 Found 564 hits with Last Name = 'vyas' and Initial = 'dm'
Found 564 hits with Last Name = 'vyas' and Initial = 'dm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM107016
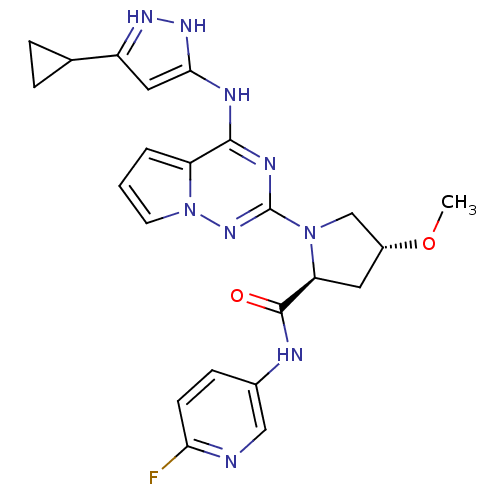
(US8592579, 219)Show SMILES CO[C@@H]1C[C@H](N(C1)c1nc(Nc2cc(n[nH]2)C2CC2)c2cccn2n1)C(=O)Nc1ccc(F)nc1 |r| Show InChI InChI=1S/C23H24FN9O2/c1-35-15-9-18(22(34)26-14-6-7-19(24)25-11-14)32(12-15)23-28-21(17-3-2-8-33(17)31-23)27-20-10-16(29-30-20)13-4-5-13/h2-3,6-8,10-11,13,15,18H,4-5,9,12H2,1H3,(H,26,34)(H2,27,28,29,30,31)/t15-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Kinase assay using IGF1-receptor. |
US Patent US8592579 (2013)
BindingDB Entry DOI: 10.7270/Q2SQ8Z12 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM107013

(US8592579, 211)Show SMILES O[C@H]1C[C@H](N(C1)c1nc(Nc2cc(n[nH]2)C2CC2)c2cccn2n1)C(=O)Nc1cccnc1 |r| Show InChI InChI=1S/C22H23N9O2/c32-15-9-18(21(33)24-14-3-1-7-23-11-14)30(12-15)22-26-20(17-4-2-8-31(17)29-22)25-19-10-16(27-28-19)13-5-6-13/h1-4,7-8,10-11,13,15,18,32H,5-6,9,12H2,(H,24,33)(H2,25,26,27,28,29)/t15-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Kinase assay using IGF1-receptor. |
US Patent US8592579 (2013)
BindingDB Entry DOI: 10.7270/Q2SQ8Z12 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50325424

((S)-1-(4-(5-cyclopropyl-1H-pyrazol-3-ylamino)pyrro...)Show SMILES Fc1ccc(NC(=O)[C@@H]2CCCN2c2nc(Nc3cc([nH]n3)C3CC3)c3cccn3n2)cn1 |r| Show InChI InChI=1S/C22H22FN9O/c23-18-8-7-14(12-24-18)25-21(33)17-4-1-9-31(17)22-27-20(16-3-2-10-32(16)30-22)26-19-11-15(28-29-19)13-5-6-13/h2-3,7-8,10-13,17H,1,4-6,9H2,(H,25,33)(H2,26,27,28,29,30)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R in IGF1R-SAL cells |
Bioorg Med Chem Lett 20: 5027-30 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.045
BindingDB Entry DOI: 10.7270/Q2WH2Q5J |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM107018

(US8592579, 254)Show SMILES CO[C@H]1C[C@H](N(C1)c1nc(Nc2cc(n[nH]2)C2CC2)c2cccn2n1)C(=O)Nc1ccc(F)nc1 |r| Show InChI InChI=1S/C23H24FN9O2/c1-35-15-9-18(22(34)26-14-6-7-19(24)25-11-14)32(12-15)23-28-21(17-3-2-8-33(17)31-23)27-20-10-16(29-30-20)13-4-5-13/h2-3,6-8,10-11,13,15,18H,4-5,9,12H2,1H3,(H,26,34)(H2,27,28,29,30,31)/t15-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Kinase assay using IGF1-receptor. |
US Patent US8592579 (2013)
BindingDB Entry DOI: 10.7270/Q2SQ8Z12 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM107023

(US8592579, 288)Show SMILES CC1(CC1)c1cc(Nc2nc(nn3cccc23)N2C[C@@H](O)C[C@H]2C(=O)Nc2ncns2)[nH]n1 |r| Show InChI InChI=1S/C20H22N10O2S/c1-20(4-5-20)14-8-15(27-26-14)23-16-12-3-2-6-30(12)28-18(24-16)29-9-11(31)7-13(29)17(32)25-19-21-10-22-33-19/h2-3,6,8,10-11,13,31H,4-5,7,9H2,1H3,(H,21,22,25,32)(H2,23,24,26,27,28)/t11-,13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Kinase assay using IGF1-receptor. |
US Patent US8592579 (2013)
BindingDB Entry DOI: 10.7270/Q2SQ8Z12 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM107020

(US8592579, 256)Show SMILES CO[C@H]1C[C@H](N(C1)c1nc(Nc2cc(n[nH]2)C2CC2)c2cccn2n1)C(=O)Nc1ncns1 |r| Show InChI InChI=1S/C20H22N10O2S/c1-32-12-7-15(18(31)25-20-21-10-22-33-20)29(9-12)19-24-17(14-3-2-6-30(14)28-19)23-16-8-13(26-27-16)11-4-5-11/h2-3,6,8,10-12,15H,4-5,7,9H2,1H3,(H,21,22,25,31)(H2,23,24,26,27,28)/t12-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Kinase assay using IGF1-receptor. |
US Patent US8592579 (2013)
BindingDB Entry DOI: 10.7270/Q2SQ8Z12 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM107021

(US8592579, 259)Show SMILES CC1(CC1)c1cc(Nc2nc(nn3cccc23)N2C[C@@H](O)C[C@H]2C(=O)Nc2cnccn2)[nH]n1 |r| Show InChI InChI=1S/C22H24N10O2/c1-22(4-5-22)16-10-17(29-28-16)25-19-14-3-2-8-32(14)30-21(27-19)31-12-13(33)9-15(31)20(34)26-18-11-23-6-7-24-18/h2-3,6-8,10-11,13,15,33H,4-5,9,12H2,1H3,(H,24,26,34)(H2,25,27,28,29,30)/t13-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Kinase assay using IGF1-receptor. |
US Patent US8592579 (2013)
BindingDB Entry DOI: 10.7270/Q2SQ8Z12 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50299138
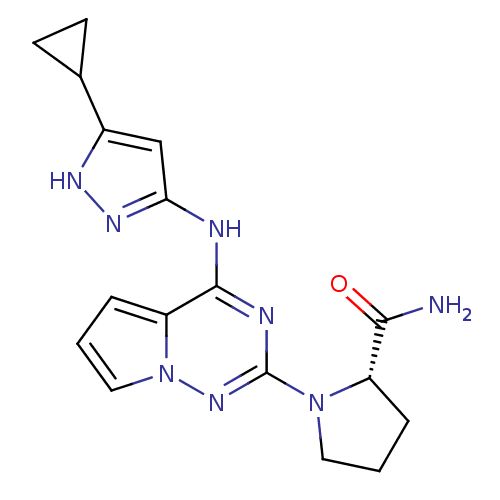
((S)-1-(4-(5-cyclopropyl-1H-pyrazol-3-ylamino)pyrro...)Show SMILES NC(=O)[C@@H]1CCCN1c1nc(Nc2cc([nH]n2)C2CC2)c2cccn2n1 |r| Show InChI InChI=1S/C17H20N8O/c18-15(26)12-3-1-7-24(12)17-20-16(13-4-2-8-25(13)23-17)19-14-9-11(21-22-14)10-5-6-10/h2,4,8-10,12H,1,3,5-7H2,(H2,18,26)(H2,19,20,21,22,23)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin E after 60 mins by fluorescence electrophoresis |
J Med Chem 52: 7360-3 (2009)
Article DOI: 10.1021/jm900786r
BindingDB Entry DOI: 10.7270/Q2P27022 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50299148
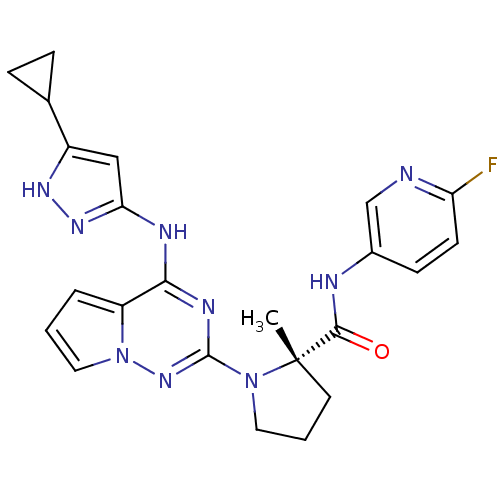
((S)-1-(4-(5-cyclopropyl-1H-pyrazol-3-ylamino)pyrro...)Show SMILES C[C@]1(CCCN1c1nc(Nc2cc([nH]n2)C2CC2)c2cccn2n1)C(=O)Nc1ccc(F)nc1 |r| Show InChI InChI=1S/C23H24FN9O/c1-23(21(34)26-15-7-8-18(24)25-13-15)9-3-10-32(23)22-28-20(17-4-2-11-33(17)31-22)27-19-12-16(29-30-19)14-5-6-14/h2,4,7-8,11-14H,3,5-6,9-10H2,1H3,(H,26,34)(H2,27,28,29,30,31)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R after 60 mins by fluorescence electrophoresis |
J Med Chem 52: 7360-3 (2009)
Article DOI: 10.1021/jm900786r
BindingDB Entry DOI: 10.7270/Q2P27022 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM107003
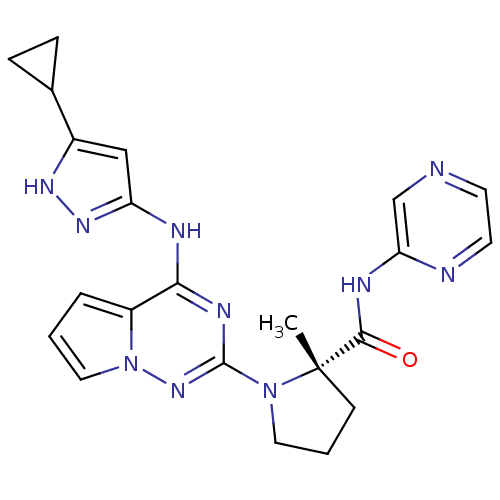
(CHEMBL575447 | US8592579, 110)Show SMILES C[C@]1(CCCN1c1nc(Nc2cc([nH]n2)C2CC2)c2cccn2n1)C(=O)Nc1cnccn1 |r| Show InChI InChI=1S/C22H24N10O/c1-22(20(33)26-18-13-23-8-9-24-18)7-3-10-31(22)21-27-19(16-4-2-11-32(16)30-21)25-17-12-15(28-29-17)14-5-6-14/h2,4,8-9,11-14H,3,5-7,10H2,1H3,(H,24,26,33)(H2,25,27,28,29,30)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Kinase assay using IGF1-receptor. |
US Patent US8592579 (2013)
BindingDB Entry DOI: 10.7270/Q2SQ8Z12 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM107024

(US8592579, 293)Show SMILES F[C@@H]1C[C@H](N(C1)c1nc(Nc2cc(n[nH]2)C2CC2)c2cccn2n1)C(=O)Nc1ncns1 |r| Show InChI InChI=1S/C19H19FN10OS/c20-11-6-14(17(31)25-19-21-9-22-32-19)29(8-11)18-24-16(13-2-1-5-30(13)28-18)23-15-7-12(26-27-15)10-3-4-10/h1-2,5,7,9-11,14H,3-4,6,8H2,(H,21,22,25,31)(H2,23,24,26,27,28)/t11-,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Kinase assay using IGF1-receptor. |
US Patent US8592579 (2013)
BindingDB Entry DOI: 10.7270/Q2SQ8Z12 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM107000

(US8592579, 104)Show SMILES C[C@]1(CCCN1c1nc(Nc2cc(n[nH]2)C2CC2)c2cccn2n1)C(=O)Nc1ccc(F)nc1 |r| Show InChI InChI=1S/C23H24FN9O/c1-23(21(34)26-15-7-8-18(24)25-13-15)9-3-10-32(23)22-28-20(17-4-2-11-33(17)31-22)27-19-12-16(29-30-19)14-5-6-14/h2,4,7-8,11-14H,3,5-6,9-10H2,1H3,(H,26,34)(H2,27,28,29,30,31)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| PDB
US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Kinase assay using IGF1-receptor. |
US Patent US8592579 (2013)
BindingDB Entry DOI: 10.7270/Q2SQ8Z12 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM107012

(US8592579, 209)Show SMILES O[C@H]1C[C@H](N(C1)c1nc(Nc2cc(n[nH]2)C2CC2)c2cccn2n1)C(=O)Nc1cnccn1 |r| Show InChI InChI=1S/C21H22N10O2/c32-13-8-16(20(33)25-18-10-22-5-6-23-18)30(11-13)21-26-19(15-2-1-7-31(15)29-21)24-17-9-14(27-28-17)12-3-4-12/h1-2,5-7,9-10,12-13,16,32H,3-4,8,11H2,(H,23,25,33)(H2,24,26,27,28,29)/t13-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Kinase assay using IGF1-receptor. |
US Patent US8592579 (2013)
BindingDB Entry DOI: 10.7270/Q2SQ8Z12 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM107026
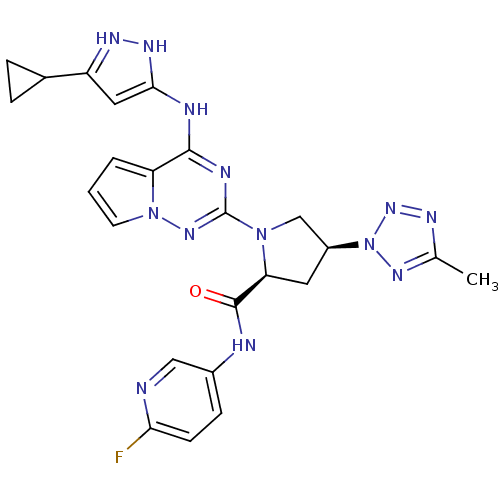
(US8592579, 301)Show SMILES Cc1nnn(n1)[C@H]1C[C@H](N(C1)c1nc(Nc2cc(n[nH]2)C2CC2)c2cccn2n1)C(=O)Nc1ccc(F)nc1 |r| Show InChI InChI=1S/C24H24FN13O/c1-13-30-35-38(33-13)16-9-19(23(39)27-15-6-7-20(25)26-11-15)36(12-16)24-29-22(18-3-2-8-37(18)34-24)28-21-10-17(31-32-21)14-4-5-14/h2-3,6-8,10-11,14,16,19H,4-5,9,12H2,1H3,(H,27,39)(H2,28,29,31,32,34)/t16-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Kinase assay using IGF1-receptor. |
US Patent US8592579 (2013)
BindingDB Entry DOI: 10.7270/Q2SQ8Z12 |
More data for this
Ligand-Target Pair | |
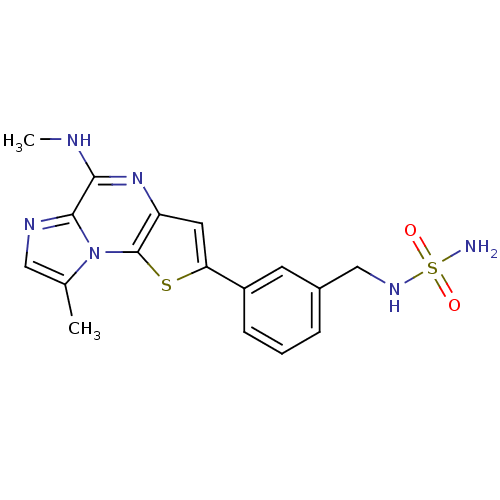
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50299143
((S)-1-(4-(5-cyclopropyl-1H-pyrazol-3-ylamino)pyrro...)Show SMILES O=C(Nc1nccs1)[C@@H]1CCCN1c1nc(Nc2cc([nH]n2)C2CC2)c2cccn2n1 |r| Show InChI InChI=1S/C20H21N9OS/c30-18(24-20-21-7-10-31-20)15-4-1-8-28(15)19-23-17(14-3-2-9-29(14)27-19)22-16-11-13(25-26-16)12-5-6-12/h2-3,7,9-12,15H,1,4-6,8H2,(H,21,24,30)(H2,22,23,25,26,27)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R after 60 mins by fluorescence electrophoresis |
J Med Chem 52: 7360-3 (2009)
Article DOI: 10.1021/jm900786r
BindingDB Entry DOI: 10.7270/Q2P27022 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50299148

((S)-1-(4-(5-cyclopropyl-1H-pyrazol-3-ylamino)pyrro...)Show SMILES C[C@]1(CCCN1c1nc(Nc2cc([nH]n2)C2CC2)c2cccn2n1)C(=O)Nc1ccc(F)nc1 |r| Show InChI InChI=1S/C23H24FN9O/c1-23(21(34)26-15-7-8-18(24)25-13-15)9-3-10-32(23)22-28-20(17-4-2-11-33(17)31-22)27-19-12-16(29-30-19)14-5-6-14/h2,4,7-8,11-14H,3,5-6,9-10H2,1H3,(H,26,34)(H2,27,28,29,30,31)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R in IGF1R-SAL cells |
Bioorg Med Chem Lett 20: 5027-30 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.045
BindingDB Entry DOI: 10.7270/Q2WH2Q5J |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50325424

((S)-1-(4-(5-cyclopropyl-1H-pyrazol-3-ylamino)pyrro...)Show SMILES Fc1ccc(NC(=O)[C@@H]2CCCN2c2nc(Nc3cc([nH]n3)C3CC3)c3cccn3n2)cn1 |r| Show InChI InChI=1S/C22H22FN9O/c23-18-8-7-14(12-24-18)25-21(33)17-4-1-9-31(17)22-27-20(16-3-2-10-32(16)30-22)26-19-11-15(28-29-19)13-5-6-13/h2-3,7-8,10-13,17H,1,4-6,9H2,(H,25,33)(H2,26,27,28,29,30)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 20: 5027-30 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.045
BindingDB Entry DOI: 10.7270/Q2WH2Q5J |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM107028

(US8592579, 318)Show SMILES F[C@H]1C[C@H](N(C1)c1nc(Nc2cc(n[nH]2)C2CC2)c2cccn2n1)C(=O)Nc1ncns1 |r| Show InChI InChI=1S/C19H19FN10OS/c20-11-6-14(17(31)25-19-21-9-22-32-19)29(8-11)18-24-16(13-2-1-5-30(13)28-18)23-15-7-12(26-27-15)10-3-4-10/h1-2,5,7,9-11,14H,3-4,6,8H2,(H,21,22,25,31)(H2,23,24,26,27,28)/t11-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Kinase assay using IGF1-receptor. |
US Patent US8592579 (2013)
BindingDB Entry DOI: 10.7270/Q2SQ8Z12 |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
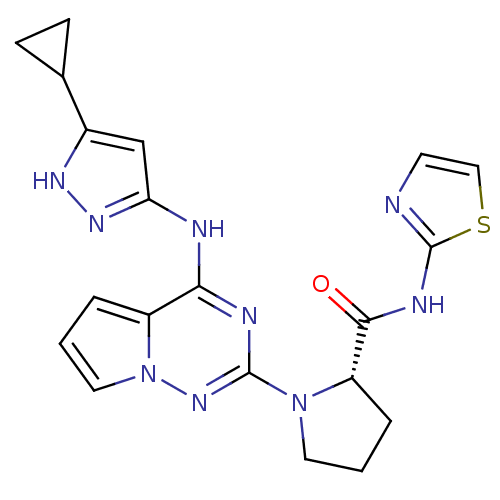
(Homo sapiens (Human)) | BDBM25960
(amino-N-({3-[12-methyl-8-(methylamino)-3-thia-1,7,...)Show SMILES CNc1nc2cc(sc2n2c(C)cnc12)-c1cccc(CNS(N)(=O)=O)c1 Show InChI InChI=1S/C17H18N6O2S2/c1-10-8-20-16-15(19-2)22-13-7-14(26-17(13)23(10)16)12-5-3-4-11(6-12)9-21-27(18,24)25/h3-8,21H,9H2,1-2H3,(H,19,22)(H2,18,24,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
| Assay Description
Assays measuring the enzyme-catalyzed phosphorylation of GST-I kappa B alpha were performed. The phosphorylated substrate was detected using a Phosph... |
Bioorg Med Chem Lett 17: 4284-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.031
BindingDB Entry DOI: 10.7270/Q2QF8R50 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM107022
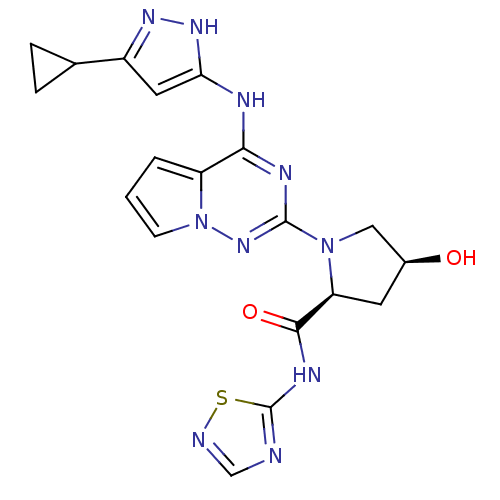
(US8592579, 287)Show SMILES O[C@H]1C[C@H](N(C1)c1nc(Nc2cc(n[nH]2)C2CC2)c2cccn2n1)C(=O)Nc1ncns1 |r| Show InChI InChI=1S/C19H20N10O2S/c30-11-6-14(17(31)24-19-20-9-21-32-19)28(8-11)18-23-16(13-2-1-5-29(13)27-18)22-15-7-12(25-26-15)10-3-4-10/h1-2,5,7,9-11,14,30H,3-4,6,8H2,(H,20,21,24,31)(H2,22,23,25,26,27)/t11-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Kinase assay using IGF1-receptor. |
US Patent US8592579 (2013)
BindingDB Entry DOI: 10.7270/Q2SQ8Z12 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM107003

(CHEMBL575447 | US8592579, 110)Show SMILES C[C@]1(CCCN1c1nc(Nc2cc([nH]n2)C2CC2)c2cccn2n1)C(=O)Nc1cnccn1 |r| Show InChI InChI=1S/C22H24N10O/c1-22(20(33)26-18-13-23-8-9-24-18)7-3-10-31(22)21-27-19(16-4-2-11-32(16)30-21)25-17-12-15(28-29-17)14-5-6-14/h2,4,8-9,11-14H,3,5-7,10H2,1H3,(H,24,26,33)(H2,25,27,28,29,30)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R after 60 mins by fluorescence electrophoresis |
J Med Chem 52: 7360-3 (2009)
Article DOI: 10.1021/jm900786r
BindingDB Entry DOI: 10.7270/Q2P27022 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM107001

(US8592579, 105)Show SMILES C[C@]1(CCCN1c1nc(Nc2cc(n[nH]2)C2CC2)c2cccn2n1)C(=O)Nc1nccs1 |r| Show InChI InChI=1S/C21H23N9OS/c1-21(18(31)25-20-22-8-11-32-20)7-3-9-29(21)19-24-17(15-4-2-10-30(15)28-19)23-16-12-14(26-27-16)13-5-6-13/h2,4,8,10-13H,3,5-7,9H2,1H3,(H,22,25,31)(H2,23,24,26,27,28)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Kinase assay using IGF1-receptor. |
US Patent US8592579 (2013)
BindingDB Entry DOI: 10.7270/Q2SQ8Z12 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50299145
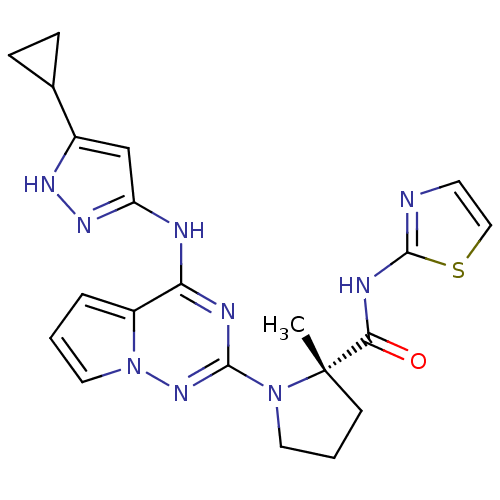
((S)-1-(4-(5-cyclopropyl-1H-pyrazol-3-ylamino)pyrro...)Show SMILES C[C@]1(CCCN1c1nc(Nc2cc([nH]n2)C2CC2)c2cccn2n1)C(=O)Nc1nccs1 |r| Show InChI InChI=1S/C21H23N9OS/c1-21(18(31)25-20-22-8-11-32-20)7-3-9-29(21)19-24-17(15-4-2-10-30(15)28-19)23-16-12-14(26-27-16)13-5-6-13/h2,4,8,10-13H,3,5-7,9H2,1H3,(H,22,25,31)(H2,23,24,26,27,28)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R after 60 mins by fluorescence electrophoresis |
J Med Chem 52: 7360-3 (2009)
Article DOI: 10.1021/jm900786r
BindingDB Entry DOI: 10.7270/Q2P27022 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM107015
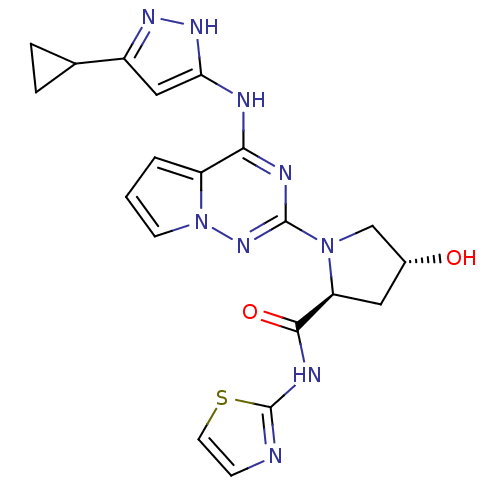
(US8592579, 216)Show SMILES O[C@@H]1C[C@H](N(C1)c1nc(Nc2cc(n[nH]2)C2CC2)c2cccn2n1)C(=O)Nc1nccs1 |r| Show InChI InChI=1S/C20H21N9O2S/c30-12-8-15(18(31)24-20-21-5-7-32-20)28(10-12)19-23-17(14-2-1-6-29(14)27-19)22-16-9-13(25-26-16)11-3-4-11/h1-2,5-7,9,11-12,15,30H,3-4,8,10H2,(H,21,24,31)(H2,22,23,25,26,27)/t12-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Kinase assay using IGF1-receptor. |
US Patent US8592579 (2013)
BindingDB Entry DOI: 10.7270/Q2SQ8Z12 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM107025
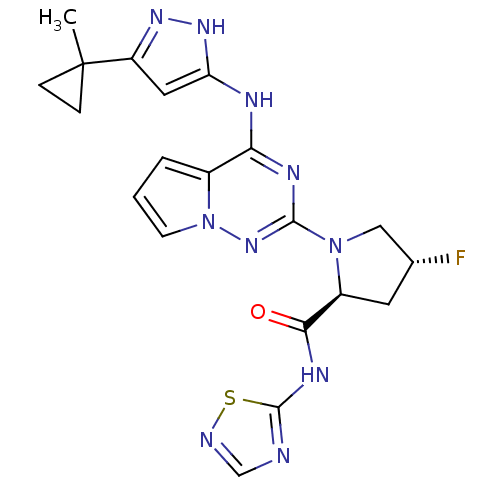
(US8592579, 294)Show SMILES CC1(CC1)c1cc(Nc2nc(nn3cccc23)N2C[C@H](F)C[C@H]2C(=O)Nc2ncns2)[nH]n1 |r| Show InChI InChI=1S/C20H21FN10OS/c1-20(4-5-20)14-8-15(28-27-14)24-16-12-3-2-6-31(12)29-18(25-16)30-9-11(21)7-13(30)17(32)26-19-22-10-23-33-19/h2-3,6,8,10-11,13H,4-5,7,9H2,1H3,(H,22,23,26,32)(H2,24,25,27,28,29)/t11-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Kinase assay using IGF1-receptor. |
US Patent US8592579 (2013)
BindingDB Entry DOI: 10.7270/Q2SQ8Z12 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM107017
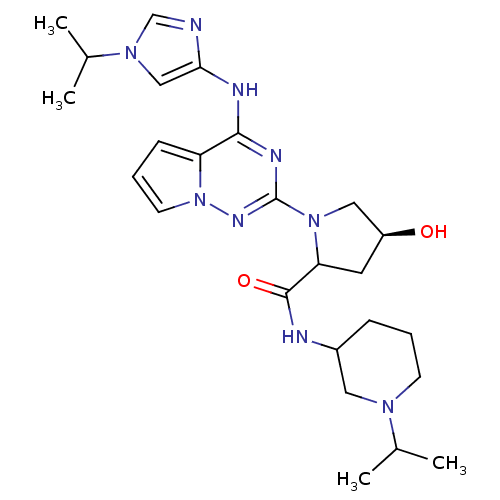
(US8592579, 243)Show SMILES CC(C)N1CCCC(C1)NC(=O)C1C[C@H](O)CN1c1nc(Nc2cn(cn2)C(C)C)c2cccn2n1 |r| Show InChI InChI=1S/C25H37N9O2/c1-16(2)31-9-5-7-18(12-31)27-24(36)21-11-19(35)13-33(21)25-29-23(20-8-6-10-34(20)30-25)28-22-14-32(15-26-22)17(3)4/h6,8,10,14-19,21,35H,5,7,9,11-13H2,1-4H3,(H,27,36)(H,28,29,30)/t18?,19-,21?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Kinase assay using IGF1-receptor. |
US Patent US8592579 (2013)
BindingDB Entry DOI: 10.7270/Q2SQ8Z12 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50325430

(CHEMBL1222855 | N-((4-(5-cyclopropyl-1H-pyrazol-3-...)Show SMILES Fc1ccc(cn1)C(=O)NCc1nc(Nc2cc(n[nH]2)C2CC2)c2cccn2n1 Show InChI InChI=1S/C19H17FN8O/c20-15-6-5-12(9-21-15)19(29)22-10-17-24-18(14-2-1-7-28(14)27-17)23-16-8-13(25-26-16)11-3-4-11/h1-2,5-9,11H,3-4,10H2,(H,22,29)(H2,23,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 20: 5027-30 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.045
BindingDB Entry DOI: 10.7270/Q2WH2Q5J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50299146
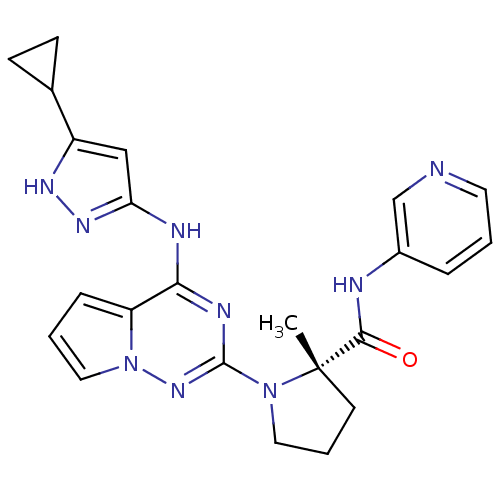
((S)-1-(4-(5-cyclopropyl-1H-pyrazol-3-ylamino)pyrro...)Show SMILES C[C@]1(CCCN1c1nc(Nc2cc([nH]n2)C2CC2)c2cccn2n1)C(=O)Nc1cccnc1 |r| Show InChI InChI=1S/C23H25N9O/c1-23(21(33)25-16-5-2-10-24-14-16)9-4-11-31(23)22-27-20(18-6-3-12-32(18)30-22)26-19-13-17(28-29-19)15-7-8-15/h2-3,5-6,10,12-15H,4,7-9,11H2,1H3,(H,25,33)(H2,26,27,28,29,30)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R after 60 mins by fluorescence electrophoresis |
J Med Chem 52: 7360-3 (2009)
Article DOI: 10.1021/jm900786r
BindingDB Entry DOI: 10.7270/Q2P27022 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50325425

((S)-(2-(4-(5-cyclopropyl-1H-pyrazol-3-ylamino)pyrr...)Show SMILES Fc1ccc(cn1)C(=O)N1CCC[C@H]1c1nc(Nc2cc(n[nH]2)C2CC2)c2cccn2n1 |r| Show InChI InChI=1S/C22H21FN8O/c23-18-8-7-14(12-24-18)22(32)30-9-1-3-16(30)21-26-20(17-4-2-10-31(17)29-21)25-19-11-15(27-28-19)13-5-6-13/h2,4,7-8,10-13,16H,1,3,5-6,9H2,(H2,25,26,27,28,29)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 20: 5027-30 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.045
BindingDB Entry DOI: 10.7270/Q2WH2Q5J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM25965

(amino-N-(2-{3-[12-methyl-8-(methylamino)-3-thia-1,...)Show SMILES CNc1nc2cc(sc2n2c(C)cnc12)-c1cccc(CCNS(N)(=O)=O)c1 Show InChI InChI=1S/C18H20N6O2S2/c1-11-10-21-17-16(20-2)23-14-9-15(27-18(14)24(11)17)13-5-3-4-12(8-13)6-7-22-28(19,25)26/h3-5,8-10,22H,6-7H2,1-2H3,(H,20,23)(H2,19,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
| Assay Description
Assays measuring the enzyme-catalyzed phosphorylation of GST-I kappa B alpha were performed. The phosphorylated substrate was detected using a Phosph... |
Bioorg Med Chem Lett 17: 4284-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.031
BindingDB Entry DOI: 10.7270/Q2QF8R50 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM107004

(US8592579, 111)Show SMILES C[C@]1(CCCN1c1nc(Nc2cc([nH]n2)C2CC2)c2cccn2n1)C(=O)Nc1ccncn1 |r| Show InChI InChI=1S/C22H24N10O/c1-22(20(33)26-17-7-9-23-13-24-17)8-3-10-31(22)21-27-19(16-4-2-11-32(16)30-21)25-18-12-15(28-29-18)14-5-6-14/h2,4,7,9,11-14H,3,5-6,8,10H2,1H3,(H,23,24,26,33)(H2,25,27,28,29,30)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Kinase assay using IGF1-receptor. |
US Patent US8592579 (2013)
BindingDB Entry DOI: 10.7270/Q2SQ8Z12 |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM25970

(2-{3-[12-methyl-8-(methylamino)-3-thia-1,7,10-tria...)Show SMILES CNc1nc2cc(sc2n2c(C)cnc12)-c1cccc(CC(N)=O)c1 Show InChI InChI=1S/C18H17N5OS/c1-10-9-21-17-16(20-2)22-13-8-14(25-18(13)23(10)17)12-5-3-4-11(6-12)7-15(19)24/h3-6,8-9H,7H2,1-2H3,(H2,19,24)(H,20,22) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
| Assay Description
Assays measuring the enzyme-catalyzed phosphorylation of GST-I kappa B alpha were performed. The phosphorylated substrate was detected using a Phosph... |
Bioorg Med Chem Lett 17: 4284-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.031
BindingDB Entry DOI: 10.7270/Q2QF8R50 |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM25942

((3-{8-[(2-aminoethyl)amino]-12-methyl-3-thia-1,7,1...)Show InChI InChI=1S/C18H19N5OS/c1-11-9-21-17-16(20-6-5-19)22-14-8-15(25-18(14)23(11)17)13-4-2-3-12(7-13)10-24/h2-4,7-9,24H,5-6,10,19H2,1H3,(H,20,22) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
| Assay Description
Assays measuring the enzyme-catalyzed phosphorylation of GST-I kappa B alpha were performed. The phosphorylated substrate was detected using a Phosph... |
Bioorg Med Chem Lett 17: 4284-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.031
BindingDB Entry DOI: 10.7270/Q2QF8R50 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50299148

((S)-1-(4-(5-cyclopropyl-1H-pyrazol-3-ylamino)pyrro...)Show SMILES C[C@]1(CCCN1c1nc(Nc2cc([nH]n2)C2CC2)c2cccn2n1)C(=O)Nc1ccc(F)nc1 |r| Show InChI InChI=1S/C23H24FN9O/c1-23(21(34)26-15-7-8-18(24)25-13-15)9-3-10-32(23)22-28-20(17-4-2-11-33(17)31-22)27-19-12-16(29-30-19)14-5-6-14/h2,4,7-8,11-14H,3,5-6,9-10H2,1H3,(H,26,34)(H2,27,28,29,30,31)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 20: 5027-30 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.045
BindingDB Entry DOI: 10.7270/Q2WH2Q5J |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM25957

(4-[3-(aminomethyl)phenyl]-N,12-dimethyl-3-thia-1,7...)Show InChI InChI=1S/C17H17N5S/c1-10-9-20-16-15(19-2)21-13-7-14(23-17(13)22(10)16)12-5-3-4-11(6-12)8-18/h3-7,9H,8,18H2,1-2H3,(H,19,21) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
| Assay Description
Assays measuring the enzyme-catalyzed phosphorylation of GST-I kappa B alpha were performed. The phosphorylated substrate was detected using a Phosph... |
Bioorg Med Chem Lett 17: 4284-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.031
BindingDB Entry DOI: 10.7270/Q2QF8R50 |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM25971

(3-{3-[12-methyl-8-(methylamino)-3-thia-1,7,10-tria...)Show SMILES CNc1nc2cc(sc2n2c(C)cnc12)-c1cccc(CCC(O)=O)c1 Show InChI InChI=1S/C19H18N4O2S/c1-11-10-21-18-17(20-2)22-14-9-15(26-19(14)23(11)18)13-5-3-4-12(8-13)6-7-16(24)25/h3-5,8-10H,6-7H2,1-2H3,(H,20,22)(H,24,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <9 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
| Assay Description
Assays measuring the enzyme-catalyzed phosphorylation of GST-I kappa B alpha were performed. The phosphorylated substrate was detected using a Phosph... |
Bioorg Med Chem Lett 17: 4284-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.031
BindingDB Entry DOI: 10.7270/Q2QF8R50 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50299144

((S)-1-(4-(5-cyclopropyl-1H-pyrazol-3-ylamino)pyrro...)Show SMILES O=C(Nc1cccnc1)[C@@H]1CCCN1c1nc(Nc2cc([nH]n2)C2CC2)c2cccn2n1 |r| Show InChI InChI=1S/C22H23N9O/c32-21(24-15-4-1-9-23-13-15)18-6-2-10-30(18)22-26-20(17-5-3-11-31(17)29-22)25-19-12-16(27-28-19)14-7-8-14/h1,3-5,9,11-14,18H,2,6-8,10H2,(H,24,32)(H2,25,26,27,28,29)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R after 60 mins by fluorescence electrophoresis |
J Med Chem 52: 7360-3 (2009)
Article DOI: 10.1021/jm900786r
BindingDB Entry DOI: 10.7270/Q2P27022 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50325428
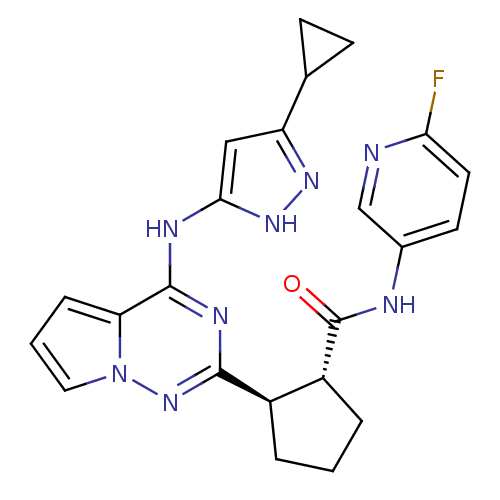
(CHEMBL1222791 | trans-2-(4-(5-cyclopropyl-1H-pyraz...)Show SMILES Fc1ccc(NC(=O)[C@@H]2CCC[C@H]2c2nc(Nc3cc(n[nH]3)C3CC3)c3cccn3n2)cn1 |r| Show InChI InChI=1S/C23H23FN8O/c24-19-9-8-14(12-25-19)26-23(33)16-4-1-3-15(16)21-28-22(18-5-2-10-32(18)31-21)27-20-11-17(29-30-20)13-6-7-13/h2,5,8-13,15-16H,1,3-4,6-7H2,(H,26,33)(H2,27,28,29,30,31)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 20: 5027-30 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.045
BindingDB Entry DOI: 10.7270/Q2WH2Q5J |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50325428

(CHEMBL1222791 | trans-2-(4-(5-cyclopropyl-1H-pyraz...)Show SMILES Fc1ccc(NC(=O)[C@@H]2CCC[C@H]2c2nc(Nc3cc(n[nH]3)C3CC3)c3cccn3n2)cn1 |r| Show InChI InChI=1S/C23H23FN8O/c24-19-9-8-14(12-25-19)26-23(33)16-4-1-3-15(16)21-28-22(18-5-2-10-32(18)31-21)27-20-11-17(29-30-20)13-6-7-13/h2,5,8-13,15-16H,1,3-4,6-7H2,(H,26,33)(H2,27,28,29,30,31)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 20: 5027-30 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.045
BindingDB Entry DOI: 10.7270/Q2WH2Q5J |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM25934
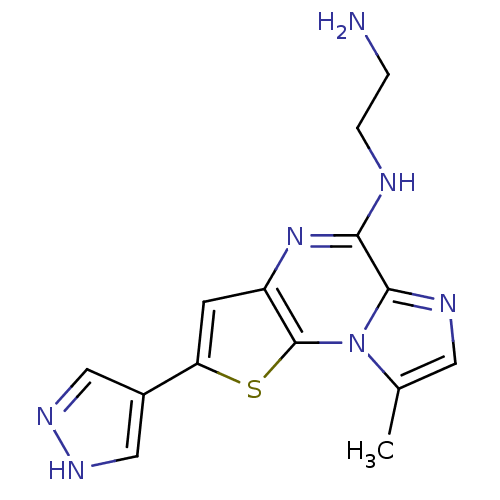
(N-(2-aminoethyl)-12-methyl-4-(1H-pyrazol-4-yl)-3-t...)Show InChI InChI=1S/C14H15N7S/c1-8-5-17-13-12(16-3-2-15)20-10-4-11(9-6-18-19-7-9)22-14(10)21(8)13/h4-7H,2-3,15H2,1H3,(H,16,20)(H,18,19) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
| Assay Description
Assays measuring the enzyme-catalyzed phosphorylation of GST-I kappa B alpha were performed. The phosphorylated substrate was detected using a Phosph... |
Bioorg Med Chem Lett 17: 4284-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.031
BindingDB Entry DOI: 10.7270/Q2QF8R50 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50325427
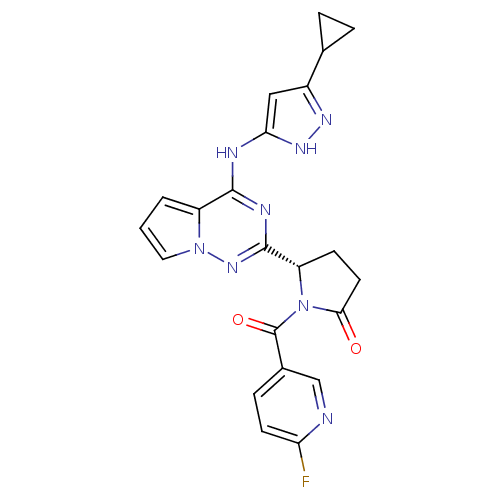
((S)-5-(4-(5-cyclopropyl-1H-pyrazol-3-ylamino)pyrro...)Show SMILES Fc1ccc(cn1)C(=O)N1[C@@H](CCC1=O)c1nc(Nc2cc(n[nH]2)C2CC2)c2cccn2n1 |r| Show InChI InChI=1S/C22H19FN8O2/c23-17-7-5-13(11-24-17)22(33)31-16(6-8-19(31)32)21-26-20(15-2-1-9-30(15)29-21)25-18-10-14(27-28-18)12-3-4-12/h1-2,5,7,9-12,16H,3-4,6,8H2,(H2,25,26,27,28,29)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 20: 5027-30 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.045
BindingDB Entry DOI: 10.7270/Q2WH2Q5J |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Mus musculus) | BDBM50299148

((S)-1-(4-(5-cyclopropyl-1H-pyrazol-3-ylamino)pyrro...)Show SMILES C[C@]1(CCCN1c1nc(Nc2cc([nH]n2)C2CC2)c2cccn2n1)C(=O)Nc1ccc(F)nc1 |r| Show InChI InChI=1S/C23H24FN9O/c1-23(21(34)26-15-7-8-18(24)25-13-15)9-3-10-32(23)22-28-20(17-4-2-11-33(17)31-22)27-19-12-16(29-30-19)14-5-6-14/h2,4,7-8,11-14H,3,5-6,9-10H2,1H3,(H,26,34)(H2,27,28,29,30,31)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R phosphorylation in mouse Sal cells by western blotting |
J Med Chem 52: 7360-3 (2009)
Article DOI: 10.1021/jm900786r
BindingDB Entry DOI: 10.7270/Q2P27022 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50299140
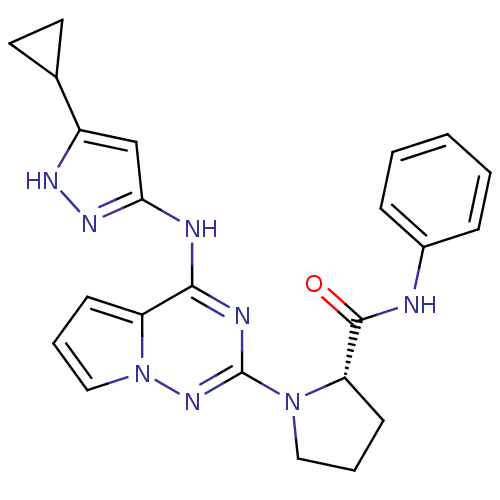
((S)-1-(4-(5-cyclopropyl-1H-pyrazol-3-ylamino)pyrro...)Show SMILES O=C(Nc1ccccc1)[C@@H]1CCCN1c1nc(Nc2cc([nH]n2)C2CC2)c2cccn2n1 |r| Show InChI InChI=1S/C23H24N8O/c32-22(24-16-6-2-1-3-7-16)19-9-4-12-30(19)23-26-21(18-8-5-13-31(18)29-23)25-20-14-17(27-28-20)15-10-11-15/h1-3,5-8,13-15,19H,4,9-12H2,(H,24,32)(H2,25,26,27,28,29)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R after 60 mins by fluorescence electrophoresis |
J Med Chem 52: 7360-3 (2009)
Article DOI: 10.1021/jm900786r
BindingDB Entry DOI: 10.7270/Q2P27022 |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM25973

(2-amino-3-{3-[12-methyl-8-(methylamino)-3-thia-1,7...)Show SMILES CNc1nc2cc(sc2n2c(C)cnc12)-c1cccc(CC(N)C(O)=O)c1 Show InChI InChI=1S/C19H19N5O2S/c1-10-9-22-17-16(21-2)23-14-8-15(27-18(14)24(10)17)12-5-3-4-11(6-12)7-13(20)19(25)26/h3-6,8-9,13H,7,20H2,1-2H3,(H,21,23)(H,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
| Assay Description
Assays measuring the enzyme-catalyzed phosphorylation of GST-I kappa B alpha were performed. The phosphorylated substrate was detected using a Phosph... |
Bioorg Med Chem Lett 17: 4284-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.031
BindingDB Entry DOI: 10.7270/Q2QF8R50 |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM25966
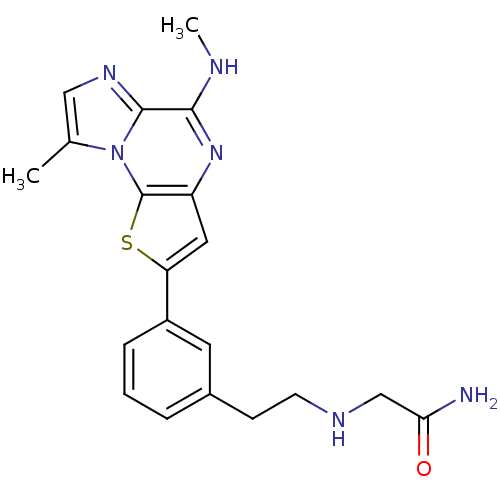
(2-[(2-{3-[12-methyl-8-(methylamino)-3-thia-1,7,10-...)Show SMILES CNc1nc2cc(sc2n2c(C)cnc12)-c1cccc(CCNCC(N)=O)c1 Show InChI InChI=1S/C20H22N6OS/c1-12-10-24-19-18(22-2)25-15-9-16(28-20(15)26(12)19)14-5-3-4-13(8-14)6-7-23-11-17(21)27/h3-5,8-10,23H,6-7,11H2,1-2H3,(H2,21,27)(H,22,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
| Assay Description
Assays measuring the enzyme-catalyzed phosphorylation of GST-I kappa B alpha were performed. The phosphorylated substrate was detected using a Phosph... |
Bioorg Med Chem Lett 17: 4284-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.031
BindingDB Entry DOI: 10.7270/Q2QF8R50 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50217195
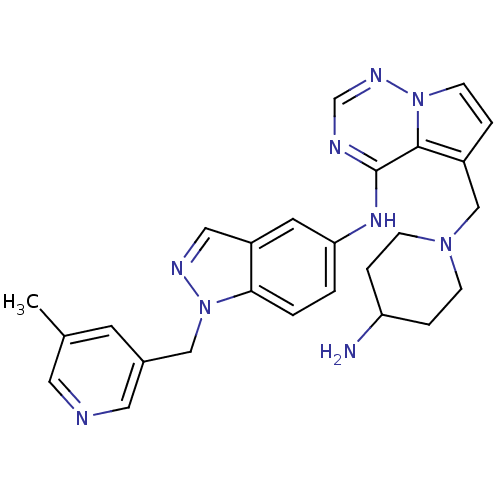
(5-((4-aminopiperidin-1-yl)methyl)-N-(1-((5-methylp...)Show SMILES Cc1cncc(Cn2ncc3cc(Nc4ncnn5ccc(CN6CCC(N)CC6)c45)ccc23)c1 Show InChI InChI=1S/C26H29N9/c1-18-10-19(13-28-12-18)15-35-24-3-2-23(11-21(24)14-30-35)32-26-25-20(4-9-34(25)31-17-29-26)16-33-7-5-22(27)6-8-33/h2-4,9-14,17,22H,5-8,15-16,27H2,1H3,(H,29,31,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HER2 expressed in insect Sf9 cells |
Bioorg Med Chem Lett 17: 4947-54 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.019
BindingDB Entry DOI: 10.7270/Q22R3RC0 |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM25975
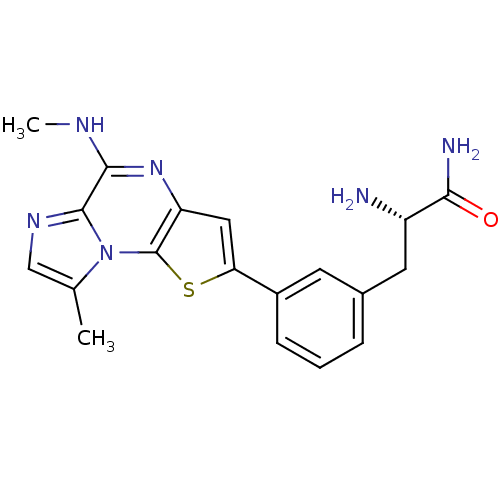
((2S)-2-amino-3-{3-[12-methyl-8-(methylamino)-3-thi...)Show SMILES CNc1nc2cc(sc2n2c(C)cnc12)-c1cccc(C[C@H](N)C(N)=O)c1 |r| Show InChI InChI=1S/C19H20N6OS/c1-10-9-23-18-17(22-2)24-14-8-15(27-19(14)25(10)18)12-5-3-4-11(6-12)7-13(20)16(21)26/h3-6,8-9,13H,7,20H2,1-2H3,(H2,21,26)(H,22,24)/t13-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
| Assay Description
Assays measuring the enzyme-catalyzed phosphorylation of GST-I kappa B alpha were performed. The phosphorylated substrate was detected using a Phosph... |
Bioorg Med Chem Lett 17: 4284-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.031
BindingDB Entry DOI: 10.7270/Q2QF8R50 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM107027

(US8592579, 317)Show SMILES C[C@]1(CCCN1c1nc(Nc2ncc(s2)C(N)=O)c2cccn2n1)C(=O)Nc1ccc(F)nc1 |r| Show InChI InChI=1S/C21H20FN9O2S/c1-21(18(33)26-12-5-6-15(22)24-10-12)7-3-8-30(21)19-27-17(13-4-2-9-31(13)29-19)28-20-25-11-14(34-20)16(23)32/h2,4-6,9-11H,3,7-8H2,1H3,(H2,23,32)(H,26,33)(H,25,27,28,29)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Kinase assay using IGF1-receptor. |
US Patent US8592579 (2013)
BindingDB Entry DOI: 10.7270/Q2SQ8Z12 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM107002

(US8592579, 107)Show SMILES C[C@]1(CCCN1c1nc(Nc2cc(n[nH]2)C2CC2)c2cccn2n1)C(=O)Nc1ncc(Cl)s1 |r| Show InChI InChI=1S/C21H22ClN9OS/c1-21(18(32)26-20-23-11-15(22)33-20)7-3-8-30(21)19-25-17(14-4-2-9-31(14)29-19)24-16-10-13(27-28-16)12-5-6-12/h2,4,9-12H,3,5-8H2,1H3,(H,23,26,32)(H2,24,25,27,28,29)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Kinase assay using IGF1-receptor. |
US Patent US8592579 (2013)
BindingDB Entry DOI: 10.7270/Q2SQ8Z12 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50208409
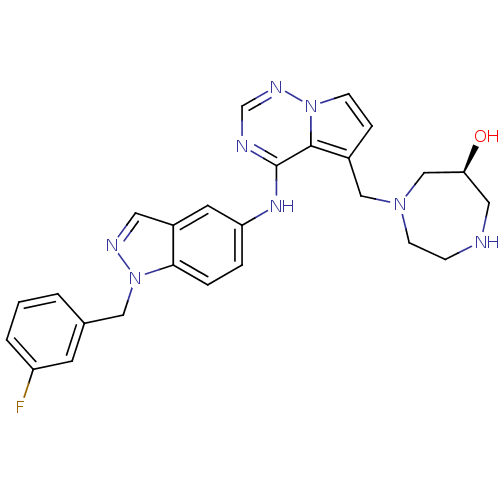
((S)-1-((4-(1-(3-fluorobenzyl)-1H-indazol-5-ylamino...)Show SMILES O[C@H]1CNCCN(Cc2ccn3ncnc(Nc4ccc5n(Cc6cccc(F)c6)ncc5c4)c23)C1 Show InChI InChI=1S/C26H27FN8O/c27-21-3-1-2-18(10-21)14-35-24-5-4-22(11-20(24)12-30-35)32-26-25-19(6-8-34(25)31-17-29-26)15-33-9-7-28-13-23(36)16-33/h1-6,8,10-12,17,23,28,36H,7,9,13-16H2,(H,29,31,32)/t23-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human HER2 |
Bioorg Med Chem Lett 17: 2828-33 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.050
BindingDB Entry DOI: 10.7270/Q2TM79SZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data