

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50075724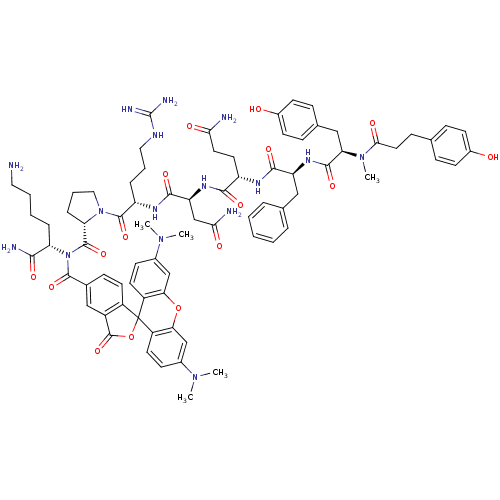 (4HOPh(CH2)2CO-DTyr(Me)-Phe-Gln-Asn-Arg-Pro-Lys(5C-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Displacement of [125I]- HO-LVA from human Vasopressin V1a receptor in membranes of CHO cells | J Med Chem 42: 1312-9 (1999) Article DOI: 10.1021/jm9804782 BindingDB Entry DOI: 10.7270/Q2057F3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50075720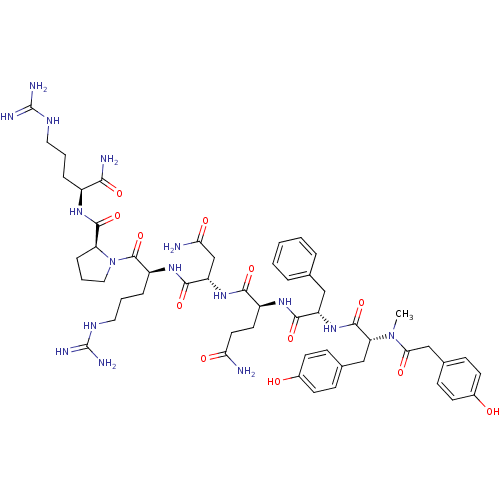 ((HO-LVA23)2-{2-[3-(4-Hydroxy-phenyl)-2-(methyl-4-H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Displacement of [125I]- HO-LVA from human Vasopressin V1a receptor in membranes of CHO cells | J Med Chem 42: 1312-9 (1999) Article DOI: 10.1021/jm9804782 BindingDB Entry DOI: 10.7270/Q2057F3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50469874 (CHEMBL59780) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Institute for Molecular Biology Curated by ChEMBL | Assay Description Binding affinity against human NK2 receptors expressed in CHO cells using [3H]GR-100679 as radioligand | J Med Chem 37: 1991-5 (1994) Article DOI: 10.1021/jm00039a012 BindingDB Entry DOI: 10.7270/Q2DZ0C10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50075722 (4HOPh(CH2)2CO-DTyr(Me)-Phe-Gln-Asn-Arg-Pro-Lys(5C-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Displacement of [125I]- HO-LVA from human Vasopressin V1a receptor in membranes of CHO cells | J Med Chem 42: 1312-9 (1999) Article DOI: 10.1021/jm9804782 BindingDB Entry DOI: 10.7270/Q2057F3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50075725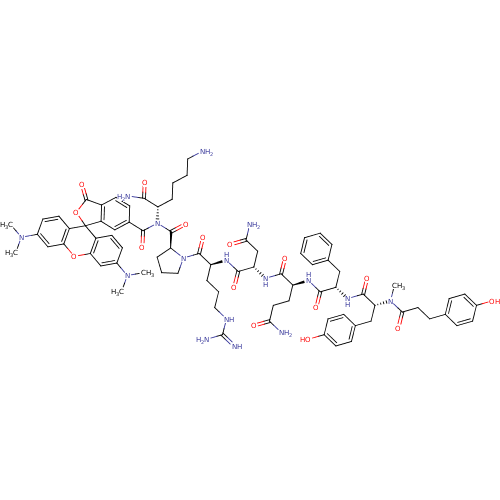 (4HOPh(CH2)2CO-DTyr(Me)-Phe-Gln-Asn-Arg-Pro-Lys(6C-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Displacement of [125I]- HO-LVA from human Vasopressin V1a receptor in membranes of CHO cells | J Med Chem 42: 1312-9 (1999) Article DOI: 10.1021/jm9804782 BindingDB Entry DOI: 10.7270/Q2057F3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50075728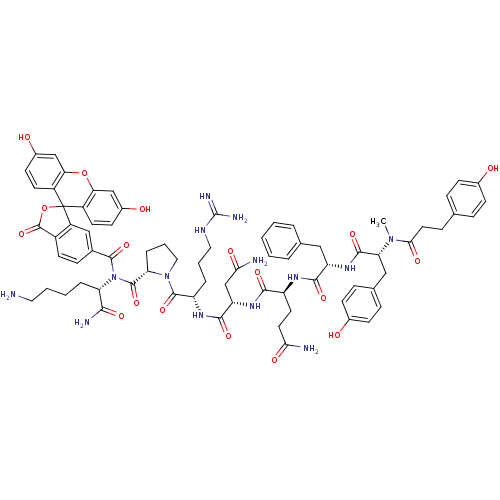 (4HOPh(CH2)2CO-DTyr(Me)-Phe-Gln-Asn-Arg-Pro-Lys(6C-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Displacement of [125I]- HO-LVA from human Vasopressin V1a receptor in membranes of CHO cells | J Med Chem 42: 1312-9 (1999) Article DOI: 10.1021/jm9804782 BindingDB Entry DOI: 10.7270/Q2057F3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50075721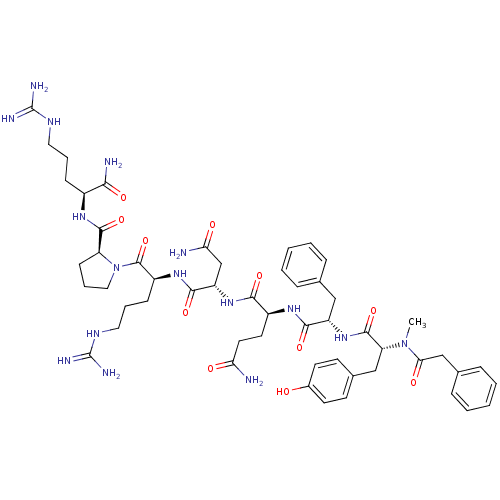 ((LVA)2-{2-[3-(4-Hydroxy-phenyl)-2-(methyl-phenylac...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Displacement of [125I]- HO-LVA from human Vasopressin V1a receptor in membranes of CHO cells | J Med Chem 42: 1312-9 (1999) Article DOI: 10.1021/jm9804782 BindingDB Entry DOI: 10.7270/Q2057F3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50075721 ((LVA)2-{2-[3-(4-Hydroxy-phenyl)-2-(methyl-phenylac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description The inhibition constant (Ki(nM)) of the compound was determined by displacement of [125I]- HO-LVA radiolabeled ligand using membranes of CHO cells of... | J Med Chem 42: 1312-9 (1999) Article DOI: 10.1021/jm9804782 BindingDB Entry DOI: 10.7270/Q2057F3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50075724 (4HOPh(CH2)2CO-DTyr(Me)-Phe-Gln-Asn-Arg-Pro-Lys(5C-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description The inhibition constant (Ki(nM)) of the compound was determined by displacement of [125I]- HO-LVA radiolabeled ligand using membranes of CHO cells of... | J Med Chem 42: 1312-9 (1999) Article DOI: 10.1021/jm9804782 BindingDB Entry DOI: 10.7270/Q2057F3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50469872 (CHEMBL413650) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Institute for Molecular Biology Curated by ChEMBL | Assay Description Binding affinity against human NK2 receptors expressed in CHO cells using [3H]GR-100679 as radioligand | J Med Chem 37: 1991-5 (1994) Article DOI: 10.1021/jm00039a012 BindingDB Entry DOI: 10.7270/Q2DZ0C10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50075727 (4HOPh(CH2)2CO-DTyr(Me)-Phe-Gln-Asn-Lys(5C-Rhm)-Pro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Displacement of [125I]- HO-LVA from human Vasopressin V1a receptor in membranes of CHO cells | J Med Chem 42: 1312-9 (1999) Article DOI: 10.1021/jm9804782 BindingDB Entry DOI: 10.7270/Q2057F3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50469869 (CHEMBL217548) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Institute for Molecular Biology Curated by ChEMBL | Assay Description Binding affinity against human NK2 receptors expressed in CHO cells using [3H]GR-100679 as radioligand | J Med Chem 37: 1991-5 (1994) Article DOI: 10.1021/jm00039a012 BindingDB Entry DOI: 10.7270/Q2DZ0C10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50469867 (CHEMBL268783) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Institute for Molecular Biology Curated by ChEMBL | Assay Description Binding affinity against human NK2 receptors expressed in CHO cells using [3H]GR-100679 as radioligand | J Med Chem 37: 1991-5 (1994) Article DOI: 10.1021/jm00039a012 BindingDB Entry DOI: 10.7270/Q2DZ0C10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50075722 (4HOPh(CH2)2CO-DTyr(Me)-Phe-Gln-Asn-Arg-Pro-Lys(5C-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description The inhibition constant (Ki(nM)) of the compound was determined by displacement of [125I]- HO-LVA radiolabeled ligand using membranes of CHO cells of... | J Med Chem 42: 1312-9 (1999) Article DOI: 10.1021/jm9804782 BindingDB Entry DOI: 10.7270/Q2057F3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50075720 ((HO-LVA23)2-{2-[3-(4-Hydroxy-phenyl)-2-(methyl-4-H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description The inhibition constant (Ki(nM)) of the compound was determined by displacement of [125I]- HO-LVA radiolabeled ligand using membranes of CHO cells of... | J Med Chem 42: 1312-9 (1999) Article DOI: 10.1021/jm9804782 BindingDB Entry DOI: 10.7270/Q2057F3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50075729 (4HOPh(CH2)2CO-DTyr(Me)-Phe-Gln-Asn-Lys(6C-Rhm)-Pro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Displacement of [125I]- HO-LVA from human Vasopressin V1a receptor in membranes of CHO cells | J Med Chem 42: 1312-9 (1999) Article DOI: 10.1021/jm9804782 BindingDB Entry DOI: 10.7270/Q2057F3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50075723 (4HOPh(CH2)2CO-DTyr(Me)-Phe-Gln-Asn-Lys(5C-Flu)-Pro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Displacement of [125I]- HO-LVA from human Vasopressin V1a receptor in membranes of CHO cells | J Med Chem 42: 1312-9 (1999) Article DOI: 10.1021/jm9804782 BindingDB Entry DOI: 10.7270/Q2057F3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM50075720 ((HO-LVA23)2-{2-[3-(4-Hydroxy-phenyl)-2-(methyl-4-H...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Compound was tested for its ability to displace [125I]- HO-LVA ligand from human Vasopressin V1b receptor in the membranes of CHO cells | J Med Chem 42: 1312-9 (1999) Article DOI: 10.1021/jm9804782 BindingDB Entry DOI: 10.7270/Q2057F3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50469870 (CHEMBL275499) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Institute for Molecular Biology Curated by ChEMBL | Assay Description Binding affinity against human NK2 receptors expressed in CHO cells using [3H]GR-100679 as radioligand | J Med Chem 37: 1991-5 (1994) Article DOI: 10.1021/jm00039a012 BindingDB Entry DOI: 10.7270/Q2DZ0C10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM50075721 ((LVA)2-{2-[3-(4-Hydroxy-phenyl)-2-(methyl-phenylac...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description The inhibition constant (Ki(nM)) of the compound was determined by displacement of [125I]- HO-LVA radiolabeled ligand using membranes of CHO cells of... | J Med Chem 42: 1312-9 (1999) Article DOI: 10.1021/jm9804782 BindingDB Entry DOI: 10.7270/Q2057F3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50469868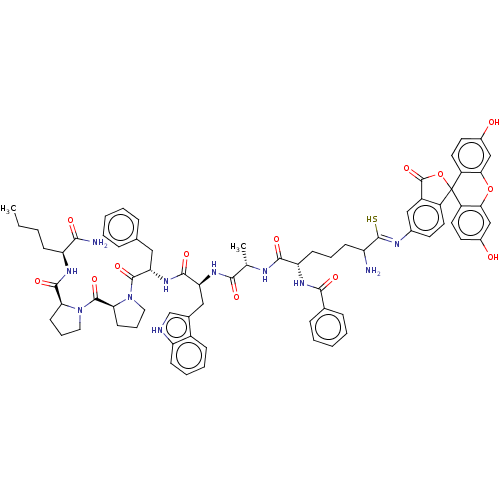 (CHEMBL413205) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Institute for Molecular Biology Curated by ChEMBL | Assay Description Binding affinity against human NK2 receptors expressed in CHO cells using [3H]GR-100679 as radioligand | J Med Chem 37: 1991-5 (1994) Article DOI: 10.1021/jm00039a012 BindingDB Entry DOI: 10.7270/Q2DZ0C10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM50075724 (4HOPh(CH2)2CO-DTyr(Me)-Phe-Gln-Asn-Arg-Pro-Lys(5C-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description The inhibition constant (Ki(nM)) of the compound was determined by displacement of [125I]- HO-LVA radiolabeled ligand using membranes of CHO cells of... | J Med Chem 42: 1312-9 (1999) Article DOI: 10.1021/jm9804782 BindingDB Entry DOI: 10.7270/Q2057F3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50469871 (CHEMBL386783) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Institute for Molecular Biology Curated by ChEMBL | Assay Description Binding affinity against human NK2 receptors expressed in CHO cells using [3H]GR-100679 as radioligand | J Med Chem 37: 1991-5 (1994) Article DOI: 10.1021/jm00039a012 BindingDB Entry DOI: 10.7270/Q2DZ0C10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50075720 ((HO-LVA23)2-{2-[3-(4-Hydroxy-phenyl)-2-(methyl-4-H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 137 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Compound was tested for its ability to of [125I]- HO-LVA radiolabeled ligand using membranes of CHO cells of human Vasopressin V2 receptor | J Med Chem 42: 1312-9 (1999) Article DOI: 10.1021/jm9804782 BindingDB Entry DOI: 10.7270/Q2057F3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM50075722 (4HOPh(CH2)2CO-DTyr(Me)-Phe-Gln-Asn-Arg-Pro-Lys(5C-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 142 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description The inhibition constant (Ki(nM)) of the compound was determined by displacement of [125I]- HO-LVA radiolabeled ligand using membranes of CHO cells of... | J Med Chem 42: 1312-9 (1999) Article DOI: 10.1021/jm9804782 BindingDB Entry DOI: 10.7270/Q2057F3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50075721 ((LVA)2-{2-[3-(4-Hydroxy-phenyl)-2-(methyl-phenylac...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 282 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description The inhibition constant (Ki(nM)) of the compound was determined by displacement of [125I]- HO-LVA radiolabeled ligand using membranes of CHO cells of... | J Med Chem 42: 1312-9 (1999) Article DOI: 10.1021/jm9804782 BindingDB Entry DOI: 10.7270/Q2057F3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50075726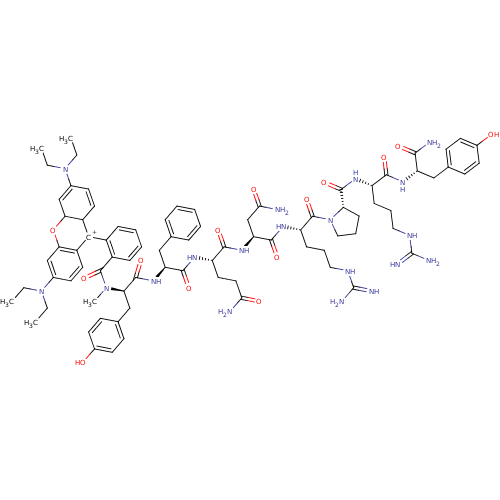 (CHEMBL409756 | tetraethylrhodamyl-DTyr(Me)-Phe-Gln...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Displacement of [125I]- HO-LVA from human Vasopressin V1a receptor in membranes of CHO cells | J Med Chem 42: 1312-9 (1999) Article DOI: 10.1021/jm9804782 BindingDB Entry DOI: 10.7270/Q2057F3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50469873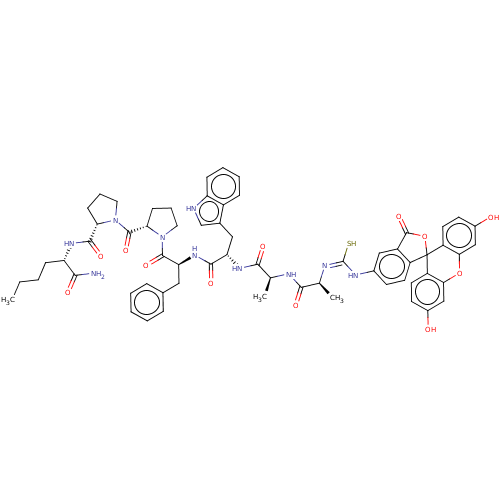 (CHEMBL411905) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Institute for Molecular Biology Curated by ChEMBL | Assay Description Binding affinity against human NK2 receptors expressed in CHO cells using [3H]GR-100679 as radioligand | J Med Chem 37: 1991-5 (1994) Article DOI: 10.1021/jm00039a012 BindingDB Entry DOI: 10.7270/Q2DZ0C10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50075724 (4HOPh(CH2)2CO-DTyr(Me)-Phe-Gln-Asn-Arg-Pro-Lys(5C-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description The inhibition constant (Ki(nM)) of the compound was determined by displacement of [125I]- HO-LVA radiolabeled ligand using membranes of CHO cells of... | J Med Chem 42: 1312-9 (1999) Article DOI: 10.1021/jm9804782 BindingDB Entry DOI: 10.7270/Q2057F3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50075722 (4HOPh(CH2)2CO-DTyr(Me)-Phe-Gln-Asn-Arg-Pro-Lys(5C-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description The inhibition constant (Ki(nM)) of the compound was determined by displacement of [125I]- HO-LVA radiolabeled ligand using membranes of CHO cells of... | J Med Chem 42: 1312-9 (1999) Article DOI: 10.1021/jm9804782 BindingDB Entry DOI: 10.7270/Q2057F3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50051818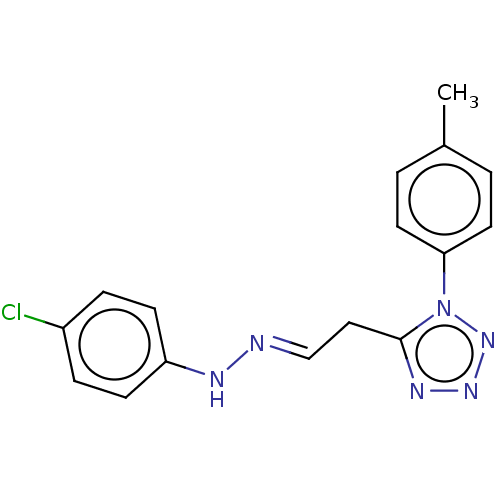 (CHEMBL3318333) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 expressed in mouse P815B cells using L-tryptophan substrate incubated for 18 hrs by HPLC based cellular assay | Eur J Med Chem 84: 284-301 (2014) Article DOI: 10.1016/j.ejmech.2014.06.078 BindingDB Entry DOI: 10.7270/Q21C1ZJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50051845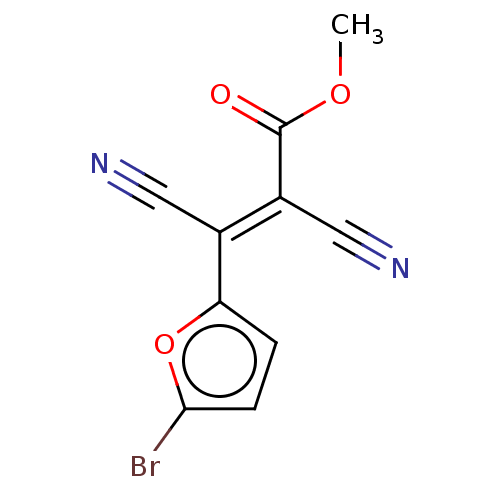 (CHEMBL3318327) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins by HPLC | Eur J Med Chem 84: 284-301 (2014) Article DOI: 10.1016/j.ejmech.2014.06.078 BindingDB Entry DOI: 10.7270/Q21C1ZJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50051846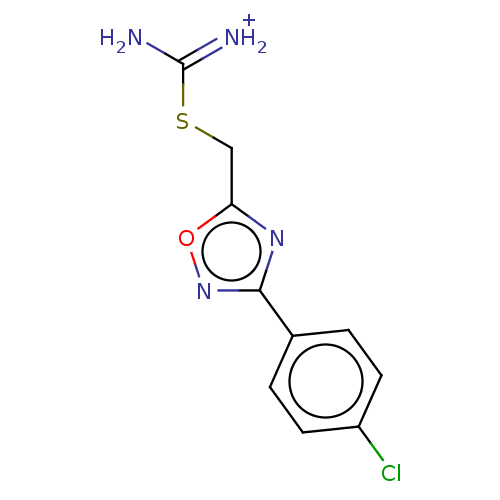 (CHEMBL3358454) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins by HPLC | Eur J Med Chem 84: 284-301 (2014) Article DOI: 10.1016/j.ejmech.2014.06.078 BindingDB Entry DOI: 10.7270/Q21C1ZJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50051847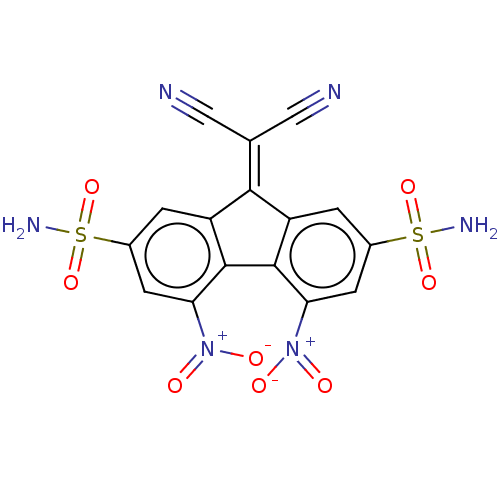 (CHEMBL3318328) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins in presence of 0.01% Triton-X by HPLC | Eur J Med Chem 84: 284-301 (2014) Article DOI: 10.1016/j.ejmech.2014.06.078 BindingDB Entry DOI: 10.7270/Q21C1ZJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50051847 (CHEMBL3318328) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins by HPLC | Eur J Med Chem 84: 284-301 (2014) Article DOI: 10.1016/j.ejmech.2014.06.078 BindingDB Entry DOI: 10.7270/Q21C1ZJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50051846 (CHEMBL3358454) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins in presence of 0.01% Triton-X by HPLC | Eur J Med Chem 84: 284-301 (2014) Article DOI: 10.1016/j.ejmech.2014.06.078 BindingDB Entry DOI: 10.7270/Q21C1ZJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50051847 (CHEMBL3318328) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins in presence of 5 mM GSH by HPLC | Eur J Med Chem 84: 284-301 (2014) Article DOI: 10.1016/j.ejmech.2014.06.078 BindingDB Entry DOI: 10.7270/Q21C1ZJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50051848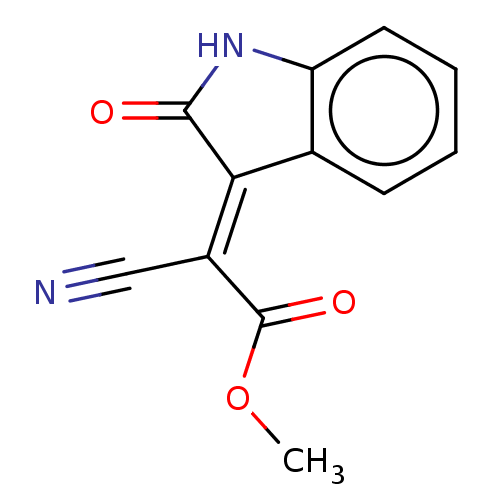 (CHEMBL3318329) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins by HPLC | Eur J Med Chem 84: 284-301 (2014) Article DOI: 10.1016/j.ejmech.2014.06.078 BindingDB Entry DOI: 10.7270/Q21C1ZJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50051846 (CHEMBL3358454) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 expressed in mouse P815B cells using L-tryptophan substrate incubated for 18 hrs by HPLC based cellular assay | Eur J Med Chem 84: 284-301 (2014) Article DOI: 10.1016/j.ejmech.2014.06.078 BindingDB Entry DOI: 10.7270/Q21C1ZJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM31772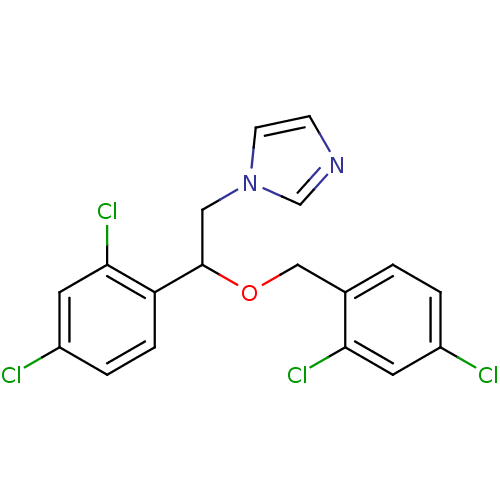 (1-[2-(2,4-dichlorobenzyl)oxy-2-(2,4-dichlorophenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins in presence of 0.01% Triton-X by HPLC | Eur J Med Chem 84: 284-301 (2014) Article DOI: 10.1016/j.ejmech.2014.06.078 BindingDB Entry DOI: 10.7270/Q21C1ZJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Mus musculus) | BDBM50051818 (CHEMBL3318333) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of mouse TDO expressed in mouse P815B cells using L-tryptophan substrate incubated for 24 hrs by HPLC based cellular assay | Eur J Med Chem 84: 284-301 (2014) Article DOI: 10.1016/j.ejmech.2014.06.078 BindingDB Entry DOI: 10.7270/Q21C1ZJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50247616 (2-(3-chloro-2-fluorophenyl)isothiazol-3(2H)-one | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins in presence of 0.01% Triton-X by HPLC | Eur J Med Chem 84: 284-301 (2014) Article DOI: 10.1016/j.ejmech.2014.06.078 BindingDB Entry DOI: 10.7270/Q21C1ZJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM92508 (DP 00477, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins in presence of 0.01% Triton-X by HPLC | Eur J Med Chem 84: 284-301 (2014) Article DOI: 10.1016/j.ejmech.2014.06.078 BindingDB Entry DOI: 10.7270/Q21C1ZJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM31770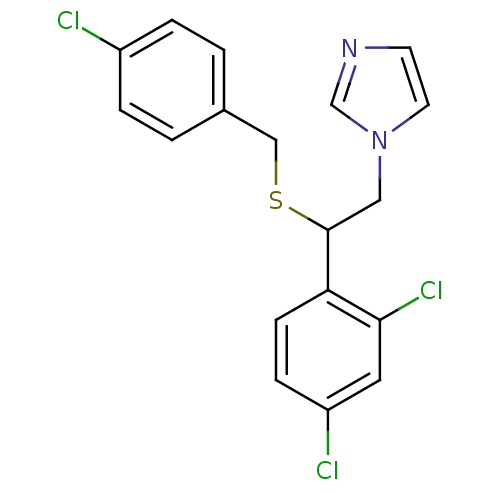 (Exelderm | Sulconazole Nitrate | cid_65495 | sulco...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins by HPLC | Eur J Med Chem 84: 284-301 (2014) Article DOI: 10.1016/j.ejmech.2014.06.078 BindingDB Entry DOI: 10.7270/Q21C1ZJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM31772 (1-[2-(2,4-dichlorobenzyl)oxy-2-(2,4-dichlorophenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins by HPLC | Eur J Med Chem 84: 284-301 (2014) Article DOI: 10.1016/j.ejmech.2014.06.078 BindingDB Entry DOI: 10.7270/Q21C1ZJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM92508 (DP 00477, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins by HPLC | Eur J Med Chem 84: 284-301 (2014) Article DOI: 10.1016/j.ejmech.2014.06.078 BindingDB Entry DOI: 10.7270/Q21C1ZJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50051845 (CHEMBL3318327) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins in presence of 5 mM GSH by HPLC | Eur J Med Chem 84: 284-301 (2014) Article DOI: 10.1016/j.ejmech.2014.06.078 BindingDB Entry DOI: 10.7270/Q21C1ZJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM31773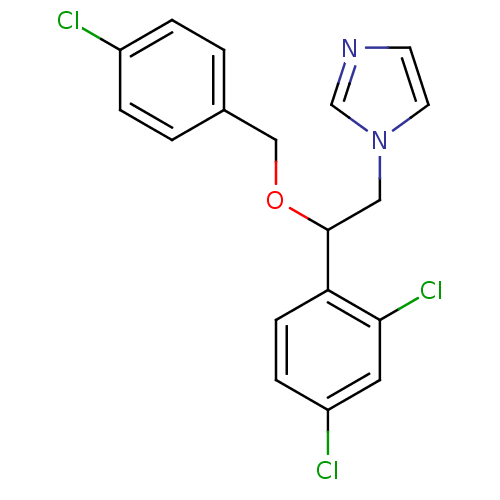 (ECONAZOLE | Econazole nitrate | Gyno-pevaryl | Pev...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins by HPLC | Eur J Med Chem 84: 284-301 (2014) Article DOI: 10.1016/j.ejmech.2014.06.078 BindingDB Entry DOI: 10.7270/Q21C1ZJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50051842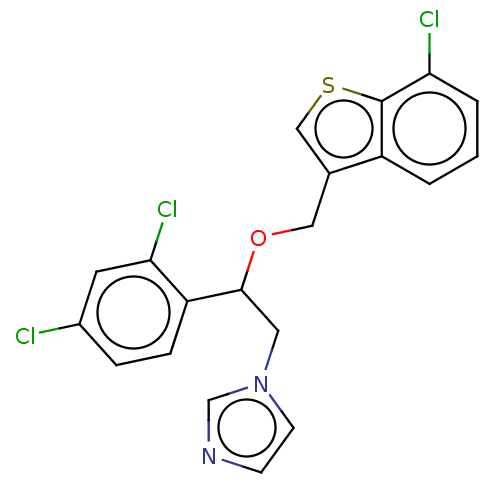 (CHEBI:83682 | Demofix | Ertaczo | Sertaconazole) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins by HPLC | Eur J Med Chem 84: 284-301 (2014) Article DOI: 10.1016/j.ejmech.2014.06.078 BindingDB Entry DOI: 10.7270/Q21C1ZJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50247616 (2-(3-chloro-2-fluorophenyl)isothiazol-3(2H)-one | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins by HPLC | Eur J Med Chem 84: 284-301 (2014) Article DOI: 10.1016/j.ejmech.2014.06.078 BindingDB Entry DOI: 10.7270/Q21C1ZJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 75 total ) | Next | Last >> |