

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 11-beta-hydroxysteroid dehydrogenase type 2 (Rattus norvegicus) | BDBM50147505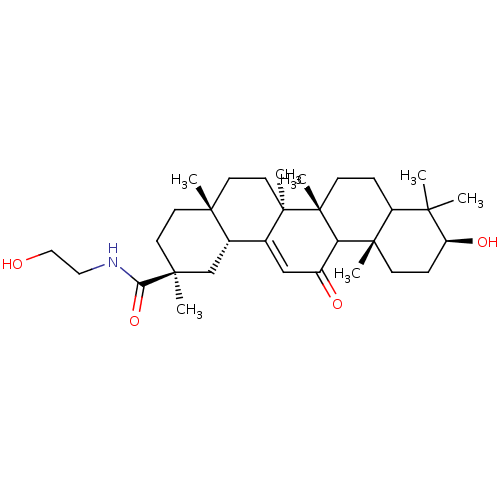 ((2S,4aS,6aS,6bR,10S,12aS,14bR)-10-Hydroxy-2,4a,6a,...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Percent inhibition against 11-beta-hydroxysteroid dehydrogenase 2 of wistar rat kidney at 10 microM was determined using [3H]-cortisol | Bioorg Med Chem Lett 14: 3263-7 (2004) Article DOI: 10.1016/j.bmcl.2004.03.107 BindingDB Entry DOI: 10.7270/Q2H41QWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3',5'-cyclic-AMP phosphodiesterase (Sus scrofa) | BDBM50041849 (3-(Bicyclo[2.2.1]hept-2-yloxy)-N-(3,5-dichloro-pyr...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Inhibitory potency against pig aortic PDE IV | J Med Chem 37: 1696-703 (1994) BindingDB Entry DOI: 10.7270/Q2CF9P5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3',5'-cyclic-AMP phosphodiesterase (Sus scrofa) | BDBM14775 (3-(cyclopentyloxy)-N-(3,5-dichloropyridin-4-yl)-4-...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Inhibitory potency against pig aortic PDE IV | J Med Chem 37: 1696-703 (1994) BindingDB Entry DOI: 10.7270/Q2CF9P5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3',5'-cyclic-AMP phosphodiesterase (Sus scrofa) | BDBM50041849 (3-(Bicyclo[2.2.1]hept-2-yloxy)-N-(3,5-dichloro-pyr...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Inhibitory potency against pig aortic PDE IV | J Med Chem 37: 1696-703 (1994) BindingDB Entry DOI: 10.7270/Q2CF9P5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3',5'-cyclic-AMP phosphodiesterase (Sus scrofa) | BDBM50041853 (3-Cyclopentyloxy-N-(3,5-dichloro-1-oxy-pyridin-4-y...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Inhibitory potency against pig aortic PDE IV | J Med Chem 37: 1696-703 (1994) BindingDB Entry DOI: 10.7270/Q2CF9P5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3',5'-cyclic-AMP phosphodiesterase (Sus scrofa) | BDBM50041831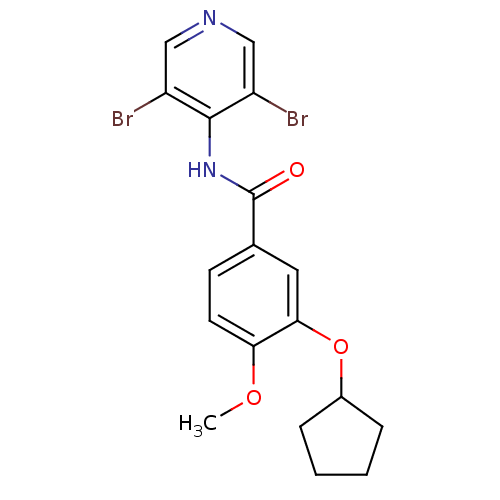 (3-Cyclopentyloxy-N-(3,5-dibromo-pyridin-4-yl)-4-me...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Inhibitory potency against pig aortic PDE IV | J Med Chem 37: 1696-703 (1994) BindingDB Entry DOI: 10.7270/Q2CF9P5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM13058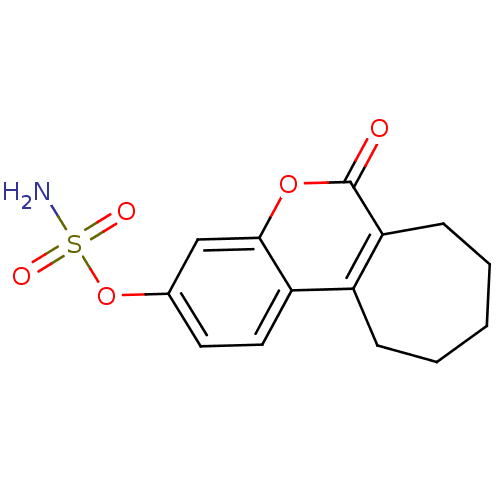 (6-oxo-6,7,8,9,10,11-hexahydrocyclohepta[c]chromen-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Maryamppound39s Hospital | Assay Description The extent of in vitro inhibition of sulfatase activities was assessed using intact monolayers of JEG-3 cells. Sulfatase activity was measured using ... | Biochem Biophys Res Commun 305: 909-14 (2003) Article DOI: 10.1016/s0006-291x(03)00865-9 BindingDB Entry DOI: 10.7270/Q2TD9VKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3',5'-cyclic-AMP phosphodiesterase (Sus scrofa) | BDBM50041846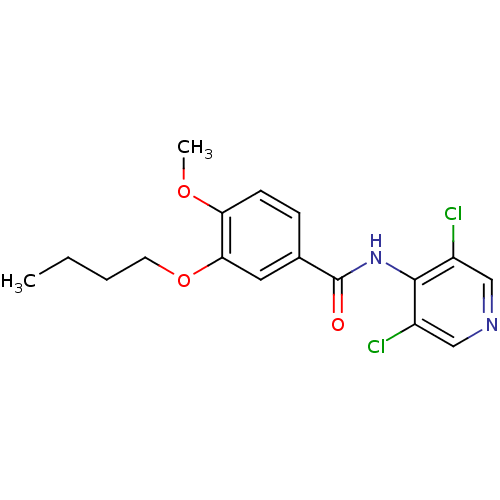 (3-Butoxy-N-(3,5-dichloro-pyridin-4-yl)-4-methoxy-b...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Inhibitory potency against pig aortic PDE IV | J Med Chem 37: 1696-703 (1994) BindingDB Entry DOI: 10.7270/Q2CF9P5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50134329 (CHEMBL122708 | Sulfamic acid (11R,12S,15S,16S)-13-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Compound was tested for inhibition of human carbonic anhydrase (hCA II) | Bioorg Med Chem Lett 13: 863-5 (2003) BindingDB Entry DOI: 10.7270/Q2319WFW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| 3',5'-cyclic-AMP phosphodiesterase (Sus scrofa) | BDBM50041886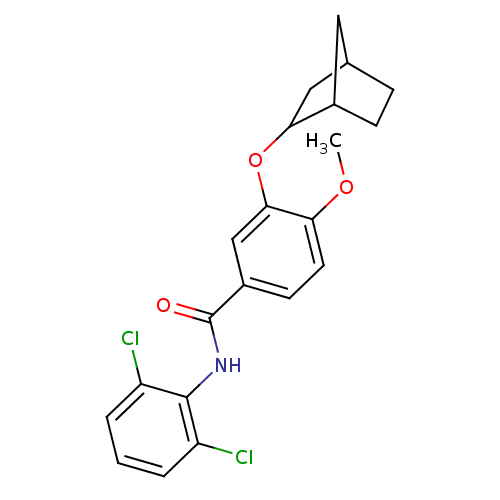 (3-(Bicyclo[2.2.1]hept-2-yloxy)-N-(2,6-dichloro-phe...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Inhibitory potency against pig aortic PDE IV | J Med Chem 37: 1696-703 (1994) BindingDB Entry DOI: 10.7270/Q2CF9P5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Compound was tested for inhibition of human carbonic anhydrase (hCA II) | Bioorg Med Chem Lett 13: 863-5 (2003) BindingDB Entry DOI: 10.7270/Q2319WFW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM13423 (CHEMBL167368 | STX 118 | [3-(2-cyclohexylethyl)-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Compound was tested for inhibition of human carbonic anhydrase (hCA II) | Bioorg Med Chem Lett 13: 863-5 (2003) BindingDB Entry DOI: 10.7270/Q2319WFW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| 3',5'-cyclic-AMP phosphodiesterase (Sus scrofa) | BDBM50041847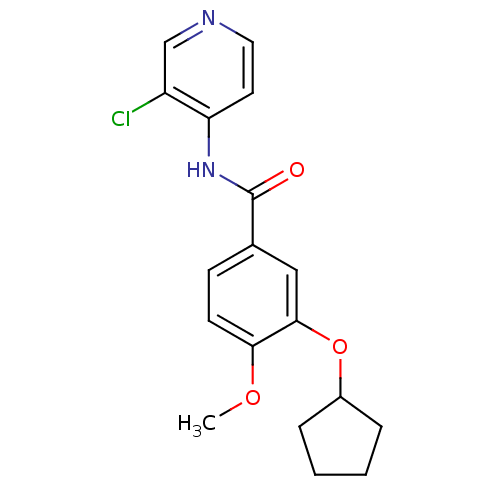 (CHEMBL295976 | N-(3-Chloro-pyridin-4-yl)-3-cyclope...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Inhibitory potency against pig aortic PDE IV | J Med Chem 37: 1696-703 (1994) BindingDB Entry DOI: 10.7270/Q2CF9P5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM13058 (6-oxo-6,7,8,9,10,11-hexahydrocyclohepta[c]chromen-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Compound was tested for inhibition of human carbonic anhydrase (hCA II) | Bioorg Med Chem Lett 13: 863-5 (2003) BindingDB Entry DOI: 10.7270/Q2319WFW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 3',5'-cyclic-AMP phosphodiesterase (Sus scrofa) | BDBM50041875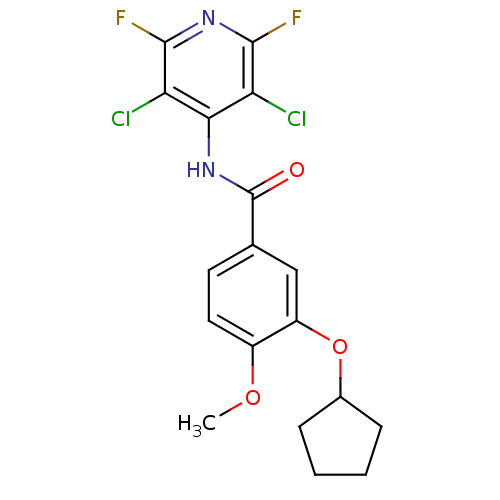 (3-Cyclopentyloxy-N-(3,5-dichloro-2,6-difluoro-pyri...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Inhibitory potency against pig aortic PDE IV | J Med Chem 37: 1696-703 (1994) BindingDB Entry DOI: 10.7270/Q2CF9P5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM11637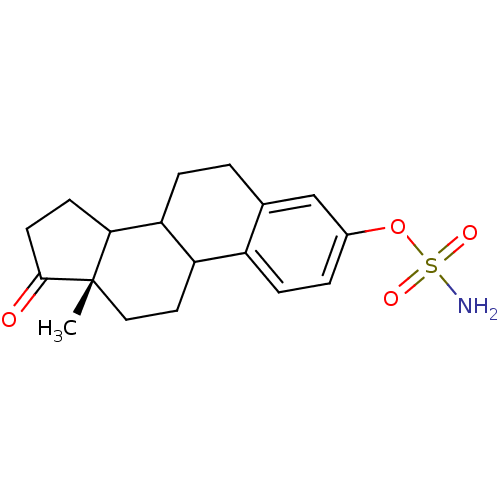 ((15S)-15-methyl-14-oxotetracyclo[8.7.0.0^{2,7}.0^{...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Maryamppound39s Hospital | Assay Description The extent of in vitro inhibition of sulfatase activities was assessed using intact monolayers of JEG-3 cells. Sulfatase activity was measured using ... | Biochem Biophys Res Commun 305: 909-14 (2003) Article DOI: 10.1016/s0006-291x(03)00865-9 BindingDB Entry DOI: 10.7270/Q2TD9VKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3',5'-cyclic-AMP phosphodiesterase (Sus scrofa) | BDBM50041838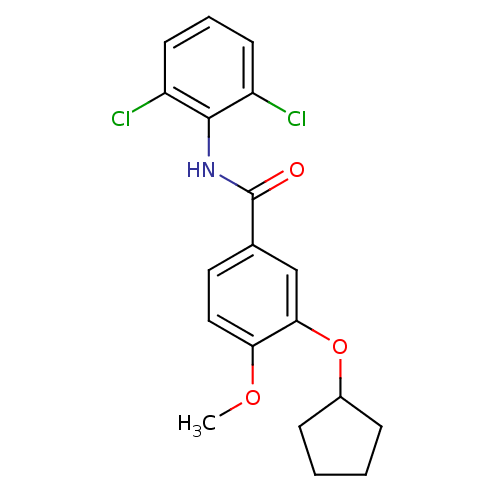 (3-(cyclopentyloxy)-N-(2,6-dichlorophenyl)-4-methox...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Inhibitory potency against pig aortic PDE IV | J Med Chem 37: 1696-703 (1994) BindingDB Entry DOI: 10.7270/Q2CF9P5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | 7.8 | 20 |
St. Maryamppound39s Hospital | Assay Description The in vitro inhibition of carbonic anhydrase was assessed by a colorimetric assay. Carbonic anhydrase-catalysed hydrolysis of p-nitrophenyl acetate ... | Biochem Biophys Res Commun 305: 909-14 (2003) Article DOI: 10.1016/s0006-291x(03)00865-9 BindingDB Entry DOI: 10.7270/Q2TD9VKK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM13058 (6-oxo-6,7,8,9,10,11-hexahydrocyclohepta[c]chromen-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | 7.8 | 20 |
St. Maryamppound39s Hospital | Assay Description The in vitro inhibition of carbonic anhydrase was assessed by a colorimetric assay. Carbonic anhydrase-catalysed hydrolysis of p-nitrophenyl acetate ... | Biochem Biophys Res Commun 305: 909-14 (2003) Article DOI: 10.1016/s0006-291x(03)00865-9 BindingDB Entry DOI: 10.7270/Q2TD9VKK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50237104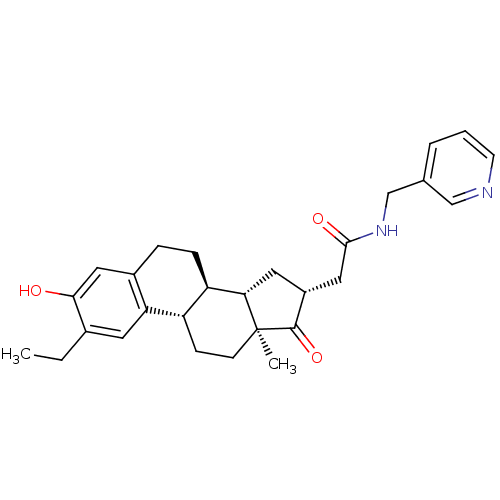 (2-((8R,9S,13S,14S,16R)-2-ethyl-3-hydroxy-13-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibitory activity against 17 beta hydroxysteroid dehydrogenase type 1 in T47D cells | J Med Chem 48: 2759-62 (2005) Article DOI: 10.1021/jm049045r BindingDB Entry DOI: 10.7270/Q2BG2PSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50237104 (2-((8R,9S,13S,14S,16R)-2-ethyl-3-hydroxy-13-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD1 expressed in intact human T47D cells after 30 mins | Bioorg Med Chem 16: 4438-56 (2008) Article DOI: 10.1016/j.bmc.2008.02.059 BindingDB Entry DOI: 10.7270/Q20002Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM13424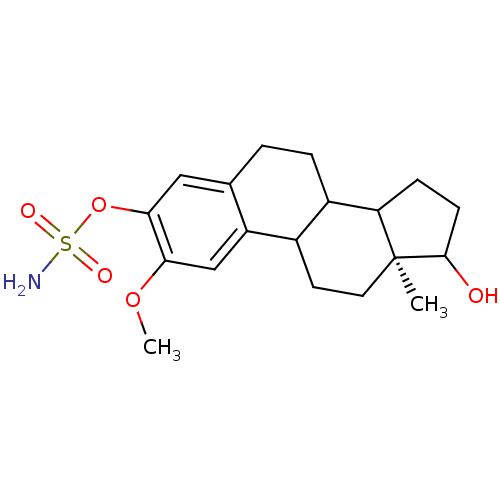 ((15S)-14-hydroxy-4-methoxy-15-methyltetracyclo[8.7...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Maryamppound39s Hospital | Assay Description The extent of in vitro inhibition of sulfatase activities was assessed using intact monolayers of JEG-3 cells. Sulfatase activity was measured using ... | Biochem Biophys Res Commun 305: 909-14 (2003) Article DOI: 10.1016/s0006-291x(03)00865-9 BindingDB Entry DOI: 10.7270/Q2TD9VKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3',5'-cyclic-AMP phosphodiesterase (Sus scrofa) | BDBM50041869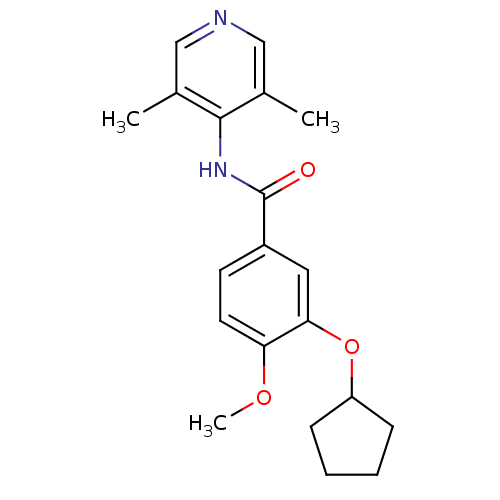 (3-Cyclopentyloxy-N-(3,5-dimethyl-pyridin-4-yl)-4-m...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Inhibitory potency against pig aortic PDE IV | J Med Chem 37: 1696-703 (1994) BindingDB Entry DOI: 10.7270/Q2CF9P5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM13057 (CHEMBL417656 | Compound 9 | Cyclic Sulfate Analogu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Compound was tested for inhibition of human carbonic anhydrase (hCA II) | Bioorg Med Chem Lett 13: 863-5 (2003) BindingDB Entry DOI: 10.7270/Q2319WFW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50370656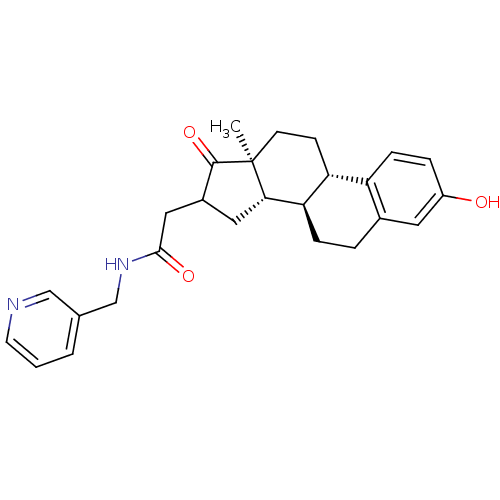 (CHEMBL1627749) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibitory activity against 17 beta hydroxysteroid dehydrogenase type 1 in T47D cells | J Med Chem 48: 2759-62 (2005) Article DOI: 10.1021/jm049045r BindingDB Entry DOI: 10.7270/Q2BG2PSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50370656 (CHEMBL1627749) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of 17-beta HSD1 in T47D cells | J Med Chem 49: 1325-45 (2006) Article DOI: 10.1021/jm050830t BindingDB Entry DOI: 10.7270/Q2C24X7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM13426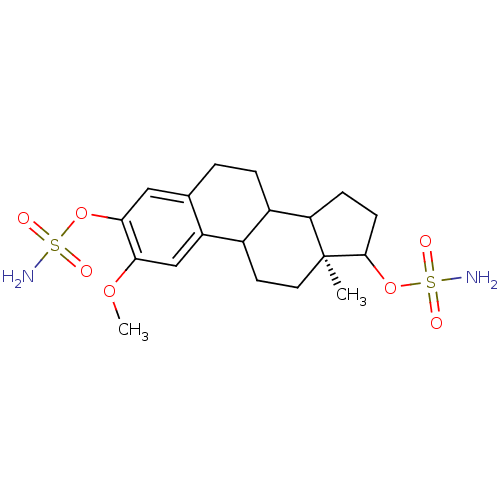 ((15S)-4-methoxy-15-methyl-5-(sulfamoyloxy)tetracyc...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Maryamppound39s Hospital | Assay Description The extent of in vitro inhibition of sulfatase activities was assessed using intact monolayers of JEG-3 cells. Sulfatase activity was measured using ... | Biochem Biophys Res Commun 305: 909-14 (2003) Article DOI: 10.1016/s0006-291x(03)00865-9 BindingDB Entry DOI: 10.7270/Q2TD9VKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM11637 ((15S)-15-methyl-14-oxotetracyclo[8.7.0.0^{2,7}.0^{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | 7.8 | 20 |
St. Maryamppound39s Hospital | Assay Description The in vitro inhibition of carbonic anhydrase was assessed by a colorimetric assay. Carbonic anhydrase-catalysed hydrolysis of p-nitrophenyl acetate ... | Biochem Biophys Res Commun 305: 909-14 (2003) Article DOI: 10.1016/s0006-291x(03)00865-9 BindingDB Entry DOI: 10.7270/Q2TD9VKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance-associated protein 1 (Homo sapiens (Human)) | BDBM50140820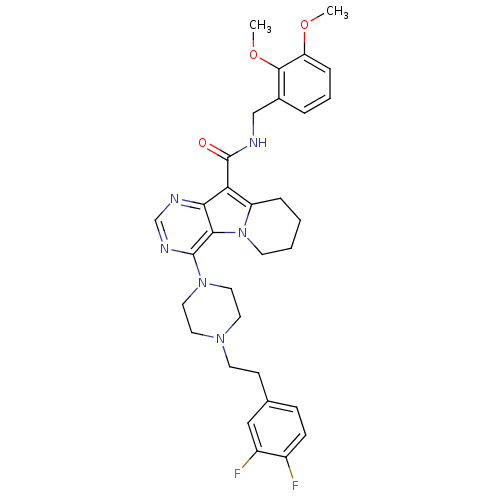 (4-{4-[2-(3,4-Difluoro-phenyl)-ethyl]-piperazin-1-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenova Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human transporter MRP1 (Multidrug resistance associated protein 1) expressed in COR.L23/R cell line | J Med Chem 47: 1329-38 (2004) Article DOI: 10.1021/jm031011g BindingDB Entry DOI: 10.7270/Q25Q4VH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM13423 (CHEMBL167368 | STX 118 | [3-(2-cyclohexylethyl)-4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Maryamppound39s Hospital | Assay Description The extent of in vitro inhibition of sulfatase activities was assessed using intact monolayers of JEG-3 cells. Sulfatase activity was measured using ... | Biochem Biophys Res Commun 305: 909-14 (2003) Article DOI: 10.1016/s0006-291x(03)00865-9 BindingDB Entry DOI: 10.7270/Q2TD9VKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3',5'-cyclic-AMP phosphodiesterase (Sus scrofa) | BDBM50041824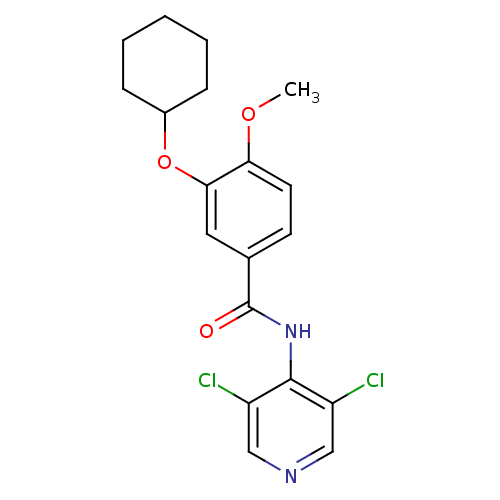 (3-Cyclohexyloxy-N-(3,5-dichloro-pyridin-4-yl)-4-me...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Inhibitory potency against pig aortic PDE IV | J Med Chem 37: 1696-703 (1994) BindingDB Entry DOI: 10.7270/Q2CF9P5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM13423 (CHEMBL167368 | STX 118 | [3-(2-cyclohexylethyl)-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | 7.8 | 20 |
St. Maryamppound39s Hospital | Assay Description The in vitro inhibition of carbonic anhydrase was assessed by a colorimetric assay. Carbonic anhydrase-catalysed hydrolysis of p-nitrophenyl acetate ... | Biochem Biophys Res Commun 305: 909-14 (2003) Article DOI: 10.1016/s0006-291x(03)00865-9 BindingDB Entry DOI: 10.7270/Q2TD9VKK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Multidrug resistance-associated protein 1 (Homo sapiens (Human)) | BDBM50140825 (4-{4-[2-(3,4-Difluoro-phenyl)-ethyl]-piperazin-1-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenova Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human transporter MRP1 (Multidrug resistance associated protein 1) expressed in COR.L23/R cell line | J Med Chem 47: 1329-38 (2004) Article DOI: 10.1021/jm031011g BindingDB Entry DOI: 10.7270/Q25Q4VH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3',5'-cyclic-AMP phosphodiesterase (Sus scrofa) | BDBM50041879 (3-Butoxy-N-(3,5-dibromo-pyridin-4-yl)-4-methoxy-be...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Inhibitory potency against pig aortic PDE IV | J Med Chem 37: 1696-703 (1994) BindingDB Entry DOI: 10.7270/Q2CF9P5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3',5'-cyclic-AMP phosphodiesterase (Sus scrofa) | BDBM50041844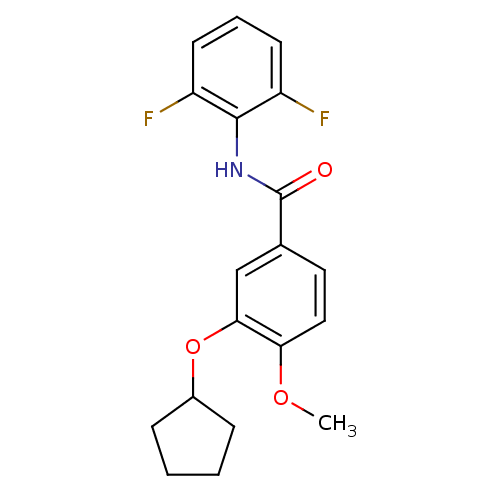 (3-(cyclopentyloxy)-N-(2,6-difluorophenyl)-4-methox...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Inhibitory potency against pig aortic PDE IV | J Med Chem 37: 1696-703 (1994) BindingDB Entry DOI: 10.7270/Q2CF9P5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance-associated protein 1 (Homo sapiens (Human)) | BDBM50140803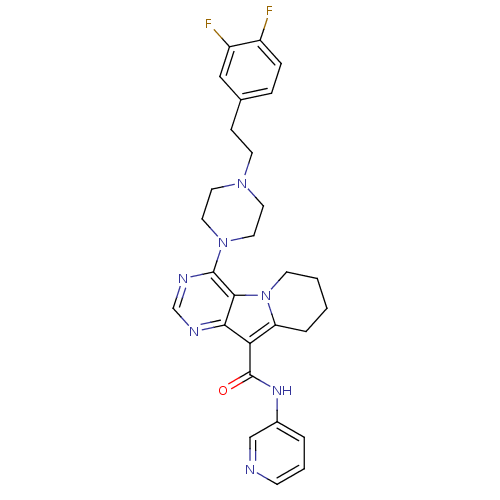 (4-{4-[2-(3,4-Difluoro-phenyl)-ethyl]-piperazin-1-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenova Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human transporter MRP1 (Multidrug resistance associated protein 1) expressed in COR.L23/R cell line | J Med Chem 47: 1329-38 (2004) Article DOI: 10.1021/jm031011g BindingDB Entry DOI: 10.7270/Q25Q4VH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3',5'-cyclic-AMP phosphodiesterase (Sus scrofa) | BDBM50041877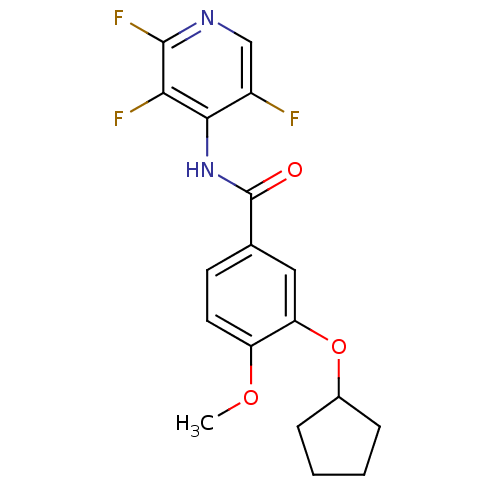 (3-Cyclopentyloxy-4-methoxy-N-(2,3,5-trifluoro-pyri...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Inhibitory potency against pig aortic PDE IV | J Med Chem 37: 1696-703 (1994) BindingDB Entry DOI: 10.7270/Q2CF9P5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM13419 ((15S)-15-methyl-14-oxotetracyclo[8.7.0.0^{2,7}.0^{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | 7.8 | 20 |
St. Maryamppound39s Hospital | Assay Description The in vitro inhibition of carbonic anhydrase was assessed by a colorimetric assay. Carbonic anhydrase-catalysed hydrolysis of p-nitrophenyl acetate ... | Biochem Biophys Res Commun 305: 909-14 (2003) Article DOI: 10.1016/s0006-291x(03)00865-9 BindingDB Entry DOI: 10.7270/Q2TD9VKK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| 3',5'-cyclic-AMP phosphodiesterase (Sus scrofa) | BDBM50041854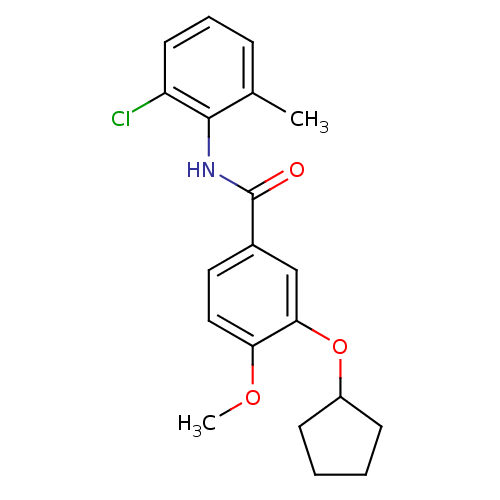 (CHEMBL294949 | N-(2-Chloro-6-methyl-phenyl)-3-cycl...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Inhibitory potency against pig aortic PDE IV | J Med Chem 37: 1696-703 (1994) BindingDB Entry DOI: 10.7270/Q2CF9P5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3',5'-cyclic-AMP phosphodiesterase (Sus scrofa) | BDBM50041826 (3-Cyclopentyloxy-N-(2,6-dimethyl-phenyl)-4-methoxy...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Inhibitory potency against pig aortic PDE IV | J Med Chem 37: 1696-703 (1994) BindingDB Entry DOI: 10.7270/Q2CF9P5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3',5'-cyclic-AMP phosphodiesterase (Sus scrofa) | BDBM50041873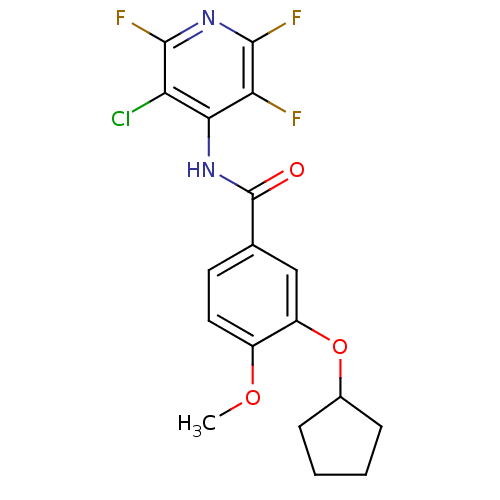 (CHEMBL47286 | N-(3-Chloro-2,5,6-trifluoro-pyridin-...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Inhibitory potency against pig aortic PDE IV | J Med Chem 37: 1696-703 (1994) BindingDB Entry DOI: 10.7270/Q2CF9P5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3',5'-cyclic-AMP phosphodiesterase (Sus scrofa) | BDBM50041866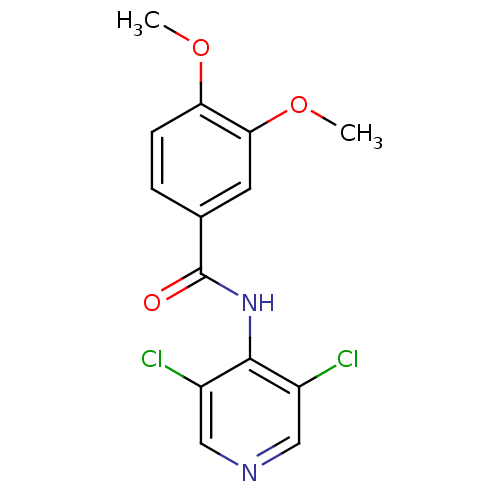 (CHEMBL48017 | N-(3,5-Dichloro-pyridin-4-yl)-3,4-di...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Inhibitory potency against pig aortic PDE IV | J Med Chem 37: 1696-703 (1994) BindingDB Entry DOI: 10.7270/Q2CF9P5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance-associated protein 1 (Homo sapiens (Human)) | BDBM50140804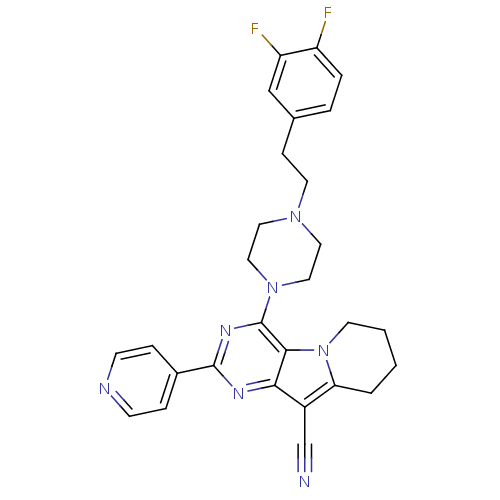 (4-{4-[2-(3,4-Difluoro-phenyl)-ethyl]-piperazin-1-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenova Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human transporter MRP1 (Multidrug resistance associated protein 1) expressed in COR.L23/R cell line | J Med Chem 47: 1329-38 (2004) Article DOI: 10.1021/jm031011g BindingDB Entry DOI: 10.7270/Q25Q4VH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50172493 ((13S)-3-hydroxy-16-(hydroxymethylene)-13-methyl-7,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estrone | J Med Chem 48: 5749-70 (2005) Article DOI: 10.1021/jm050348a BindingDB Entry DOI: 10.7270/Q2QJ7J38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50172493 ((13S)-3-hydroxy-16-(hydroxymethylene)-13-methyl-7,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of 17-beta HSD1 in T47D cells | J Med Chem 49: 1325-45 (2006) Article DOI: 10.1021/jm050830t BindingDB Entry DOI: 10.7270/Q2C24X7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50394437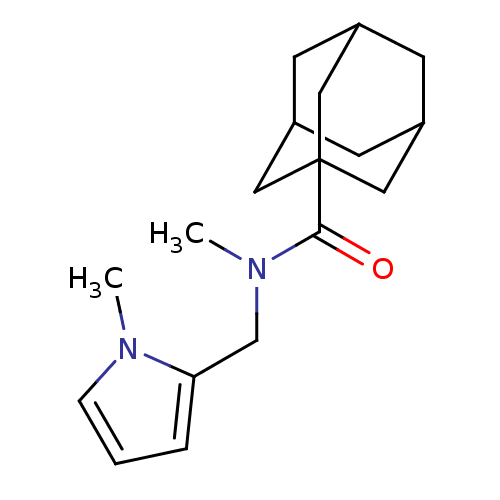 (CHEMBL2159084) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in HEK293 cells assessed as conversion of [3H]cortisone to [3H]cortisol after 2 hrs by scintillation counti... | Bioorg Med Chem 20: 6394-402 (2012) Article DOI: 10.1016/j.bmc.2012.08.056 BindingDB Entry DOI: 10.7270/Q27D2W81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50394435 (CHEMBL2159638) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 118 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in HEK293 cells assessed as conversion of [3H]cortisone to [3H]cortisol after 2 hrs by scintillation counti... | Bioorg Med Chem 20: 6394-402 (2012) Article DOI: 10.1016/j.bmc.2012.08.056 BindingDB Entry DOI: 10.7270/Q27D2W81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3',5'-cyclic-AMP phosphodiesterase (Sus scrofa) | BDBM50041856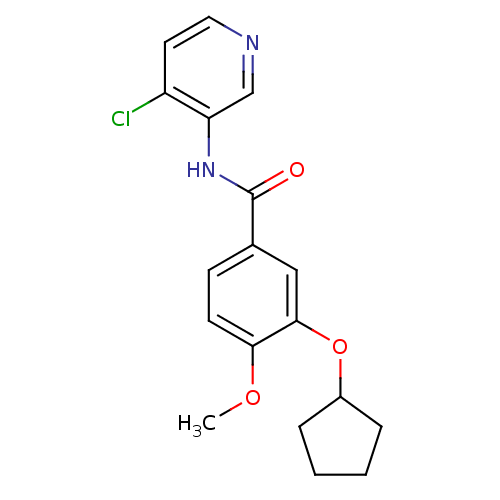 (CHEMBL48048 | N-(4-Chloro-pyridin-3-yl)-3-cyclopen...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Inhibitory potency against pig aortic PDE IV | J Med Chem 37: 1696-703 (1994) BindingDB Entry DOI: 10.7270/Q2CF9P5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance-associated protein 1 (Homo sapiens (Human)) | BDBM50140798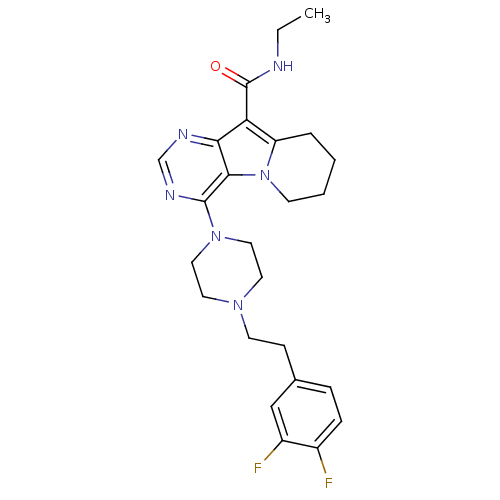 (4-{4-[2-(3,4-Difluoro-phenyl)-ethyl]-piperazin-1-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenova Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human transporter MRP1 (Multidrug resistance associated protein 1) expressed in COR.L23/R cell line | J Med Chem 47: 1329-38 (2004) Article DOI: 10.1021/jm031011g BindingDB Entry DOI: 10.7270/Q25Q4VH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50394439 (CHEMBL2159082) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in HEK293 cells assessed as conversion of [3H]cortisone to [3H]cortisol after 2 hrs by scintillation counti... | Bioorg Med Chem 20: 6394-402 (2012) Article DOI: 10.1016/j.bmc.2012.08.056 BindingDB Entry DOI: 10.7270/Q27D2W81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 366 total ) | Next | Last >> |