 Found 264 hits with Last Name = 'turcaud' and Initial = 's'
Found 264 hits with Last Name = 'turcaud' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neprilysin
(Homo sapiens (Human)) | BDBM50407297
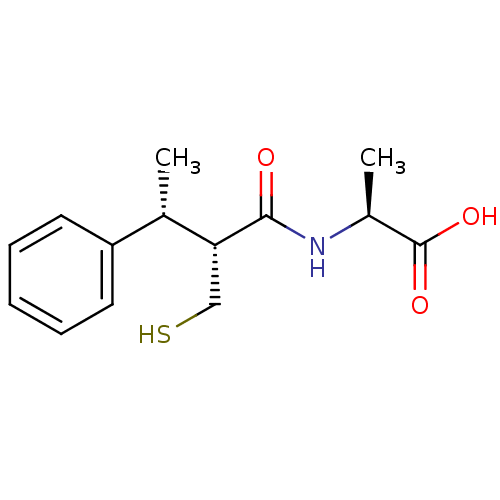
(CHEMBL2052008)Show SMILES C[C@H](NC(=O)[C@H](CS)[C@@H](C)c1ccccc1)C(O)=O |r| Show InChI InChI=1S/C14H19NO3S/c1-9(11-6-4-3-5-7-11)12(8-19)13(16)15-10(2)14(17)18/h3-7,9-10,12,19H,8H2,1-2H3,(H,15,16)(H,17,18)/t9-,10-,12+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vivo inhibitory potency against neutral endopeptidase by displacement of [3H]-HACBOGly binding in mouse kidney |
J Med Chem 37: 1070-83 (1994)
BindingDB Entry DOI: 10.7270/Q26W995T |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50407297

(CHEMBL2052008)Show SMILES C[C@H](NC(=O)[C@H](CS)[C@@H](C)c1ccccc1)C(O)=O |r| Show InChI InChI=1S/C14H19NO3S/c1-9(11-6-4-3-5-7-11)12(8-19)13(16)15-10(2)14(17)18/h3-7,9-10,12,19H,8H2,1-2H3,(H,15,16)(H,17,18)/t9-,10-,12+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vivo inhibitory potency against neutral endopeptidase by displacement of [3H]-HACBOGly binding in mouse kidney |
J Med Chem 37: 1070-83 (1994)
BindingDB Entry DOI: 10.7270/Q26W995T |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50407299
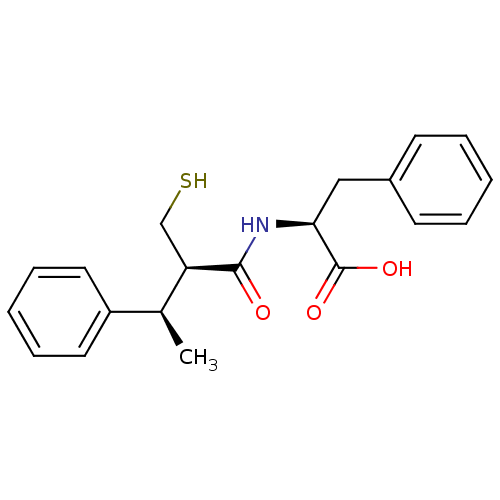
(CHEMBL2052007)Show SMILES C[C@H]([C@@H](CS)C(=O)N[C@@H](Cc1ccccc1)C(O)=O)c1ccccc1 |r| Show InChI InChI=1S/C20H23NO3S/c1-14(16-10-6-3-7-11-16)17(13-25)19(22)21-18(20(23)24)12-15-8-4-2-5-9-15/h2-11,14,17-18,25H,12-13H2,1H3,(H,21,22)(H,23,24)/t14-,17+,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vivo inhibitory potency against neutral endopeptidase by displacement of [3H]-HACBOGly binding in mouse kidney |
J Med Chem 37: 1070-83 (1994)
BindingDB Entry DOI: 10.7270/Q26W995T |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50407299

(CHEMBL2052007)Show SMILES C[C@H]([C@@H](CS)C(=O)N[C@@H](Cc1ccccc1)C(O)=O)c1ccccc1 |r| Show InChI InChI=1S/C20H23NO3S/c1-14(16-10-6-3-7-11-16)17(13-25)19(22)21-18(20(23)24)12-15-8-4-2-5-9-15/h2-11,14,17-18,25H,12-13H2,1H3,(H,21,22)(H,23,24)/t14-,17+,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vivo inhibitory potency against neutral endopeptidase by displacement of [3H]-HACBOGly binding in mouse kidney |
J Med Chem 37: 1070-83 (1994)
BindingDB Entry DOI: 10.7270/Q26W995T |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Mus musculus) | BDBM50407299

(CHEMBL2052007)Show SMILES C[C@H]([C@@H](CS)C(=O)N[C@@H](Cc1ccccc1)C(O)=O)c1ccccc1 |r| Show InChI InChI=1S/C20H23NO3S/c1-14(16-10-6-3-7-11-16)17(13-25)19(22)21-18(20(23)24)12-15-8-4-2-5-9-15/h2-11,14,17-18,25H,12-13H2,1H3,(H,21,22)(H,23,24)/t14-,17+,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung |
J Med Chem 37: 1070-83 (1994)
BindingDB Entry DOI: 10.7270/Q26W995T |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Mus musculus) | BDBM50407299

(CHEMBL2052007)Show SMILES C[C@H]([C@@H](CS)C(=O)N[C@@H](Cc1ccccc1)C(O)=O)c1ccccc1 |r| Show InChI InChI=1S/C20H23NO3S/c1-14(16-10-6-3-7-11-16)17(13-25)19(22)21-18(20(23)24)12-15-8-4-2-5-9-15/h2-11,14,17-18,25H,12-13H2,1H3,(H,21,22)(H,23,24)/t14-,17+,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung |
J Med Chem 37: 1070-83 (1994)
BindingDB Entry DOI: 10.7270/Q26W995T |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50407298
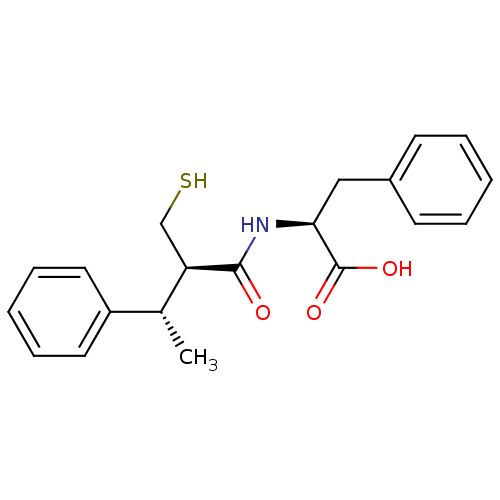
(CHEMBL2051772)Show SMILES C[C@@H]([C@@H](CS)C(=O)N[C@@H](Cc1ccccc1)C(O)=O)c1ccccc1 |r| Show InChI InChI=1S/C20H23NO3S/c1-14(16-10-6-3-7-11-16)17(13-25)19(22)21-18(20(23)24)12-15-8-4-2-5-9-15/h2-11,14,17-18,25H,12-13H2,1H3,(H,21,22)(H,23,24)/t14-,17-,18+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vivo inhibitory potency against neutral endopeptidase by displacement of [3H]-HACBOGly binding in mouse kidney |
J Med Chem 37: 1070-83 (1994)
BindingDB Entry DOI: 10.7270/Q26W995T |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Mus musculus) | BDBM50407298

(CHEMBL2051772)Show SMILES C[C@@H]([C@@H](CS)C(=O)N[C@@H](Cc1ccccc1)C(O)=O)c1ccccc1 |r| Show InChI InChI=1S/C20H23NO3S/c1-14(16-10-6-3-7-11-16)17(13-25)19(22)21-18(20(23)24)12-15-8-4-2-5-9-15/h2-11,14,17-18,25H,12-13H2,1H3,(H,21,22)(H,23,24)/t14-,17-,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung |
J Med Chem 37: 1070-83 (1994)
BindingDB Entry DOI: 10.7270/Q26W995T |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Mus musculus) | BDBM50407297

(CHEMBL2052008)Show SMILES C[C@H](NC(=O)[C@H](CS)[C@@H](C)c1ccccc1)C(O)=O |r| Show InChI InChI=1S/C14H19NO3S/c1-9(11-6-4-3-5-7-11)12(8-19)13(16)15-10(2)14(17)18/h3-7,9-10,12,19H,8H2,1-2H3,(H,15,16)(H,17,18)/t9-,10-,12+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung |
J Med Chem 37: 1070-83 (1994)
BindingDB Entry DOI: 10.7270/Q26W995T |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Mus musculus) | BDBM50407297

(CHEMBL2052008)Show SMILES C[C@H](NC(=O)[C@H](CS)[C@@H](C)c1ccccc1)C(O)=O |r| Show InChI InChI=1S/C14H19NO3S/c1-9(11-6-4-3-5-7-11)12(8-19)13(16)15-10(2)14(17)18/h3-7,9-10,12,19H,8H2,1-2H3,(H,15,16)(H,17,18)/t9-,10-,12+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung |
J Med Chem 37: 1070-83 (1994)
BindingDB Entry DOI: 10.7270/Q26W995T |
More data for this
Ligand-Target Pair | |
Tetanus toxin
(Clostridium tetani) | BDBM50073671
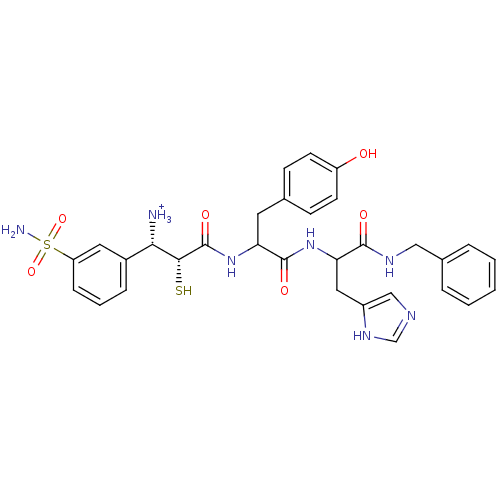
((1S,2R)-2-[1-[1-Benzylcarbamoyl-2-(1H-imidazol-4-y...)Show SMILES NS(=O)(=O)c1cccc(c1)[C@H]([NH3+])[C@@H](S)C(=O)NC(Cc1ccc(O)cc1)C(=O)NC(Cc1cnc[nH]1)C(=O)NCc1ccccc1 Show InChI InChI=1S/C31H35N7O6S2/c32-27(21-7-4-8-24(14-21)46(33,43)44)28(45)31(42)38-25(13-19-9-11-23(39)12-10-19)30(41)37-26(15-22-17-34-18-36-22)29(40)35-16-20-5-2-1-3-6-20/h1-12,14,17-18,25-28,39,45H,13,15-16,32H2,(H,34,36)(H,35,40)(H,37,41)(H,38,42)(H2,33,43,44)/p+1/t25?,26?,27-,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of tetanus neurotoxin (TeNt) |
J Med Chem 42: 515-25 (1999)
Article DOI: 10.1021/jm981066w
BindingDB Entry DOI: 10.7270/Q2BC4075 |
More data for this
Ligand-Target Pair | |
Tetanus toxin
(Clostridium tetani) | BDBM50073669

((S)-1-{(R)-[(S)-1-[(S)-1-Benzylcarbamoyl-2-(3H-imi...)Show SMILES NS(=O)(=O)CC[C@H]([NH3+])[C@@H](S)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)NCc1ccccc1 Show InChI InChI=1S/C27H35N7O6S2/c28-21(10-11-42(29,39)40)24(41)27(38)34-22(12-17-6-8-20(35)9-7-17)26(37)33-23(13-19-15-30-16-32-19)25(36)31-14-18-4-2-1-3-5-18/h1-9,15-16,21-24,35,41H,10-14,28H2,(H,30,32)(H,31,36)(H,33,37)(H,34,38)(H2,29,39,40)/p+1/t21-,22-,23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of tetanus neurotoxin (TeNt) |
J Med Chem 42: 515-25 (1999)
Article DOI: 10.1021/jm981066w
BindingDB Entry DOI: 10.7270/Q2BC4075 |
More data for this
Ligand-Target Pair | |
Tetanus toxin
(Clostridium tetani) | BDBM50073670

((1R,2S)-2-[1-[1-Benzylcarbamoyl-2-(1H-imidazol-4-y...)Show SMILES NS(=O)(=O)c1cccc(c1)[C@@H]([NH3+])[C@H](S)C(=O)NC(Cc1ccc(O)cc1)C(=O)NC(Cc1cnc[nH]1)C(=O)NCc1ccccc1 Show InChI InChI=1S/C31H35N7O6S2/c32-27(21-7-4-8-24(14-21)46(33,43)44)28(45)31(42)38-25(13-19-9-11-23(39)12-10-19)30(41)37-26(15-22-17-34-18-36-22)29(40)35-16-20-5-2-1-3-6-20/h1-12,14,17-18,25-28,39,45H,13,15-16,32H2,(H,34,36)(H,35,40)(H,37,41)(H,38,42)(H2,33,43,44)/p+1/t25?,26?,27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of tetanus neurotoxin (TeNt) |
J Med Chem 42: 515-25 (1999)
Article DOI: 10.1021/jm981066w
BindingDB Entry DOI: 10.7270/Q2BC4075 |
More data for this
Ligand-Target Pair | |
Tetanus toxin
(Clostridium tetani) | BDBM50073668

((S)-1-{(R)-[(S)-1-[(S)-1-Carboxy-2-(3H-imidazol-4-...)Show SMILES NS(=O)(=O)CC[C@H]([NH3+])[C@@H](S)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(O)=O Show InChI InChI=1S/C20H28N6O7S2/c21-14(5-6-35(22,32)33)17(34)19(29)25-15(7-11-1-3-13(27)4-2-11)18(28)26-16(20(30)31)8-12-9-23-10-24-12/h1-4,9-10,14-17,27,34H,5-8,21H2,(H,23,24)(H,25,29)(H,26,28)(H,30,31)(H2,22,32,33)/p+1/t14-,15-,16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of tetanus neurotoxin (TeNt) |
J Med Chem 42: 515-25 (1999)
Article DOI: 10.1021/jm981066w
BindingDB Entry DOI: 10.7270/Q2BC4075 |
More data for this
Ligand-Target Pair | |
Tetanus toxin
(Clostridium tetani) | BDBM50073678

((1R,2R)-2-[1-[1-Benzylcarbamoyl-2-(1H-imidazol-4-y...)Show SMILES NS(=O)(=O)c1cccc(c1)[C@@H]([NH3+])[C@@H](S)C(=O)NC(Cc1ccc(O)cc1)C(=O)NC(Cc1cnc[nH]1)C(=O)NCc1ccccc1 Show InChI InChI=1S/C31H35N7O6S2/c32-27(21-7-4-8-24(14-21)46(33,43)44)28(45)31(42)38-25(13-19-9-11-23(39)12-10-19)30(41)37-26(15-22-17-34-18-36-22)29(40)35-16-20-5-2-1-3-6-20/h1-12,14,17-18,25-28,39,45H,13,15-16,32H2,(H,34,36)(H,35,40)(H,37,41)(H,38,42)(H2,33,43,44)/p+1/t25?,26?,27-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of tetanus neurotoxin (TeNt) |
J Med Chem 42: 515-25 (1999)
Article DOI: 10.1021/jm981066w
BindingDB Entry DOI: 10.7270/Q2BC4075 |
More data for this
Ligand-Target Pair | |
Tetanus toxin
(Clostridium tetani) | BDBM50073679

((S)-1-{(S)-[(S)-1-[(S)-1-Benzylcarbamoyl-2-(3H-imi...)Show SMILES NS(=O)(=O)CC[C@H]([NH3+])[C@H](S)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)NCc1ccccc1 Show InChI InChI=1S/C27H35N7O6S2/c28-21(10-11-42(29,39)40)24(41)27(38)34-22(12-17-6-8-20(35)9-7-17)26(37)33-23(13-19-15-30-16-32-19)25(36)31-14-18-4-2-1-3-5-18/h1-9,15-16,21-24,35,41H,10-14,28H2,(H,30,32)(H,31,36)(H,33,37)(H,34,38)(H2,29,39,40)/p+1/t21-,22-,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of tetanus neurotoxin (TeNt) |
J Med Chem 42: 515-25 (1999)
Article DOI: 10.1021/jm981066w
BindingDB Entry DOI: 10.7270/Q2BC4075 |
More data for this
Ligand-Target Pair | |
Tetanus toxin
(Clostridium tetani) | BDBM50073675
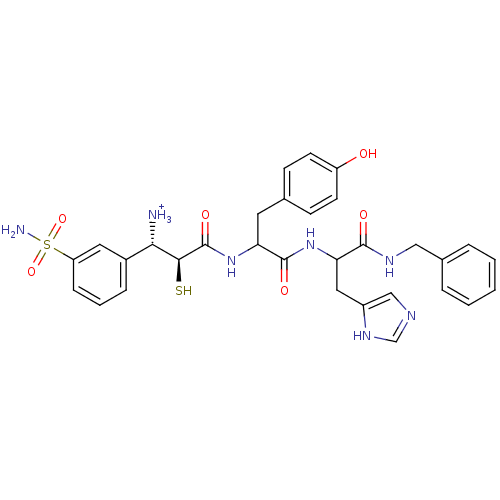
((1S,2S)-2-[1-[1-Benzylcarbamoyl-2-(1H-imidazol-4-y...)Show SMILES NS(=O)(=O)c1cccc(c1)[C@H]([NH3+])[C@H](S)C(=O)NC(Cc1ccc(O)cc1)C(=O)NC(Cc1cnc[nH]1)C(=O)NCc1ccccc1 Show InChI InChI=1S/C31H35N7O6S2/c32-27(21-7-4-8-24(14-21)46(33,43)44)28(45)31(42)38-25(13-19-9-11-23(39)12-10-19)30(41)37-26(15-22-17-34-18-36-22)29(40)35-16-20-5-2-1-3-6-20/h1-12,14,17-18,25-28,39,45H,13,15-16,32H2,(H,34,36)(H,35,40)(H,37,41)(H,38,42)(H2,33,43,44)/p+1/t25?,26?,27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of tetanus neurotoxin (TeNt) |
J Med Chem 42: 515-25 (1999)
Article DOI: 10.1021/jm981066w
BindingDB Entry DOI: 10.7270/Q2BC4075 |
More data for this
Ligand-Target Pair | |
Tetanus toxin
(Clostridium tetani) | BDBM50073674
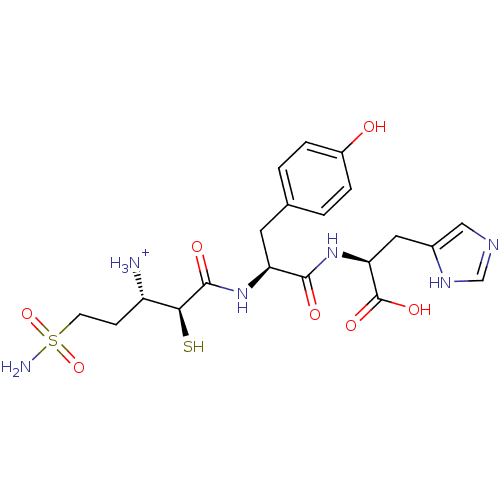
((S)-1-{(S)-[(S)-1-[(S)-1-Carboxy-2-(3H-imidazol-4-...)Show SMILES NS(=O)(=O)CC[C@H]([NH3+])[C@H](S)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(O)=O Show InChI InChI=1S/C20H28N6O7S2/c21-14(5-6-35(22,32)33)17(34)19(29)25-15(7-11-1-3-13(27)4-2-11)18(28)26-16(20(30)31)8-12-9-23-10-24-12/h1-4,9-10,14-17,27,34H,5-8,21H2,(H,23,24)(H,25,29)(H,26,28)(H,30,31)(H2,22,32,33)/p+1/t14-,15-,16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of tetanus neurotoxin (TeNt) |
J Med Chem 42: 515-25 (1999)
Article DOI: 10.1021/jm981066w
BindingDB Entry DOI: 10.7270/Q2BC4075 |
More data for this
Ligand-Target Pair | |
Tetanus toxin
(Clostridium tetani) | BDBM50073680
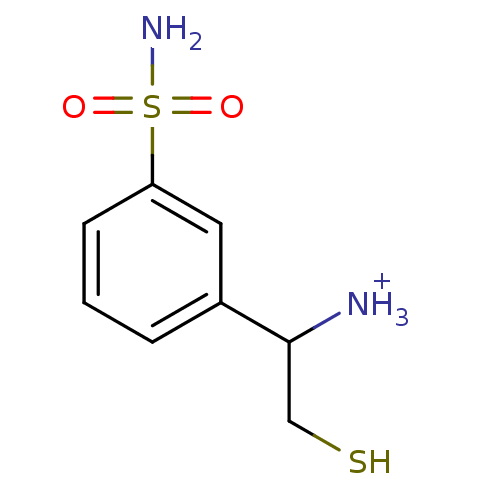
(2-Mercapto-1-(3-sulfamoyl-phenyl)-ethyl-ammonium)Show InChI InChI=1S/C8H12N2O2S2/c9-8(5-13)6-2-1-3-7(4-6)14(10,11)12/h1-4,8,13H,5,9H2,(H2,10,11,12)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of tetanus neurotoxin (TeNt) |
J Med Chem 42: 515-25 (1999)
Article DOI: 10.1021/jm981066w
BindingDB Entry DOI: 10.7270/Q2BC4075 |
More data for this
Ligand-Target Pair | |
Tetanus toxin
(Clostridium tetani) | BDBM50073673

(N-[(2SR,3S)-3-Amino-5-carbamoyl-2-sulfanylpentanoy...)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](Cc1ccccc1)NC(=O)C(S)[C@@H]([NH3+])CCC(=O)ON)C(=O)N[C@@H](CO)C(O)=O Show InChI InChI=1S/C27H40N6O12S/c1-13(35)21(25(41)32-18(12-34)27(43)44)33-23(39)16(8-9-19(36)37)30-24(40)17(11-14-5-3-2-4-6-14)31-26(42)22(46)15(28)7-10-20(38)45-29/h2-6,13,15-18,21-22,34-35,46H,7-12,28-29H2,1H3,(H,30,40)(H,31,42)(H,32,41)(H,33,39)(H,36,37)(H,43,44)/p+1/t13-,15+,16+,17-,18+,21+,22?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of tetanus neurotoxin (TeNt) |
J Med Chem 42: 515-25 (1999)
Article DOI: 10.1021/jm981066w
BindingDB Entry DOI: 10.7270/Q2BC4075 |
More data for this
Ligand-Target Pair | |
Tetanus toxin
(Clostridium tetani) | BDBM50073677

(N-[(2SR,3S)-3-Amino-5-carbamoyl-2-sulfanylpentanoy...)Show SMILES NOC(=O)CC[C@H]([NH3+])C(S)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O Show InChI InChI=1S/C20H28N4O8S/c21-12(6-9-16(27)32-22)17(33)19(29)24-14(10-11-4-2-1-3-5-11)18(28)23-13(20(30)31)7-8-15(25)26/h1-5,12-14,17,33H,6-10,21-22H2,(H,23,28)(H,24,29)(H,25,26)(H,30,31)/p+1/t12-,13-,14+,17?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of tetanus neurotoxin (TeNt) |
J Med Chem 42: 515-25 (1999)
Article DOI: 10.1021/jm981066w
BindingDB Entry DOI: 10.7270/Q2BC4075 |
More data for this
Ligand-Target Pair | |
Tetanus toxin
(Clostridium tetani) | BDBM50073676
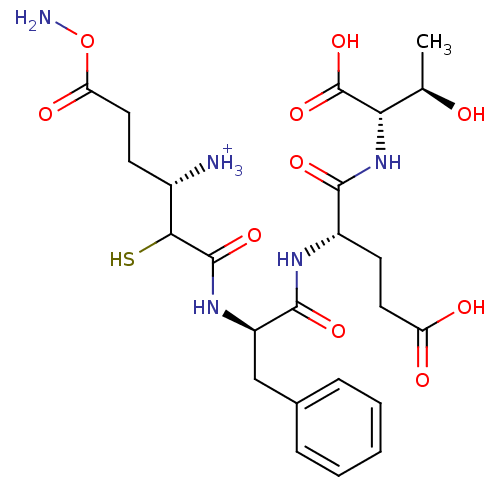
(N-[(2SR,3S)-3-Amino-5-carbamoyl-2-sulfanylpentanoy...)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](Cc1ccccc1)NC(=O)C(S)[C@@H]([NH3+])CCC(=O)ON)C(O)=O Show InChI InChI=1S/C24H35N5O10S/c1-12(30)19(24(37)38)29-21(34)15(8-9-17(31)32)27-22(35)16(11-13-5-3-2-4-6-13)28-23(36)20(40)14(25)7-10-18(33)39-26/h2-6,12,14-16,19-20,30,40H,7-11,25-26H2,1H3,(H,27,35)(H,28,36)(H,29,34)(H,31,32)(H,37,38)/p+1/t12-,14+,15+,16-,19+,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of tetanus neurotoxin (TeNt) |
J Med Chem 42: 515-25 (1999)
Article DOI: 10.1021/jm981066w
BindingDB Entry DOI: 10.7270/Q2BC4075 |
More data for this
Ligand-Target Pair | |
Tetanus toxin
(Clostridium tetani) | BDBM50073667
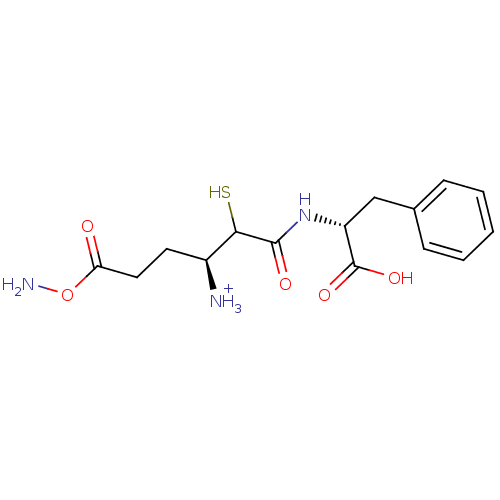
(N-[(2SR,3S)-3-Amino-5-carbamoyl-2-sulfanylpentanoy...)Show SMILES NOC(=O)CC[C@H]([NH3+])C(S)C(=O)N[C@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C15H21N3O5S/c16-10(6-7-12(19)23-17)13(24)14(20)18-11(15(21)22)8-9-4-2-1-3-5-9/h1-5,10-11,13,24H,6-8,16-17H2,(H,18,20)(H,21,22)/p+1/t10-,11+,13?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of tetanus neurotoxin (TeNt) |
J Med Chem 42: 515-25 (1999)
Article DOI: 10.1021/jm981066w
BindingDB Entry DOI: 10.7270/Q2BC4075 |
More data for this
Ligand-Target Pair | |
Tetanus toxin
(Clostridium tetani) | BDBM50073666
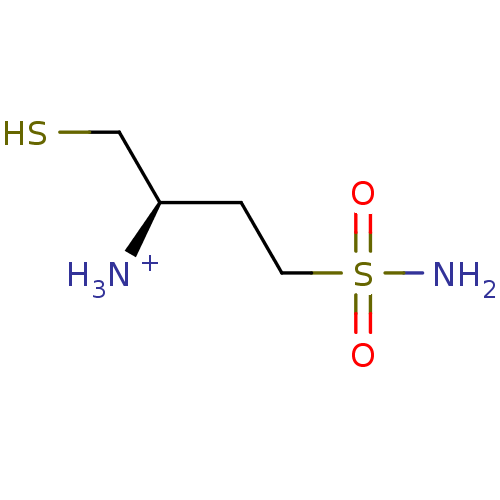
((R)-1-Mercaptomethyl-3-sulfamoyl-propyl-ammonium)Show InChI InChI=1S/C4H12N2O2S2/c5-4(3-9)1-2-10(6,7)8/h4,9H,1-3,5H2,(H2,6,7,8)/p+1/t4-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of tetanus neurotoxin (TeNt) |
J Med Chem 42: 515-25 (1999)
Article DOI: 10.1021/jm981066w
BindingDB Entry DOI: 10.7270/Q2BC4075 |
More data for this
Ligand-Target Pair | |
Tetanus toxin
(Clostridium tetani) | BDBM50073672
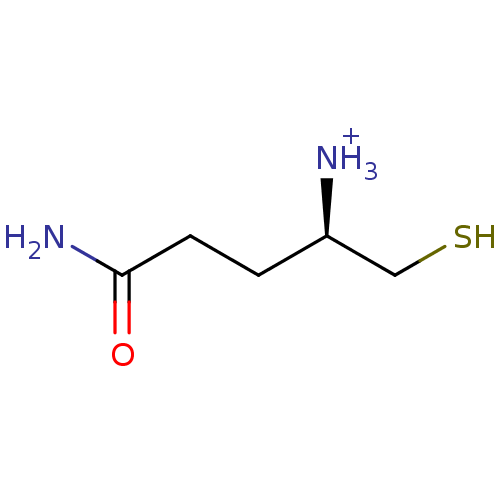
((R)-3-Carbamoyl-1-mercaptomethyl-propyl-ammonium)Show InChI InChI=1S/C5H12N2OS/c6-4(3-9)1-2-5(7)8/h4,9H,1-3,6H2,(H2,7,8)/p+1/t4-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of tetanus neurotoxin (TeNt) |
J Med Chem 42: 515-25 (1999)
Article DOI: 10.1021/jm981066w
BindingDB Entry DOI: 10.7270/Q2BC4075 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50050127

((S)-3-(4-Hydroxy-phenyl)-2-[(2S,3R)-2-((S)-2-merca...)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H](S)C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C20H30N2O5S/c1-5-12(4)16(22-19(25)17(28)11(2)3)18(24)21-15(20(26)27)10-13-6-8-14(23)9-7-13/h6-9,11-12,15-17,23,28H,5,10H2,1-4H3,(H,21,24)(H,22,25)(H,26,27)/t12-,15+,16+,17+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin I converting enzyme |
J Med Chem 39: 1210-9 (1996)
Article DOI: 10.1021/jm950590p
BindingDB Entry DOI: 10.7270/Q2DF6RWV |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50050151

(1-[(S)-2-((S)-2-Mercapto-3-phenyl-propionylamino)-...)Show SMILES C[C@H](NC(=O)[C@@H](S)Cc1ccccc1)C(=O)N1CCCC1C(O)=O Show InChI InChI=1S/C17H22N2O4S/c1-11(16(21)19-9-5-8-13(19)17(22)23)18-15(20)14(24)10-12-6-3-2-4-7-12/h2-4,6-7,11,13-14,24H,5,8-10H2,1H3,(H,18,20)(H,22,23)/t11-,13?,14-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin I converting enzyme |
J Med Chem 39: 1210-9 (1996)
Article DOI: 10.1021/jm950590p
BindingDB Entry DOI: 10.7270/Q2DF6RWV |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50050149

(1-[(S)-2-((S)-2-Mercapto-4-phenyl-butyrylamino)-pr...)Show SMILES C[C@H](NC(=O)[C@@H](S)CCc1ccccc1)C(=O)N1CCCC1C(O)=O Show InChI InChI=1S/C18H24N2O4S/c1-12(17(22)20-11-5-8-14(20)18(23)24)19-16(21)15(25)10-9-13-6-3-2-4-7-13/h2-4,6-7,12,14-15,25H,5,8-11H2,1H3,(H,19,21)(H,23,24)/t12-,14?,15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin I converting enzyme |
J Med Chem 39: 1210-9 (1996)
Article DOI: 10.1021/jm950590p
BindingDB Entry DOI: 10.7270/Q2DF6RWV |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50050150
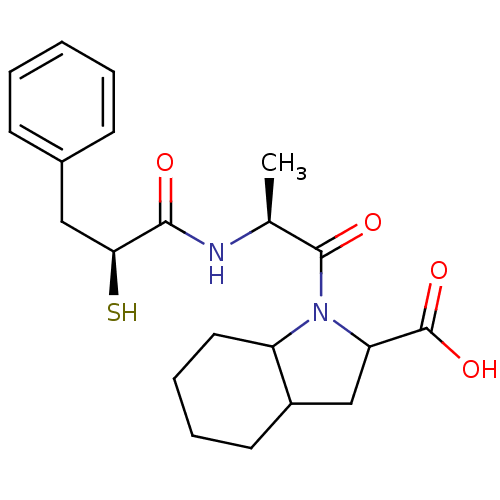
(1-[(S)-2-((S)-2-Mercapto-3-phenyl-propionylamino)-...)Show SMILES C[C@H](NC(=O)[C@@H](S)Cc1ccccc1)C(=O)N1C(CC2CCCCC12)C(O)=O Show InChI InChI=1S/C21H28N2O4S/c1-13(22-19(24)18(28)11-14-7-3-2-4-8-14)20(25)23-16-10-6-5-9-15(16)12-17(23)21(26)27/h2-4,7-8,13,15-18,28H,5-6,9-12H2,1H3,(H,22,24)(H,26,27)/t13-,15?,16?,17?,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin I converting enzyme |
J Med Chem 39: 1210-9 (1996)
Article DOI: 10.1021/jm950590p
BindingDB Entry DOI: 10.7270/Q2DF6RWV |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50051796

((2S,5R)-5-(2-Hydroxy-phenyl)-1-{2-[(S)-2-mercapto-...)Show SMILES COc1ccc(C[C@H](S)C(=O)NCC(=O)N2[C@@H](CC[C@@H]2c2ccccc2O)C(O)=O)cc1 Show InChI InChI=1S/C23H26N2O6S/c1-31-15-8-6-14(7-9-15)12-20(32)22(28)24-13-21(27)25-17(10-11-18(25)23(29)30)16-4-2-3-5-19(16)26/h2-9,17-18,20,26,32H,10-13H2,1H3,(H,24,28)(H,29,30)/t17-,18+,20+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat angiotensin I converting enzyme |
J Med Chem 39: 2594-608 (1996)
Article DOI: 10.1021/jm950783c
BindingDB Entry DOI: 10.7270/Q25B0353 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50051798
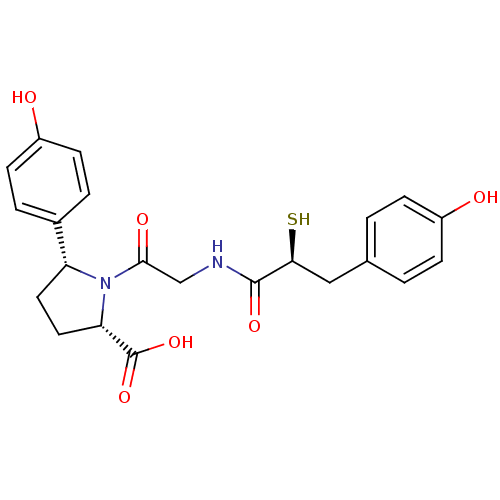
((2S,5R)-5-(4-Hydroxy-phenyl)-1-{2-[(S)-3-(4-hydrox...)Show SMILES OC(=O)[C@@H]1CC[C@@H](N1C(=O)CNC(=O)[C@@H](S)Cc1ccc(O)cc1)c1ccc(O)cc1 Show InChI InChI=1S/C22H24N2O6S/c25-15-5-1-13(2-6-15)11-19(31)21(28)23-12-20(27)24-17(9-10-18(24)22(29)30)14-3-7-16(26)8-4-14/h1-8,17-19,25-26,31H,9-12H2,(H,23,28)(H,29,30)/t17-,18+,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat angiotensin I converting enzyme |
J Med Chem 39: 2594-608 (1996)
Article DOI: 10.1021/jm950783c
BindingDB Entry DOI: 10.7270/Q25B0353 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50051784
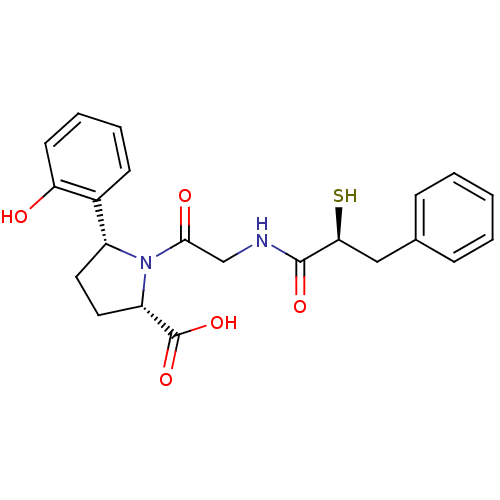
((2S,5R)-5-(2-Hydroxy-phenyl)-1-[2-((S)-2-mercapto-...)Show SMILES OC(=O)[C@@H]1CC[C@@H](N1C(=O)CNC(=O)[C@@H](S)Cc1ccccc1)c1ccccc1O Show InChI InChI=1S/C22H24N2O5S/c25-18-9-5-4-8-15(18)16-10-11-17(22(28)29)24(16)20(26)13-23-21(27)19(30)12-14-6-2-1-3-7-14/h1-9,16-17,19,25,30H,10-13H2,(H,23,27)(H,28,29)/t16-,17+,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat angiotensin I converting enzyme |
J Med Chem 39: 2594-608 (1996)
Article DOI: 10.1021/jm950783c
BindingDB Entry DOI: 10.7270/Q25B0353 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50051785
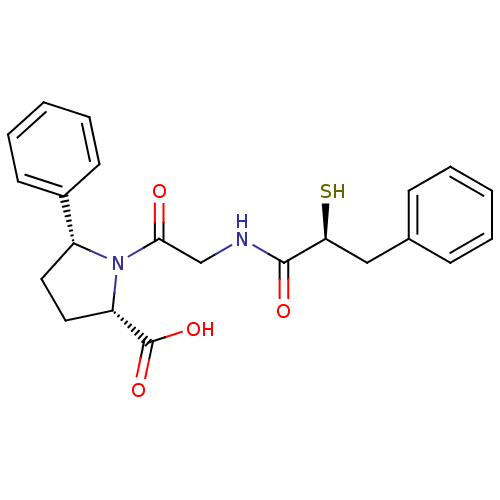
((2S,5R)-1-[2-((S)-2-Mercapto-3-phenyl-propionylami...)Show SMILES OC(=O)[C@@H]1CC[C@@H](N1C(=O)CNC(=O)[C@@H](S)Cc1ccccc1)c1ccccc1 Show InChI InChI=1S/C22H24N2O4S/c25-20(14-23-21(26)19(29)13-15-7-3-1-4-8-15)24-17(11-12-18(24)22(27)28)16-9-5-2-6-10-16/h1-10,17-19,29H,11-14H2,(H,23,26)(H,27,28)/t17-,18+,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat angiotensin I converting enzyme |
J Med Chem 39: 2594-608 (1996)
Article DOI: 10.1021/jm950783c
BindingDB Entry DOI: 10.7270/Q25B0353 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50050156

((S)-1-[(S)-2-((2S,3S)-2-Mercapto-3-methyl-pentanoy...)Show SMILES CC[C@H](C)[C@H](S)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(O)=O Show InChI InChI=1S/C16H28N2O4S/c1-5-10(4)13(23)14(19)17-12(9(2)3)15(20)18-8-6-7-11(18)16(21)22/h9-13,23H,5-8H2,1-4H3,(H,17,19)(H,21,22)/t10-,11-,12-,13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin I converting enzyme |
J Med Chem 39: 1210-9 (1996)
Article DOI: 10.1021/jm950590p
BindingDB Entry DOI: 10.7270/Q2DF6RWV |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50051780
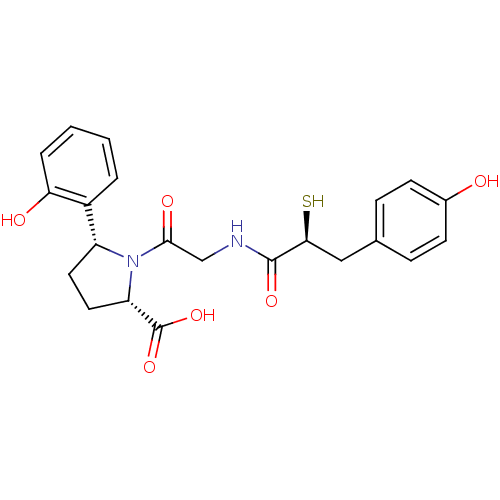
((2S,5R)-5-(2-Hydroxy-phenyl)-1-{2-[(S)-3-(4-hydrox...)Show SMILES OC(=O)[C@@H]1CC[C@@H](N1C(=O)CNC(=O)[C@@H](S)Cc1ccc(O)cc1)c1ccccc1O Show InChI InChI=1S/C22H24N2O6S/c25-14-7-5-13(6-8-14)11-19(31)21(28)23-12-20(27)24-16(9-10-17(24)22(29)30)15-3-1-2-4-18(15)26/h1-8,16-17,19,25-26,31H,9-12H2,(H,23,28)(H,29,30)/t16-,17+,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat angiotensin I converting enzyme |
J Med Chem 39: 2594-608 (1996)
Article DOI: 10.1021/jm950783c
BindingDB Entry DOI: 10.7270/Q25B0353 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50051791
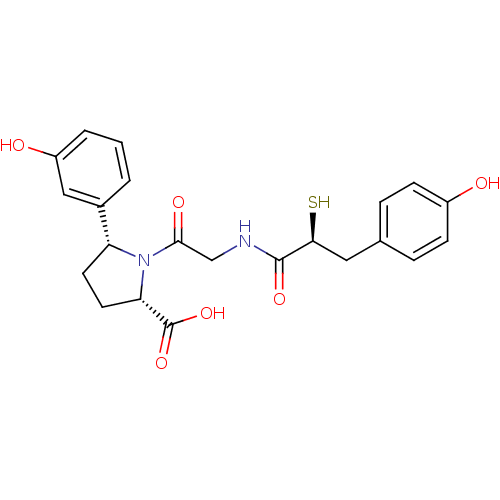
((2S,5R)-5-(3-Hydroxy-phenyl)-1-{2-[(S)-3-(4-hydrox...)Show SMILES OC(=O)[C@@H]1CC[C@@H](N1C(=O)CNC(=O)[C@@H](S)Cc1ccc(O)cc1)c1cccc(O)c1 Show InChI InChI=1S/C22H24N2O6S/c25-15-6-4-13(5-7-15)10-19(31)21(28)23-12-20(27)24-17(8-9-18(24)22(29)30)14-2-1-3-16(26)11-14/h1-7,11,17-19,25-26,31H,8-10,12H2,(H,23,28)(H,29,30)/t17-,18+,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat angiotensin I converting enzyme |
J Med Chem 39: 2594-608 (1996)
Article DOI: 10.1021/jm950783c
BindingDB Entry DOI: 10.7270/Q25B0353 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50050144
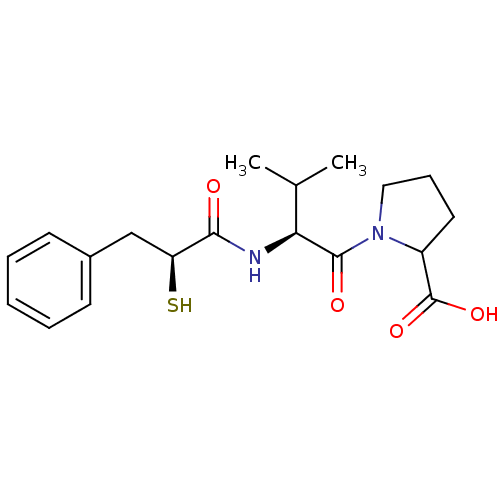
(1-[(S)-2-((S)-2-Mercapto-3-phenyl-propionylamino)-...)Show SMILES CC(C)[C@H](NC(=O)[C@@H](S)Cc1ccccc1)C(=O)N1CCCC1C(O)=O Show InChI InChI=1S/C19H26N2O4S/c1-12(2)16(18(23)21-10-6-9-14(21)19(24)25)20-17(22)15(26)11-13-7-4-3-5-8-13/h3-5,7-8,12,14-16,26H,6,9-11H2,1-2H3,(H,20,22)(H,24,25)/t14?,15-,16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin I converting enzyme |
J Med Chem 39: 1210-9 (1996)
Article DOI: 10.1021/jm950590p
BindingDB Entry DOI: 10.7270/Q2DF6RWV |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50051803
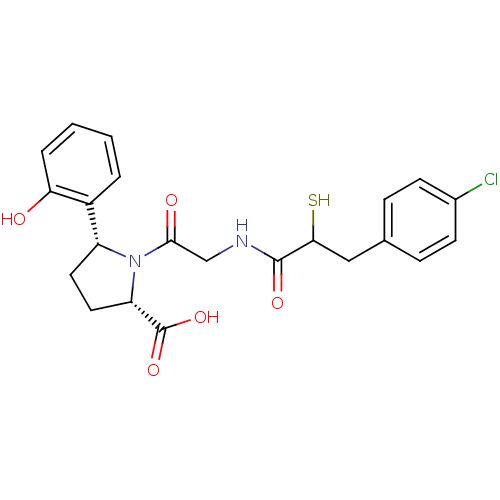
((2S,5R)-1-{2-[3-(4-Chloro-phenyl)-2-mercapto-propi...)Show SMILES OC(=O)[C@@H]1CC[C@@H](N1C(=O)CNC(=O)C(S)Cc1ccc(Cl)cc1)c1ccccc1O Show InChI InChI=1S/C22H23ClN2O5S/c23-14-7-5-13(6-8-14)11-19(31)21(28)24-12-20(27)25-16(9-10-17(25)22(29)30)15-3-1-2-4-18(15)26/h1-8,16-17,19,26,31H,9-12H2,(H,24,28)(H,29,30)/t16-,17+,19?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat angiotensin I converting enzyme |
J Med Chem 39: 2594-608 (1996)
Article DOI: 10.1021/jm950783c
BindingDB Entry DOI: 10.7270/Q25B0353 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Oryctolagus cuniculus (rabbit)) | BDBM50051784

((2S,5R)-5-(2-Hydroxy-phenyl)-1-[2-((S)-2-mercapto-...)Show SMILES OC(=O)[C@@H]1CC[C@@H](N1C(=O)CNC(=O)[C@@H](S)Cc1ccccc1)c1ccccc1O Show InChI InChI=1S/C22H24N2O5S/c25-18-9-5-4-8-15(18)16-10-11-17(22(28)29)24(16)20(26)13-23-21(27)19(30)12-14-6-2-1-3-7-14/h1-9,16-17,19,25,30H,10-13H2,(H,23,27)(H,28,29)/t16-,17+,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vitro inhibition of neutral endopeptidase |
J Med Chem 39: 2594-608 (1996)
Article DOI: 10.1021/jm950783c
BindingDB Entry DOI: 10.7270/Q25B0353 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50050125

(1-[(S)-2-((S)-2-Mercapto-3-phenyl-propionylamino)-...)Show SMILES OC(=O)C1CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](S)Cc1ccccc1 Show InChI InChI=1S/C23H26N2O4S/c26-21(20(30)15-17-10-5-2-6-11-17)24-18(14-16-8-3-1-4-9-16)22(27)25-13-7-12-19(25)23(28)29/h1-6,8-11,18-20,30H,7,12-15H2,(H,24,26)(H,28,29)/t18-,19?,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin I converting enzyme |
J Med Chem 39: 1210-9 (1996)
Article DOI: 10.1021/jm950590p
BindingDB Entry DOI: 10.7270/Q2DF6RWV |
More data for this
Ligand-Target Pair | |
Neprilysin
(Oryctolagus cuniculus (rabbit)) | BDBM50051803

((2S,5R)-1-{2-[3-(4-Chloro-phenyl)-2-mercapto-propi...)Show SMILES OC(=O)[C@@H]1CC[C@@H](N1C(=O)CNC(=O)C(S)Cc1ccc(Cl)cc1)c1ccccc1O Show InChI InChI=1S/C22H23ClN2O5S/c23-14-7-5-13(6-8-14)11-19(31)21(28)24-12-20(27)25-16(9-10-17(25)22(29)30)15-3-1-2-4-18(15)26/h1-8,16-17,19,26,31H,9-12H2,(H,24,28)(H,29,30)/t16-,17+,19?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vitro inhibition of neutral endopeptidase |
J Med Chem 39: 2594-608 (1996)
Article DOI: 10.1021/jm950783c
BindingDB Entry DOI: 10.7270/Q25B0353 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50041071
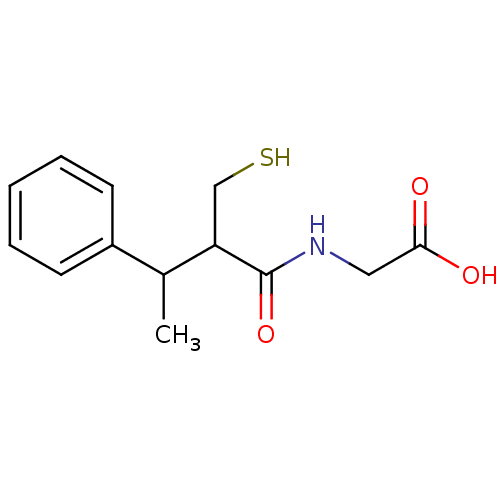
((2-Mercaptomethyl-3-phenyl-butyrylamino)-acetic ac...)Show InChI InChI=1S/C13H17NO3S/c1-9(10-5-3-2-4-6-10)11(8-18)13(17)14-7-12(15)16/h2-6,9,11,18H,7-8H2,1H3,(H,14,17)(H,15,16) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vivo inhibitory potency against neutral endopeptidase by displacement of [3H]-HACBOGly binding in mouse kidney |
J Med Chem 37: 1070-83 (1994)
BindingDB Entry DOI: 10.7270/Q26W995T |
More data for this
Ligand-Target Pair | |
Neprilysin
(Oryctolagus cuniculus (rabbit)) | BDBM50050158
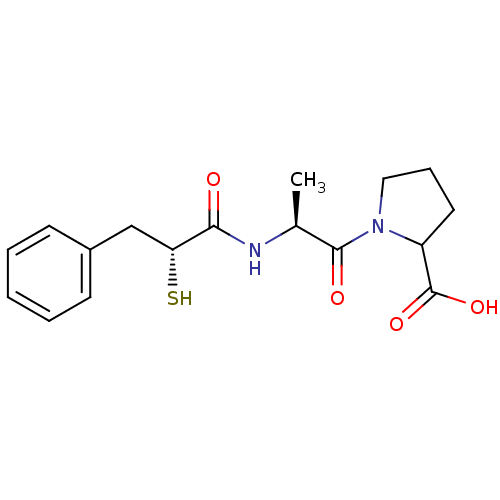
(1-[(S)-2-((R)-2-Mercapto-3-phenyl-propionylamino)-...)Show SMILES C[C@H](NC(=O)[C@H](S)Cc1ccccc1)C(=O)N1CCCC1C(O)=O Show InChI InChI=1S/C17H22N2O4S/c1-11(16(21)19-9-5-8-13(19)17(22)23)18-15(20)14(24)10-12-6-3-2-4-7-12/h2-4,6-7,11,13-14,24H,5,8-10H2,1H3,(H,18,20)(H,22,23)/t11-,13?,14+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity was tested against neutral endopeptidase |
J Med Chem 39: 1210-9 (1996)
Article DOI: 10.1021/jm950590p
BindingDB Entry DOI: 10.7270/Q2DF6RWV |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50002022
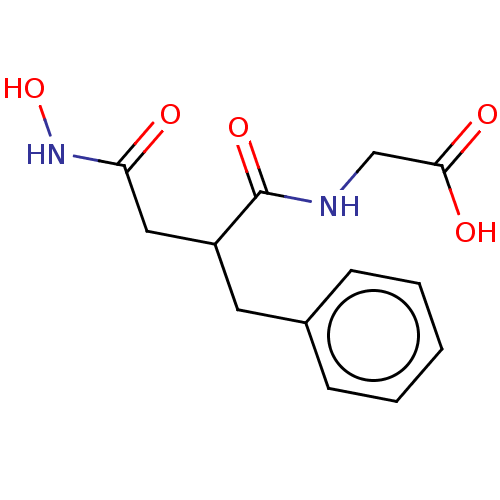
((2-Benzyl-3-hydroxycarbamoyl-propionylamino)-aceti...)Show InChI InChI=1S/C13H16N2O5/c16-11(15-20)7-10(13(19)14-8-12(17)18)6-9-4-2-1-3-5-9/h1-5,10,20H,6-8H2,(H,14,19)(H,15,16)(H,17,18) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory potency against neutral endopeptidase |
J Med Chem 35: 1259-66 (1992)
BindingDB Entry DOI: 10.7270/Q2125T81 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Oryctolagus cuniculus (rabbit)) | BDBM50050127

((S)-3-(4-Hydroxy-phenyl)-2-[(2S,3R)-2-((S)-2-merca...)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H](S)C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C20H30N2O5S/c1-5-12(4)16(22-19(25)17(28)11(2)3)18(24)21-15(20(26)27)10-13-6-8-14(23)9-7-13/h6-9,11-12,15-17,23,28H,5,10H2,1-4H3,(H,21,24)(H,22,25)(H,26,27)/t12-,15+,16+,17+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity was tested against neutral endopeptidase |
J Med Chem 39: 1210-9 (1996)
Article DOI: 10.1021/jm950590p
BindingDB Entry DOI: 10.7270/Q2DF6RWV |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50050162
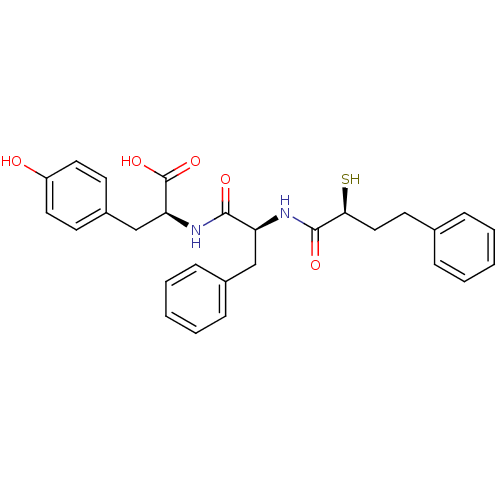
((S)-3-(4-Hydroxy-phenyl)-2-[(S)-2-((S)-2-mercapto-...)Show SMILES OC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](S)CCc1ccccc1 Show InChI InChI=1S/C28H30N2O5S/c31-22-14-11-21(12-15-22)18-24(28(34)35)30-26(32)23(17-20-9-5-2-6-10-20)29-27(33)25(36)16-13-19-7-3-1-4-8-19/h1-12,14-15,23-25,31,36H,13,16-18H2,(H,29,33)(H,30,32)(H,34,35)/t23-,24-,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin I converting enzyme |
J Med Chem 39: 1210-9 (1996)
Article DOI: 10.1021/jm950590p
BindingDB Entry DOI: 10.7270/Q2DF6RWV |
More data for this
Ligand-Target Pair | |
Neprilysin
(Oryctolagus cuniculus (rabbit)) | BDBM50051800
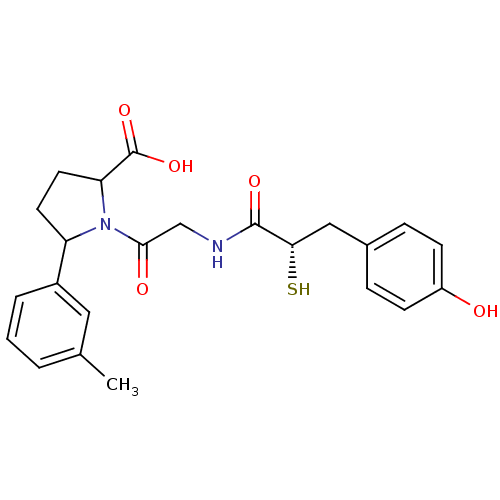
(1-{2-[(S)-3-(4-Hydroxy-phenyl)-2-mercapto-propiony...)Show SMILES Cc1cccc(c1)C1CCC(N1C(=O)CNC(=O)[C@@H](S)Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C23H26N2O5S/c1-14-3-2-4-16(11-14)18-9-10-19(23(29)30)25(18)21(27)13-24-22(28)20(31)12-15-5-7-17(26)8-6-15/h2-8,11,18-20,26,31H,9-10,12-13H2,1H3,(H,24,28)(H,29,30)/t18?,19?,20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vitro inhibition of neutral endopeptidase |
J Med Chem 39: 2594-608 (1996)
Article DOI: 10.1021/jm950783c
BindingDB Entry DOI: 10.7270/Q25B0353 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50051790
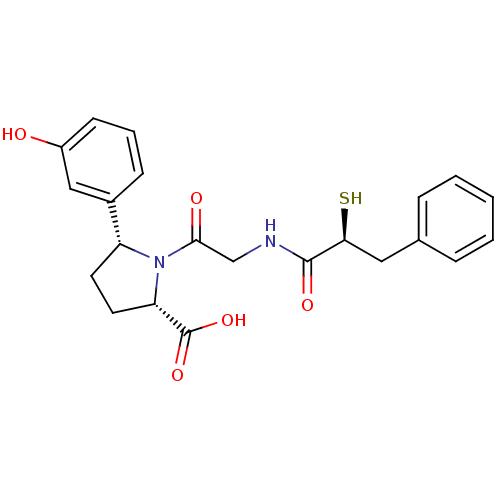
((2S,5R)-5-(3-Hydroxy-phenyl)-1-[2-((S)-2-mercapto-...)Show SMILES OC(=O)[C@@H]1CC[C@@H](N1C(=O)CNC(=O)[C@@H](S)Cc1ccccc1)c1cccc(O)c1 Show InChI InChI=1S/C22H24N2O5S/c25-16-8-4-7-15(12-16)17-9-10-18(22(28)29)24(17)20(26)13-23-21(27)19(30)11-14-5-2-1-3-6-14/h1-8,12,17-19,25,30H,9-11,13H2,(H,23,27)(H,28,29)/t17-,18+,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat angiotensin I converting enzyme |
J Med Chem 39: 2594-608 (1996)
Article DOI: 10.1021/jm950783c
BindingDB Entry DOI: 10.7270/Q25B0353 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Oryctolagus cuniculus (rabbit)) | BDBM50051785

((2S,5R)-1-[2-((S)-2-Mercapto-3-phenyl-propionylami...)Show SMILES OC(=O)[C@@H]1CC[C@@H](N1C(=O)CNC(=O)[C@@H](S)Cc1ccccc1)c1ccccc1 Show InChI InChI=1S/C22H24N2O4S/c25-20(14-23-21(26)19(29)13-15-7-3-1-4-8-15)24-17(11-12-18(24)22(27)28)16-9-5-2-6-10-16/h1-10,17-19,29H,11-14H2,(H,23,26)(H,27,28)/t17-,18+,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vitro inhibition of neutral endopeptidase |
J Med Chem 39: 2594-608 (1996)
Article DOI: 10.1021/jm950783c
BindingDB Entry DOI: 10.7270/Q25B0353 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50050148

((S)-3-(4-Hydroxy-phenyl)-2-[(S)-2-((S)-2-mercapto-...)Show SMILES CC(C)[C@H](NC(=O)[C@@H](S)CCc1ccccc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C24H30N2O5S/c1-15(2)21(26-22(28)20(32)13-10-16-6-4-3-5-7-16)23(29)25-19(24(30)31)14-17-8-11-18(27)12-9-17/h3-9,11-12,15,19-21,27,32H,10,13-14H2,1-2H3,(H,25,29)(H,26,28)(H,30,31)/t19-,20-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin I converting enzyme |
J Med Chem 39: 1210-9 (1996)
Article DOI: 10.1021/jm950590p
BindingDB Entry DOI: 10.7270/Q2DF6RWV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data