Chem 51: 6280-92 (2008)
Article DOI: 10.1021/jm8005405
BindingDB Entry DOI: 10.7270/Q218369B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50252777

(CHEMBL523374 | N,6-Dimethyl-7-(1-((S)-3-methylmorp...)Show SMILES CNc1noc2c(c(C)ccc12)-c1ccc2c(nncc2c1)N1CCOC[C@@H]1C |r| Show InChI InChI=1S/C22H23N5O2/c1-13-4-6-18-20(29-26-21(18)23-3)19(13)15-5-7-17-16(10-15)11-24-25-22(17)27-8-9-28-12-14(27)2/h4-7,10-11,14H,8-9,12H2,1-3H3,(H,23,26)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged p38alphaMAPK-induced ATF2 phosphorylation |
J Med Chem 51: 6280-92 (2008)
Article DOI: 10.1021/jm8005405
BindingDB Entry DOI: 10.7270/Q218369B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50253103

(7-(1-Isopropoxyphthalazin-6-yl)-N,6-dimethylbenzo[...)Show SMILES CNc1noc2c(c(C)ccc12)-c1ccc2c(OC(C)C)nncc2c1 Show InChI InChI=1S/C20H20N4O2/c1-11(2)25-20-15-8-6-13(9-14(15)10-22-23-20)17-12(3)5-7-16-18(17)26-24-19(16)21-4/h5-11H,1-4H3,(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged p38alphaMAPK-induced ATF2 phosphorylation |
J Med Chem 51: 6280-92 (2008)
Article DOI: 10.1021/jm8005405
BindingDB Entry DOI: 10.7270/Q218369B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50252735
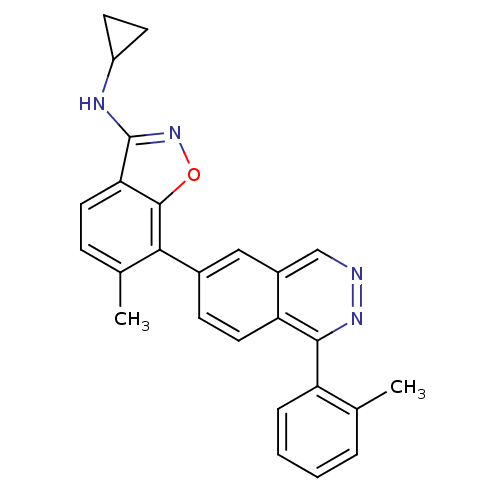
(CHEMBL495084 | N-Cyclopropyl-6-methyl-7-(1-o-tolyl...)Show SMILES Cc1ccccc1-c1nncc2cc(ccc12)-c1c(C)ccc2c(NC3CC3)noc12 Show InChI InChI=1S/C26H22N4O/c1-15-5-3-4-6-20(15)24-21-12-8-17(13-18(21)14-27-29-24)23-16(2)7-11-22-25(23)31-30-26(22)28-19-9-10-19/h3-8,11-14,19H,9-10H2,1-2H3,(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged p38alphaMAPK-induced ATF2 phosphorylation |
J Med Chem 51: 6280-92 (2008)
Article DOI: 10.1021/jm8005405
BindingDB Entry DOI: 10.7270/Q218369B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50253149

(6-Chloro-N-isopropyl-7-(1-((S)-3-methylmorpholino)...)Show SMILES CC(C)Nc1noc2c(c(Cl)ccc12)-c1ccc2c(nncc2c1)N1CCOC[C@@H]1C |r| Show InChI InChI=1S/C23H24ClN5O2/c1-13(2)26-22-18-6-7-19(24)20(21(18)31-28-22)15-4-5-17-16(10-15)11-25-27-23(17)29-8-9-30-12-14(29)3/h4-7,10-11,13-14H,8-9,12H2,1-3H3,(H,26,28)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged p38alphaMAPK-induced ATF2 phosphorylation |
J Med Chem 51: 6280-92 (2008)
Article DOI: 10.1021/jm8005405
BindingDB Entry DOI: 10.7270/Q218369B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50252775
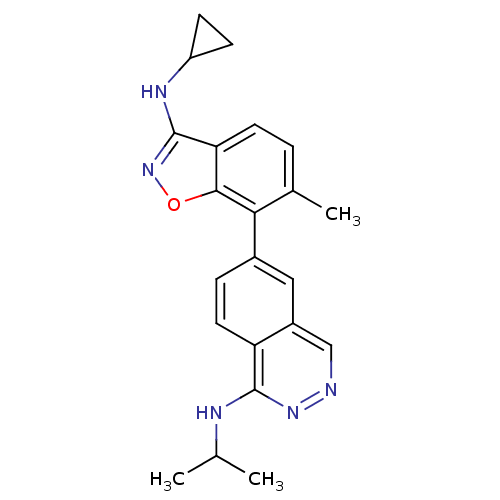
(6-(3-(cyclopropylamino)-6-methylbenzo[d]isoxazol-7...)Show SMILES CC(C)Nc1nncc2cc(ccc12)-c1c(C)ccc2c(NC3CC3)noc12 Show InChI InChI=1S/C22H23N5O/c1-12(2)24-21-17-9-5-14(10-15(17)11-23-26-21)19-13(3)4-8-18-20(19)28-27-22(18)25-16-6-7-16/h4-5,8-12,16H,6-7H2,1-3H3,(H,24,26)(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged p38alphaMAPK-induced ATF2 phosphorylation |
J Med Chem 51: 6280-92 (2008)
Article DOI: 10.1021/jm8005405
BindingDB Entry DOI: 10.7270/Q218369B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50253147
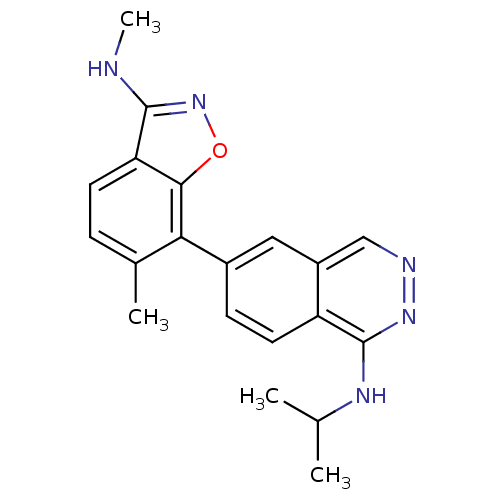
(CHEMBL494305 | N-Isopropyl-6-(6-methyl-3-(methylam...)Show SMILES CNc1noc2c(c(C)ccc12)-c1ccc2c(NC(C)C)nncc2c1 Show InChI InChI=1S/C20H21N5O/c1-11(2)23-20-15-8-6-13(9-14(15)10-22-24-20)17-12(3)5-7-16-18(17)26-25-19(16)21-4/h5-11H,1-4H3,(H,21,25)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged p38alphaMAPK-induced ATF2 phosphorylation |
J Med Chem 51: 6280-92 (2008)
Article DOI: 10.1021/jm8005405
BindingDB Entry DOI: 10.7270/Q218369B |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50005463

((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)c2cc3ccccc3[nH]2)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C25H20N4O2/c1-29-21-14-8-6-12-18(21)22(16-9-3-2-4-10-16)27-23(25(29)31)28-24(30)20-15-17-11-5-7-13-19(17)26-20/h2-15,23,26H,1H3,(H,28,30)/t23-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 269: 725-31 (1994)
BindingDB Entry DOI: 10.7270/Q2WD3Z2F |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50253104
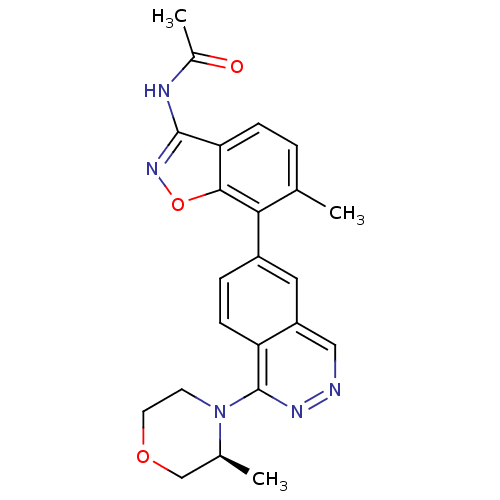
(CHEMBL521860 | N-(6-Methyl-7-(1-((S)-3-methylmorph...)Show SMILES C[C@H]1COCCN1c1nncc2cc(ccc12)-c1c(C)ccc2c(NC(C)=O)noc12 |r| Show InChI InChI=1S/C23H23N5O3/c1-13-4-6-19-21(31-27-22(19)25-15(3)29)20(13)16-5-7-18-17(10-16)11-24-26-23(18)28-8-9-30-12-14(28)2/h4-7,10-11,14H,8-9,12H2,1-3H3,(H,25,27,29)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged p38alphaMAPK-induced ATF2 phosphorylation |
J Med Chem 51: 6280-92 (2008)
Article DOI: 10.1021/jm8005405
BindingDB Entry DOI: 10.7270/Q218369B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50252736
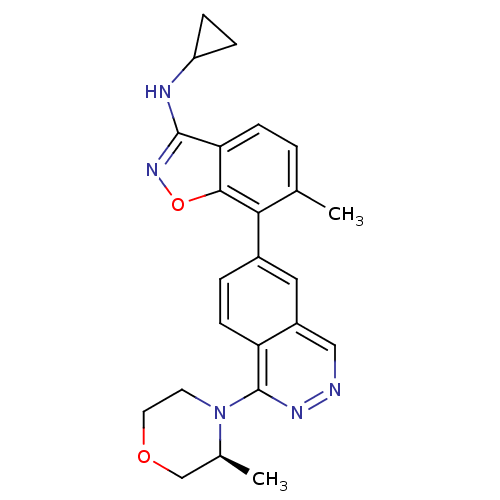
(CHEMBL522877 | N-Cyclopropyl-6-methyl-7-(1-((S)-3-...)Show SMILES C[C@H]1COCCN1c1nncc2cc(ccc12)-c1c(C)ccc2c(NC3CC3)noc12 |r| Show InChI InChI=1S/C24H25N5O2/c1-14-3-7-20-22(31-28-23(20)26-18-5-6-18)21(14)16-4-8-19-17(11-16)12-25-27-24(19)29-9-10-30-13-15(29)2/h3-4,7-8,11-12,15,18H,5-6,9-10,13H2,1-2H3,(H,26,28)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged p38alphaMAPK-induced ATF2 phosphorylation |
J Med Chem 51: 6280-92 (2008)
Article DOI: 10.1021/jm8005405
BindingDB Entry DOI: 10.7270/Q218369B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50252774
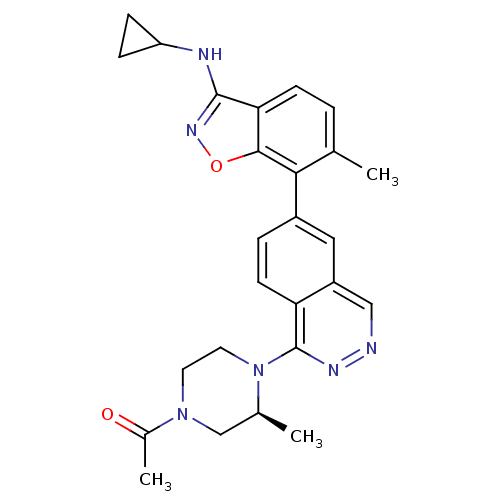
(1-((S)-4-(6-(3-(Cyclopropylamino)-6-methylbenzo[d]...)Show SMILES C[C@H]1CN(CCN1c1nncc2cc(ccc12)-c1c(C)ccc2c(NC3CC3)noc12)C(C)=O |r| Show InChI InChI=1S/C26H28N6O2/c1-15-4-8-22-24(34-30-25(22)28-20-6-7-20)23(15)18-5-9-21-19(12-18)13-27-29-26(21)32-11-10-31(17(3)33)14-16(32)2/h4-5,8-9,12-13,16,20H,6-7,10-11,14H2,1-3H3,(H,28,30)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged p38alphaMAPK-induced ATF2 phosphorylation |
J Med Chem 51: 6280-92 (2008)
Article DOI: 10.1021/jm8005405
BindingDB Entry DOI: 10.7270/Q218369B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50253101

(7-(1-Isopropylphthalazin-6-yl)-N,6-dimethylbenzo[d...)Show InChI InChI=1S/C20H20N4O/c1-11(2)18-15-8-6-13(9-14(15)10-22-23-18)17-12(3)5-7-16-19(17)25-24-20(16)21-4/h5-11H,1-4H3,(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged p38alphaMAPK-induced ATF2 phosphorylation |
J Med Chem 51: 6280-92 (2008)
Article DOI: 10.1021/jm8005405
BindingDB Entry DOI: 10.7270/Q218369B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50252777

(CHEMBL523374 | N,6-Dimethyl-7-(1-((S)-3-methylmorp...)Show SMILES CNc1noc2c(c(C)ccc12)-c1ccc2c(nncc2c1)N1CCOC[C@@H]1C |r| Show InChI InChI=1S/C22H23N5O2/c1-13-4-6-18-20(29-26-21(18)23-3)19(13)15-5-7-17-16(10-15)11-24-25-22(17)27-8-9-28-12-14(27)2/h4-7,10-11,14H,8-9,12H2,1-3H3,(H,23,26)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged p38beta-induced ATF2 phosphorylation |
J Med Chem 51: 6280-92 (2008)
Article DOI: 10.1021/jm8005405
BindingDB Entry DOI: 10.7270/Q218369B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50252738
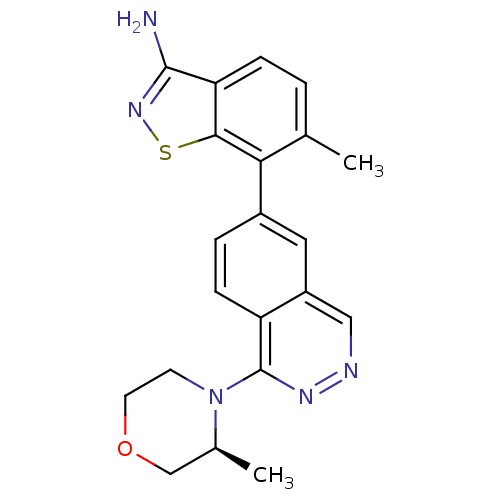
(6-Methyl-7-(1-((S)-3-methylmorpholino)phthalazin-6...)Show SMILES C[C@H]1COCCN1c1nncc2cc(ccc12)-c1c(C)ccc2c(N)nsc12 |r| Show InChI InChI=1S/C21H21N5OS/c1-12-3-5-17-19(28-25-20(17)22)18(12)14-4-6-16-15(9-14)10-23-24-21(16)26-7-8-27-11-13(26)2/h3-6,9-10,13H,7-8,11H2,1-2H3,(H2,22,25)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged p38alphaMAPK-induced ATF2 phosphorylation |
J Med Chem 51: 6280-92 (2008)
Article DOI: 10.1021/jm8005405
BindingDB Entry DOI: 10.7270/Q218369B |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM82235
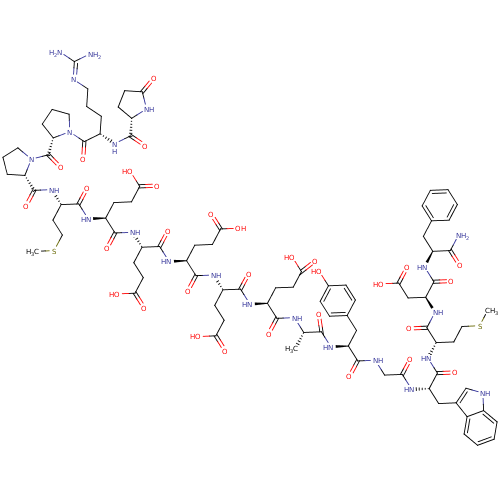
(Gastrin I | Gastrin-17 | Gastrin-I-(1-17))Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CNC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H]1CCC(=O)N1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,wU:4.4,138.143,38.41,52.60,70.78,88.95,96.98,wD:8.20,130.135,26.37,43.51,61.69,79.87,103.106,110.122,121.125,(26,6.14,;24.67,5.35,;24.67,3.8,;23.32,3.01,;23.32,1.49,;22,.73,;20.68,1.49,;20.68,3.04,;19.35,.73,;19.35,-.76,;20.68,-1.52,;21.94,-.7,;23.15,-1.69,;22.59,-3.1,;23.29,-4.48,;22.45,-5.77,;20.9,-5.71,;20.2,-4.31,;21.04,-2.98,;18,1.49,;16.68,.7,;16.68,-.76,;15.36,1.49,;14.03,.7,;12.68,1.46,;12.68,3.01,;11.36,.68,;11.36,-.79,;12.71,-1.55,;12.71,-3.1,;14.03,-3.86,;15.36,-3.07,;16.71,-3.86,;15.36,-1.55,;14.03,-.79,;10.01,1.44,;8.66,.68,;8.66,-.82,;7.33,1.44,;7.3,2.98,;6.01,.65,;4.66,1.44,;4.66,2.98,;3.34,.65,;3.34,-.84,;4.66,-1.6,;4.66,-3.15,;6.01,-3.91,;3.34,-3.91,;2.01,1.44,;.66,.65,;.66,-.84,;-.61,1.44,;-.61,2.96,;.66,3.74,;.66,5.32,;2.01,6.11,;-.61,6.11,;-1.93,.65,;-3.28,1.41,;-3.28,2.96,;-4.6,.65,;-4.6,-.84,;-3.28,-1.6,;-3.28,-3.15,;-1.93,-3.94,;-4.6,-3.94,;-5.93,1.41,;-7.28,.65,;-7.28,-.84,;-8.6,1.44,;-8.6,2.96,;-7.28,3.74,;-7.28,5.32,;-5.93,6.11,;-8.6,6.11,;-9.95,.65,;-11.33,1.44,;-11.33,2.98,;-12.65,.65,;-12.65,-.82,;-11.33,-1.6,;-11.33,-3.15,;-9.95,-3.94,;-12.65,-3.91,;-13.98,1.44,;-15.33,.68,;-15.33,-.79,;-16.65,1.44,;-16.65,2.96,;-15.3,3.77,;-15.3,5.29,;-13.98,6.08,;-17.97,.68,;-19.32,1.44,;-19.32,2.98,;-20.62,.68,;-19.69,1.83,;-20.39,3.26,;-21.91,2.96,;-21.91,1.44,;-23.24,.68,;-23.24,-.96,;-24.7,1.52,;-23.97,.34,;-24.56,-.98,;-26.16,-.7,;-26.16,.68,;-27.52,1.44,;-27.52,2.98,;-28.84,.68,;-28.84,-.82,;-27.52,-1.55,;-27.52,-3.1,;-26.14,-3.86,;-26.11,-5.4,;-27.52,-6.16,;-24.78,-6.16,;-30.19,1.44,;-31.51,.68,;-31.51,-.82,;-32.84,1.41,;-31.85,.28,;-32.55,-1.1,;-34.07,-.79,;-35.37,-1.58,;-34.07,.68,;24.67,.73,;24.67,-.73,;26,1.49,;27.35,.7,;27.35,-.76,;28.7,-1.55,;30.02,-.76,;28.7,-3.07,;28.7,1.49,;28.7,3.04,;30.02,.7,;31.34,1.49,;31.34,3.01,;32.7,3.83,;32.7,5.38,;34.02,6.16,;35.37,5.38,;35.37,3.83,;34.02,3.01,;32.7,.7,;34.02,1.49,;32.7,-.76,)| Show InChI InChI=1S/C94H128N22O31S2/c1-48(79(133)113-65(43-50-19-21-52(117)22-20-50)80(134)100-47-71(119)103-66(44-51-46-99-54-15-8-7-14-53(51)54)89(143)109-61(35-40-148-2)88(142)114-67(45-77(130)131)90(144)112-64(78(95)132)42-49-12-5-4-6-13-49)101-81(135)56(24-30-72(120)121)104-83(137)57(25-31-73(122)123)105-84(138)58(26-32-74(124)125)106-85(139)59(27-33-75(126)127)107-86(140)60(28-34-76(128)129)108-87(141)62(36-41-149-3)110-91(145)68-17-10-38-115(68)93(147)69-18-11-39-116(69)92(146)63(16-9-37-98-94(96)97)111-82(136)55-23-29-70(118)102-55/h4-8,12-15,19-22,46,48,55-69,99,117H,9-11,16-18,23-45,47H2,1-3H3,(H2,95,132)(H,100,134)(H,101,135)(H,102,118)(H,103,119)(H,104,137)(H,105,138)(H,106,139)(H,107,140)(H,108,141)(H,109,143)(H,110,145)(H,111,136)(H,112,144)(H,113,133)(H,114,142)(H,120,121)(H,122,123)(H,124,125)(H,126,127)(H,128,129)(H,130,131)(H4,96,97,98)/t48-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 269: 725-31 (1994)
BindingDB Entry DOI: 10.7270/Q2WD3Z2F |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM82403
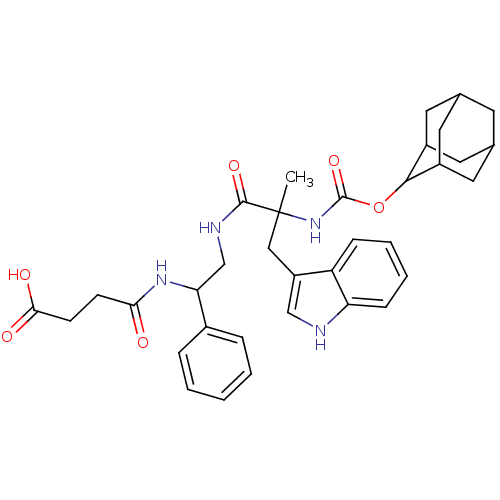
(CAS_108186 | CI-988 | NSC_108186)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NCC(NC(=O)CCC(O)=O)c1ccccc1 |TLB:25:24:18.19.20:22,THB:15:16:18.19.20:22,20:19:16:21.22.23,20:21:16:18.19.25,25:19:22:16.23.24,(.76,2.29,;.32,.82,;-.11,-.66,;.95,-1.78,;.68,-3.29,;2.03,-4.02,;3.15,-2.96,;4.68,-3.07,;5.55,-1.8,;4.88,-.42,;3.35,-.3,;2.48,-1.57,;1.8,.38,;2.92,1.45,;2.55,2.94,;4.39,1.01,;5.51,2.08,;6.88,2.77,;6.88,4.17,;7.72,5.64,;9.09,4.95,;9.09,3.55,;8.25,2.08,;7.72,2.85,;6.35,3.55,;6.35,4.95,;-1.15,1.25,;-1.52,2.75,;-2.27,.19,;-3.75,.62,;-4.86,-.44,;-6.34,-.01,;-7.45,-1.07,;-7.09,-2.57,;-8.93,-.64,;-10.04,-1.7,;-11.52,-1.27,;-11.89,.23,;-12.64,-2.33,;-4.5,-1.94,;-3.02,-2.37,;-2.65,-3.87,;-3.77,-4.93,;-5.25,-4.5,;-5.61,-3,)| Show InChI InChI=1S/C35H42N4O6/c1-35(18-26-19-36-28-10-6-5-9-27(26)28,39-34(44)45-32-24-14-21-13-22(16-24)17-25(32)15-21)33(43)37-20-29(23-7-3-2-4-8-23)38-30(40)11-12-31(41)42/h2-10,19,21-22,24-25,29,32,36H,11-18,20H2,1H3,(H,37,43)(H,38,40)(H,39,44)(H,41,42) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 269: 725-31 (1994)
BindingDB Entry DOI: 10.7270/Q2WD3Z2F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10882

(6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...)Show InChI InChI=1S/C9H10N2O3S2/c1-2-14-6-3-4-7-8(5-6)15-9(11-7)16(10,12)13/h3-5H,2H2,1H3,(H2,10,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Balikesir University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 2 after 15 mins by CO2 hydration method |
Bioorg Med Chem 18: 5498-503 (2010)
Article DOI: 10.1016/j.bmc.2010.06.056
BindingDB Entry DOI: 10.7270/Q2CZ37B6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10887

(Sulfamate 7 | Topiramate (TPM) | US11535599, Examp...)Show SMILES CC1(C)O[C@@H]2CO[C@@]3(COS(N)(=O)=O)OC(C)(C)O[C@H]3[C@@H]2O1 |r| Show InChI InChI=1S/C12H21NO8S/c1-10(2)18-7-5-16-12(6-17-22(13,14)15)9(8(7)19-10)20-11(3,4)21-12/h7-9H,5-6H2,1-4H3,(H2,13,14,15)/t7-,8-,9+,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Balikesir University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 2 after 15 mins by CO2 hydration method |
Bioorg Med Chem 18: 5498-503 (2010)
Article DOI: 10.1016/j.bmc.2010.06.056
BindingDB Entry DOI: 10.7270/Q2CZ37B6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Balikesir University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 2 after 15 mins by CO2 hydration method |
Bioorg Med Chem 18: 5498-503 (2010)
Article DOI: 10.1016/j.bmc.2010.06.056
BindingDB Entry DOI: 10.7270/Q2CZ37B6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50253151
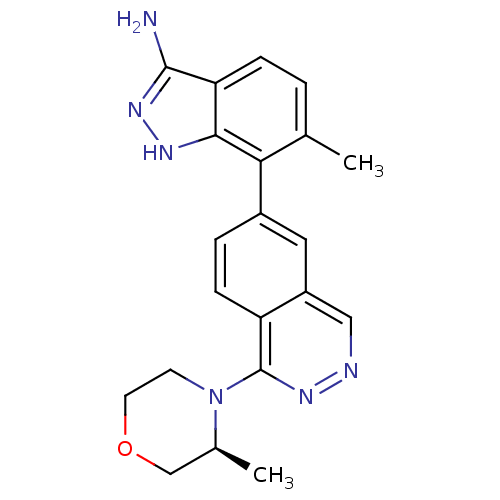
(6-Methyl-7-(1-((S)-3-methylmorpholino)phthalazin-6...)Show SMILES C[C@H]1COCCN1c1nncc2cc(ccc12)-c1c(C)ccc2c(N)n[nH]c12 |r| Show InChI InChI=1S/C21H22N6O/c1-12-3-5-17-19(24-25-20(17)22)18(12)14-4-6-16-15(9-14)10-23-26-21(16)27-7-8-28-11-13(27)2/h3-6,9-10,13H,7-8,11H2,1-2H3,(H3,22,24,25)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged p38alphaMAPK-induced ATF2 phosphorylation |
J Med Chem 51: 6280-92 (2008)
Article DOI: 10.1021/jm8005405
BindingDB Entry DOI: 10.7270/Q218369B |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50061220
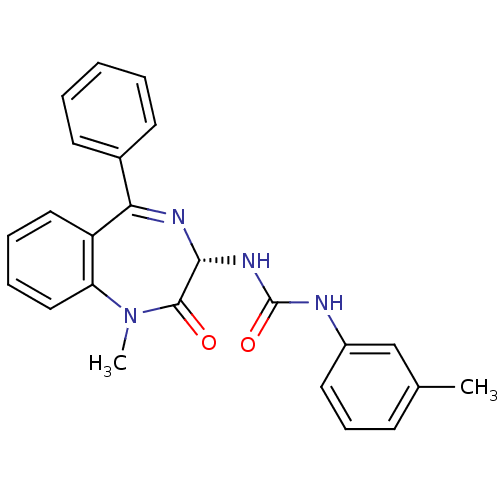
(1-((S)-1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)Nc2cccc(C)c2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C24H22N4O2/c1-16-9-8-12-18(15-16)25-24(30)27-22-23(29)28(2)20-14-7-6-13-19(20)21(26-22)17-10-4-3-5-11-17/h3-15,22H,1-2H3,(H2,25,27,30)/t22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 269: 725-31 (1994)
BindingDB Entry DOI: 10.7270/Q2WD3Z2F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Balikesir University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 2 after 15 mins by CO2 hydration method |
Bioorg Med Chem 18: 5498-503 (2010)
Article DOI: 10.1016/j.bmc.2010.06.056
BindingDB Entry DOI: 10.7270/Q2CZ37B6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10882

(6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...)Show InChI InChI=1S/C9H10N2O3S2/c1-2-14-6-3-4-7-8(5-6)15-9(11-7)16(10,12)13/h3-5H,2H2,1H3,(H2,10,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Balikesir University
Curated by ChEMBL
| Assay Description
Inhibition of wild type human recombinant carbonic anhydrase 1 expressed in Escherichia coli BL21 (DE3) after 15 mins by CO2 hydration method |
Bioorg Med Chem 18: 5498-503 (2010)
Article DOI: 10.1016/j.bmc.2010.06.056
BindingDB Entry DOI: 10.7270/Q2CZ37B6 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 269: 725-31 (1994)
BindingDB Entry DOI: 10.7270/Q2WD3Z2F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10888
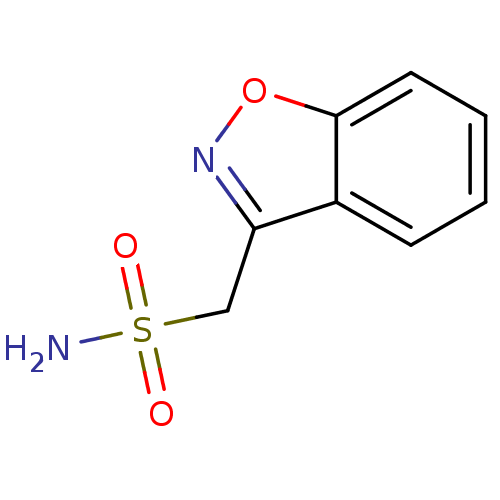
(1,2-benzoxazol-3-ylmethanesulfonamide | CHEMBL750 ...)Show InChI InChI=1S/C8H8N2O3S/c9-14(11,12)5-7-6-3-1-2-4-8(6)13-10-7/h1-4H,5H2,(H2,9,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Balikesir University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 2 after 15 mins by CO2 hydration method |
Bioorg Med Chem 18: 5498-503 (2010)
Article DOI: 10.1016/j.bmc.2010.06.056
BindingDB Entry DOI: 10.7270/Q2CZ37B6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10888

(1,2-benzoxazol-3-ylmethanesulfonamide | CHEMBL750 ...)Show InChI InChI=1S/C8H8N2O3S/c9-14(11,12)5-7-6-3-1-2-4-8(6)13-10-7/h1-4H,5H2,(H2,9,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Balikesir University
Curated by ChEMBL
| Assay Description
Inhibition of wild type human recombinant carbonic anhydrase 1 expressed in Escherichia coli BL21 (DE3) after 15 mins by CO2 hydration method |
Bioorg Med Chem 18: 5498-503 (2010)
Article DOI: 10.1016/j.bmc.2010.06.056
BindingDB Entry DOI: 10.7270/Q2CZ37B6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10868
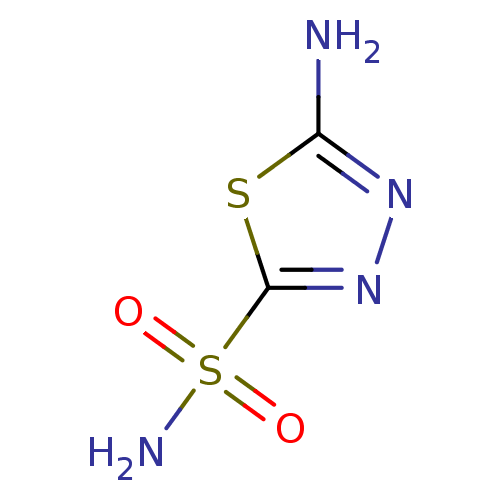
(1,3,4-Thiadiazole-2-sulfonamide, 6 | 1,3,4-thiadia...)Show InChI InChI=1S/C2H4N4O2S2/c3-1-5-6-2(9-1)10(4,7)8/h(H2,3,5)(H2,4,7,8) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Balikesir University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 2 after 15 mins by CO2 hydration method |
Bioorg Med Chem 18: 5498-503 (2010)
Article DOI: 10.1016/j.bmc.2010.06.056
BindingDB Entry DOI: 10.7270/Q2CZ37B6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10862
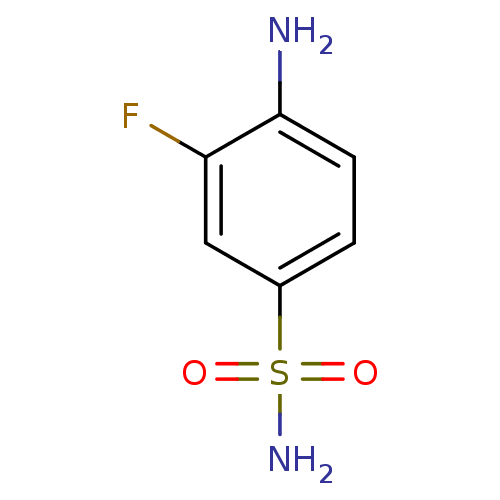
(4-Amino-3-fluorobenzenesulfonamide | 4-amino-3-flu...)Show InChI InChI=1S/C6H7FN2O2S/c7-5-3-4(12(9,10)11)1-2-6(5)8/h1-3H,8H2,(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Balikesir University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 2 after 15 mins by CO2 hydration method |
Bioorg Med Chem 18: 5498-503 (2010)
Article DOI: 10.1016/j.bmc.2010.06.056
BindingDB Entry DOI: 10.7270/Q2CZ37B6 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50056101

(1-[(R)-2-Oxo-1-(2-oxo-2-o-tolyl-ethyl)-5-phenyl-2,...)Show SMILES Cc1cccc(NC(=O)N[C@@H]2N=C(c3ccccc3)c3ccccc3N(CC(=O)c3ccccc3C)C2=O)c1 |t:11| Show InChI InChI=1S/C32H28N4O3/c1-21-11-10-15-24(19-21)33-32(39)35-30-31(38)36(20-28(37)25-16-7-6-12-22(25)2)27-18-9-8-17-26(27)29(34-30)23-13-4-3-5-14-23/h3-19,30H,20H2,1-2H3,(H2,33,35,39)/t30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 269: 725-31 (1994)
BindingDB Entry DOI: 10.7270/Q2WD3Z2F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10867
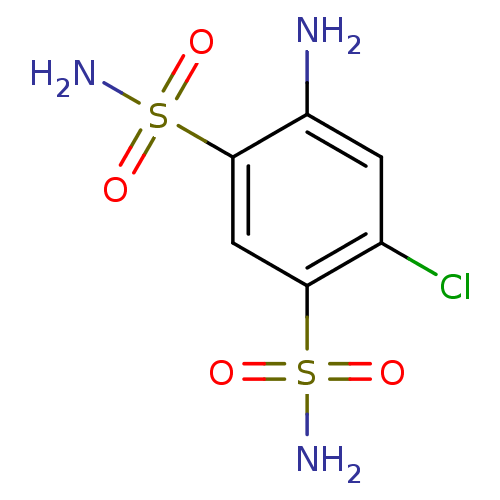
(4-amino-6-chlorobenzene-1,3-disulfonamide | CHEMBL...)Show InChI InChI=1S/C6H8ClN3O4S2/c7-3-1-4(8)6(16(10,13)14)2-5(3)15(9,11)12/h1-2H,8H2,(H2,9,11,12)(H2,10,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Balikesir University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 2 after 15 mins by CO2 hydration method |
Bioorg Med Chem 18: 5498-503 (2010)
Article DOI: 10.1016/j.bmc.2010.06.056
BindingDB Entry DOI: 10.7270/Q2CZ37B6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10877
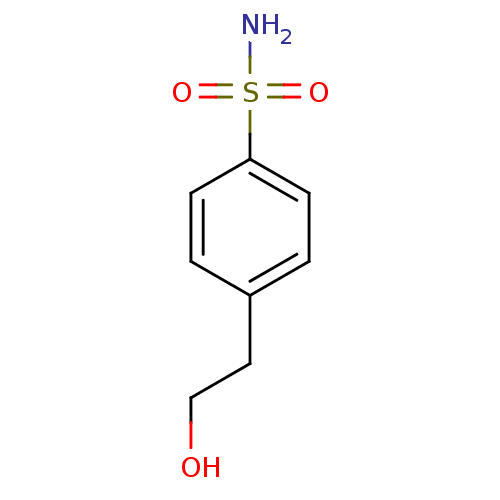
(4-(2-hydroxyethyl)benzene-1-sulfonamide | CHEMBL67...)Show InChI InChI=1S/C8H11NO3S/c9-13(11,12)8-3-1-7(2-4-8)5-6-10/h1-4,10H,5-6H2,(H2,9,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Balikesir University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 2 after 15 mins by CO2 hydration method |
Bioorg Med Chem 18: 5498-503 (2010)
Article DOI: 10.1016/j.bmc.2010.06.056
BindingDB Entry DOI: 10.7270/Q2CZ37B6 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM82480

(YM022 S-isomer)Show SMILES Cc1cccc(NC(=O)N[C@H]2N=C(c3ccccc3)c3ccccc3N(CC(=O)c3ccccc3C)C2=O)c1 |r,t:11| Show InChI InChI=1S/C32H28N4O3/c1-21-11-10-15-24(19-21)33-32(39)35-30-31(38)36(20-28(37)25-16-7-6-12-22(25)2)27-18-9-8-17-26(27)29(34-30)23-13-4-3-5-14-23/h3-19,30H,20H2,1-2H3,(H2,33,35,39)/t30-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 269: 725-31 (1994)
BindingDB Entry DOI: 10.7270/Q2WD3Z2F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10861
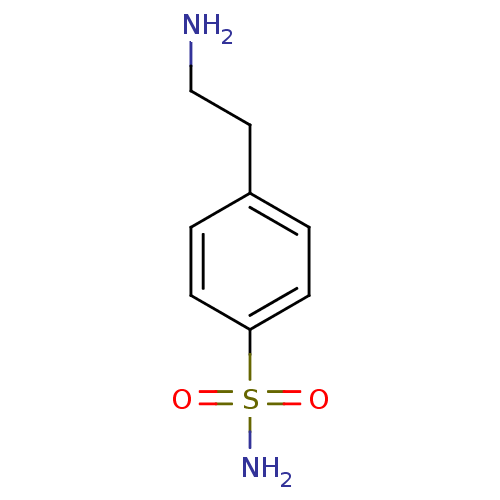
(4-(2-aminoethyl)benzene-1-sulfonamide | CHEMBL7087...)Show InChI InChI=1S/C8H12N2O2S/c9-6-5-7-1-3-8(4-2-7)13(10,11)12/h1-4H,5-6,9H2,(H2,10,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Balikesir University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 2 after 15 mins by CO2 hydration method |
Bioorg Med Chem 18: 5498-503 (2010)
Article DOI: 10.1016/j.bmc.2010.06.056
BindingDB Entry DOI: 10.7270/Q2CZ37B6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10857
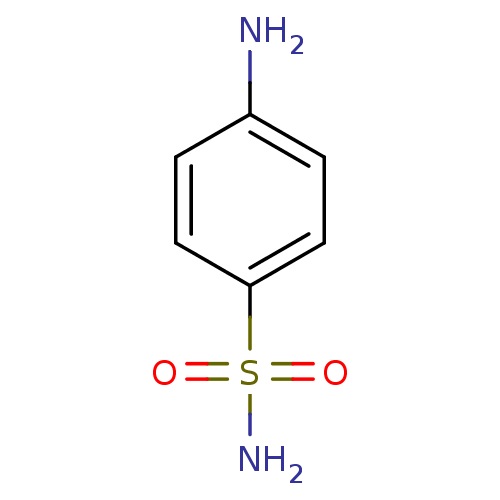
(4-aminobenzene-1-sulfonamide | CHEMBL21 | Sulfanil...)Show InChI InChI=1S/C6H8N2O2S/c7-5-1-3-6(4-2-5)11(8,9)10/h1-4H,7H2,(H2,8,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Balikesir University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 2 after 15 mins by CO2 hydration method |
Bioorg Med Chem 18: 5498-503 (2010)
Article DOI: 10.1016/j.bmc.2010.06.056
BindingDB Entry DOI: 10.7270/Q2CZ37B6 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10887

(Sulfamate 7 | Topiramate (TPM) | US11535599, Examp...)Show SMILES CC1(C)O[C@@H]2CO[C@@]3(COS(N)(=O)=O)OC(C)(C)O[C@H]3[C@@H]2O1 |r| Show InChI InChI=1S/C12H21NO8S/c1-10(2)18-7-5-16-12(6-17-22(13,14)15)9(8(7)19-10)20-11(3,4)21-12/h7-9H,5-6H2,1-4H3,(H2,13,14,15)/t7-,8-,9+,12+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Balikesir University
Curated by ChEMBL
| Assay Description
Inhibition of wild type human recombinant carbonic anhydrase 1 expressed in Escherichia coli BL21 (DE3) after 15 mins by CO2 hydration method |
Bioorg Med Chem 18: 5498-503 (2010)
Article DOI: 10.1016/j.bmc.2010.06.056
BindingDB Entry DOI: 10.7270/Q2CZ37B6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Balikesir University
Curated by ChEMBL
| Assay Description
Inhibition of wild type human recombinant carbonic anhydrase 1 expressed in Escherichia coli BL21 (DE3) after 15 mins by CO2 hydration method |
Bioorg Med Chem 18: 5498-503 (2010)
Article DOI: 10.1016/j.bmc.2010.06.056
BindingDB Entry DOI: 10.7270/Q2CZ37B6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50056101

(1-[(R)-2-Oxo-1-(2-oxo-2-o-tolyl-ethyl)-5-phenyl-2,...)Show SMILES Cc1cccc(NC(=O)N[C@@H]2N=C(c3ccccc3)c3ccccc3N(CC(=O)c3ccccc3C)C2=O)c1 |t:11| Show InChI InChI=1S/C32H28N4O3/c1-21-11-10-15-24(19-21)33-32(39)35-30-31(38)36(20-28(37)25-16-7-6-12-22(25)2)27-18-9-8-17-26(27)29(34-30)23-13-4-3-5-14-23/h3-19,30H,20H2,1-2H3,(H2,33,35,39)/t30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 269: 725-31 (1994)
BindingDB Entry DOI: 10.7270/Q2WD3Z2F |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM82480

(YM022 S-isomer)Show SMILES Cc1cccc(NC(=O)N[C@H]2N=C(c3ccccc3)c3ccccc3N(CC(=O)c3ccccc3C)C2=O)c1 |r,t:11| Show InChI InChI=1S/C32H28N4O3/c1-21-11-10-15-24(19-21)33-32(39)35-30-31(38)36(20-28(37)25-16-7-6-12-22(25)2)27-18-9-8-17-26(27)29(34-30)23-13-4-3-5-14-23/h3-19,30H,20H2,1-2H3,(H2,33,35,39)/t30-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 269: 725-31 (1994)
BindingDB Entry DOI: 10.7270/Q2WD3Z2F |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50505208
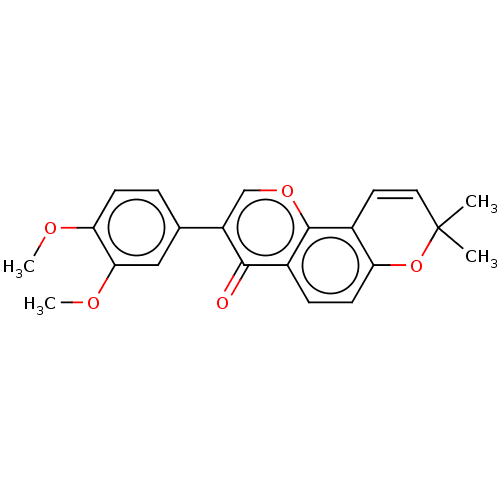
(CHEMBL3311040)Show SMILES COc1ccc(cc1OC)-c1coc2c3C=CC(C)(C)Oc3ccc2c1=O |c:16| Show InChI InChI=1S/C22H20O5/c1-22(2)10-9-14-17(27-22)8-6-15-20(23)16(12-26-21(14)15)13-5-7-18(24-3)19(11-13)25-4/h5-12H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Mixed type inhibition of BChE (unknown origin) using butyrylthiocholine iodide as substrate by Ellman's method based Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 29: 1194-1198 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.024
BindingDB Entry DOI: 10.7270/Q2HH6PBG |
More data for this
Ligand-Target Pair | |
C-terminal processing protease of the D1 protein
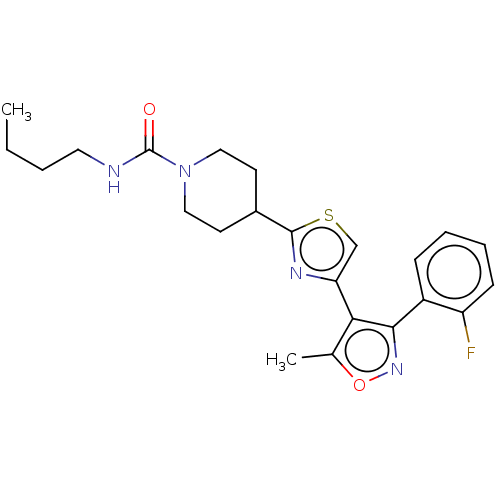
(Spinacia oleracea) | BDBM50488040
(CHEMBL2270666)Show SMILES CCCCNC(=O)N1CCC(CC1)c1nc(cs1)-c1c(C)onc1-c1ccccc1F Show InChI InChI=1S/C23H27FN4O2S/c1-3-4-11-25-23(29)28-12-9-16(10-13-28)22-26-19(14-31-22)20-15(2)30-27-21(20)17-7-5-6-8-18(17)24/h5-8,14,16H,3-4,9-13H2,1-2H3,(H,25,29) | NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University
Curated by ChEMBL
| Assay Description
Competitive inhibition of CtpA in Spinacia oleracea (spinach) thylakoids using S24 substrate incubated for 30 min by HPLC method based double-recipro... |
Molecules 14: 1288-303 (2009)
Article DOI: 10.3390/molecules14031288
BindingDB Entry DOI: 10.7270/Q26H4M9N |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50005463

((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)c2cc3ccccc3[nH]2)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C25H20N4O2/c1-29-21-14-8-6-12-18(21)22(16-9-3-2-4-10-16)27-23(25(29)31)28-24(30)20-15-17-11-5-7-13-19(17)26-20/h2-15,23,26H,1H3,(H,28,30)/t23-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 269: 725-31 (1994)
BindingDB Entry DOI: 10.7270/Q2WD3Z2F |
More data for this
Ligand-Target Pair | |
Cholinesterase
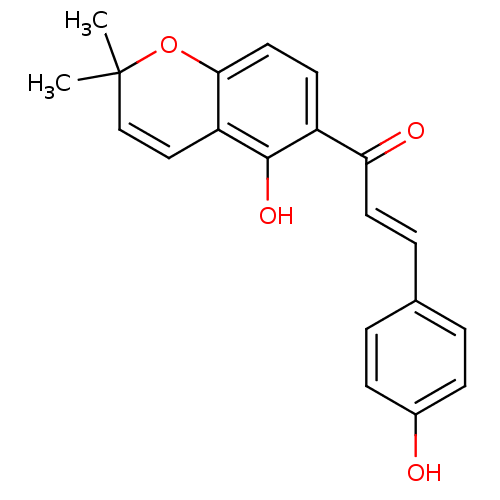
(Homo sapiens (Human)) | BDBM50240945
((E)-1-(5-hydroxy-2,2-dimethyl-2H-chromen-6-yl)-3-(...)Show SMILES CC1(C)Oc2ccc(C(=O)\C=C\c3ccc(O)cc3)c(O)c2C=C1 |c:24| Show InChI InChI=1S/C20H18O4/c1-20(2)12-11-16-18(24-20)10-8-15(19(16)23)17(22)9-5-13-3-6-14(21)7-4-13/h3-12,21,23H,1-2H3/b9-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Mixed type inhibition of BChE (unknown origin) using butyrylthiocholine iodide as substrate by Ellman's method based Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 29: 1194-1198 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.024
BindingDB Entry DOI: 10.7270/Q2HH6PBG |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50505209
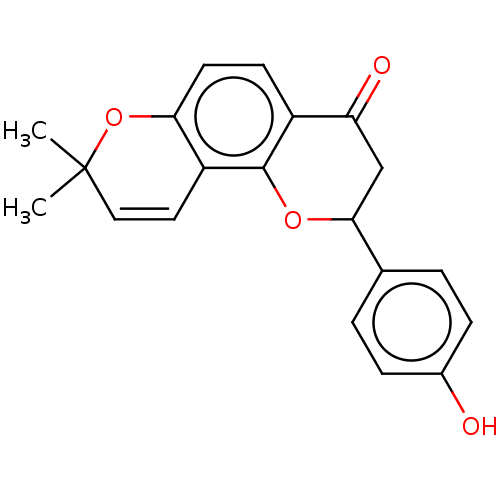
(CHEMBL4550657)Show SMILES CC1(C)Oc2ccc3C(=O)CC(Oc3c2C=C1)c1ccc(O)cc1 |c:17| Show InChI InChI=1S/C20H18O4/c1-20(2)10-9-15-17(24-20)8-7-14-16(22)11-18(23-19(14)15)12-3-5-13(21)6-4-12/h3-10,18,21H,11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Mixed type inhibition of BChE (unknown origin) using butyrylthiocholine iodide as substrate by Ellman's method based Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 29: 1194-1198 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.024
BindingDB Entry DOI: 10.7270/Q2HH6PBG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data