

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50383315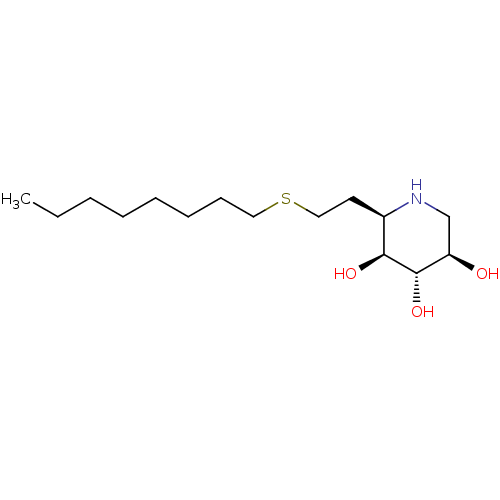 (CHEMBL2029773) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 4-methylumbelliferyl beta-D-glucopyranoside as substrate after 15 mins by Dixon an... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50254115 (CHEMBL4080589) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graz University of Technology Curated by ChEMBL | Assay Description Competitive inhibition of recombinant beta-glucocerebrosidase (unknown origin) using 4-nitrophenyl beta-D-galactopyranoside as substrate pre-incubate... | Bioorg Med Chem Lett 27: 3431-3435 (2017) Article DOI: 10.1016/j.bmcl.2017.05.086 BindingDB Entry DOI: 10.7270/Q29889D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50018014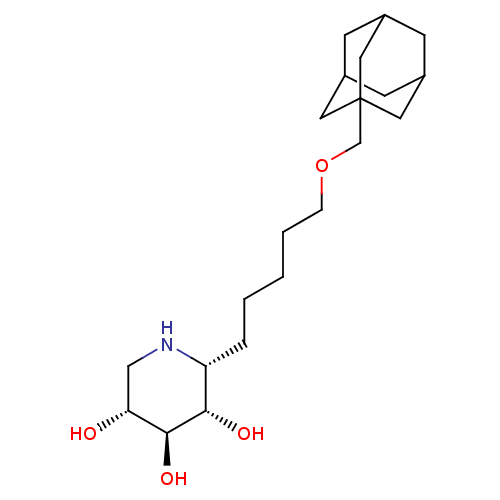 (CHEMBL3289676) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University Graz Curated by ChEMBL | Assay Description Inhibition of human lysosomal beta-glucocerebrosidase | Bioorg Med Chem Lett 24: 2777-80 (2014) Article DOI: 10.1016/j.bmcl.2014.03.069 BindingDB Entry DOI: 10.7270/Q2RV0Q8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Homo sapiens (Human)) | BDBM223314 (YPYSCWARHVRIREN | piHA-D1) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
The University of Tokyo | Assay Description The release of 2-chloro-4-nitrophenol resulting from the HPA-catalyzed hydrolysis of 2-chloro-4-nitrophenyl a-maltotrioside (CNPG3) was monitored at ... | Cell Chem Biol 24: 381-390 (2017) Article DOI: 10.1016/j.chembiol.2017.02.001 BindingDB Entry DOI: 10.7270/Q27D2T0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50383327 (CHEMBL2029772) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 4-methylumbelliferyl beta-D-glucopyranoside as substrate after 15 mins by Dixon an... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50315250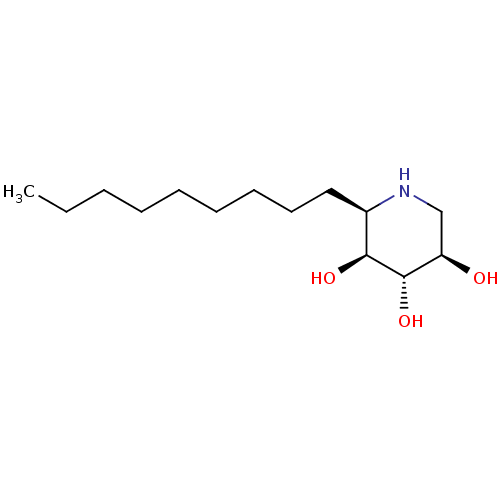 ((2R,3S,4S,5R)-2-nonylpiperidine-3,4,5-triol | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University Graz Curated by ChEMBL | Assay Description Inhibition of human lysosomal beta-glucocerebrosidase | Bioorg Med Chem Lett 24: 2777-80 (2014) Article DOI: 10.1016/j.bmcl.2014.03.069 BindingDB Entry DOI: 10.7270/Q2RV0Q8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Homo sapiens (Human)) | BDBM223315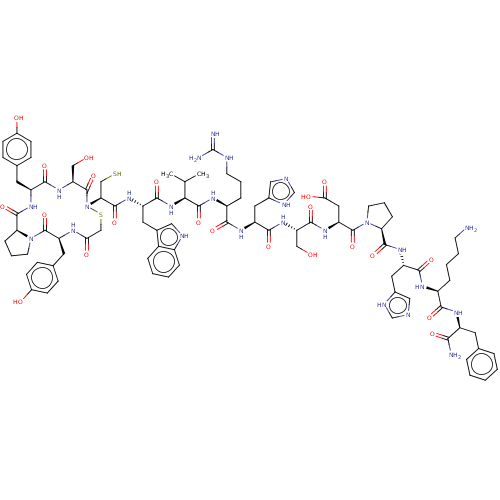 (YPYSCWVRHSDPHKF | piHA-D3) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
The University of Tokyo | Assay Description The release of 2-chloro-4-nitrophenol resulting from the HPA-catalyzed hydrolysis of 2-chloro-4-nitrophenyl a-maltotrioside (CNPG3) was monitored at ... | Cell Chem Biol 24: 381-390 (2017) Article DOI: 10.1016/j.chembiol.2017.02.001 BindingDB Entry DOI: 10.7270/Q27D2T0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50383321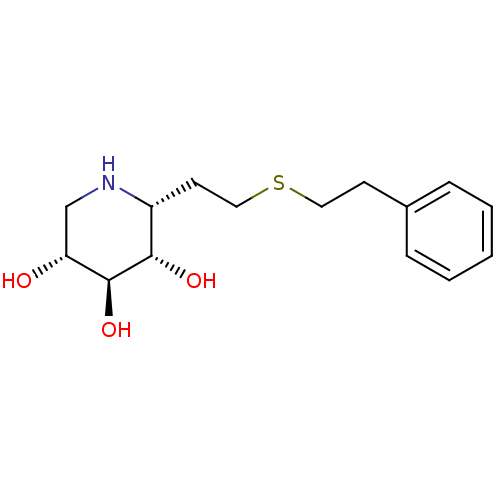 (CHEMBL2029780) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 4-methylumbelliferyl beta-D-glucopyranoside as substrate after 15 mins by Dixon an... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50383323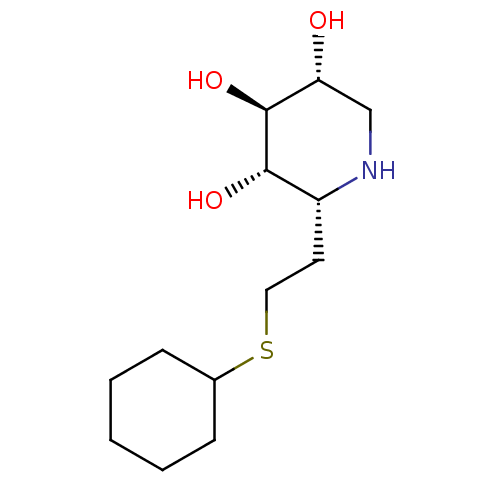 (CHEMBL2029778) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 4-methylumbelliferyl beta-D-glucopyranoside as substrate after 15 mins by Dixon an... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50383325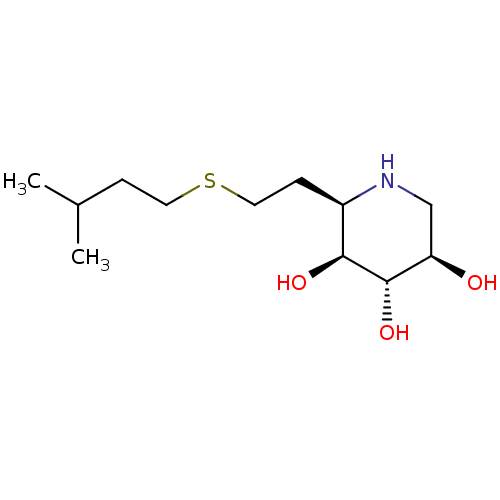 (CHEMBL2029776) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 4-methylumbelliferyl beta-D-glucopyranoside as substrate after 15 mins by Dixon an... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Bos taurus (Bovine)) | BDBM50254109 (CHEMBL4069909) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graz University of Technology Curated by ChEMBL | Assay Description Competitive inhibition of Bovine liver beta-galactosidase using 4-nitrophenyl beta-D-galactopyranoside as substrate pre-incubated for up to 5 mins be... | Bioorg Med Chem Lett 27: 3431-3435 (2017) Article DOI: 10.1016/j.bmcl.2017.05.086 BindingDB Entry DOI: 10.7270/Q29889D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50383324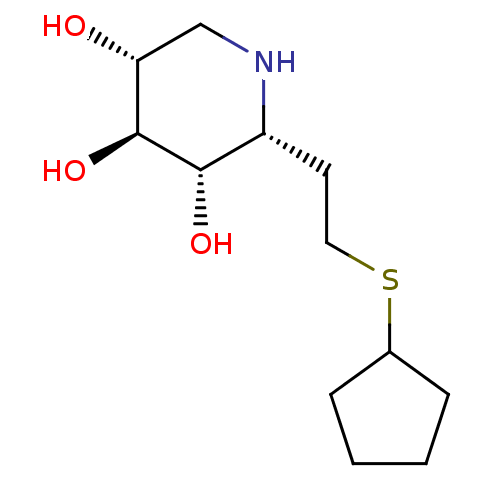 (CHEMBL2029777) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 4-methylumbelliferyl beta-D-glucopyranoside as substrate after 15 mins by Dixon an... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Homo sapiens (Human)) | BDBM223316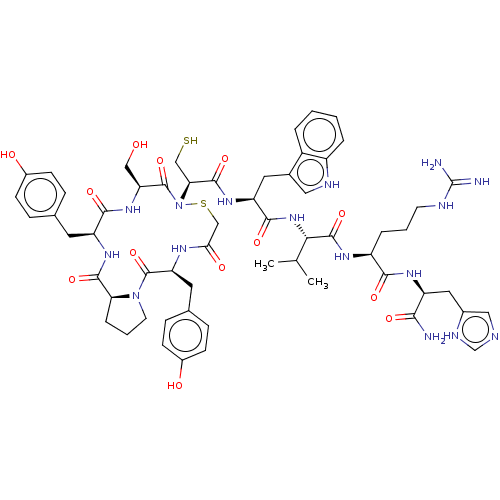 (YPYSCWVRH | piHA-Dm) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
The University of Tokyo | Assay Description The release of 2-chloro-4-nitrophenol resulting from the HPA-catalyzed hydrolysis of 2-chloro-4-nitrophenyl a-maltotrioside (CNPG3) was monitored at ... | Cell Chem Biol 24: 381-390 (2017) Article DOI: 10.1016/j.chembiol.2017.02.001 BindingDB Entry DOI: 10.7270/Q27D2T0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50018018 (CHEMBL3289679) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University Graz Curated by ChEMBL | Assay Description Inhibition of human lysosomal beta-glucocerebrosidase using 2,4-dinitrophenyl-beta-D-glucopyranoside as substrate by UV spectrophotometric analysis | Bioorg Med Chem Lett 24: 2777-80 (2014) Article DOI: 10.1016/j.bmcl.2014.03.069 BindingDB Entry DOI: 10.7270/Q2RV0Q8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Homo sapiens (Human)) | BDBM163646 (Montbretin A (MbA)) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.10 | -47.0 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
University of British Columbia | Assay Description Inhibitor concentrations varied from 0.25 to 5 times the Ki value in 50 mM sodium phosphate, 100 mM sodium chloride buffer, pH 7.0, at 30 °C. 2-Chlor... | Nat Chem Biol 11: 691-6 (2015) Article DOI: 10.1038/nchembio.1865 BindingDB Entry DOI: 10.7270/Q2KD1WP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Homo sapiens (Human)) | BDBM163647 (MbA-G (1)) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 9.10 | -46.7 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
University of British Columbia | Assay Description Inhibitor concentrations varied from 0.25 to 5 times the Ki value in 50 mM sodium phosphate, 100 mM sodium chloride buffer, pH 7.0, at 30 °C. 2-Chlor... | Nat Chem Biol 11: 691-6 (2015) Article DOI: 10.1038/nchembio.1865 BindingDB Entry DOI: 10.7270/Q2KD1WP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50383319 (CHEMBL2029782) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 4-methylumbelliferyl beta-D-glucopyranoside as substrate after 15 mins by Dixon an... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50383328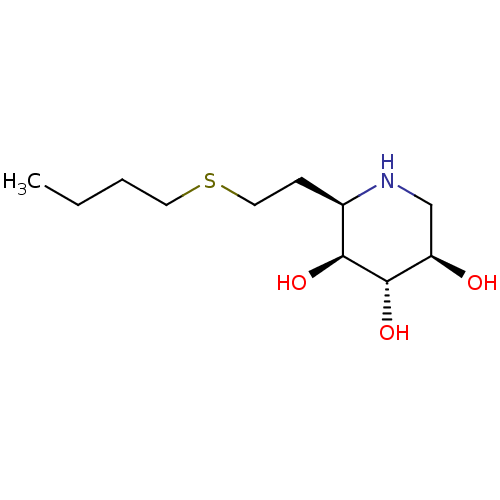 (CHEMBL2029771) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 4-methylumbelliferyl beta-D-glucopyranoside as substrate after 15 mins by Dixon an... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50383322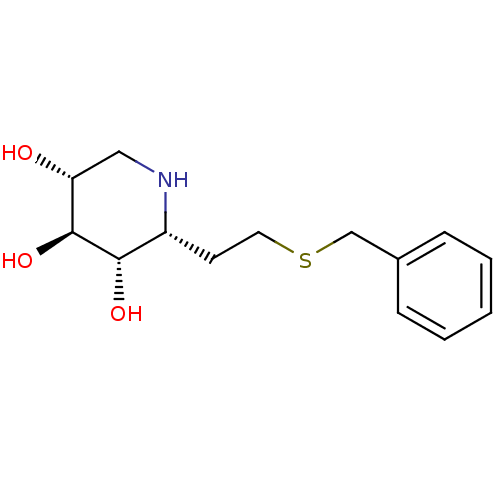 (CHEMBL2029779) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 4-methylumbelliferyl beta-D-glucopyranoside as substrate after 15 mins by Dixon an... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50254109 (CHEMBL4069909) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graz University of Technology Curated by ChEMBL | Assay Description Competitive inhibition of recombinant beta-glucocerebrosidase (unknown origin) using 4-nitrophenyl beta-D-galactopyranoside as substrate pre-incubate... | Bioorg Med Chem Lett 27: 3431-3435 (2017) Article DOI: 10.1016/j.bmcl.2017.05.086 BindingDB Entry DOI: 10.7270/Q29889D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Homo sapiens (Human)) | BDBM163648 (MbA-R (2)) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 21.3 | -44.5 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
University of British Columbia | Assay Description Inhibitor concentrations varied from 0.25 to 5 times the Ki value in 50 mM sodium phosphate, 100 mM sodium chloride buffer, pH 7.0, at 30 °C. 2-Chlor... | Nat Chem Biol 11: 691-6 (2015) Article DOI: 10.1038/nchembio.1865 BindingDB Entry DOI: 10.7270/Q2KD1WP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50383326 (CHEMBL2029775) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 2,4-dinitrophenyl beta-D-glucopyranoside as substrate after 3 mins by Dixon and Li... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50383318 (CHEMBL2029783) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 2,4-dinitrophenyl beta-D-glucopyranoside as substrate after 3 mins by Dixon and Li... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Homo sapiens (Human)) | BDBM163649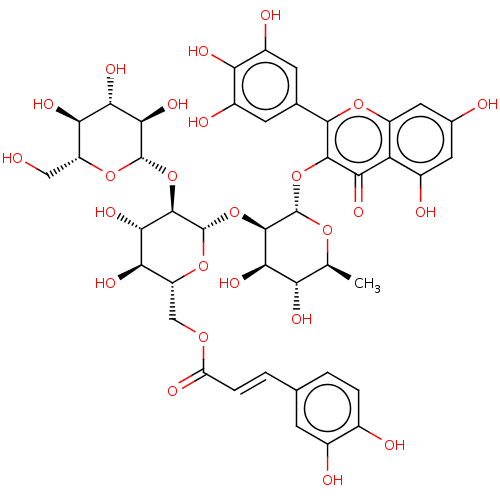 (MbA-RX (3)) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 42.4 | -42.8 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
University of British Columbia | Assay Description Inhibitor concentrations varied from 0.25 to 5 times the Ki value in 50 mM sodium phosphate, 100 mM sodium chloride buffer, pH 7.0, at 30 °C. 2-Chlor... | Nat Chem Biol 11: 691-6 (2015) Article DOI: 10.1038/nchembio.1865 BindingDB Entry DOI: 10.7270/Q2KD1WP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50383314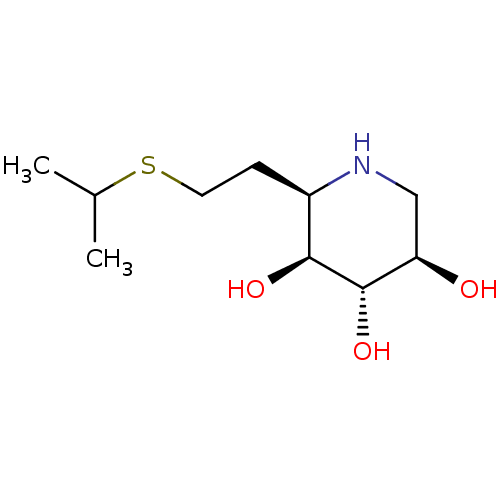 (CHEMBL2029774) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 2,4-dinitrophenyl beta-D-glucopyranoside as substrate after 3 mins by Dixon and Li... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Homo sapiens (Human)) | BDBM163650 (MbA-GR (4)) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 79.3 | -41.2 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
University of British Columbia | Assay Description Inhibitor concentrations varied from 0.25 to 5 times the Ki value in 50 mM sodium phosphate, 100 mM sodium chloride buffer, pH 7.0, at 30 °C. 2-Chlor... | Nat Chem Biol 11: 691-6 (2015) Article DOI: 10.1038/nchembio.1865 BindingDB Entry DOI: 10.7270/Q2KD1WP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Homo sapiens (Human)) | BDBM163651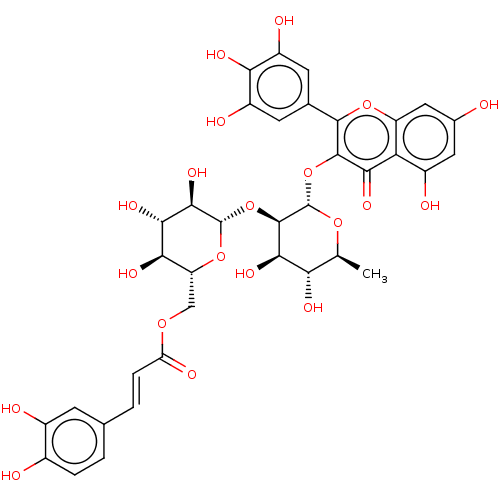 (MbA-GRX (5) | mini-MbA) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 93.3 | -40.8 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
University of British Columbia | Assay Description Inhibitor concentrations varied from 0.25 to 5 times the Ki value in 50 mM sodium phosphate, 100 mM sodium chloride buffer, pH 7.0, at 30 °C. 2-Chlor... | Nat Chem Biol 11: 691-6 (2015) Article DOI: 10.1038/nchembio.1865 BindingDB Entry DOI: 10.7270/Q2KD1WP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-galactosidase (Bos taurus (Bovine)) | BDBM50254116 (CHEMBL4083115) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graz University of Technology Curated by ChEMBL | Assay Description Competitive inhibition of Bovine liver beta-galactosidase using 4-nitrophenyl beta-D-galactopyranoside as substrate pre-incubated for up to 5 mins be... | Bioorg Med Chem Lett 27: 3431-3435 (2017) Article DOI: 10.1016/j.bmcl.2017.05.086 BindingDB Entry DOI: 10.7270/Q29889D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Bos taurus (Bovine)) | BDBM50254117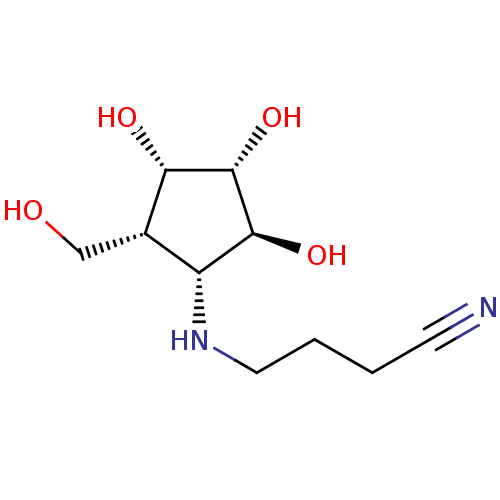 (CHEMBL4061246) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graz University of Technology Curated by ChEMBL | Assay Description Competitive inhibition of Bovine liver beta-galactosidase using 4-nitrophenyl beta-D-galactopyranoside as substrate pre-incubated for up to 5 mins be... | Bioorg Med Chem Lett 27: 3431-3435 (2017) Article DOI: 10.1016/j.bmcl.2017.05.086 BindingDB Entry DOI: 10.7270/Q29889D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50383329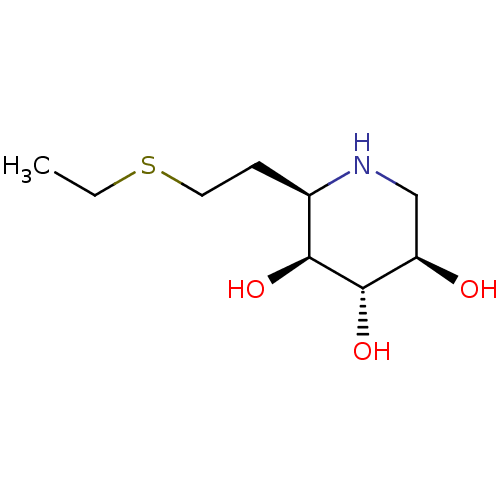 (CHEMBL2029770) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 2,4-dinitrophenyl beta-D-glucopyranoside as substrate after 3 mins by Dixon and Li... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50383320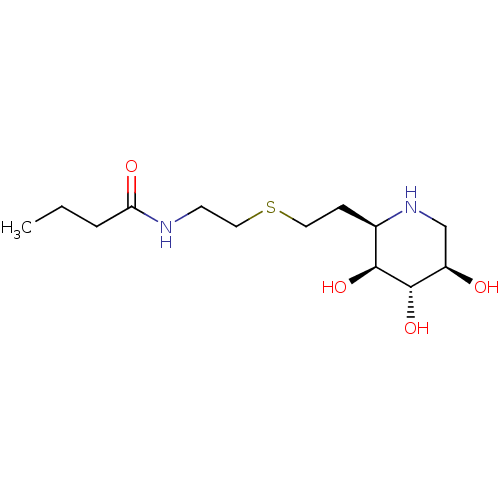 (CHEMBL2029781) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 2,4-dinitrophenyl beta-D-glucopyranoside as substrate after 3 mins by Dixon and Li... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50383317 (CHEMBL2029784) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 2,4-dinitrophenyl beta-D-glucopyranoside as substrate after 3 mins by Dixon and Li... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Homo sapiens (Human)) | BDBM50254109 (CHEMBL4069909) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graz University of Technology Curated by ChEMBL | Assay Description Competitive inhibition of human fibroblast lysosomal beta-galactosidase using 4-methylumbelliferyl-beta-D-galactopyranoside as substrate pre-incubate... | Bioorg Med Chem Lett 27: 3431-3435 (2017) Article DOI: 10.1016/j.bmcl.2017.05.086 BindingDB Entry DOI: 10.7270/Q29889D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50383316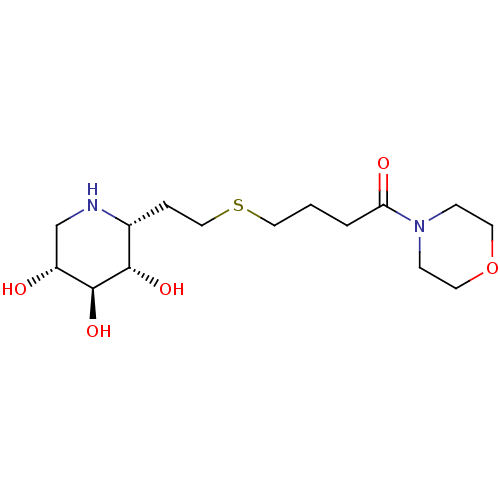 (CHEMBL2029785) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 2,4-dinitrophenyl beta-D-glucopyranoside as substrate after 3 mins by Dixon and Li... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Homo sapiens (Human)) | BDBM50358321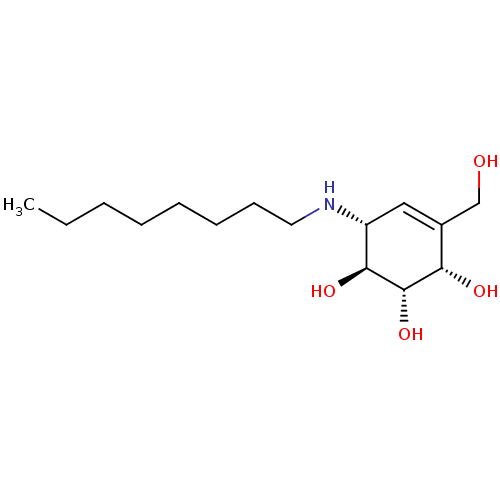 (CHEMBL1922579 | CHEMBL1922581) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graz University of Technology Curated by ChEMBL | Assay Description Competitive inhibition of human fibroblast lysosomal beta-galactosidase using 4-methylumbelliferyl-beta-D-galactopyranoside as substrate pre-incubate... | Bioorg Med Chem Lett 27: 3431-3435 (2017) Article DOI: 10.1016/j.bmcl.2017.05.086 BindingDB Entry DOI: 10.7270/Q29889D9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Pancreatic alpha-amylase (Homo sapiens (Human)) | BDBM163652 (MbA-C (6)) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 730 | -35.6 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
University of British Columbia | Assay Description Inhibitor concentrations varied from 0.25 to 5 times the Ki value in 50 mM sodium phosphate, 100 mM sodium chloride buffer, pH 7.0, at 30 °C. 2-Chlor... | Nat Chem Biol 11: 691-6 (2015) Article DOI: 10.1038/nchembio.1865 BindingDB Entry DOI: 10.7270/Q2KD1WP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Bos taurus (Bovine)) | BDBM50358321 (CHEMBL1922579 | CHEMBL1922581) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 873 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graz University of Technology Curated by ChEMBL | Assay Description Competitive inhibition of Bovine liver beta-galactosidase using 4-nitrophenyl beta-D-galactopyranoside as substrate pre-incubated for up to 5 mins be... | Bioorg Med Chem Lett 27: 3431-3435 (2017) Article DOI: 10.1016/j.bmcl.2017.05.086 BindingDB Entry DOI: 10.7270/Q29889D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50383330 (CHEMBL2029769) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 2,4-dinitrophenyl beta-D-glucopyranoside as substrate after 3 mins by Dixon and Li... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50182798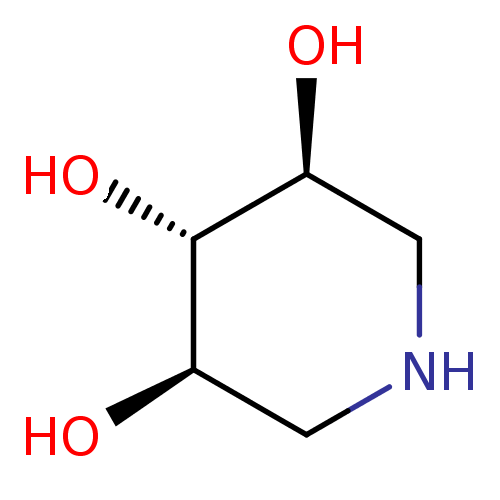 ((3R,4r,5S)-piperidine-3,4,5-triol | 1,5-Dideoxy-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University Graz Curated by ChEMBL | Assay Description Inhibition of human lysosomal beta-glucocerebrosidase | Bioorg Med Chem Lett 24: 2777-80 (2014) Article DOI: 10.1016/j.bmcl.2014.03.069 BindingDB Entry DOI: 10.7270/Q2RV0Q8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Homo sapiens (Human)) | BDBM163653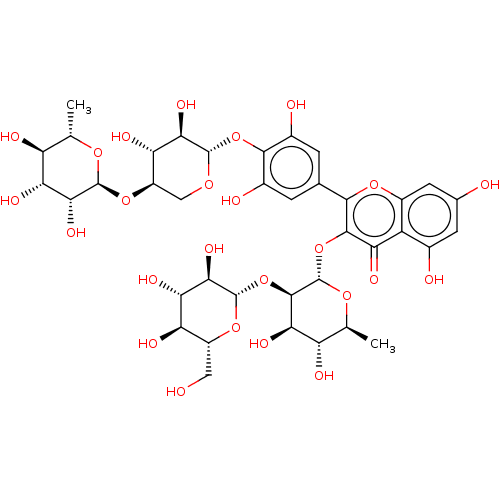 (MbA-CG (7)) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.24E+3 | -32.8 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
University of British Columbia | Assay Description Inhibitor concentrations varied from 0.25 to 5 times the Ki value in 50 mM sodium phosphate, 100 mM sodium chloride buffer, pH 7.0, at 30 °C. 2-Chlor... | Nat Chem Biol 11: 691-6 (2015) Article DOI: 10.1038/nchembio.1865 BindingDB Entry DOI: 10.7270/Q2KD1WP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50383331 (CHEMBL2029768) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 2,4-dinitrophenyl beta-D-glucopyranoside as substrate after 3 mins by Dixon and Li... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50182798 ((3R,4r,5S)-piperidine-3,4,5-triol | 1,5-Dideoxy-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 2,4-dinitrophenyl beta-D-glucopyranoside as substrate after 3 mins by Dixon and Li... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Homo sapiens (Human)) | BDBM50254118 (CHEMBL4090899) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graz University of Technology Curated by ChEMBL | Assay Description Competitive inhibition of human fibroblast lysosomal beta-galactosidase using 4-methylumbelliferyl-beta-D-galactopyranoside as substrate pre-incubate... | Bioorg Med Chem Lett 27: 3431-3435 (2017) Article DOI: 10.1016/j.bmcl.2017.05.086 BindingDB Entry DOI: 10.7270/Q29889D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50254117 (CHEMBL4061246) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graz University of Technology Curated by ChEMBL | Assay Description Competitive inhibition of recombinant beta-glucocerebrosidase (unknown origin) using 4-nitrophenyl beta-D-galactopyranoside as substrate pre-incubate... | Bioorg Med Chem Lett 27: 3431-3435 (2017) Article DOI: 10.1016/j.bmcl.2017.05.086 BindingDB Entry DOI: 10.7270/Q29889D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50254116 (CHEMBL4083115) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graz University of Technology Curated by ChEMBL | Assay Description Competitive inhibition of recombinant beta-glucocerebrosidase (unknown origin) using 4-nitrophenyl beta-D-galactopyranoside as substrate pre-incubate... | Bioorg Med Chem Lett 27: 3431-3435 (2017) Article DOI: 10.1016/j.bmcl.2017.05.086 BindingDB Entry DOI: 10.7270/Q29889D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucosidase (Agrobacterium tumefaciens) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University Graz Curated by ChEMBL | Assay Description Inhibition of Agrobacterium sp. beta glucosidase | Bioorg Med Chem Lett 24: 2777-80 (2014) Article DOI: 10.1016/j.bmcl.2014.03.069 BindingDB Entry DOI: 10.7270/Q2RV0Q8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Homo sapiens (Human)) | BDBM163656 (3-O-(6-(4-(Caffeamidomethyl)-triazolyl)hexyl)querc...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.39E+4 | -28.2 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
University of British Columbia | Assay Description Inhibitor concentrations varied from 0.25 to 5 times the Ki value in 50 mM sodium phosphate, 100 mM sodium chloride buffer, pH 7.0, at 30 °C. 2-Chlor... | Nat Chem Biol 11: 691-6 (2015) Article DOI: 10.1038/nchembio.1865 BindingDB Entry DOI: 10.7270/Q2KD1WP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Bos taurus (Bovine)) | BDBM50254118 (CHEMBL4090899) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graz University of Technology Curated by ChEMBL | Assay Description Competitive inhibition of Bovine liver beta-galactosidase using 4-nitrophenyl beta-D-galactopyranoside as substrate pre-incubated for up to 5 mins be... | Bioorg Med Chem Lett 27: 3431-3435 (2017) Article DOI: 10.1016/j.bmcl.2017.05.086 BindingDB Entry DOI: 10.7270/Q29889D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50018016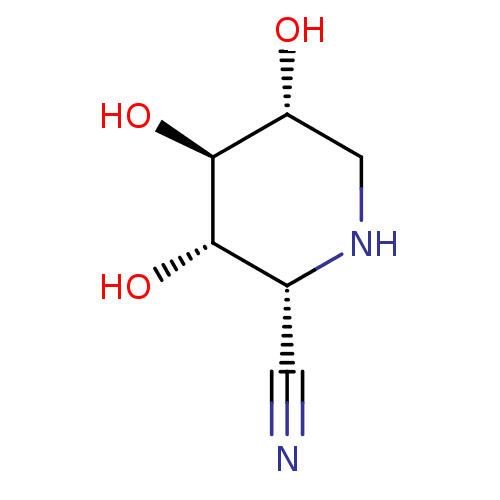 (CHEMBL3289677) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University Graz Curated by ChEMBL | Assay Description Inhibition of human lysosomal beta-glucocerebrosidase using 2,4-dinitrophenyl-beta-D-glucopyranoside as substrate by UV spectrophotometric analysis | Bioorg Med Chem Lett 24: 2777-80 (2014) Article DOI: 10.1016/j.bmcl.2014.03.069 BindingDB Entry DOI: 10.7270/Q2RV0Q8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucosidase (Agrobacterium tumefaciens) | BDBM50018018 (CHEMBL3289679) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University Graz Curated by ChEMBL | Assay Description Inhibition of Agrobacterium sp. beta glucosidase using 2,4-dinitrophenyl-beta-D-glucopyranoside as substrate measured for 3 mins | Bioorg Med Chem Lett 24: 2777-80 (2014) Article DOI: 10.1016/j.bmcl.2014.03.069 BindingDB Entry DOI: 10.7270/Q2RV0Q8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 65 total ) | Next | Last >> |