 Found 232 hits with Last Name = 'voliva' and Initial = 'c'
Found 232 hits with Last Name = 'voliva' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Collagenase 3
(Homo sapiens (Human)) | BDBM50076589

(3-(4-Phenoxy-benzenesulfonyl)-propane-1-thiol | CH...)Show InChI InChI=1S/C15H16O3S2/c16-20(17,12-4-11-19)15-9-7-14(8-10-15)18-13-5-2-1-3-6-13/h1-3,5-10,19H,4,11-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-13 (MMP-13) |
Bioorg Med Chem Lett 9: 943-8 (1999)
BindingDB Entry DOI: 10.7270/Q2B8578V |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50076600

(3-(4-Methoxy-benzenesulfonyl)-4-phenyl-butane-1-th...)Show InChI InChI=1S/C17H20O3S2/c1-20-15-7-9-16(10-8-15)22(18,19)17(11-12-21)13-14-5-3-2-4-6-14/h2-10,17,21H,11-13H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-13 (MMP-13) |
Bioorg Med Chem Lett 9: 943-8 (1999)
BindingDB Entry DOI: 10.7270/Q2B8578V |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50112349
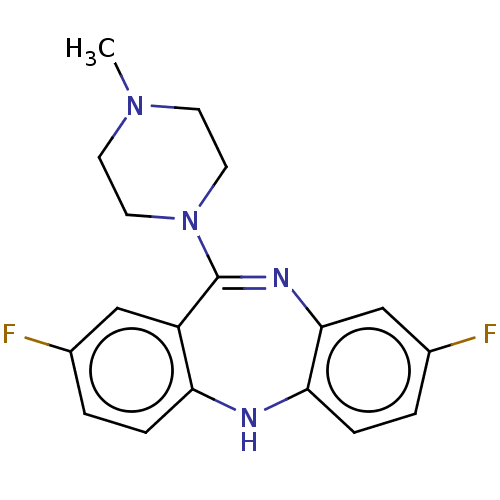
(CHEMBL3609328)Show SMILES CN1CCN(CC1)C1=Nc2cc(F)ccc2Nc2ccc(F)cc12 |t:8| Show InChI InChI=1S/C18H18F2N4/c1-23-6-8-24(9-7-23)18-14-10-12(19)2-4-15(14)21-16-5-3-13(20)11-17(16)22-18/h2-5,10-11,21H,6-9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human histamine H1 receptor |
ACS Med Chem Lett 6: 776-81 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00102
BindingDB Entry DOI: 10.7270/Q25X2BQS |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50076593
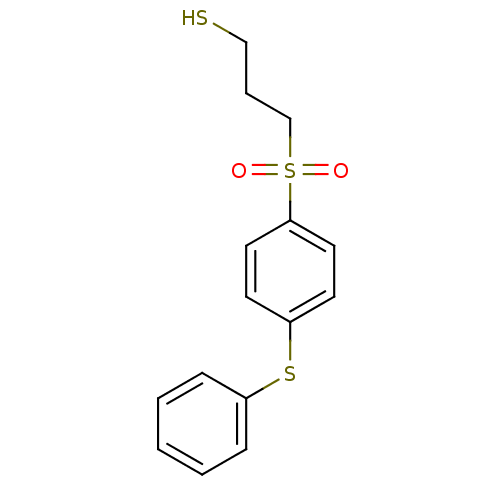
(3-(4-Phenylsulfanyl-benzenesulfonyl)-propane-1-thi...)Show InChI InChI=1S/C15H16O2S3/c16-20(17,12-4-11-18)15-9-7-14(8-10-15)19-13-5-2-1-3-6-13/h1-3,5-10,18H,4,11-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-13 (MMP-13) |
Bioorg Med Chem Lett 9: 943-8 (1999)
BindingDB Entry DOI: 10.7270/Q2B8578V |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50439721
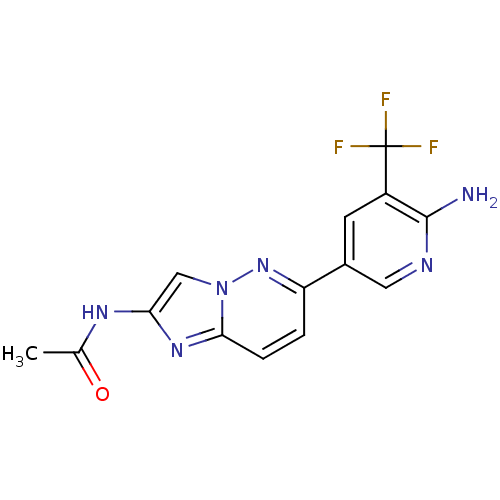
(CHEMBL2418953)Show SMILES CC(=O)Nc1cn2nc(ccc2n1)-c1cnc(N)c(c1)C(F)(F)F Show InChI InChI=1S/C14H11F3N6O/c1-7(24)20-11-6-23-12(21-11)3-2-10(22-23)8-4-9(14(15,16)17)13(18)19-5-8/h2-6H,1H3,(H2,18,19)(H,20,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140266
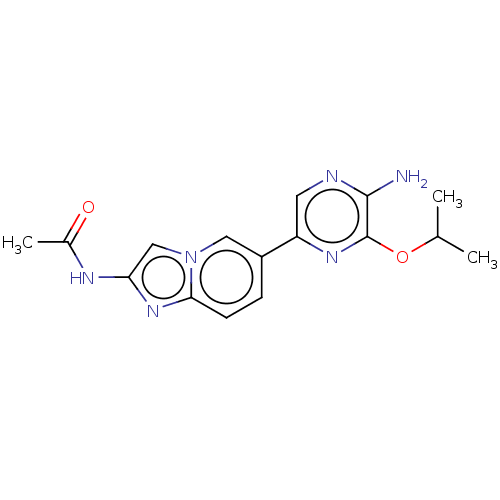
(CHEMBL3753366)Show InChI InChI=1S/C16H18N6O2/c1-9(2)24-16-15(17)18-6-12(20-16)11-4-5-14-21-13(19-10(3)23)8-22(14)7-11/h4-9H,1-3H3,(H2,17,18)(H,19,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50439721

(CHEMBL2418953)Show SMILES CC(=O)Nc1cn2nc(ccc2n1)-c1cnc(N)c(c1)C(F)(F)F Show InChI InChI=1S/C14H11F3N6O/c1-7(24)20-11-6-23-12(21-11)3-2-10(22-23)8-4-9(14(15,16)17)13(18)19-5-8/h2-6H,1H3,(H2,18,19)(H,20,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using [gamma33P]ATP as substrate by top counting analysis |
Bioorg Med Chem Lett 23: 4652-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.010
BindingDB Entry DOI: 10.7270/Q2NP25T0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50380371
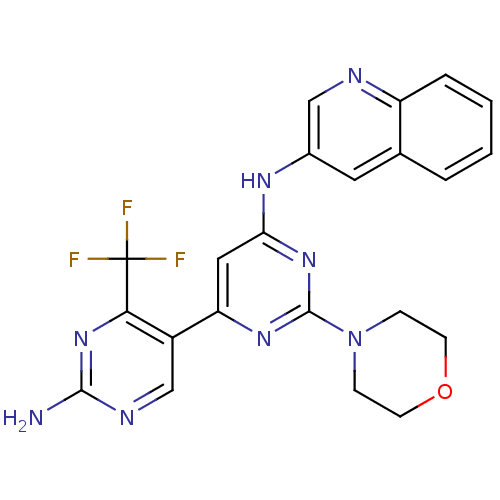
(CHEMBL2017970)Show SMILES Nc1ncc(-c2cc(Nc3cnc4ccccc4c3)nc(n2)N2CCOCC2)c(n1)C(F)(F)F Show InChI InChI=1S/C22H19F3N8O/c23-22(24,25)19-15(12-28-20(26)32-19)17-10-18(31-21(30-17)33-5-7-34-8-6-33)29-14-9-13-3-1-2-4-16(13)27-11-14/h1-4,9-12H,5-8H2,(H2,26,28,32)(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha using 1-alpha-phosphotidylinositol as substrate by ATP depletion assay |
ACS Med Chem Lett 2: 774-779 (2011)
Article DOI: 10.1021/ml200156t
BindingDB Entry DOI: 10.7270/Q2N29XXW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50380366
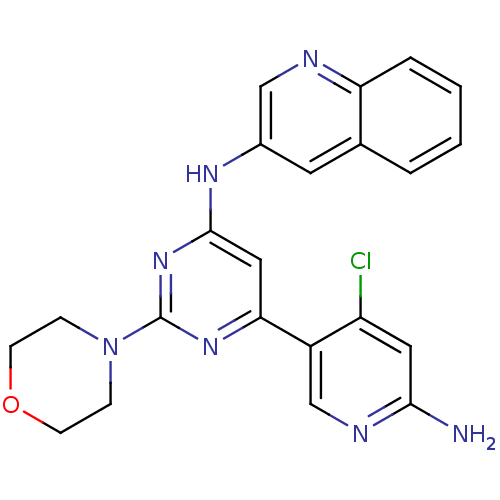
(CHEMBL2017965)Show SMILES Nc1cc(Cl)c(cn1)-c1cc(Nc2cnc3ccccc3c2)nc(n1)N1CCOCC1 Show InChI InChI=1S/C22H20ClN7O/c23-17-10-20(24)26-13-16(17)19-11-21(29-22(28-19)30-5-7-31-8-6-30)27-15-9-14-3-1-2-4-18(14)25-12-15/h1-4,9-13H,5-8H2,(H2,24,26)(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha using 1-alpha-phosphotidylinositol as substrate by ATP depletion assay |
ACS Med Chem Lett 2: 774-779 (2011)
Article DOI: 10.1021/ml200156t
BindingDB Entry DOI: 10.7270/Q2N29XXW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50380369

(CHEMBL2017968)Show SMILES Nc1ncc(c(N)n1)-c1cc(Nc2cnc3ccccc3c2)nc(n1)N1CCOCC1 Show InChI InChI=1S/C21H21N9O/c22-19-15(12-25-20(23)29-19)17-10-18(28-21(27-17)30-5-7-31-8-6-30)26-14-9-13-3-1-2-4-16(13)24-11-14/h1-4,9-12H,5-8H2,(H,26,27,28)(H4,22,23,25,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha using 1-alpha-phosphotidylinositol as substrate by ATP depletion assay |
ACS Med Chem Lett 2: 774-779 (2011)
Article DOI: 10.1021/ml200156t
BindingDB Entry DOI: 10.7270/Q2N29XXW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140269
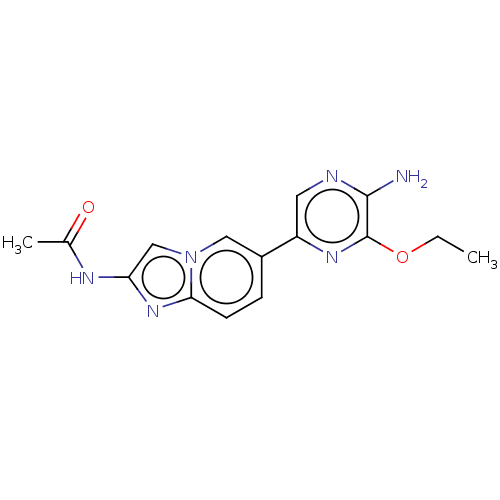
(CHEMBL3752653)Show InChI InChI=1S/C15H16N6O2/c1-3-23-15-14(16)17-6-11(19-15)10-4-5-13-20-12(18-9(2)22)8-21(13)7-10/h4-8H,3H2,1-2H3,(H2,16,17)(H,18,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140267
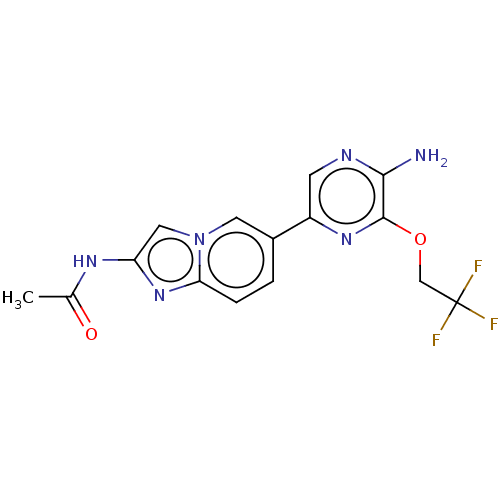
(CHEMBL3752019)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cnc(N)c(OCC(F)(F)F)n1 Show InChI InChI=1S/C15H13F3N6O2/c1-8(25)21-11-6-24-5-9(2-3-12(24)23-11)10-4-20-13(19)14(22-10)26-7-15(16,17)18/h2-6H,7H2,1H3,(H2,19,20)(H,21,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140265
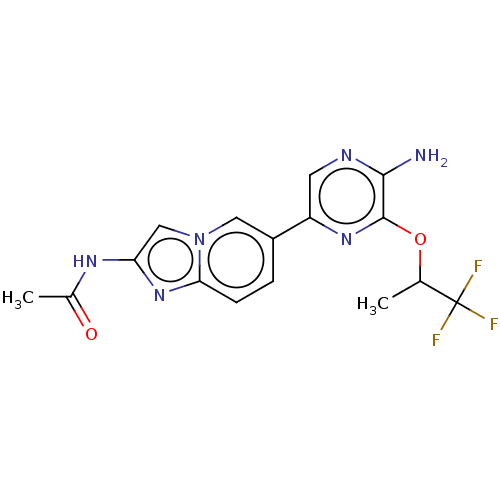
(CHEMBL3753450)Show SMILES CC(Oc1nc(cnc1N)-c1ccc2nc(NC(C)=O)cn2c1)C(F)(F)F Show InChI InChI=1S/C16H15F3N6O2/c1-8(16(17,18)19)27-15-14(20)21-5-11(23-15)10-3-4-13-24-12(22-9(2)26)7-25(13)6-10/h3-8H,1-2H3,(H2,20,21)(H,22,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140270
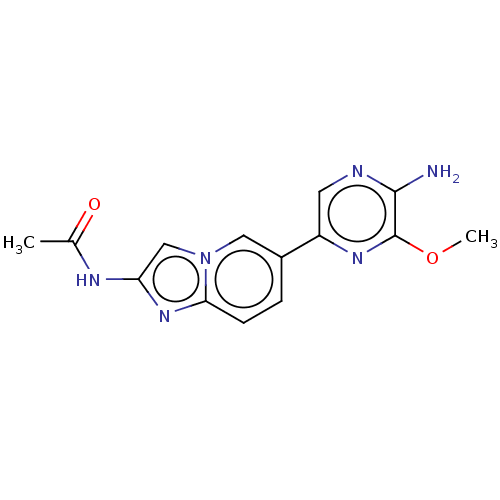
(CHEMBL3752503)Show InChI InChI=1S/C14H14N6O2/c1-8(21)17-11-7-20-6-9(3-4-12(20)19-11)10-5-16-13(15)14(18-10)22-2/h3-7H,1-2H3,(H2,15,16)(H,17,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50439711
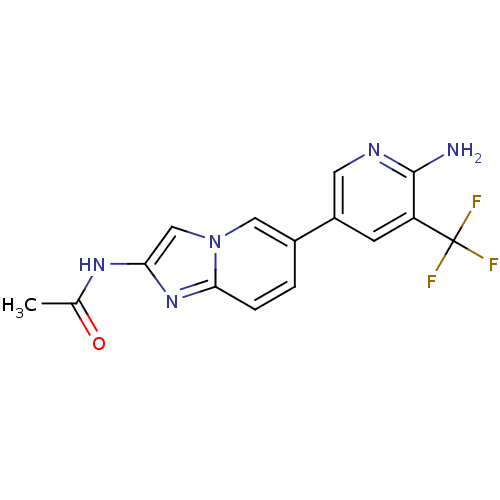
(CHEMBL2418954)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cnc(N)c(c1)C(F)(F)F Show InChI InChI=1S/C15H12F3N5O/c1-8(24)21-12-7-23-6-9(2-3-13(23)22-12)10-4-11(15(16,17)18)14(19)20-5-10/h2-7H,1H3,(H2,19,20)(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1alpha-phosphotidylinositol by luminescence assay |
Bioorg Med Chem Lett 23: 4652-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.010
BindingDB Entry DOI: 10.7270/Q2NP25T0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50439711

(CHEMBL2418954)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cnc(N)c(c1)C(F)(F)F Show InChI InChI=1S/C15H12F3N5O/c1-8(24)21-12-7-23-6-9(2-3-13(23)22-12)10-4-11(15(16,17)18)14(19)20-5-10/h2-7H,1H3,(H2,19,20)(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50076596

(3-(4-Methoxy-benzenesulfonyl)-pentane-1-thiol | CH...)Show InChI InChI=1S/C12H18O3S2/c1-3-11(8-9-16)17(13,14)12-6-4-10(15-2)5-7-12/h4-7,11,16H,3,8-9H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-13 (MMP-13) |
Bioorg Med Chem Lett 9: 943-8 (1999)
BindingDB Entry DOI: 10.7270/Q2B8578V |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50439711

(CHEMBL2418954)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cnc(N)c(c1)C(F)(F)F Show InChI InChI=1S/C15H12F3N5O/c1-8(24)21-12-7-23-6-9(2-3-13(23)22-12)10-4-11(15(16,17)18)14(19)20-5-10/h2-7H,1H3,(H2,19,20)(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using [gamma33P]ATP as substrate by top counting analysis |
Bioorg Med Chem Lett 23: 4652-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.010
BindingDB Entry DOI: 10.7270/Q2NP25T0 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50076589

(3-(4-Phenoxy-benzenesulfonyl)-propane-1-thiol | CH...)Show InChI InChI=1S/C15H16O3S2/c16-20(17,12-4-11-19)15-9-7-14(8-10-15)18-13-5-2-1-3-6-13/h1-3,5-10,19H,4,11-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-8 |
Bioorg Med Chem Lett 9: 943-8 (1999)
BindingDB Entry DOI: 10.7270/Q2B8578V |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50380367
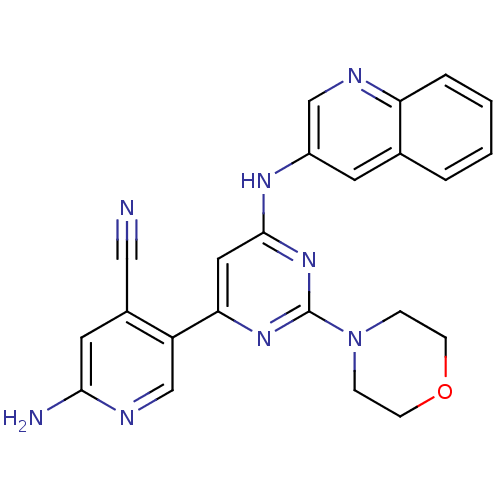
(CHEMBL2017966)Show SMILES Nc1cc(C#N)c(cn1)-c1cc(Nc2cnc3ccccc3c2)nc(n1)N1CCOCC1 Show InChI InChI=1S/C23H20N8O/c24-12-16-10-21(25)27-14-18(16)20-11-22(30-23(29-20)31-5-7-32-8-6-31)28-17-9-15-3-1-2-4-19(15)26-13-17/h1-4,9-11,13-14H,5-8H2,(H2,25,27)(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha using 1-alpha-phosphotidylinositol as substrate by ATP depletion assay |
ACS Med Chem Lett 2: 774-779 (2011)
Article DOI: 10.1021/ml200156t
BindingDB Entry DOI: 10.7270/Q2N29XXW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140273
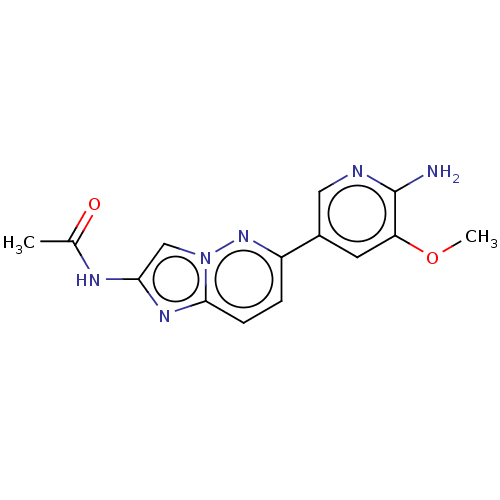
(CHEMBL3752760)Show InChI InChI=1S/C14H14N6O2/c1-8(21)17-12-7-20-13(18-12)4-3-10(19-20)9-5-11(22-2)14(15)16-6-9/h3-7H,1-2H3,(H2,15,16)(H,17,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50112348

(CHEMBL3609372)Show SMILES CC(C)NC(=O)N1CC[C@@H](C1)NC1=Nc2cc(F)ccc2N(CC(F)F)c2ccc(Cl)cc12 |r,t:13| Show InChI InChI=1S/C23H25ClF3N5O/c1-13(2)28-23(33)31-8-7-16(11-31)29-22-17-9-14(24)3-5-19(17)32(12-21(26)27)20-6-4-15(25)10-18(20)30-22/h3-6,9-10,13,16,21H,7-8,11-12H2,1-2H3,(H,28,33)(H,29,30)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of wild type dephosphorylated form of PAK1 (249 to 545) (unknown origin) expressed in Escherichia coli using 5-Fluo-Ahx-AKRRRLSSLRA-COOH a... |
ACS Med Chem Lett 6: 776-81 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00102
BindingDB Entry DOI: 10.7270/Q25X2BQS |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50076594
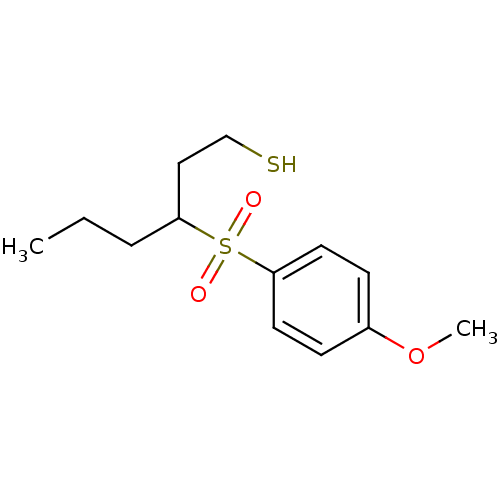
(3-(4-Methoxy-benzenesulfonyl)-hexane-1-thiol | CHE...)Show InChI InChI=1S/C13H20O3S2/c1-3-4-12(9-10-17)18(14,15)13-7-5-11(16-2)6-8-13/h5-8,12,17H,3-4,9-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-13 (MMP-13) |
Bioorg Med Chem Lett 9: 943-8 (1999)
BindingDB Entry DOI: 10.7270/Q2B8578V |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50112349

(CHEMBL3609328)Show SMILES CN1CCN(CC1)C1=Nc2cc(F)ccc2Nc2ccc(F)cc12 |t:8| Show InChI InChI=1S/C18H18F2N4/c1-23-6-8-24(9-7-23)18-14-10-12(19)2-4-15(14)21-16-5-3-13(20)11-17(16)22-18/h2-5,10-11,21H,6-9H2,1H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human muscarinic M1 receptor |
ACS Med Chem Lett 6: 776-81 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00102
BindingDB Entry DOI: 10.7270/Q25X2BQS |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50331592

(3-(6-(6-methoxypyridin-3-ylamino)-2-morpholinopyri...)Show SMILES COc1ccc(Nc2cc(nc(n2)N2CCOCC2)-c2cccc(O)c2)cn1 Show InChI InChI=1S/C20H21N5O3/c1-27-19-6-5-15(13-21-19)22-18-12-17(14-3-2-4-16(26)11-14)23-20(24-18)25-7-9-28-10-8-25/h2-6,11-13,26H,7-10H2,1H3,(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta |
Bioorg Med Chem Lett 20: 6895-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.021
BindingDB Entry DOI: 10.7270/Q2QZ2B6C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50336877
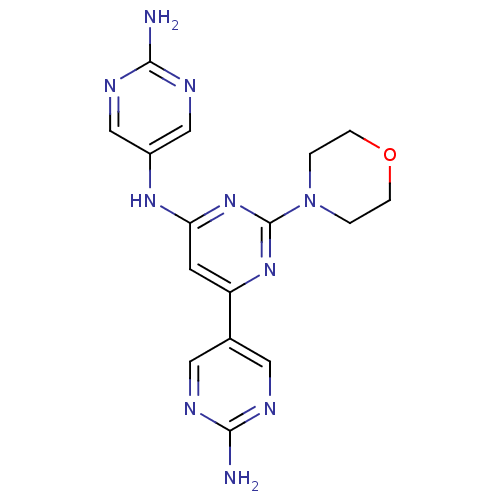
(5,5'-(2-morpholinopyrimidine-4,6-diyl)dipyrimidin-...)Show SMILES Nc1ncc(Nc2cc(nc(n2)N2CCOCC2)-c2cnc(N)nc2)cn1 Show InChI InChI=1S/C16H18N10O/c17-14-19-6-10(7-20-14)12-5-13(23-11-8-21-15(18)22-9-11)25-16(24-12)26-1-3-27-4-2-26/h5-9H,1-4H2,(H2,17,19,20)(H2,18,21,22)(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha after 15 mins |
ACS Med Chem Lett 2: 34-38 (2011)
Article DOI: 10.1021/ml1001932
BindingDB Entry DOI: 10.7270/Q2B56K0R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140271
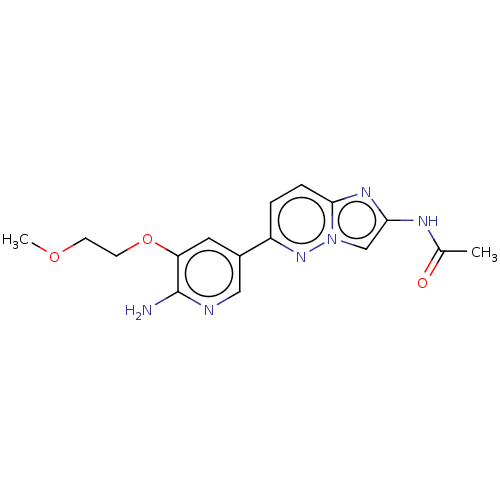
(CHEMBL3751961)Show InChI InChI=1S/C16H18N6O3/c1-10(23)19-14-9-22-15(20-14)4-3-12(21-22)11-7-13(16(17)18-8-11)25-6-5-24-2/h3-4,7-9H,5-6H2,1-2H3,(H2,17,18)(H,19,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50140265

(CHEMBL3753450)Show SMILES CC(Oc1nc(cnc1N)-c1ccc2nc(NC(C)=O)cn2c1)C(F)(F)F Show InChI InChI=1S/C16H15F3N6O2/c1-8(16(17,18)19)27-15-14(20)21-5-11(23-15)10-3-4-13-24-12(22-9(2)26)7-25(13)6-10/h3-8H,1-2H3,(H2,20,21)(H,22,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50336875
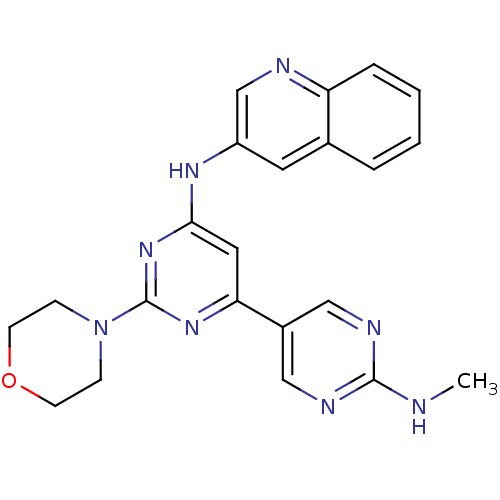
(CHEMBL1672324 | N2'-methyl-2-morpholino-N6-(quinol...)Show SMILES CNc1ncc(cn1)-c1cc(Nc2cnc3ccccc3c2)nc(n1)N1CCOCC1 Show InChI InChI=1S/C22H22N8O/c1-23-21-25-12-16(13-26-21)19-11-20(29-22(28-19)30-6-8-31-9-7-30)27-17-10-15-4-2-3-5-18(15)24-14-17/h2-5,10-14H,6-9H2,1H3,(H,23,25,26)(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha after 15 mins |
ACS Med Chem Lett 2: 34-38 (2011)
Article DOI: 10.1021/ml1001932
BindingDB Entry DOI: 10.7270/Q2B56K0R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50380370

(CHEMBL2017969)Show SMILES Nc1ncc(-c2cc(Nc3cnc4ccccc4c3)nc(n2)N2CCOCC2)c(=O)[nH]1 Show InChI InChI=1S/C21H20N8O2/c22-20-24-12-15(19(30)28-20)17-10-18(27-21(26-17)29-5-7-31-8-6-29)25-14-9-13-3-1-2-4-16(13)23-11-14/h1-4,9-12H,5-8H2,(H,25,26,27)(H3,22,24,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha using 1-alpha-phosphotidylinositol as substrate by ATP depletion assay |
ACS Med Chem Lett 2: 774-779 (2011)
Article DOI: 10.1021/ml200156t
BindingDB Entry DOI: 10.7270/Q2N29XXW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50112347
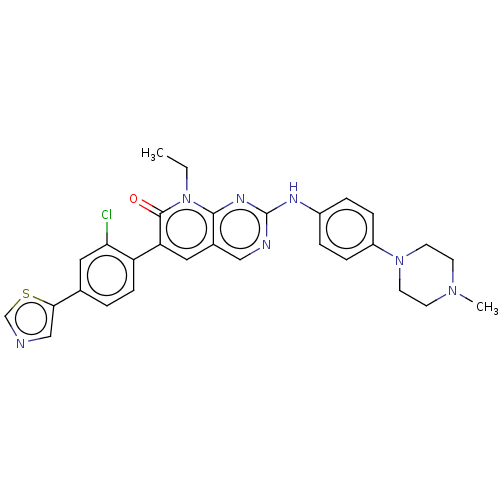
(CHEMBL3609327 | FRAX597)Show SMILES CCn1c2nc(Nc3ccc(cc3)N3CCN(C)CC3)ncc2cc(-c2ccc(cc2Cl)-c2cncs2)c1=O Show InChI InChI=1S/C29H28ClN7OS/c1-3-37-27-20(14-24(28(37)38)23-9-4-19(15-25(23)30)26-17-31-18-39-26)16-32-29(34-27)33-21-5-7-22(8-6-21)36-12-10-35(2)11-13-36/h4-9,14-18H,3,10-13H2,1-2H3,(H,32,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PAK1 by Z'-LYTE assay |
ACS Med Chem Lett 6: 776-81 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00102
BindingDB Entry DOI: 10.7270/Q25X2BQS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50439726
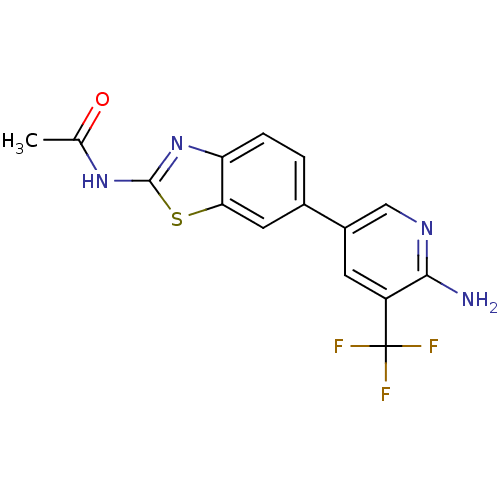
(CHEMBL2418948)Show SMILES CC(=O)Nc1nc2ccc(cc2s1)-c1cnc(N)c(c1)C(F)(F)F Show InChI InChI=1S/C15H11F3N4OS/c1-7(23)21-14-22-11-3-2-8(5-12(11)24-14)9-4-10(15(16,17)18)13(19)20-6-9/h2-6H,1H3,(H2,19,20)(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using [gamma33P]ATP as substrate by top counting analysis |
Bioorg Med Chem Lett 23: 4652-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.010
BindingDB Entry DOI: 10.7270/Q2NP25T0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50380372
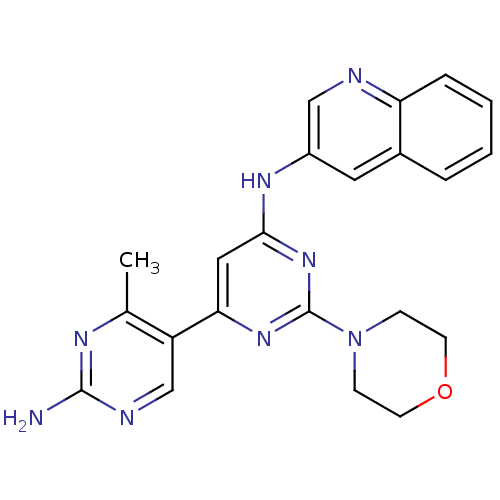
(CHEMBL2016592)Show SMILES Cc1nc(N)ncc1-c1cc(Nc2cnc3ccccc3c2)nc(n1)N1CCOCC1 Show InChI InChI=1S/C22H22N8O/c1-14-17(13-25-21(23)26-14)19-11-20(29-22(28-19)30-6-8-31-9-7-30)27-16-10-15-4-2-3-5-18(15)24-12-16/h2-5,10-13H,6-9H2,1H3,(H2,23,25,26)(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha using 1-alpha-phosphotidylinositol as substrate by ATP depletion assay |
ACS Med Chem Lett 2: 774-779 (2011)
Article DOI: 10.1021/ml200156t
BindingDB Entry DOI: 10.7270/Q2N29XXW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50336873
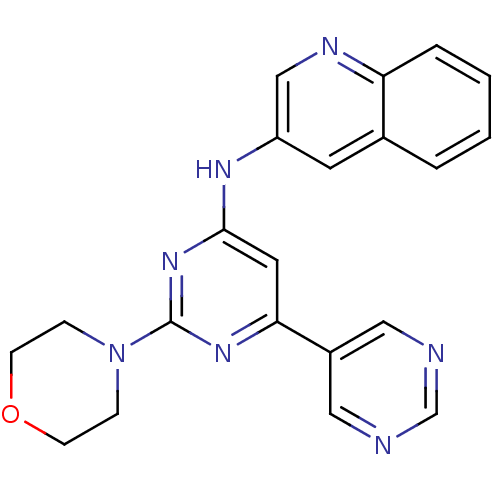
(CHEMBL1672321 | N-(2-morpholino-4,5'-bipyrimidin-6...)Show SMILES C1CN(CCO1)c1nc(Nc2cnc3ccccc3c2)cc(n1)-c1cncnc1 Show InChI InChI=1S/C21H19N7O/c1-2-4-18-15(3-1)9-17(13-24-18)25-20-10-19(16-11-22-14-23-12-16)26-21(27-20)28-5-7-29-8-6-28/h1-4,9-14H,5-8H2,(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha after 15 mins |
ACS Med Chem Lett 2: 34-38 (2011)
Article DOI: 10.1021/ml1001932
BindingDB Entry DOI: 10.7270/Q2B56K0R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50140265

(CHEMBL3753450)Show SMILES CC(Oc1nc(cnc1N)-c1ccc2nc(NC(C)=O)cn2c1)C(F)(F)F Show InChI InChI=1S/C16H15F3N6O2/c1-8(16(17,18)19)27-15-14(20)21-5-11(23-15)10-3-4-13-24-12(22-9(2)26)7-25(13)6-10/h3-8H,1-2H3,(H2,20,21)(H,22,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140268

(CHEMBL3753085)Show InChI InChI=1S/C16H18N6O3/c1-10(23)19-13-9-22-8-11(3-4-14(22)21-13)12-7-18-15(17)16(20-12)25-6-5-24-2/h3-4,7-9H,5-6H2,1-2H3,(H2,17,18)(H,19,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50439711

(CHEMBL2418954)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cnc(N)c(c1)C(F)(F)F Show InChI InChI=1S/C15H12F3N5O/c1-8(24)21-12-7-23-6-9(2-3-13(23)22-12)10-4-11(15(16,17)18)14(19)20-5-10/h2-7H,1H3,(H2,19,20)(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using 1alpha-phosphotidylinositol by luminescence assay |
Bioorg Med Chem Lett 23: 4652-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.010
BindingDB Entry DOI: 10.7270/Q2NP25T0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50112355
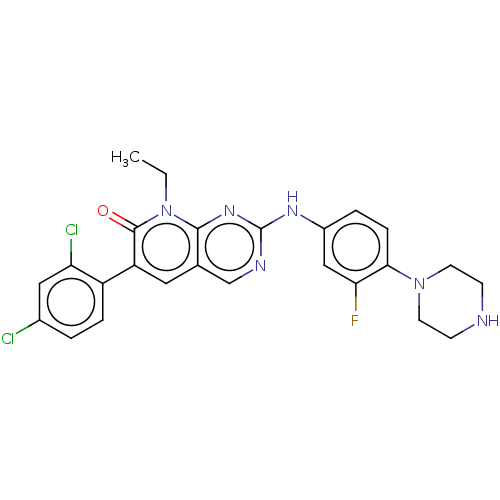
(CHEMBL3609326)Show SMILES CCn1c2nc(Nc3ccc(N4CCNCC4)c(F)c3)ncc2cc(-c2ccc(Cl)cc2Cl)c1=O Show InChI InChI=1S/C25H23Cl2FN6O/c1-2-34-23-15(11-19(24(34)35)18-5-3-16(26)12-20(18)27)14-30-25(32-23)31-17-4-6-22(21(28)13-17)33-9-7-29-8-10-33/h3-6,11-14,29H,2,7-10H2,1H3,(H,30,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of full length PAK1 (unknown origin) by Z'-LYTE assay |
ACS Med Chem Lett 6: 776-81 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00102
BindingDB Entry DOI: 10.7270/Q25X2BQS |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50336865
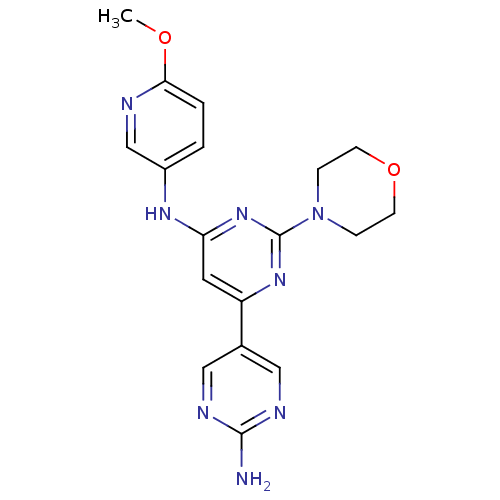
(CHEMBL1672328 | N6-(6-methoxypyridin-3-yl)-2-morph...)Show SMILES COc1ccc(Nc2cc(nc(n2)N2CCOCC2)-c2cnc(N)nc2)cn1 Show InChI InChI=1S/C18H20N8O2/c1-27-16-3-2-13(11-20-16)23-15-8-14(12-9-21-17(19)22-10-12)24-18(25-15)26-4-6-28-7-5-26/h2-3,8-11H,4-7H2,1H3,(H2,19,21,22)(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma after 15 mins |
ACS Med Chem Lett 2: 34-38 (2011)
Article DOI: 10.1021/ml1001932
BindingDB Entry DOI: 10.7270/Q2B56K0R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140272
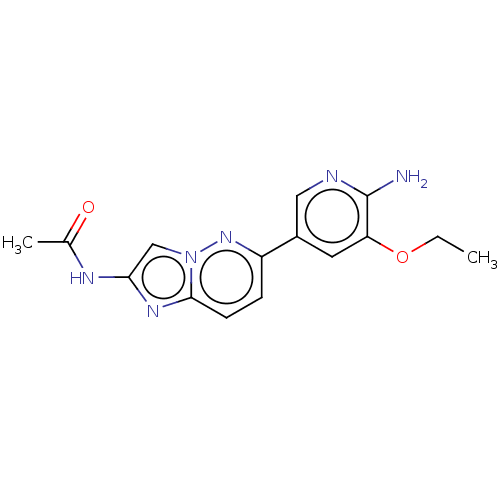
(CHEMBL3754572)Show InChI InChI=1S/C15H16N6O2/c1-3-23-12-6-10(7-17-15(12)16)11-4-5-14-19-13(18-9(2)22)8-21(14)20-11/h4-8H,3H2,1-2H3,(H2,16,17)(H,18,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50336878
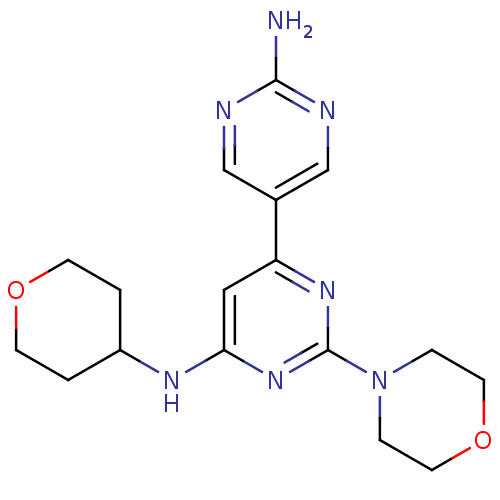
(2-morpholino-N6-(tetrahydro-2H-pyran-4-yl)-4,5'-bi...)Show InChI InChI=1S/C17H23N7O2/c18-16-19-10-12(11-20-16)14-9-15(21-13-1-5-25-6-2-13)23-17(22-14)24-3-7-26-8-4-24/h9-11,13H,1-8H2,(H2,18,19,20)(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha after 15 mins |
ACS Med Chem Lett 2: 34-38 (2011)
Article DOI: 10.1021/ml1001932
BindingDB Entry DOI: 10.7270/Q2B56K0R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140275

(CHEMBL3752775)Show InChI InChI=1S/C15H15N5O2/c1-9(21)18-13-8-20-7-10(3-4-14(20)19-13)11-5-12(22-2)15(16)17-6-11/h3-8H,1-2H3,(H2,16,17)(H,18,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 2
(Homo sapiens (Human)) | BDBM50112347

(CHEMBL3609327 | FRAX597)Show SMILES CCn1c2nc(Nc3ccc(cc3)N3CCN(C)CC3)ncc2cc(-c2ccc(cc2Cl)-c2cncs2)c1=O Show InChI InChI=1S/C29H28ClN7OS/c1-3-37-27-20(14-24(28(37)38)23-9-4-19(15-25(23)30)26-17-31-18-39-26)16-32-29(34-27)33-21-5-7-22(8-6-21)36-12-10-35(2)11-13-36/h4-9,14-18H,3,10-13H2,1-2H3,(H,32,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PAK2 by Z'-LYTE assay |
ACS Med Chem Lett 6: 776-81 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00102
BindingDB Entry DOI: 10.7270/Q25X2BQS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50380377
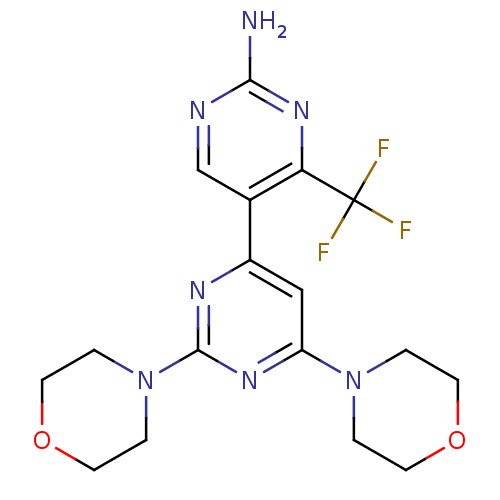
(CHEMBL2017977 | WO2007/084786, Compound 85)Show SMILES Nc1ncc(-c2cc(nc(n2)N2CCOCC2)N2CCOCC2)c(n1)C(F)(F)F Show InChI InChI=1S/C17H20F3N7O2/c18-17(19,20)14-11(10-22-15(21)25-14)12-9-13(26-1-5-28-6-2-26)24-16(23-12)27-3-7-29-8-4-27/h9-10H,1-8H2,(H2,21,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha using 1-alpha-phosphotidylinositol as substrate by ATP depletion assay |
ACS Med Chem Lett 2: 774-779 (2011)
Article DOI: 10.1021/ml200156t
BindingDB Entry DOI: 10.7270/Q2N29XXW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50336879
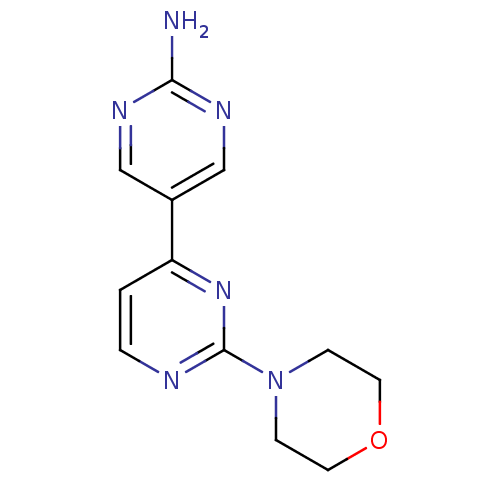
(2-morpholino-4,5'-bipyrimidin-2'-amine | CHEMBL167...)Show InChI InChI=1S/C12H14N6O/c13-11-15-7-9(8-16-11)10-1-2-14-12(17-10)18-3-5-19-6-4-18/h1-2,7-8H,3-6H2,(H2,13,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha after 15 mins |
ACS Med Chem Lett 2: 34-38 (2011)
Article DOI: 10.1021/ml1001932
BindingDB Entry DOI: 10.7270/Q2B56K0R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM101618
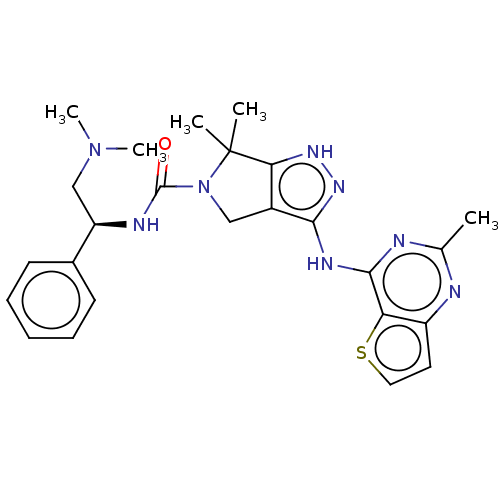
(US8530652, 114)Show SMILES CN(C)C[C@@H](NC(=O)N1Cc2c(Nc3nc(C)nc4ccsc34)n[nH]c2C1(C)C)c1ccccc1 Show InChI InChI=1S/C25H30N8OS/c1-15-26-18-11-12-35-20(18)23(27-15)29-22-17-13-33(25(2,3)21(17)30-31-22)24(34)28-19(14-32(4)5)16-9-7-6-8-10-16/h6-12,19H,13-14H2,1-5H3,(H,28,34)(H2,26,27,29,30,31)/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PAK1 (unknown origin) using Syntide2 peptide as substrate by pyruvate kinase/lactate dehydrogenase coupled assay |
ACS Med Chem Lett 6: 776-81 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00102
BindingDB Entry DOI: 10.7270/Q25X2BQS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50439723
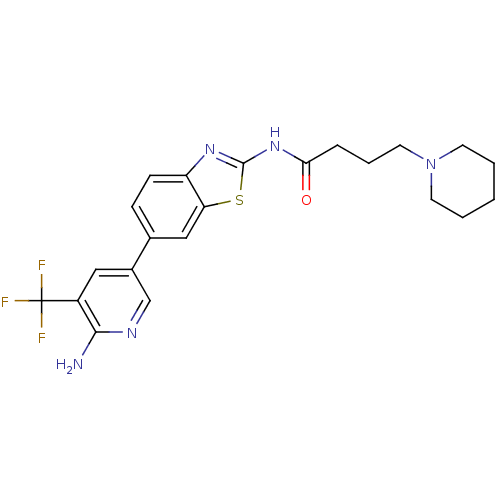
(CHEMBL2418951)Show SMILES Nc1ncc(cc1C(F)(F)F)-c1ccc2nc(NC(=O)CCCN3CCCCC3)sc2c1 Show InChI InChI=1S/C22H24F3N5OS/c23-22(24,25)16-11-15(13-27-20(16)26)14-6-7-17-18(12-14)32-21(28-17)29-19(31)5-4-10-30-8-2-1-3-9-30/h6-7,11-13H,1-5,8-10H2,(H2,26,27)(H,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using [gamma33P]ATP as substrate by top counting analysis |
Bioorg Med Chem Lett 23: 4652-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.010
BindingDB Entry DOI: 10.7270/Q2NP25T0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50380376
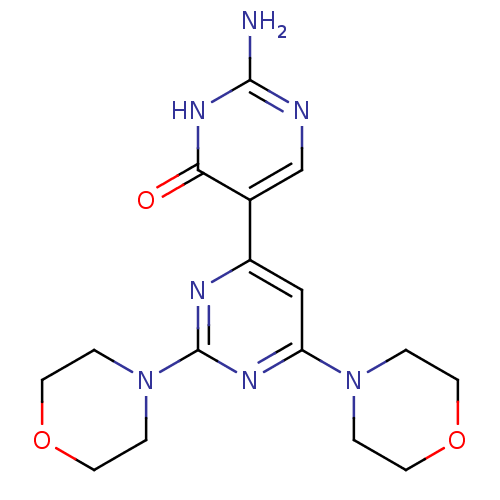
(CHEMBL2017976)Show SMILES Nc1ncc(-c2cc(nc(n2)N2CCOCC2)N2CCOCC2)c(=O)[nH]1 Show InChI InChI=1S/C16H21N7O3/c17-15-18-10-11(14(24)21-15)12-9-13(22-1-5-25-6-2-22)20-16(19-12)23-3-7-26-8-4-23/h9-10H,1-8H2,(H3,17,18,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha using 1-alpha-phosphotidylinositol as substrate by ATP depletion assay |
ACS Med Chem Lett 2: 774-779 (2011)
Article DOI: 10.1021/ml200156t
BindingDB Entry DOI: 10.7270/Q2N29XXW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140274

(CHEMBL3753665)Show InChI InChI=1S/C16H17N5O2/c1-3-23-13-6-12(7-18-16(13)17)11-4-5-15-20-14(19-10(2)22)9-21(15)8-11/h4-9H,3H2,1-2H3,(H2,17,18)(H,19,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50331592

(3-(6-(6-methoxypyridin-3-ylamino)-2-morpholinopyri...)Show SMILES COc1ccc(Nc2cc(nc(n2)N2CCOCC2)-c2cccc(O)c2)cn1 Show InChI InChI=1S/C20H21N5O3/c1-27-19-6-5-15(13-21-19)22-18-12-17(14-3-2-4-16(26)11-14)23-20(24-18)25-7-9-28-10-8-25/h2-6,11-13,26H,7-10H2,1H3,(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 6895-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.021
BindingDB Entry DOI: 10.7270/Q2QZ2B6C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data