

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50181451 (CHEMBL3818265) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal 6-His-tagged PAK1 kinase domain (249 to 545 residues) expressed in Escherichia coli BL21(DE3) assessed as ... | J Med Chem 59: 5520-41 (2016) Article DOI: 10.1021/acs.jmedchem.6b00638 BindingDB Entry DOI: 10.7270/Q23X88KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 2 (Homo sapiens (Human)) | BDBM50181451 (CHEMBL3818265) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant PAK2 assessed as phosphorylation of coumarin labeled FRET peptide substrate at Ser/Thr19 preincubated for 10 mins fol... | J Med Chem 59: 5520-41 (2016) Article DOI: 10.1021/acs.jmedchem.6b00638 BindingDB Entry DOI: 10.7270/Q23X88KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50181449 (CHEMBL3817856) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal 6-His-tagged PAK1 kinase domain (249 to 545 residues) expressed in Escherichia coli BL21(DE3) assessed as ... | J Med Chem 59: 5520-41 (2016) Article DOI: 10.1021/acs.jmedchem.6b00638 BindingDB Entry DOI: 10.7270/Q23X88KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50182295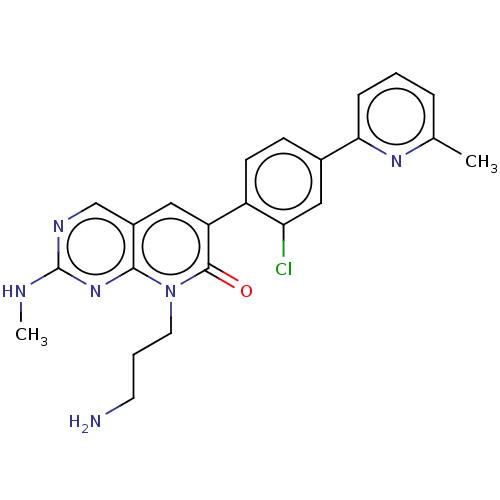 (CHEMBL3818046) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal 6-His-tagged PAK1 kinase domain (249 to 545 residues) expressed in Escherichia coli BL21(DE3) assessed as ... | J Med Chem 59: 5520-41 (2016) Article DOI: 10.1021/acs.jmedchem.6b00638 BindingDB Entry DOI: 10.7270/Q23X88KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50182290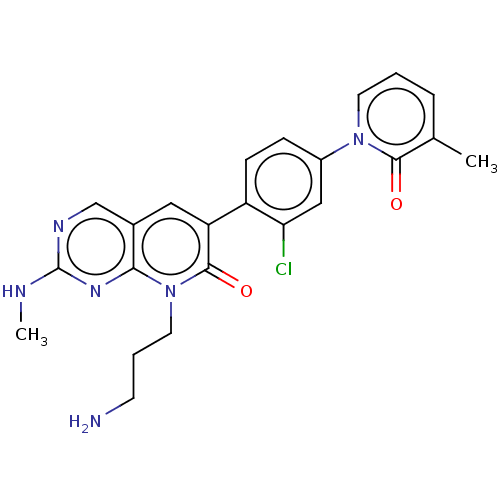 (CHEMBL3819356) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal 6-His-tagged PAK1 kinase domain (249 to 545 residues) expressed in Escherichia coli BL21(DE3) assessed as ... | J Med Chem 59: 5520-41 (2016) Article DOI: 10.1021/acs.jmedchem.6b00638 BindingDB Entry DOI: 10.7270/Q23X88KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50148921 (CHEMBL3770443) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal 6-His-tagged PAK1 kinase domain (249 to 545 residues) expressed in Escherichia coli BL21(DE3) assessed as ... | J Med Chem 59: 5520-41 (2016) Article DOI: 10.1021/acs.jmedchem.6b00638 BindingDB Entry DOI: 10.7270/Q23X88KT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50181450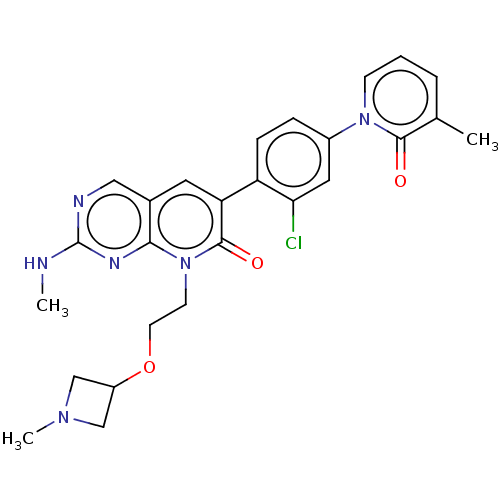 (CHEMBL3818828) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal 6-His-tagged PAK1 kinase domain (249 to 545 residues) expressed in Escherichia coli BL21(DE3) assessed as ... | J Med Chem 59: 5520-41 (2016) Article DOI: 10.1021/acs.jmedchem.6b00638 BindingDB Entry DOI: 10.7270/Q23X88KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 2 (Homo sapiens (Human)) | BDBM50182399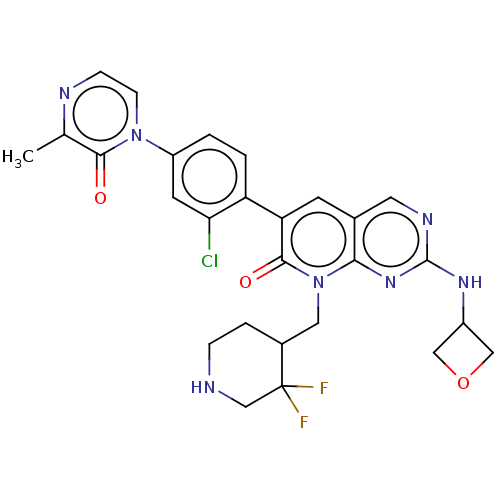 (CHEMBL3818479) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant PAK2 assessed as phosphorylation of coumarin labeled FRET peptide substrate at Ser/Thr19 preincubated for 10 mins fol... | J Med Chem 59: 5520-41 (2016) Article DOI: 10.1021/acs.jmedchem.6b00638 BindingDB Entry DOI: 10.7270/Q23X88KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50182287 (CHEMBL3818592) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal 6-His-tagged PAK1 kinase domain (249 to 545 residues) expressed in Escherichia coli BL21(DE3) assessed as ... | J Med Chem 59: 5520-41 (2016) Article DOI: 10.1021/acs.jmedchem.6b00638 BindingDB Entry DOI: 10.7270/Q23X88KT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50182399 (CHEMBL3818479) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal 6-His-tagged PAK1 kinase domain (249 to 545 residues) expressed in Escherichia coli BL21(DE3) assessed as ... | J Med Chem 59: 5520-41 (2016) Article DOI: 10.1021/acs.jmedchem.6b00638 BindingDB Entry DOI: 10.7270/Q23X88KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50182399 (CHEMBL3818479) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal 6-His-tagged PAK1 kinase domain (249 to 545 residues) expressed in Escherichia coli BL21(DE3) assessed as ... | J Med Chem 59: 5520-41 (2016) Article DOI: 10.1021/acs.jmedchem.6b00638 BindingDB Entry DOI: 10.7270/Q23X88KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50182405 (CHEMBL3818016) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal 6-His-tagged PAK1 kinase domain (249 to 545 residues) expressed in Escherichia coli BL21(DE3) assessed as ... | J Med Chem 59: 5520-41 (2016) Article DOI: 10.1021/acs.jmedchem.6b00638 BindingDB Entry DOI: 10.7270/Q23X88KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50182294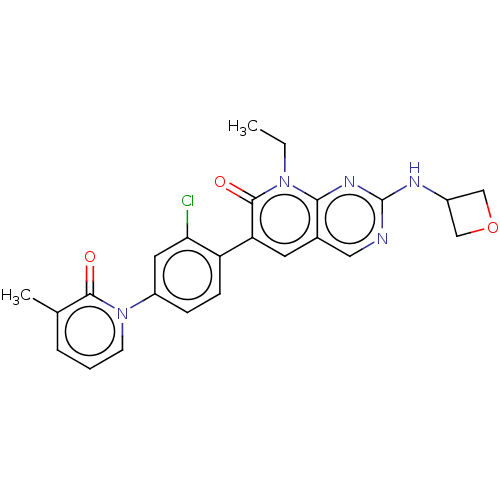 (CHEMBL3817984) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal 6-His-tagged PAK1 kinase domain (249 to 545 residues) expressed in Escherichia coli BL21(DE3) assessed as ... | J Med Chem 59: 5520-41 (2016) Article DOI: 10.1021/acs.jmedchem.6b00638 BindingDB Entry DOI: 10.7270/Q23X88KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50182288 (CHEMBL3818432) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal 6-His-tagged PAK1 kinase domain (249 to 545 residues) expressed in Escherichia coli BL21(DE3) assessed as ... | J Med Chem 59: 5520-41 (2016) Article DOI: 10.1021/acs.jmedchem.6b00638 BindingDB Entry DOI: 10.7270/Q23X88KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50182293 (CHEMBL3817890) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal 6-His-tagged PAK1 kinase domain (249 to 545 residues) expressed in Escherichia coli BL21(DE3) assessed as ... | J Med Chem 59: 5520-41 (2016) Article DOI: 10.1021/acs.jmedchem.6b00638 BindingDB Entry DOI: 10.7270/Q23X88KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50181448 (CHEMBL3818233) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal 6-His-tagged PAK1 kinase domain (249 to 545 residues) expressed in Escherichia coli BL21(DE3) assessed as ... | J Med Chem 59: 5520-41 (2016) Article DOI: 10.1021/acs.jmedchem.6b00638 BindingDB Entry DOI: 10.7270/Q23X88KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 2 (Homo sapiens (Human)) | BDBM50181452 (CHEMBL3819118) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant PAK2 assessed as phosphorylation of coumarin labeled FRET peptide substrate at Ser/Thr19 preincubated for 10 mins fol... | J Med Chem 59: 5520-41 (2016) Article DOI: 10.1021/acs.jmedchem.6b00638 BindingDB Entry DOI: 10.7270/Q23X88KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 2 (Homo sapiens (Human)) | BDBM50148921 (CHEMBL3770443) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant PAK2 assessed as phosphorylation of coumarin labeled FRET peptide substrate at Ser/Thr19 preincubated for 10 mins fol... | J Med Chem 59: 5520-41 (2016) Article DOI: 10.1021/acs.jmedchem.6b00638 BindingDB Entry DOI: 10.7270/Q23X88KT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50181452 (CHEMBL3819118) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal 6-His-tagged PAK1 kinase domain (249 to 545 residues) expressed in Escherichia coli BL21(DE3) assessed as ... | J Med Chem 59: 5520-41 (2016) Article DOI: 10.1021/acs.jmedchem.6b00638 BindingDB Entry DOI: 10.7270/Q23X88KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50182292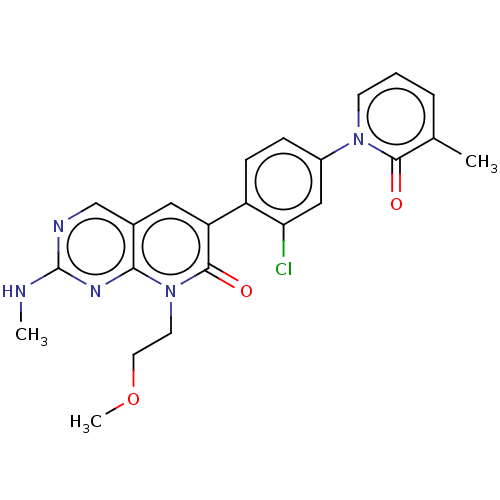 (CHEMBL3818273) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal 6-His-tagged PAK1 kinase domain (249 to 545 residues) expressed in Escherichia coli BL21(DE3) assessed as ... | J Med Chem 59: 5520-41 (2016) Article DOI: 10.1021/acs.jmedchem.6b00638 BindingDB Entry DOI: 10.7270/Q23X88KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50182297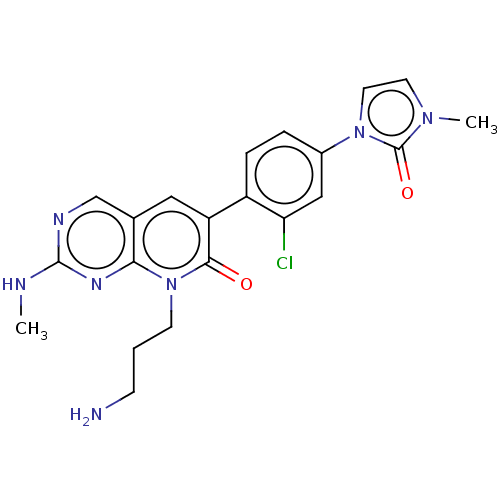 (CHEMBL3818372) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal 6-His-tagged PAK1 kinase domain (249 to 545 residues) expressed in Escherichia coli BL21(DE3) assessed as ... | J Med Chem 59: 5520-41 (2016) Article DOI: 10.1021/acs.jmedchem.6b00638 BindingDB Entry DOI: 10.7270/Q23X88KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50182291 (CHEMBL3818138) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal 6-His-tagged PAK1 kinase domain (249 to 545 residues) expressed in Escherichia coli BL21(DE3) assessed as ... | J Med Chem 59: 5520-41 (2016) Article DOI: 10.1021/acs.jmedchem.6b00638 BindingDB Entry DOI: 10.7270/Q23X88KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 2 (Homo sapiens (Human)) | BDBM50182294 (CHEMBL3817984) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant PAK2 assessed as phosphorylation of coumarin labeled FRET peptide substrate at Ser/Thr19 preincubated for 10 mins fol... | J Med Chem 59: 5520-41 (2016) Article DOI: 10.1021/acs.jmedchem.6b00638 BindingDB Entry DOI: 10.7270/Q23X88KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50182402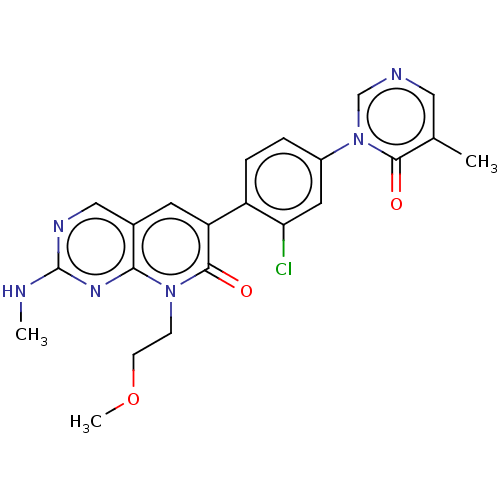 (CHEMBL3819447) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal 6-His-tagged PAK1 kinase domain (249 to 545 residues) expressed in Escherichia coli BL21(DE3) assessed as ... | J Med Chem 59: 5520-41 (2016) Article DOI: 10.1021/acs.jmedchem.6b00638 BindingDB Entry DOI: 10.7270/Q23X88KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50148931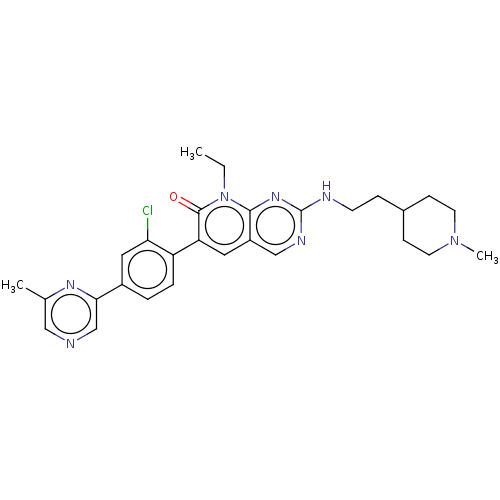 (CHEMBL3770186) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal 6-His-tagged PAK1 kinase domain (249 to 545 residues) expressed in Escherichia coli BL21(DE3) assessed as ... | J Med Chem 59: 5520-41 (2016) Article DOI: 10.1021/acs.jmedchem.6b00638 BindingDB Entry DOI: 10.7270/Q23X88KT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50182289 (CHEMBL3818326) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal 6-His-tagged PAK1 kinase domain (249 to 545 residues) expressed in Escherichia coli BL21(DE3) assessed as ... | J Med Chem 59: 5520-41 (2016) Article DOI: 10.1021/acs.jmedchem.6b00638 BindingDB Entry DOI: 10.7270/Q23X88KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 2 (Homo sapiens (Human)) | BDBM50182402 (CHEMBL3819447) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant PAK2 assessed as phosphorylation of coumarin labeled FRET peptide substrate at Ser/Thr19 preincubated for 10 mins fol... | J Med Chem 59: 5520-41 (2016) Article DOI: 10.1021/acs.jmedchem.6b00638 BindingDB Entry DOI: 10.7270/Q23X88KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 2 (Homo sapiens (Human)) | BDBM50148931 (CHEMBL3770186) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant PAK2 assessed as phosphorylation of coumarin labeled FRET peptide substrate at Ser/Thr19 preincubated for 10 mins fol... | J Med Chem 59: 5520-41 (2016) Article DOI: 10.1021/acs.jmedchem.6b00638 BindingDB Entry DOI: 10.7270/Q23X88KT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50182354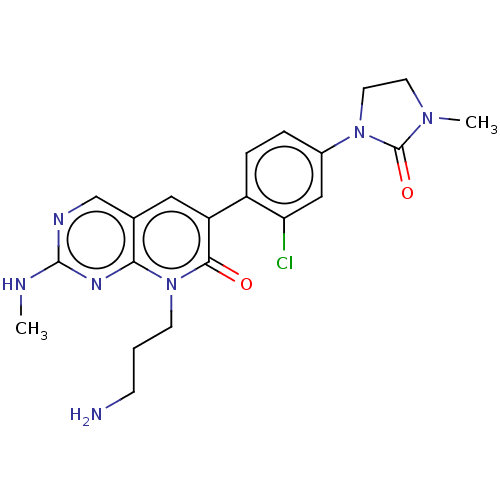 (CHEMBL3818217) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal 6-His-tagged PAK1 kinase domain (249 to 545 residues) expressed in Escherichia coli BL21(DE3) assessed as ... | J Med Chem 59: 5520-41 (2016) Article DOI: 10.1021/acs.jmedchem.6b00638 BindingDB Entry DOI: 10.7270/Q23X88KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50182309 (CHEMBL3818571) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal 6-His-tagged PAK1 kinase domain (249 to 545 residues) expressed in Escherichia coli BL21(DE3) assessed as ... | J Med Chem 59: 5520-41 (2016) Article DOI: 10.1021/acs.jmedchem.6b00638 BindingDB Entry DOI: 10.7270/Q23X88KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50182404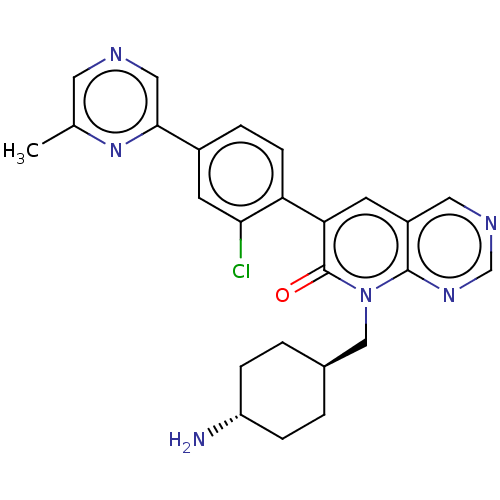 (CHEMBL3819371) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 372 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal 6-His-tagged PAK1 kinase domain (249 to 545 residues) expressed in Escherichia coli BL21(DE3) assessed as ... | J Med Chem 59: 5520-41 (2016) Article DOI: 10.1021/acs.jmedchem.6b00638 BindingDB Entry DOI: 10.7270/Q23X88KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50182296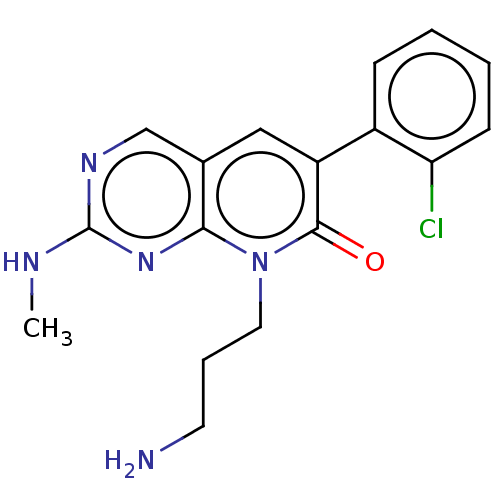 (CHEMBL3818088) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 382 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal 6-His-tagged PAK1 kinase domain (249 to 545 residues) expressed in Escherichia coli BL21(DE3) assessed as ... | J Med Chem 59: 5520-41 (2016) Article DOI: 10.1021/acs.jmedchem.6b00638 BindingDB Entry DOI: 10.7270/Q23X88KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 2 (Homo sapiens (Human)) | BDBM50182404 (CHEMBL3819371) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant PAK2 assessed as phosphorylation of coumarin labeled FRET peptide substrate at Ser/Thr19 preincubated for 10 mins fol... | J Med Chem 59: 5520-41 (2016) Article DOI: 10.1021/acs.jmedchem.6b00638 BindingDB Entry DOI: 10.7270/Q23X88KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50182401 (CHEMBL3818261) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 943 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal 6-His-tagged PAK1 kinase domain (249 to 545 residues) expressed in Escherichia coli BL21(DE3) assessed as ... | J Med Chem 59: 5520-41 (2016) Article DOI: 10.1021/acs.jmedchem.6b00638 BindingDB Entry DOI: 10.7270/Q23X88KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50182400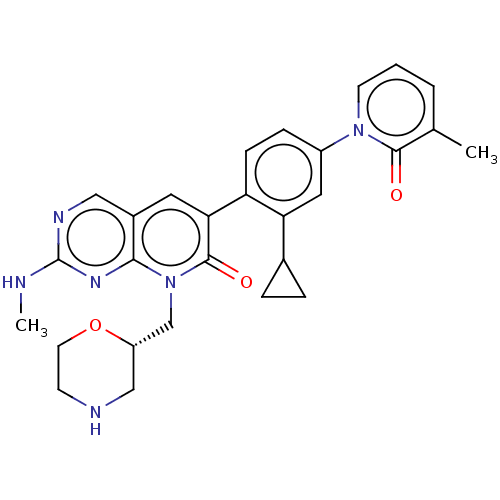 (CHEMBL3819633) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal 6-His-tagged PAK1 kinase domain (249 to 545 residues) expressed in Escherichia coli BL21(DE3) assessed as ... | J Med Chem 59: 5520-41 (2016) Article DOI: 10.1021/acs.jmedchem.6b00638 BindingDB Entry DOI: 10.7270/Q23X88KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50545751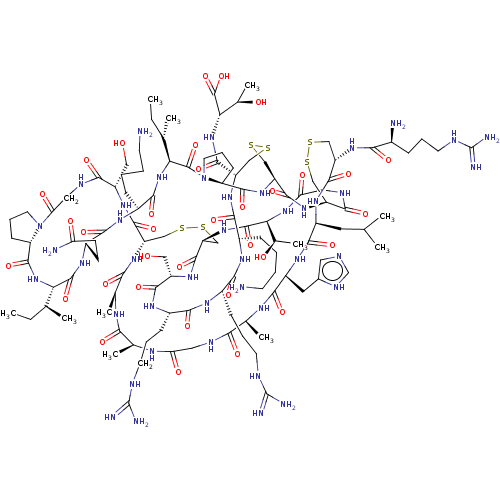 (CHEMBL4633001) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris-Sud Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-alpha-MSH from human MC1R expressed in CHO-K1 cell membranes incubated for 3 hrs by top-count beta counting | J Med Chem 63: 8250-8264 (2020) Article DOI: 10.1021/acs.jmedchem.0c00485 BindingDB Entry DOI: 10.7270/Q2T72N20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50545750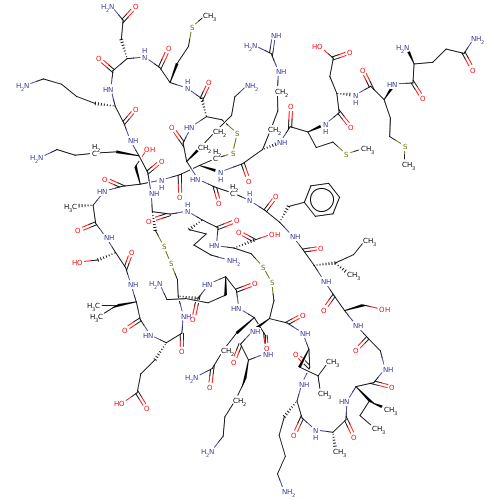 (CHEMBL4639782) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris-Sud Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-alpha-MSH from human MC5R expressed in CHO-K1 cell membranes incubated for 3 hrs by top-count beta counting | J Med Chem 63: 8250-8264 (2020) Article DOI: 10.1021/acs.jmedchem.0c00485 BindingDB Entry DOI: 10.7270/Q2T72N20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 2 (Homo sapiens (Human)) | BDBM50182401 (CHEMBL3818261) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant PAK2 assessed as phosphorylation of coumarin labeled FRET peptide substrate at Ser/Thr19 preincubated for 10 mins fol... | J Med Chem 59: 5520-41 (2016) Article DOI: 10.1021/acs.jmedchem.6b00638 BindingDB Entry DOI: 10.7270/Q23X88KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50545750 (CHEMBL4639782) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris-Sud Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-alpha-MSH from human MC4R expressed in CHO-K1 cell membranes incubated for 3 hrs by top-count beta counting | J Med Chem 63: 8250-8264 (2020) Article DOI: 10.1021/acs.jmedchem.0c00485 BindingDB Entry DOI: 10.7270/Q2T72N20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50545750 (CHEMBL4639782) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris-Sud Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-alpha-MSH from human MC1R expressed in CHO-K1 cell membranes incubated for 3 hrs by top-count beta counting | J Med Chem 63: 8250-8264 (2020) Article DOI: 10.1021/acs.jmedchem.0c00485 BindingDB Entry DOI: 10.7270/Q2T72N20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 2 (Homo sapiens (Human)) | BDBM50182400 (CHEMBL3819633) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant PAK2 assessed as phosphorylation of coumarin labeled FRET peptide substrate at Ser/Thr19 preincubated for 10 mins fol... | J Med Chem 59: 5520-41 (2016) Article DOI: 10.1021/acs.jmedchem.6b00638 BindingDB Entry DOI: 10.7270/Q23X88KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50545750 (CHEMBL4639782) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris-Sud Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-alpha-MSH from human MC3R expressed in CHO-K1 cell membranes incubated for 3 hrs by top-count beta counting | J Med Chem 63: 8250-8264 (2020) Article DOI: 10.1021/acs.jmedchem.0c00485 BindingDB Entry DOI: 10.7270/Q2T72N20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50545751 (CHEMBL4633001) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris-Sud Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-alpha-MSH from human MC4R expressed in CHO-K1 cell membranes incubated for 3 hrs by top-count beta counting | J Med Chem 63: 8250-8264 (2020) Article DOI: 10.1021/acs.jmedchem.0c00485 BindingDB Entry DOI: 10.7270/Q2T72N20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50545751 (CHEMBL4633001) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris-Sud Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-alpha-MSH from human MC5R expressed in CHO-K1 cell membranes incubated for 3 hrs by top-count beta counting | J Med Chem 63: 8250-8264 (2020) Article DOI: 10.1021/acs.jmedchem.0c00485 BindingDB Entry DOI: 10.7270/Q2T72N20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50545751 (CHEMBL4633001) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris-Sud Curated by ChEMBL | Assay Description Displacement of [125I]-NDP-alpha-MSH from human MC3R expressed in CHO-K1 cell membranes incubated for 3 hrs by top-count beta counting | J Med Chem 63: 8250-8264 (2020) Article DOI: 10.1021/acs.jmedchem.0c00485 BindingDB Entry DOI: 10.7270/Q2T72N20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324297 (4-(1-Piperazin-1-yl[2,6]naphthyridin-3-yl)pyridin-...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5400-21 (2010) Article DOI: 10.1021/jm100075z BindingDB Entry DOI: 10.7270/Q2T153T9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324315 (CHEMBL1214998 | Cyclohexyl-[4-(1-piperazin-1-yl[2,...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5400-21 (2010) Article DOI: 10.1021/jm100075z BindingDB Entry DOI: 10.7270/Q2T153T9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324315 (CHEMBL1214998 | Cyclohexyl-[4-(1-piperazin-1-yl[2,...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5422-38 (2010) Article DOI: 10.1021/jm100076w BindingDB Entry DOI: 10.7270/Q2P84C2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324322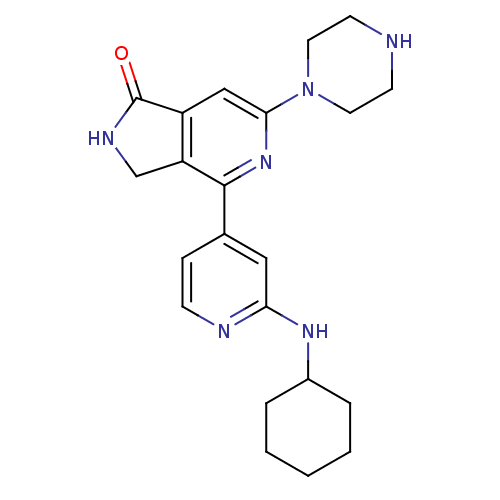 (4-(2-cyclohexylaminopyridin-4-yl)-6-(piperazin-1-y...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5422-38 (2010) Article DOI: 10.1021/jm100076w BindingDB Entry DOI: 10.7270/Q2P84C2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycystin-2 (Homo sapiens (Human)) | BDBM50324324 (2'-Phenylamino-6-piperazin-1-yl[2,4']bipyridinyl-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD2 by TR-FRET assay | J Med Chem 53: 5422-38 (2010) Article DOI: 10.1021/jm100076w BindingDB Entry DOI: 10.7270/Q2P84C2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 250 total ) | Next | Last >> |