 Found 124 hits with Last Name = 'vemulapalli' and Initial = 's'
Found 124 hits with Last Name = 'vemulapalli' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14776

(2-{2-ethoxy-5-[(4-ethylpiperazine-1-)sulfonyl]phen...)Show SMILES CCCc1nc(C)c2n1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C23H32N6O4S/c1-5-8-20-24-16(4)21-23(30)25-22(26-29(20)21)18-15-17(9-10-19(18)33-7-3)34(31,32)28-13-11-27(6-2)12-14-28/h9-10,15H,5-8,11-14H2,1-4H3,(H,25,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human phosphodiesterase 5 |
Bioorg Med Chem Lett 15: 2365-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.083
BindingDB Entry DOI: 10.7270/Q2TM79MQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50120086
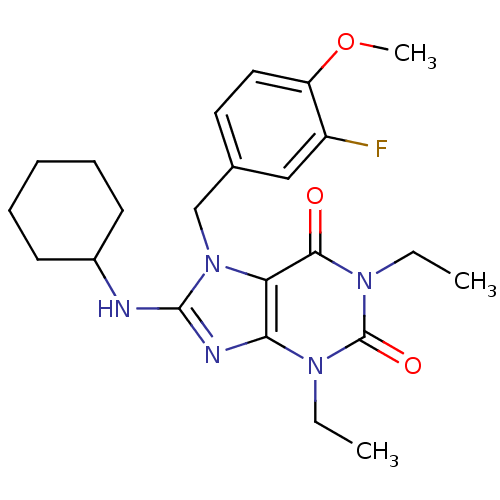
(8-Cyclohexylamino-1,3-diethyl-7-(3-fluoro-4-methox...)Show SMILES CCn1c2nc(NC3CCCCC3)n(Cc3ccc(OC)c(F)c3)c2c(=O)n(CC)c1=O Show InChI InChI=1S/C23H30FN5O3/c1-4-27-20-19(21(30)28(5-2)23(27)31)29(14-15-11-12-18(32-3)17(24)13-15)22(26-20)25-16-9-7-6-8-10-16/h11-13,16H,4-10,14H2,1-3H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined |
Bioorg Med Chem Lett 12: 3149-52 (2002)
BindingDB Entry DOI: 10.7270/Q2S46R83 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50140583

(5-ethyl-3-(4-hydroxybenzyl)-2-(2-phenyl-1-ethynyl)...)Show SMILES CCN1C2=N[C@@H]3CCC[C@@H]3N2c2nc(C#Cc3ccccc3)n(Cc3ccc(O)cc3)c2C1=O |r,t:3| Show InChI InChI=1S/C27H25N5O2/c1-2-30-26(34)24-25(32-22-10-6-9-21(22)28-27(30)32)29-23(16-13-18-7-4-3-5-8-18)31(24)17-19-11-14-20(33)15-12-19/h3-5,7-8,11-12,14-15,21-22,33H,2,6,9-10,17H2,1H3/t21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human phosphodiesterase 5 (PDE5) enzyme |
Bioorg Med Chem Lett 14: 1291-4 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.027
BindingDB Entry DOI: 10.7270/Q2BG2NF0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50120069

(8-Cyclohexylamino-1,3-diethyl-7-(3-fluoro-4-hydrox...)Show SMILES CCn1c2nc(NC3CCCCC3)n(Cc3ccc(O)c(F)c3)c2c(=O)n(CC)c1=O Show InChI InChI=1S/C22H28FN5O3/c1-3-26-19-18(20(30)27(4-2)22(26)31)28(13-14-10-11-17(29)16(23)12-14)21(25-19)24-15-8-6-5-7-9-15/h10-12,15,29H,3-9,13H2,1-2H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined |
Bioorg Med Chem Lett 12: 3149-52 (2002)
BindingDB Entry DOI: 10.7270/Q2S46R83 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50120079
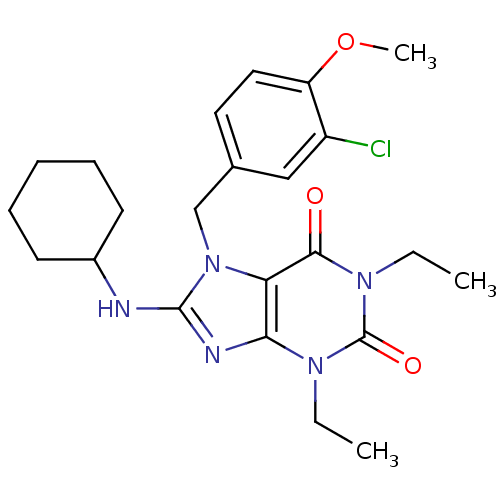
(7-(3-Chloro-4-methoxy-benzyl)-8-cyclohexylamino-1,...)Show SMILES CCn1c2nc(NC3CCCCC3)n(Cc3ccc(OC)c(Cl)c3)c2c(=O)n(CC)c1=O Show InChI InChI=1S/C23H30ClN5O3/c1-4-27-20-19(21(30)28(5-2)23(27)31)29(14-15-11-12-18(32-3)17(24)13-15)22(26-20)25-16-9-7-6-8-10-16/h11-13,16H,4-10,14H2,1-3H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined |
Bioorg Med Chem Lett 12: 3149-52 (2002)
BindingDB Entry DOI: 10.7270/Q2S46R83 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50120074

(8-Cyclohexylamino-1,3-diethyl-7-(4-hydroxy-benzyl)...)Show SMILES CCn1c2nc(NC3CCCCC3)n(Cc3ccc(O)cc3)c2c(=O)n(CC)c1=O Show InChI InChI=1S/C22H29N5O3/c1-3-25-19-18(20(29)26(4-2)22(25)30)27(14-15-10-12-17(28)13-11-15)21(24-19)23-16-8-6-5-7-9-16/h10-13,16,28H,3-9,14H2,1-2H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined |
Bioorg Med Chem Lett 12: 3149-52 (2002)
BindingDB Entry DOI: 10.7270/Q2S46R83 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50140608
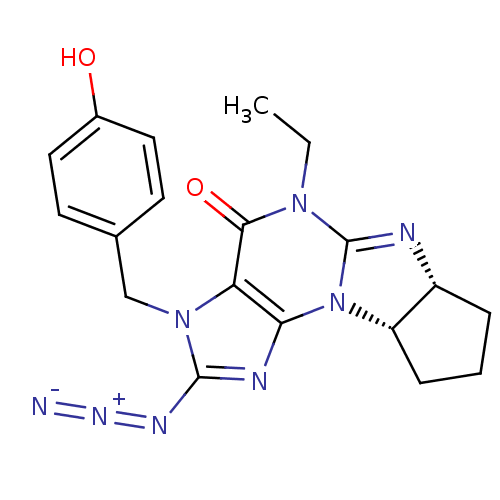
(2-azido-5-ethyl-3-(4-hydroxybenzyl)-(6aR,9aS)-3,4,...)Show SMILES CCN1C2=N[C@@H]3CCC[C@@H]3N2c2nc(N=[N+]=[N-])n(Cc3ccc(O)cc3)c2C1=O |t:3| Show InChI InChI=1S/C19H20N8O2/c1-2-25-17(29)15-16(27-14-5-3-4-13(14)21-19(25)27)22-18(23-24-20)26(15)10-11-6-8-12(28)9-7-11/h6-9,13-14,28H,2-5,10H2,1H3/t13-,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human phosphodiesterase 5 (PDE5) enzyme |
Bioorg Med Chem Lett 14: 1291-4 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.027
BindingDB Entry DOI: 10.7270/Q2BG2NF0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50120067
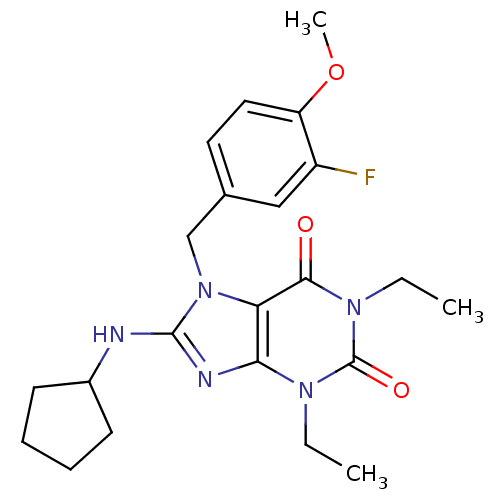
(8-Cyclopentylamino-1,3-diethyl-7-(3-fluoro-4-metho...)Show SMILES CCn1c2nc(NC3CCCC3)n(Cc3ccc(OC)c(F)c3)c2c(=O)n(CC)c1=O Show InChI InChI=1S/C22H28FN5O3/c1-4-26-19-18(20(29)27(5-2)22(26)30)28(21(25-19)24-15-8-6-7-9-15)13-14-10-11-17(31-3)16(23)12-14/h10-12,15H,4-9,13H2,1-3H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined |
Bioorg Med Chem Lett 12: 3149-52 (2002)
BindingDB Entry DOI: 10.7270/Q2S46R83 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50120072
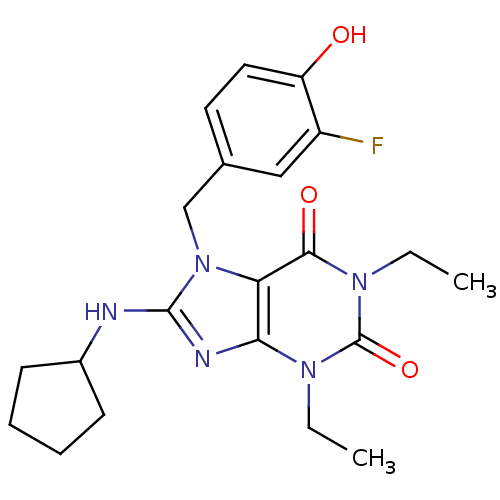
(8-Cyclopentylamino-1,3-diethyl-7-(3-fluoro-4-hydro...)Show SMILES CCn1c2nc(NC3CCCC3)n(Cc3ccc(O)c(F)c3)c2c(=O)n(CC)c1=O Show InChI InChI=1S/C21H26FN5O3/c1-3-25-18-17(19(29)26(4-2)21(25)30)27(12-13-9-10-16(28)15(22)11-13)20(24-18)23-14-7-5-6-8-14/h9-11,14,28H,3-8,12H2,1-2H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined |
Bioorg Med Chem Lett 12: 3149-52 (2002)
BindingDB Entry DOI: 10.7270/Q2S46R83 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50140619
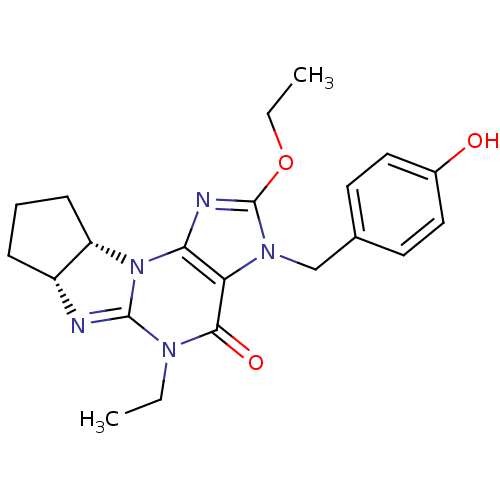
(2-ethoxy-5-ethyl-3-(4-hydroxybenzyl)-(6aR,9aS)-3,4...)Show SMILES CCOc1nc2N3[C@H]4CCC[C@H]4N=C3N(CC)C(=O)c2n1Cc1ccc(O)cc1 |r,c:13| Show InChI InChI=1S/C21H25N5O3/c1-3-24-19(28)17-18(26-16-7-5-6-15(16)22-20(24)26)23-21(29-4-2)25(17)12-13-8-10-14(27)11-9-13/h8-11,15-16,27H,3-7,12H2,1-2H3/t15-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human phosphodiesterase 5 (PDE5) enzyme |
Bioorg Med Chem Lett 14: 1291-4 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.027
BindingDB Entry DOI: 10.7270/Q2BG2NF0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50140624
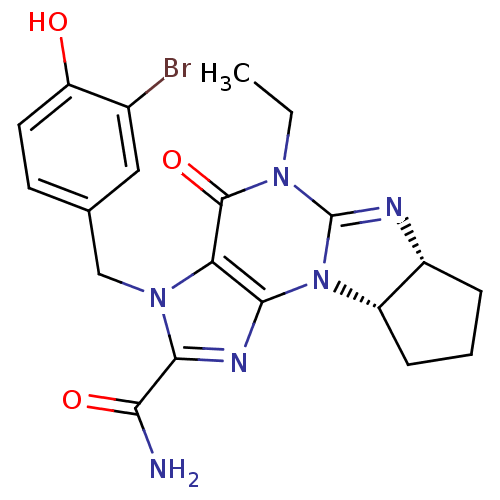
(3-(3-bromo-4-hydroxybenzyl)-5-ethyl-4-oxo-(6aR,9aS...)Show SMILES CCN1C2=N[C@@H]3CCC[C@@H]3N2c2nc(C(N)=O)n(Cc3ccc(O)c(Br)c3)c2C1=O |r,t:3| Show InChI InChI=1S/C20H21BrN6O3/c1-2-25-19(30)15-17(27-13-5-3-4-12(13)23-20(25)27)24-18(16(22)29)26(15)9-10-6-7-14(28)11(21)8-10/h6-8,12-13,28H,2-5,9H2,1H3,(H2,22,29)/t12-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human phosphodiesterase 5 (PDE5) enzyme |
Bioorg Med Chem Lett 14: 1291-4 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.027
BindingDB Entry DOI: 10.7270/Q2BG2NF0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50140593
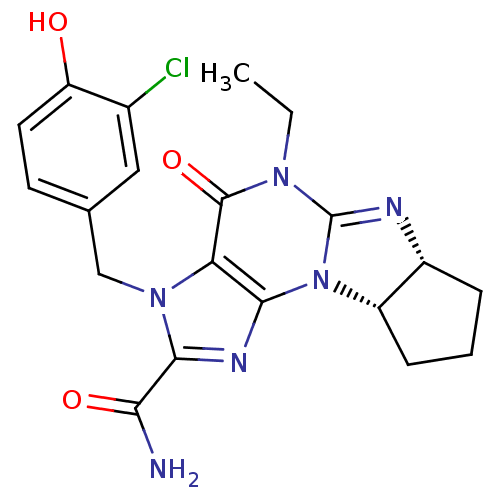
(3-(3-chloro-4-hydroxybenzyl)-5-ethyl-4-oxo-(6aR,9a...)Show SMILES CCN1C2=N[C@@H]3CCC[C@@H]3N2c2nc(C(N)=O)n(Cc3ccc(O)c(Cl)c3)c2C1=O |r,t:3| Show InChI InChI=1S/C20H21ClN6O3/c1-2-25-19(30)15-17(27-13-5-3-4-12(13)23-20(25)27)24-18(16(22)29)26(15)9-10-6-7-14(28)11(21)8-10/h6-8,12-13,28H,2-5,9H2,1H3,(H2,22,29)/t12-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human phosphodiesterase 5 (PDE5) enzyme |
Bioorg Med Chem Lett 14: 1291-4 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.027
BindingDB Entry DOI: 10.7270/Q2BG2NF0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50120071
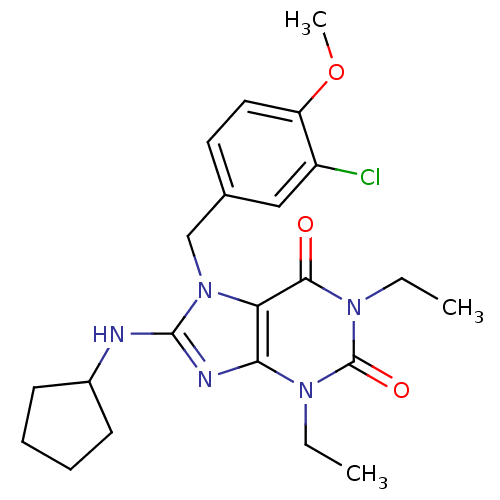
(7-(3-Chloro-4-methoxy-benzyl)-8-cyclopentylamino-1...)Show SMILES CCn1c2nc(NC3CCCC3)n(Cc3ccc(OC)c(Cl)c3)c2c(=O)n(CC)c1=O Show InChI InChI=1S/C22H28ClN5O3/c1-4-26-19-18(20(29)27(5-2)22(26)30)28(21(25-19)24-15-8-6-7-9-15)13-14-10-11-17(31-3)16(23)12-14/h10-12,15H,4-9,13H2,1-3H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined |
Bioorg Med Chem Lett 12: 3149-52 (2002)
BindingDB Entry DOI: 10.7270/Q2S46R83 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50120080
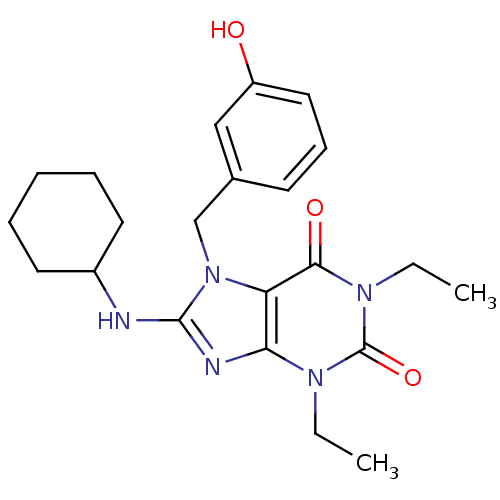
(8-Cyclohexylamino-1,3-diethyl-7-(3-hydroxy-benzyl)...)Show SMILES CCn1c2nc(NC3CCCCC3)n(Cc3cccc(O)c3)c2c(=O)n(CC)c1=O Show InChI InChI=1S/C22H29N5O3/c1-3-25-19-18(20(29)26(4-2)22(25)30)27(14-15-9-8-12-17(28)13-15)21(24-19)23-16-10-6-5-7-11-16/h8-9,12-13,16,28H,3-7,10-11,14H2,1-2H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined |
Bioorg Med Chem Lett 12: 3149-52 (2002)
BindingDB Entry DOI: 10.7270/Q2S46R83 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50140582
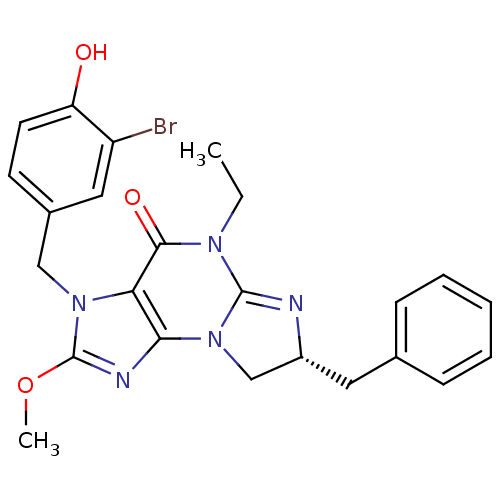
((R)-3-(3-bromo-4-hydroxybenzyl)-7-benzyl-5-ethyl-2...)Show SMILES CCN1C2=N[C@H](Cc3ccccc3)CN2c2nc(OC)n(Cc3ccc(O)c(Br)c3)c2C1=O |r,t:3| Show InChI InChI=1S/C24H24BrN5O3/c1-3-28-22(32)20-21(30-14-17(26-23(28)30)11-15-7-5-4-6-8-15)27-24(33-2)29(20)13-16-9-10-19(31)18(25)12-16/h4-10,12,17,31H,3,11,13-14H2,1-2H3/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human phosphodiesterase 5 (PDE5) enzyme |
Bioorg Med Chem Lett 14: 1291-4 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.027
BindingDB Entry DOI: 10.7270/Q2BG2NF0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50140590

(2N-methyl-3-(3-chloro-4-hydroxybenzyl)-5-ethyl-4-o...)Show SMILES CCN1C2=N[C@@H]3CCC[C@@H]3N2c2nc(C(=O)NC)n(Cc3ccc(O)c(Cl)c3)c2C1=O |r,t:3| Show InChI InChI=1S/C21H23ClN6O3/c1-3-26-20(31)16-17(28-14-6-4-5-13(14)24-21(26)28)25-18(19(30)23-2)27(16)10-11-7-8-15(29)12(22)9-11/h7-9,13-14,29H,3-6,10H2,1-2H3,(H,23,30)/t13-,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human phosphodiesterase 5 (PDE5) enzyme |
Bioorg Med Chem Lett 14: 1291-4 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.027
BindingDB Entry DOI: 10.7270/Q2BG2NF0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50120070
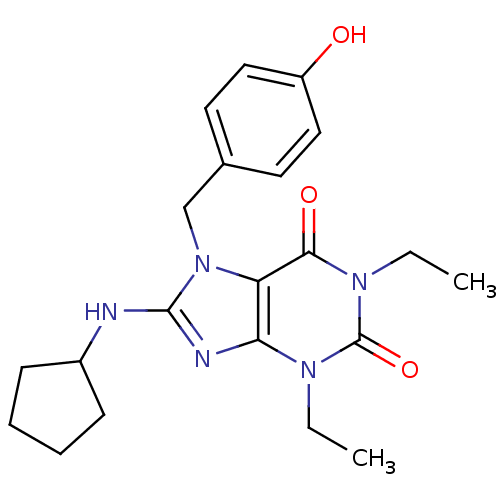
(8-Cyclopentylamino-1,3-diethyl-7-(4-methoxy-benzyl...)Show SMILES CCn1c2nc(NC3CCCC3)n(Cc3ccc(O)cc3)c2c(=O)n(CC)c1=O Show InChI InChI=1S/C21H27N5O3/c1-3-24-18-17(19(28)25(4-2)21(24)29)26(13-14-9-11-16(27)12-10-14)20(23-18)22-15-7-5-6-8-15/h9-12,15,27H,3-8,13H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined |
Bioorg Med Chem Lett 12: 3149-52 (2002)
BindingDB Entry DOI: 10.7270/Q2S46R83 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50140580
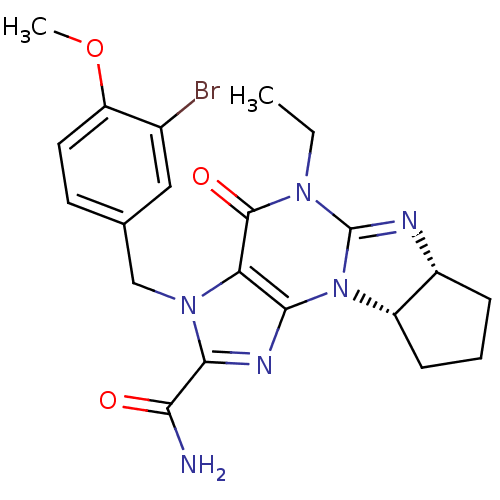
(3-(3-bromo-4-methoxybenzyl)-5-ethyl-4-oxo-(6aR,9aS...)Show SMILES CCN1C2=N[C@@H]3CCC[C@@H]3N2c2nc(C(N)=O)n(Cc3ccc(OC)c(Br)c3)c2C1=O |r,t:3| Show InChI InChI=1S/C21H23BrN6O3/c1-3-26-20(30)16-18(28-14-6-4-5-13(14)24-21(26)28)25-19(17(23)29)27(16)10-11-7-8-15(31-2)12(22)9-11/h7-9,13-14H,3-6,10H2,1-2H3,(H2,23,29)/t13-,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human phosphodiesterase 5 (PDE5) enzyme |
Bioorg Med Chem Lett 14: 1291-4 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.027
BindingDB Entry DOI: 10.7270/Q2BG2NF0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50140603

(3-(3-bromo-4-hydroxybenzyl)-5-ethyl-2-methoxy-(6aR...)Show SMILES CCN1C2=N[C@@H]3CCC[C@@H]3N2c2nc(OC)n(Cc3ccc(O)c(Br)c3)c2C1=O |r,t:3| Show InChI InChI=1S/C20H22BrN5O3/c1-3-24-18(28)16-17(26-14-6-4-5-13(14)22-19(24)26)23-20(29-2)25(16)10-11-7-8-15(27)12(21)9-11/h7-9,13-14,27H,3-6,10H2,1-2H3/t13-,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human phosphodiesterase 5 (PDE5) enzyme |
Bioorg Med Chem Lett 14: 1291-4 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.027
BindingDB Entry DOI: 10.7270/Q2BG2NF0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50140620
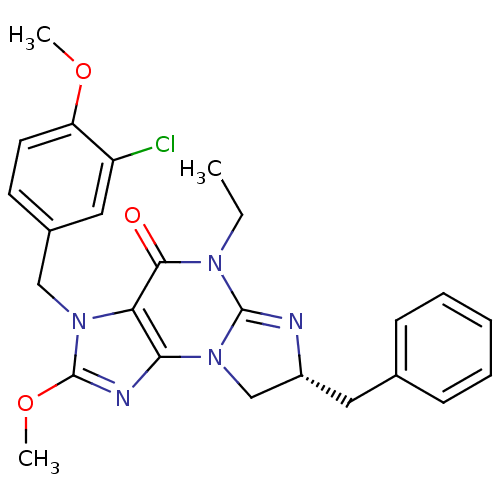
(7-benzyl-3-(3-chloro-4-methoxybenzyl)-5-ethyl-2-me...)Show SMILES CCN1C2=N[C@H](Cc3ccccc3)CN2c2nc(OC)n(Cc3ccc(OC)c(Cl)c3)c2C1=O |t:3| Show InChI InChI=1S/C25H26ClN5O3/c1-4-29-23(32)21-22(31-15-18(27-24(29)31)12-16-8-6-5-7-9-16)28-25(34-3)30(21)14-17-10-11-20(33-2)19(26)13-17/h5-11,13,18H,4,12,14-15H2,1-3H3/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human phosphodiesterase 5 (PDE5) enzyme |
Bioorg Med Chem Lett 14: 1291-4 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.027
BindingDB Entry DOI: 10.7270/Q2BG2NF0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50120081
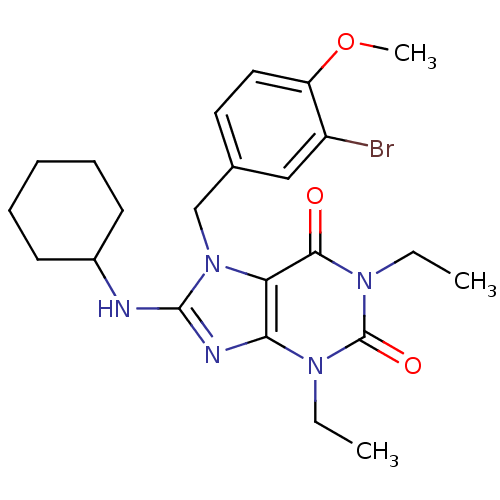
(7-(3-Bromo-4-methoxy-benzyl)-8-cyclohexylamino-1,3...)Show SMILES CCn1c2nc(NC3CCCCC3)n(Cc3ccc(OC)c(Br)c3)c2c(=O)n(CC)c1=O Show InChI InChI=1S/C23H30BrN5O3/c1-4-27-20-19(21(30)28(5-2)23(27)31)29(14-15-11-12-18(32-3)17(24)13-15)22(26-20)25-16-9-7-6-8-10-16/h11-13,16H,4-10,14H2,1-3H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined |
Bioorg Med Chem Lett 12: 3149-52 (2002)
BindingDB Entry DOI: 10.7270/Q2S46R83 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50120078

(7-(3-Chloro-4-hydroxy-benzyl)-8-cyclopentylamino-1...)Show SMILES CCn1c2nc(NC3CCCC3)n(Cc3ccc(O)c(Cl)c3)c2c(=O)n(CC)c1=O Show InChI InChI=1S/C21H26ClN5O3/c1-3-25-18-17(19(29)26(4-2)21(25)30)27(12-13-9-10-16(28)15(22)11-13)20(24-18)23-14-7-5-6-8-14/h9-11,14,28H,3-8,12H2,1-2H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined |
Bioorg Med Chem Lett 12: 3149-52 (2002)
BindingDB Entry DOI: 10.7270/Q2S46R83 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50140598
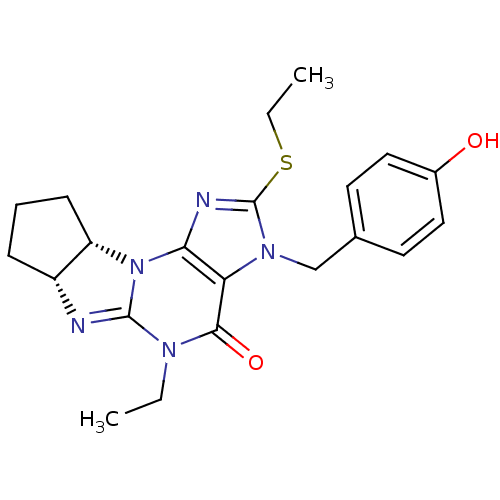
(5-ethyl-2-ethylsulfanyl-3-(4-hydroxybenzyl)-(6aR,9...)Show SMILES CCSc1nc2N3[C@H]4CCC[C@H]4N=C3N(CC)C(=O)c2n1Cc1ccc(O)cc1 |r,c:13| Show InChI InChI=1S/C21H25N5O2S/c1-3-24-19(28)17-18(26-16-7-5-6-15(16)22-20(24)26)23-21(29-4-2)25(17)12-13-8-10-14(27)11-9-13/h8-11,15-16,27H,3-7,12H2,1-2H3/t15-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human phosphodiesterase 5 (PDE5) enzyme |
Bioorg Med Chem Lett 14: 1291-4 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.027
BindingDB Entry DOI: 10.7270/Q2BG2NF0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50140585

((R)-3-(3-chloro-4-hydroxybenzyl)-7-benzyl-5-ethyl-...)Show SMILES CCN1C2=N[C@H](Cc3ccccc3)CN2c2nc(OC)n(Cc3ccc(O)c(Cl)c3)c2C1=O |r,t:3| Show InChI InChI=1S/C24H24ClN5O3/c1-3-28-22(32)20-21(30-14-17(26-23(28)30)11-15-7-5-4-6-8-15)27-24(33-2)29(20)13-16-9-10-19(31)18(25)12-16/h4-10,12,17,31H,3,11,13-14H2,1-2H3/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human phosphodiesterase 5 (PDE5) enzyme |
Bioorg Med Chem Lett 14: 1291-4 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.027
BindingDB Entry DOI: 10.7270/Q2BG2NF0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50140584
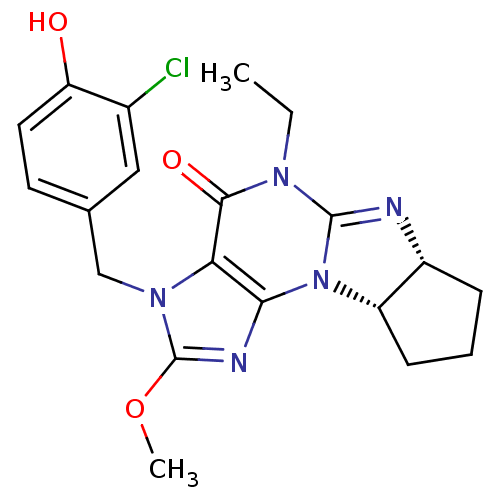
(3-(3-chloro-4-hydroxybenzyl)-5-ethyl-2-methoxy-(6a...)Show SMILES CCN1C2=N[C@@H]3CCC[C@@H]3N2c2nc(OC)n(Cc3ccc(O)c(Cl)c3)c2C1=O |r,t:3| Show InChI InChI=1S/C20H22ClN5O3/c1-3-24-18(28)16-17(26-14-6-4-5-13(14)22-19(24)26)23-20(29-2)25(16)10-11-7-8-15(27)12(21)9-11/h7-9,13-14,27H,3-6,10H2,1-2H3/t13-,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human phosphodiesterase 5 (PDE5) enzyme |
Bioorg Med Chem Lett 14: 1291-4 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.027
BindingDB Entry DOI: 10.7270/Q2BG2NF0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50120085

(7-(3-Chloro-4-hydroxy-benzyl)-8-cyclohexylamino-1,...)Show SMILES CCn1c2nc(NC3CCCCC3)n(Cc3ccc(O)c(Cl)c3)c2c(=O)n(CC)c1=O Show InChI InChI=1S/C22H28ClN5O3/c1-3-26-19-18(20(30)27(4-2)22(26)31)28(13-14-10-11-17(29)16(23)12-14)21(25-19)24-15-8-6-5-7-9-15/h10-12,15,29H,3-9,13H2,1-2H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined |
Bioorg Med Chem Lett 12: 3149-52 (2002)
BindingDB Entry DOI: 10.7270/Q2S46R83 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50120068
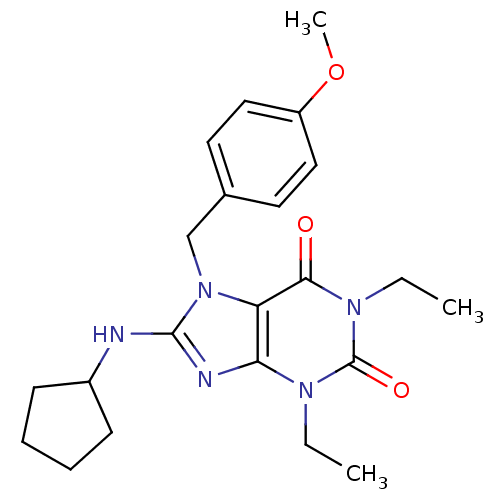
(8-Cyclopentylamino-1,3-diethyl-7-(4-methoxy-benzyl...)Show SMILES CCn1c2nc(NC3CCCC3)n(Cc3ccc(OC)cc3)c2c(=O)n(CC)c1=O Show InChI InChI=1S/C22H29N5O3/c1-4-25-19-18(20(28)26(5-2)22(25)29)27(14-15-10-12-17(30-3)13-11-15)21(24-19)23-16-8-6-7-9-16/h10-13,16H,4-9,14H2,1-3H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined |
Bioorg Med Chem Lett 12: 3149-52 (2002)
BindingDB Entry DOI: 10.7270/Q2S46R83 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50140614
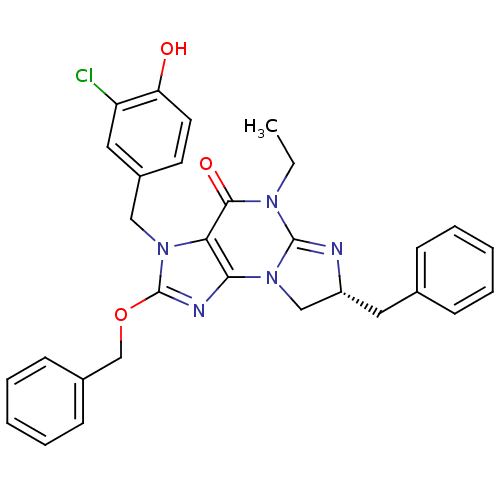
((R)-3-(3-chloro-4-hydroxybenzyl)-7-benzyl-2-(benzy...)Show SMILES CCN1C2=N[C@H](Cc3ccccc3)CN2c2nc(OCc3ccccc3)n(Cc3ccc(O)c(Cl)c3)c2C1=O |r,t:3| Show InChI InChI=1S/C30H28ClN5O3/c1-2-34-28(38)26-27(36-18-23(32-29(34)36)15-20-9-5-3-6-10-20)33-30(39-19-21-11-7-4-8-12-21)35(26)17-22-13-14-25(37)24(31)16-22/h3-14,16,23,37H,2,15,17-19H2,1H3/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human phosphodiesterase 5 (PDE5) enzyme |
Bioorg Med Chem Lett 14: 1291-4 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.027
BindingDB Entry DOI: 10.7270/Q2BG2NF0 |
More data for this
Ligand-Target Pair | |
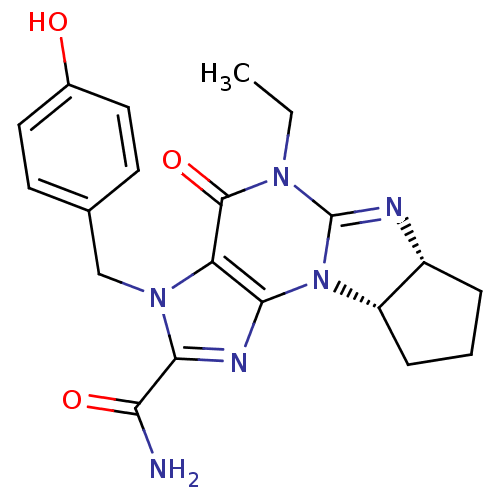
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50140579
(5-ethyl-3-(4-hydroxybenzyl)-4-oxo-(6aR,9aS)-3,4,5,...)Show SMILES CCN1C2=N[C@@H]3CCC[C@@H]3N2c2nc(C(N)=O)n(Cc3ccc(O)cc3)c2C1=O |r,t:3| Show InChI InChI=1S/C20H22N6O3/c1-2-24-19(29)15-17(26-14-5-3-4-13(14)22-20(24)26)23-18(16(21)28)25(15)10-11-6-8-12(27)9-7-11/h6-9,13-14,27H,2-5,10H2,1H3,(H2,21,28)/t13-,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human phosphodiesterase 5 (PDE5) enzyme |
Bioorg Med Chem Lett 14: 1291-4 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.027
BindingDB Entry DOI: 10.7270/Q2BG2NF0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50140605

(3-(3-chloro-4-hydroxybenzyl)-2-ethoxy-5-ethyl-(6aR...)Show SMILES CCOc1nc2N3[C@H]4CCC[C@H]4N=C3N(CC)C(=O)c2n1Cc1ccc(O)c(Cl)c1 |r,c:13| Show InChI InChI=1S/C21H24ClN5O3/c1-3-25-19(29)17-18(27-15-7-5-6-14(15)23-20(25)27)24-21(30-4-2)26(17)11-12-8-9-16(28)13(22)10-12/h8-10,14-15,28H,3-7,11H2,1-2H3/t14-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human phosphodiesterase 5 (PDE5) enzyme |
Bioorg Med Chem Lett 14: 1291-4 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.027
BindingDB Entry DOI: 10.7270/Q2BG2NF0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50140611
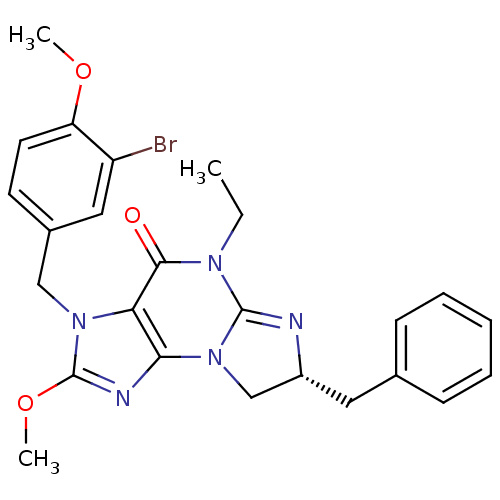
((R)-3-(3-bromo-4-methoxybenzyl)-7-benzyl-5-ethyl-2...)Show SMILES CCN1C2=N[C@H](Cc3ccccc3)CN2c2nc(OC)n(Cc3ccc(OC)c(Br)c3)c2C1=O |r,t:3| Show InChI InChI=1S/C25H26BrN5O3/c1-4-29-23(32)21-22(31-15-18(27-24(29)31)12-16-8-6-5-7-9-16)28-25(34-3)30(21)14-17-10-11-20(33-2)19(26)13-17/h5-11,13,18H,4,12,14-15H2,1-3H3/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human phosphodiesterase 5 (PDE5) enzyme |
Bioorg Med Chem Lett 14: 1291-4 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.027
BindingDB Entry DOI: 10.7270/Q2BG2NF0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50140616
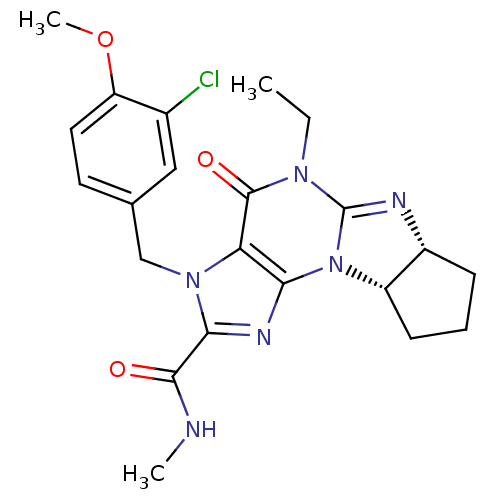
(2N-methyl-3-(3-chloro-4-methoxybenzyl)-5-ethyl-4-o...)Show SMILES CCN1C2=N[C@@H]3CCC[C@@H]3N2c2nc(C(=O)NC)n(Cc3ccc(OC)c(Cl)c3)c2C1=O |r,t:3| Show InChI InChI=1S/C22H25ClN6O3/c1-4-27-21(31)17-18(29-15-7-5-6-14(15)25-22(27)29)26-19(20(30)24-2)28(17)11-12-8-9-16(32-3)13(23)10-12/h8-10,14-15H,4-7,11H2,1-3H3,(H,24,30)/t14-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human phosphodiesterase 5 (PDE5) enzyme |
Bioorg Med Chem Lett 14: 1291-4 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.027
BindingDB Entry DOI: 10.7270/Q2BG2NF0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50165781

((R)-7-Benzyl-2-chloro-5-ethyl-3-(4-hydroxy-benzyl)...)Show SMILES CCN1C2=N[C@H](Cc3ccccc3)CN2c2nc(Cl)n(Cc3ccc(O)cc3)c2C1=O |t:3| Show InChI InChI=1S/C23H22ClN5O2/c1-2-27-21(31)19-20(26-22(24)28(19)13-16-8-10-18(30)11-9-16)29-14-17(25-23(27)29)12-15-6-4-3-5-7-15/h3-11,17,30H,2,12-14H2,1H3/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human phosphodiesterase 5 |
Bioorg Med Chem Lett 15: 2365-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.083
BindingDB Entry DOI: 10.7270/Q2TM79MQ |
More data for this
Ligand-Target Pair | |
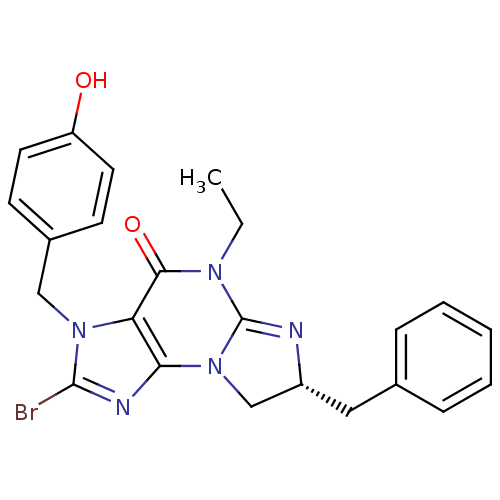
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50165771
((R)-7-Benzyl-2-bromo-5-ethyl-3-(4-hydroxy-benzyl)-...)Show SMILES CCN1C2=N[C@H](Cc3ccccc3)CN2c2nc(Br)n(Cc3ccc(O)cc3)c2C1=O |t:3| Show InChI InChI=1S/C23H22BrN5O2/c1-2-27-21(31)19-20(26-22(24)28(19)13-16-8-10-18(30)11-9-16)29-14-17(25-23(27)29)12-15-6-4-3-5-7-15/h3-11,17,30H,2,12-14H2,1H3/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human phosphodiesterase 5 |
Bioorg Med Chem Lett 15: 2365-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.083
BindingDB Entry DOI: 10.7270/Q2TM79MQ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50120083
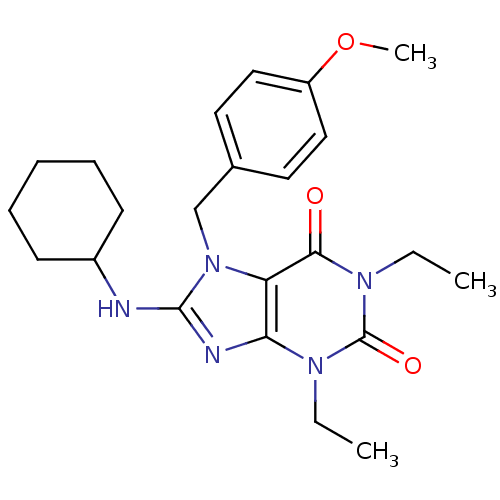
(8-Cyclohexylamino-1,3-diethyl-7-(4-methoxy-benzyl)...)Show SMILES CCn1c2nc(NC3CCCCC3)n(Cc3ccc(OC)cc3)c2c(=O)n(CC)c1=O Show InChI InChI=1S/C23H31N5O3/c1-4-26-20-19(21(29)27(5-2)23(26)30)28(15-16-11-13-18(31-3)14-12-16)22(25-20)24-17-9-7-6-8-10-17/h11-14,17H,4-10,15H2,1-3H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined |
Bioorg Med Chem Lett 12: 3149-52 (2002)
BindingDB Entry DOI: 10.7270/Q2S46R83 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50140613

(3-(3-bromo-4-methoxybenzyl)-5-ethyl-2-methoxy-(6aR...)Show SMILES CCN1C2=N[C@@H]3CCC[C@@H]3N2c2nc(OC)n(Cc3ccc(OC)c(Br)c3)c2C1=O |r,t:3| Show InChI InChI=1S/C21H24BrN5O3/c1-4-25-19(28)17-18(27-15-7-5-6-14(15)23-20(25)27)24-21(30-3)26(17)11-12-8-9-16(29-2)13(22)10-12/h8-10,14-15H,4-7,11H2,1-3H3/t14-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human phosphodiesterase 5 (PDE5) enzyme |
Bioorg Med Chem Lett 14: 1291-4 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.027
BindingDB Entry DOI: 10.7270/Q2BG2NF0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
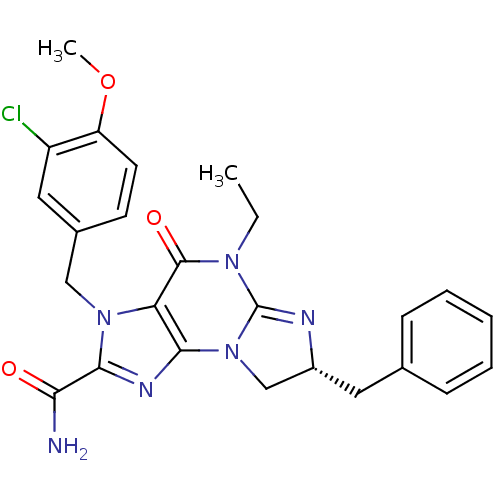
(Homo sapiens (Human)) | BDBM50140578
((R)-3-(3-chloro-4-methoxybenzyl)-7-benzyl-5-ethyl-...)Show SMILES CCN1C2=N[C@H](Cc3ccccc3)CN2c2nc(C(N)=O)n(Cc3ccc(OC)c(Cl)c3)c2C1=O |r,t:3| Show InChI InChI=1S/C25H25ClN6O3/c1-3-30-24(34)20-22(32-14-17(28-25(30)32)11-15-7-5-4-6-8-15)29-23(21(27)33)31(20)13-16-9-10-19(35-2)18(26)12-16/h4-10,12,17H,3,11,13-14H2,1-2H3,(H2,27,33)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human phosphodiesterase 5 (PDE5) enzyme |
Bioorg Med Chem Lett 14: 1291-4 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.027
BindingDB Entry DOI: 10.7270/Q2BG2NF0 |
More data for this
Ligand-Target Pair | |
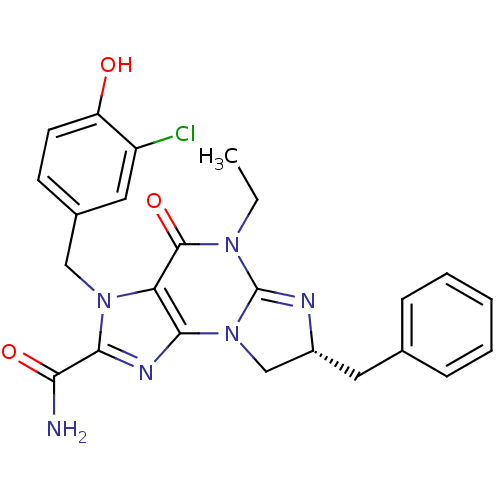
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50140623
((R)-3-(3-chloro-4-hydroxybenzyl)-7-benzyl-5-ethyl-...)Show SMILES CCN1C2=N[C@H](Cc3ccccc3)CN2c2nc(C(N)=O)n(Cc3ccc(O)c(Cl)c3)c2C1=O |r,t:3| Show InChI InChI=1S/C24H23ClN6O3/c1-2-29-23(34)19-21(31-13-16(27-24(29)31)10-14-6-4-3-5-7-14)28-22(20(26)33)30(19)12-15-8-9-18(32)17(25)11-15/h3-9,11,16,32H,2,10,12-13H2,1H3,(H2,26,33)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human phosphodiesterase 5 (PDE5) enzyme |
Bioorg Med Chem Lett 14: 1291-4 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.027
BindingDB Entry DOI: 10.7270/Q2BG2NF0 |
More data for this
Ligand-Target Pair | |
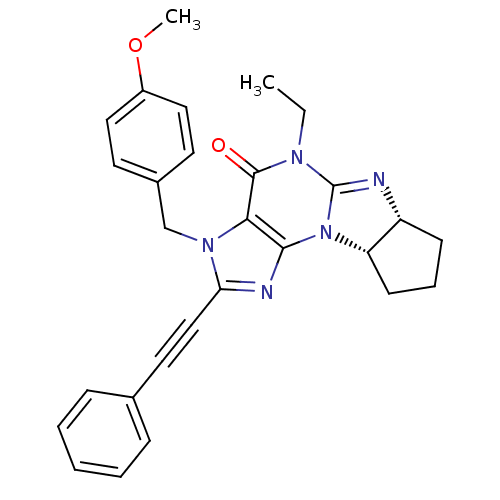
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50140622
(5-ethyl-3-(4-methoxybenzyl)-2-(2-phenyl-1-ethynyl)...)Show SMILES CCN1C2=N[C@@H]3CCC[C@@H]3N2c2nc(C#Cc3ccccc3)n(Cc3ccc(OC)cc3)c2C1=O |r,t:3| Show InChI InChI=1S/C28H27N5O2/c1-3-31-27(34)25-26(33-23-11-7-10-22(23)29-28(31)33)30-24(17-14-19-8-5-4-6-9-19)32(25)18-20-12-15-21(35-2)16-13-20/h4-6,8-9,12-13,15-16,22-23H,3,7,10-11,18H2,1-2H3/t22-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human phosphodiesterase 5 (PDE5) enzyme |
Bioorg Med Chem Lett 14: 1291-4 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.027
BindingDB Entry DOI: 10.7270/Q2BG2NF0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50165773

((R)-7-Benzyl-5-ethyl-3-(4-hydroxy-benzyl)-2-iodo-7...)Show SMILES CCN1C2=N[C@H](Cc3ccccc3)CN2c2nc(I)n(Cc3ccc(O)cc3)c2C1=O |t:3| Show InChI InChI=1S/C23H22IN5O2/c1-2-27-21(31)19-20(26-22(24)28(19)13-16-8-10-18(30)11-9-16)29-14-17(25-23(27)29)12-15-6-4-3-5-7-15/h3-11,17,30H,2,12-14H2,1H3/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human phosphodiesterase 5 |
Bioorg Med Chem Lett 15: 2365-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.083
BindingDB Entry DOI: 10.7270/Q2TM79MQ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined |
Bioorg Med Chem Lett 12: 3149-52 (2002)
BindingDB Entry DOI: 10.7270/Q2S46R83 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human phosphodiesterase 5 (PDE5) enzyme |
Bioorg Med Chem Lett 14: 1291-4 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.027
BindingDB Entry DOI: 10.7270/Q2BG2NF0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human phosphodiesterase 5 |
Bioorg Med Chem Lett 15: 2365-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.083
BindingDB Entry DOI: 10.7270/Q2TM79MQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
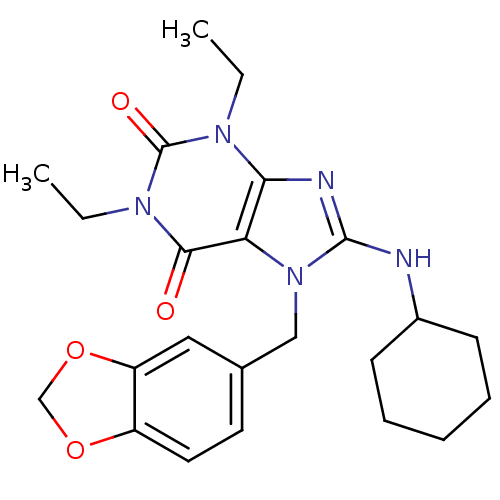
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50120075
(7-Benzo[1,3]dioxol-5-ylmethyl-8-cyclohexylamino-1,...)Show SMILES CCn1c2nc(NC3CCCCC3)n(Cc3ccc4OCOc4c3)c2c(=O)n(CC)c1=O Show InChI InChI=1S/C23H29N5O4/c1-3-26-20-19(21(29)27(4-2)23(26)30)28(22(25-20)24-16-8-6-5-7-9-16)13-15-10-11-17-18(12-15)32-14-31-17/h10-12,16H,3-9,13-14H2,1-2H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined |
Bioorg Med Chem Lett 12: 3149-52 (2002)
BindingDB Entry DOI: 10.7270/Q2S46R83 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50140587

(2-benzyl-5-ethyl-3-(4-hydroxybenzyl)-(6aR,9aS)-3,4...)Show SMILES CCN1C2=N[C@@H]3CCC[C@@H]3N2c2nc(Cc3ccccc3)n(Cc3ccc(O)cc3)c2C1=O |r,t:3| Show InChI InChI=1S/C26H27N5O2/c1-2-29-25(33)23-24(31-21-10-6-9-20(21)27-26(29)31)28-22(15-17-7-4-3-5-8-17)30(23)16-18-11-13-19(32)14-12-18/h3-5,7-8,11-14,20-21,32H,2,6,9-10,15-16H2,1H3/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human phosphodiesterase 5 (PDE5) enzyme |
Bioorg Med Chem Lett 14: 1291-4 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.027
BindingDB Entry DOI: 10.7270/Q2BG2NF0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14777

((2R,8R)-2-(2H-1,3-benzodioxol-5-yl)-6-methyl-3,6,1...)Show SMILES [H][C@]12Cc3c([nH]c4ccccc34)[C@H](N1C(=O)CN(C)C2=O)c1ccc2OCOc2c1 |r| Show InChI InChI=1S/C22H19N3O4/c1-24-10-19(26)25-16(22(24)27)9-14-13-4-2-3-5-15(13)23-20(14)21(25)12-6-7-17-18(8-12)29-11-28-17/h2-8,16,21,23H,9-11H2,1H3/t16-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human phosphodiesterase 5 |
Bioorg Med Chem Lett 15: 2365-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.083
BindingDB Entry DOI: 10.7270/Q2TM79MQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
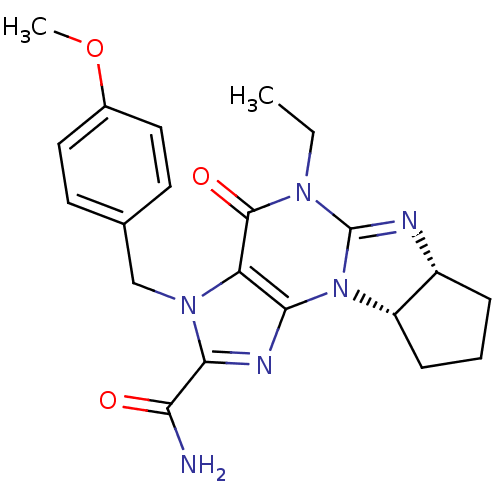
(Homo sapiens (Human)) | BDBM50140617
(5-ethyl-3-(4-methoxybenzyl)-4-oxo-(6aR,9aS)-3,4,5,...)Show SMILES CCN1C2=N[C@@H]3CCC[C@@H]3N2c2nc(C(N)=O)n(Cc3ccc(OC)cc3)c2C1=O |r,t:3| Show InChI InChI=1S/C21H24N6O3/c1-3-25-20(29)16-18(27-15-6-4-5-14(15)23-21(25)27)24-19(17(22)28)26(16)11-12-7-9-13(30-2)10-8-12/h7-10,14-15H,3-6,11H2,1-2H3,(H2,22,28)/t14-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human phosphodiesterase 5 (PDE5) enzyme |
Bioorg Med Chem Lett 14: 1291-4 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.027
BindingDB Entry DOI: 10.7270/Q2BG2NF0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50140595
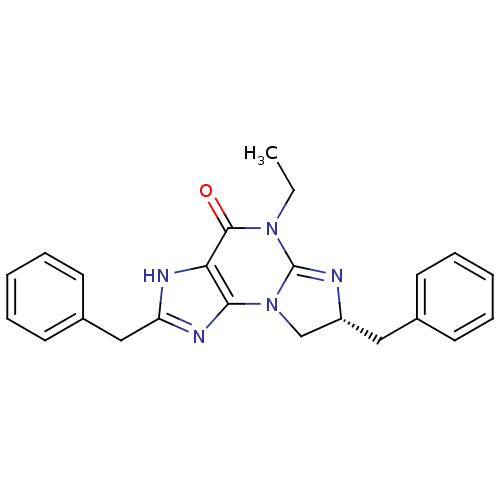
((R)-2,7-Dibenzyl-5-ethyl-7,8-dihydro-1H,5H-imidazo...)Show SMILES CCN1C2=N[C@H](Cc3ccccc3)CN2c2nc(Cc3ccccc3)[nH]c2C1=O |t:3| Show InChI InChI=1S/C23H23N5O/c1-2-27-22(29)20-21(26-19(25-20)14-17-11-7-4-8-12-17)28-15-18(24-23(27)28)13-16-9-5-3-6-10-16/h3-12,18H,2,13-15H2,1H3,(H,25,26)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human phosphodiesterase 5 (PDE5) enzyme |
Bioorg Med Chem Lett 14: 1291-4 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.027
BindingDB Entry DOI: 10.7270/Q2BG2NF0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
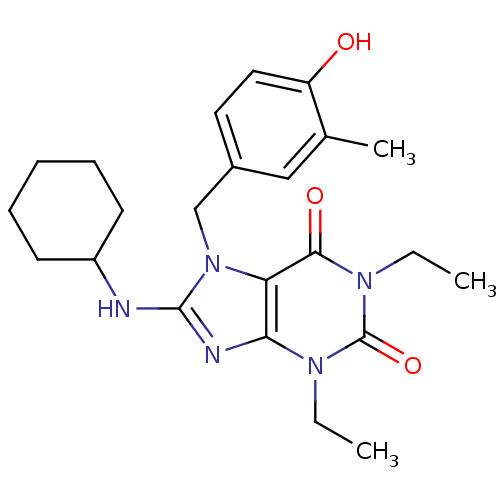
(Homo sapiens (Human)) | BDBM50120076
(8-Cyclohexylamino-1,3-diethyl-7-(4-hydroxy-3-methy...)Show SMILES CCn1c2nc(NC3CCCCC3)n(Cc3ccc(O)c(C)c3)c2c(=O)n(CC)c1=O Show InChI InChI=1S/C23H31N5O3/c1-4-26-20-19(21(30)27(5-2)23(26)31)28(14-16-11-12-18(29)15(3)13-16)22(25-20)24-17-9-7-6-8-10-17/h11-13,17,29H,4-10,14H2,1-3H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined |
Bioorg Med Chem Lett 12: 3149-52 (2002)
BindingDB Entry DOI: 10.7270/Q2S46R83 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50140595

((R)-2,7-Dibenzyl-5-ethyl-7,8-dihydro-1H,5H-imidazo...)Show SMILES CCN1C2=N[C@H](Cc3ccccc3)CN2c2nc(Cc3ccccc3)[nH]c2C1=O |t:3| Show InChI InChI=1S/C23H23N5O/c1-2-27-22(29)20-21(26-19(25-20)14-17-11-7-4-8-12-17)28-15-18(24-23(27)28)13-16-9-5-3-6-10-16/h3-12,18H,2,13-15H2,1H3,(H,25,26)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human phosphodiesterase 5 |
Bioorg Med Chem Lett 15: 2365-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.083
BindingDB Entry DOI: 10.7270/Q2TM79MQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data