 Found 1369 hits with Last Name = 'watanabe' and Initial = 'k'
Found 1369 hits with Last Name = 'watanabe' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50016326
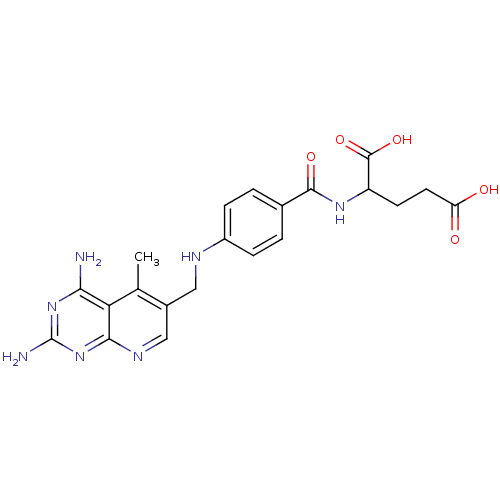
(2-{4-[(2,4-Diamino-5-methyl-pyrido[2,3-d]pyrimidin...)Show SMILES Cc1c(CNc2ccc(cc2)C(=O)NC(CCC(O)=O)C(O)=O)cnc2nc(N)nc(N)c12 Show InChI InChI=1S/C21H23N7O5/c1-10-12(9-25-18-16(10)17(22)27-21(23)28-18)8-24-13-4-2-11(3-5-13)19(31)26-14(20(32)33)6-7-15(29)30/h2-5,9,14,24H,6-8H2,1H3,(H,26,31)(H,29,30)(H,32,33)(H4,22,23,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by ChEMBL
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells |
J Med Chem 31: 1209-15 (1988)
BindingDB Entry DOI: 10.7270/Q2930S6N |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM66082

((2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methy...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by ChEMBL
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells |
J Med Chem 31: 1209-15 (1988)
BindingDB Entry DOI: 10.7270/Q2930S6N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50023681

(2-{4-[(2,4-Diamino-5,7-dimethyl-pyrido[2,3-d]pyrim...)Show SMILES Cc1nc2nc(N)nc(N)c2c(C)c1CNc1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O Show InChI InChI=1S/C22H25N7O5/c1-10-14(11(2)26-19-17(10)18(23)28-22(24)29-19)9-25-13-5-3-12(4-6-13)20(32)27-15(21(33)34)7-8-16(30)31/h3-6,15,25H,7-9H2,1-2H3,(H,27,32)(H,30,31)(H,33,34)(H4,23,24,26,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by ChEMBL
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells |
J Med Chem 31: 1209-15 (1988)
BindingDB Entry DOI: 10.7270/Q2930S6N |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50023682

(2-{4-[(2,4-Diamino-5-methyl-7-phenyl-pyrido[2,3-d]...)Show SMILES Cc1c(CNc2ccc(cc2)C(=O)NC(CCC(O)=O)C(O)=O)c(nc2nc(N)nc(N)c12)-c1ccccc1 Show InChI InChI=1S/C27H27N7O5/c1-14-18(22(15-5-3-2-4-6-15)32-24-21(14)23(28)33-27(29)34-24)13-30-17-9-7-16(8-10-17)25(37)31-19(26(38)39)11-12-20(35)36/h2-10,19,30H,11-13H2,1H3,(H,31,37)(H,35,36)(H,38,39)(H4,28,29,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by ChEMBL
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells |
J Med Chem 31: 1209-15 (1988)
BindingDB Entry DOI: 10.7270/Q2930S6N |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50023684
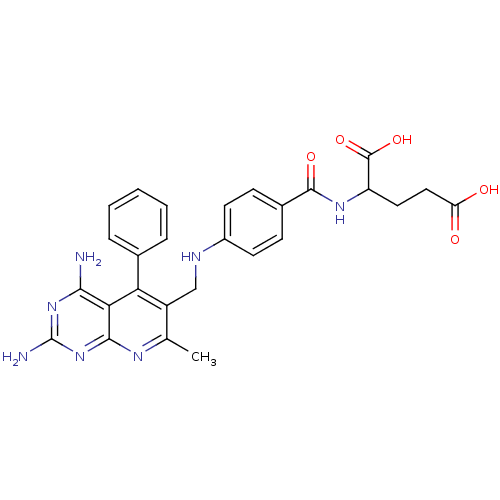
(2-{4-[(2,4-Diamino-7-methyl-5-phenyl-pyrido[2,3-d]...)Show SMILES Cc1nc2nc(N)nc(N)c2c(-c2ccccc2)c1CNc1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O Show InChI InChI=1S/C27H27N7O5/c1-14-18(21(15-5-3-2-4-6-15)22-23(28)33-27(29)34-24(22)31-14)13-30-17-9-7-16(8-10-17)25(37)32-19(26(38)39)11-12-20(35)36/h2-10,19,30H,11-13H2,1H3,(H,32,37)(H,35,36)(H,38,39)(H4,28,29,31,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by ChEMBL
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells |
J Med Chem 31: 1209-15 (1988)
BindingDB Entry DOI: 10.7270/Q2930S6N |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50023680

(2-{4-[(2,4-Diamino-7-phenyl-pyrido[2,3-d]pyrimidin...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)c(nc2n1)-c1ccccc1 Show InChI InChI=1S/C26H25N7O5/c27-22-18-12-16(21(14-4-2-1-3-5-14)31-23(18)33-26(28)32-22)13-29-17-8-6-15(7-9-17)24(36)30-19(25(37)38)10-11-20(34)35/h1-9,12,19,29H,10-11,13H2,(H,30,36)(H,34,35)(H,37,38)(H4,27,28,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by ChEMBL
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells |
J Med Chem 31: 1209-15 (1988)
BindingDB Entry DOI: 10.7270/Q2930S6N |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50023683
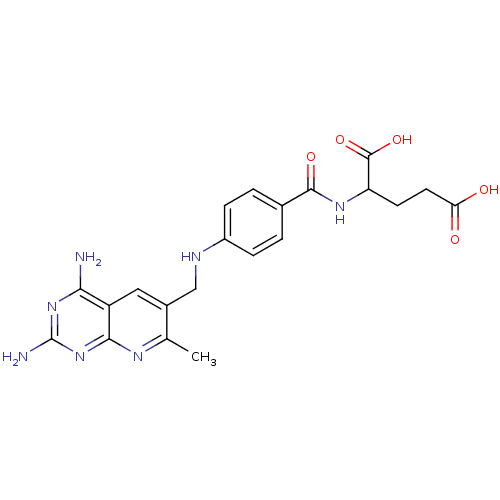
(2-{4-[(2,4-Diamino-7-methyl-pyrido[2,3-d]pyrimidin...)Show SMILES Cc1nc2nc(N)nc(N)c2cc1CNc1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O Show InChI InChI=1S/C21H23N7O5/c1-10-12(8-14-17(22)27-21(23)28-18(14)25-10)9-24-13-4-2-11(3-5-13)19(31)26-15(20(32)33)6-7-16(29)30/h2-5,8,15,24H,6-7,9H2,1H3,(H,26,31)(H,29,30)(H,32,33)(H4,22,23,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by ChEMBL
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells |
J Med Chem 31: 1209-15 (1988)
BindingDB Entry DOI: 10.7270/Q2930S6N |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50474150
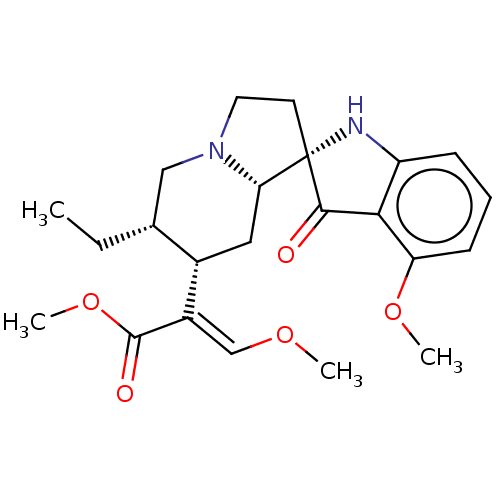
(CHEMBL58362)Show SMILES [H][C@@]12C[C@]([H])(C(=C/OC)\C(=O)OC)[C@]([H])(CC)CN1CC[C@]21Nc2cccc(OC)c2C1=O Show InChI InChI=1S/C23H30N2O5/c1-5-14-12-25-10-9-23(19(25)11-15(14)16(13-28-2)22(27)30-4)21(26)20-17(24-23)7-6-8-18(20)29-3/h6-8,13-15,19,24H,5,9-12H2,1-4H3/b16-13+/t14-,15+,19+,23+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.0871 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University
Curated by ChEMBL
| Assay Description
Binding affinity using guinea pig brain membrane preparations, towards Opioid receptor mu 1 using [3H]- DAMGO as radioligand |
J Med Chem 45: 1949-56 (2002)
Article DOI: 10.1021/jm010576e
BindingDB Entry DOI: 10.7270/Q2WS8X0T |
More data for this
Ligand-Target Pair | |
Alcohol dehydrogenase E/S chain
(Equus caballus) | BDBM50368629

(CHEMBL2368671)Show SMILES NC(=O)c1cncc(c1)[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)OC[C@@H]2O[C@@H]([C@@H](O)[C@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C21H27N7O14P2/c22-18-12-20(26-6-25-18)28(7-27-12)21-16(32)14(30)11(41-21)5-39-44(36,37)42-43(34,35)38-4-10-13(29)15(31)17(40-10)8-1-9(19(23)33)3-24-2-8/h1-3,6-7,10-11,13-17,21,29-32H,4-5H2,(H2,23,33)(H,34,35)(H,36,37)(H2,22,25,26)/t10-,11+,13-,14+,15-,16+,17+,21+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by ChEMBL
| Assay Description
Inhibition of horse liver alcohol dehydrogenase enzyme by Non-competitive inhibition |
J Med Chem 36: 1855-9 (1993)
BindingDB Entry DOI: 10.7270/Q2X63NM4 |
More data for this
Ligand-Target Pair | |
Alcohol dehydrogenase 1A
(Homo sapiens (Human)) | BDBM50368954
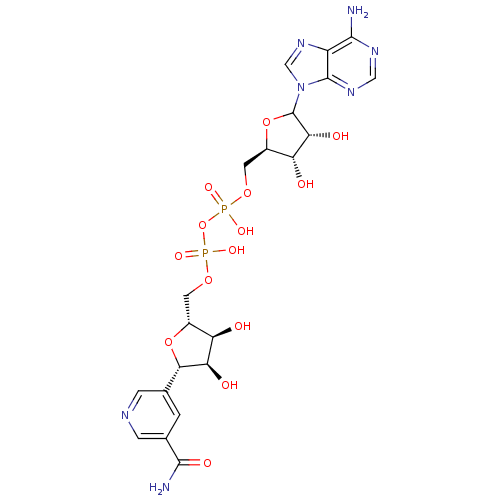
(CHEMBL610377)Show SMILES NC(=O)c1cncc(c1)[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)OC[C@H]2OC([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C21H27N7O14P2/c22-18-12-20(26-6-25-18)28(7-27-12)21-16(32)14(30)11(41-21)5-39-44(36,37)42-43(34,35)38-4-10-13(29)15(31)17(40-10)8-1-9(19(23)33)3-24-2-8/h1-3,6-7,10-11,13-17,21,29-32H,4-5H2,(H2,23,33)(H,34,35)(H,36,37)(H2,22,25,26)/t10-,11-,13-,14-,15-,16-,17+,21?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester Medical Center
Curated by ChEMBL
| Assay Description
Apparent inhibition constant against mammalian liver alcohol dehydrogenase (ADH) |
J Med Chem 37: 392-9 (1994)
BindingDB Entry DOI: 10.7270/Q2RN38H5 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50474150

(CHEMBL58362)Show SMILES [H][C@@]12C[C@]([H])(C(=C/OC)\C(=O)OC)[C@]([H])(CC)CN1CC[C@]21Nc2cccc(OC)c2C1=O Show InChI InChI=1S/C23H30N2O5/c1-5-14-12-25-10-9-23(19(25)11-15(14)16(13-28-2)22(27)30-4)21(26)20-17(24-23)7-6-8-18(20)29-3/h6-8,13-15,19,24H,5,9-12H2,1-4H3/b16-13+/t14-,15+,19+,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University
Curated by ChEMBL
| Assay Description
Binding affinity using guinea pig brain membrane preparations, towards Opioid receptor delta 1 using [3H]DPDPE as radioligand |
J Med Chem 45: 1949-56 (2002)
Article DOI: 10.1021/jm010576e
BindingDB Entry DOI: 10.7270/Q2WS8X0T |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University
Curated by ChEMBL
| Assay Description
Binding affinity using guinea pig brain membrane preparations, towards Opioid receptor mu 1 using [3H]- DAMGO as radioligand |
J Med Chem 45: 1949-56 (2002)
Article DOI: 10.1021/jm010576e
BindingDB Entry DOI: 10.7270/Q2WS8X0T |
More data for this
Ligand-Target Pair | |
Alcohol dehydrogenase 1A
(Homo sapiens (Human)) | BDBM50368954

(CHEMBL610377)Show SMILES NC(=O)c1cncc(c1)[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)OC[C@H]2OC([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C21H27N7O14P2/c22-18-12-20(26-6-25-18)28(7-27-12)21-16(32)14(30)11(41-21)5-39-44(36,37)42-43(34,35)38-4-10-13(29)15(31)17(40-10)8-1-9(19(23)33)3-24-2-8/h1-3,6-7,10-11,13-17,21,29-32H,4-5H2,(H2,23,33)(H,34,35)(H,36,37)(H2,22,25,26)/t10-,11-,13-,14-,15-,16-,17+,21?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester Medical Center
Curated by ChEMBL
| Assay Description
Inhibition constant against mammalian liver alcohol dehydrogenase (ADH) |
J Med Chem 37: 392-9 (1994)
BindingDB Entry DOI: 10.7270/Q2RN38H5 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM19264

((4E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dih...)Show InChI InChI=1S/C17H20O6/c1-9(5-7-13(18)19)4-6-11-15(20)14-12(8-23-17(14)21)10(2)16(11)22-3/h4,20H,5-8H2,1-3H3,(H,18,19)/b9-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Codon Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inosine-5'-monophosphate dehydrogenase 2 (IMPDH type II isoform); Range is 6-10 nM |
J Med Chem 41: 618-22 (1998)
Article DOI: 10.1021/jm970705k
BindingDB Entry DOI: 10.7270/Q20C4WGZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50474152
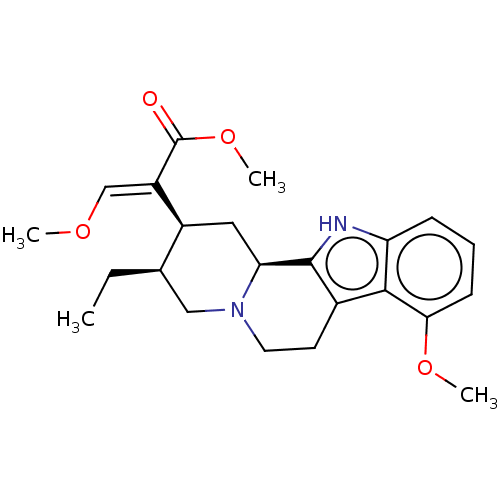
(CHEBI:6956 | CHEMBL299031)Show SMILES [H][C@@]1(CC)CN2CCc3c([nH]c4cccc(OC)c34)[C@]2([H])C[C@]1([H])C(=C/OC)\C(=O)OC Show InChI InChI=1S/C23H30N2O4/c1-5-14-12-25-10-9-15-21-18(7-6-8-20(21)28-3)24-22(15)19(25)11-16(14)17(13-27-2)23(26)29-4/h6-8,13-14,16,19,24H,5,9-12H2,1-4H3/b17-13+/t14-,16+,19+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University
Curated by ChEMBL
| Assay Description
Binding affinity using guinea pig brain membrane preparations, towards Opioid receptor mu 1 using [3H]- DAMGO as radioligand |
J Med Chem 45: 1949-56 (2002)
Article DOI: 10.1021/jm010576e
BindingDB Entry DOI: 10.7270/Q2WS8X0T |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM19264

((4E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dih...)Show InChI InChI=1S/C17H20O6/c1-9(5-7-13(18)19)4-6-11-15(20)14-12(8-23-17(14)21)10(2)16(11)22-3/h4,20H,5-8H2,1-3H3,(H,18,19)/b9-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmasset Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inosine-5'-monophosphate dehydrogenase 2 (IMPDH type II) |
J Med Chem 45: 703-12 (2002)
BindingDB Entry DOI: 10.7270/Q29S1RS4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM19264

((4E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dih...)Show InChI InChI=1S/C17H20O6/c1-9(5-7-13(18)19)4-6-11-15(20)14-12(8-23-17(14)21)10(2)16(11)22-3/h4,20H,5-8H2,1-3H3,(H,18,19)/b9-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmasset Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inosine-5'-monophosphate dehydrogenase 2 (IMPDH type II) |
J Med Chem 45: 703-12 (2002)
BindingDB Entry DOI: 10.7270/Q29S1RS4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50474151

(CHEMBL292521)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)CN1CCc1c2[nH]c2cccc(O)c12)C(=C/OC)\C(=O)OC Show InChI InChI=1S/C22H28N2O4/c1-4-13-11-24-9-8-14-20-17(6-5-7-19(20)25)23-21(14)18(24)10-15(13)16(12-27-2)22(26)28-3/h5-7,12-13,15,18,23,25H,4,8-11H2,1-3H3/b16-12+/t13-,15+,18+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University
Curated by ChEMBL
| Assay Description
Binding affinity using guinea pig brain membrane preparations, towards Opioid receptor mu 1 using [3H]- DAMGO as radioligand |
J Med Chem 45: 1949-56 (2002)
Article DOI: 10.1021/jm010576e
BindingDB Entry DOI: 10.7270/Q2WS8X0T |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50474153

(CHEMBL61630)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)CN1CC[C@@]1(O)C2=Nc2cccc(OC)c12)C(=C/OC)\C(=O)OC |t:15| Show InChI InChI=1S/C23H30N2O5/c1-5-14-12-25-10-9-23(27)20-17(7-6-8-19(20)29-3)24-21(23)18(25)11-15(14)16(13-28-2)22(26)30-4/h6-8,13-15,18,27H,5,9-12H2,1-4H3/b16-13+/t14-,15+,18+,23+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University
Curated by ChEMBL
| Assay Description
Binding affinity using guinea pig brain membrane preparations, towards Opioid receptor mu 1 using [3H]- DAMGO as radioligand |
J Med Chem 45: 1949-56 (2002)
Article DOI: 10.1021/jm010576e
BindingDB Entry DOI: 10.7270/Q2WS8X0T |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 1
(Homo sapiens (Human)) | BDBM19264

((4E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dih...)Show InChI InChI=1S/C17H20O6/c1-9(5-7-13(18)19)4-6-11-15(20)14-12(8-23-17(14)21)10(2)16(11)22-3/h4,20H,5-8H2,1-3H3,(H,18,19)/b9-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Codon Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inosine-5'-monophosphate dehydrogenase 1 (IMPDH type I isoform); Range is 33-37 nM |
J Med Chem 41: 618-22 (1998)
Article DOI: 10.1021/jm970705k
BindingDB Entry DOI: 10.7270/Q20C4WGZ |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 1
(Homo sapiens (Human)) | BDBM19264

((4E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dih...)Show InChI InChI=1S/C17H20O6/c1-9(5-7-13(18)19)4-6-11-15(20)14-12(8-23-17(14)21)10(2)16(11)22-3/h4,20H,5-8H2,1-3H3,(H,18,19)/b9-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmasset Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inosine-5'-monophosphate dehydrogenase 1 (IMPDH type I) |
J Med Chem 45: 703-12 (2002)
BindingDB Entry DOI: 10.7270/Q29S1RS4 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 1
(Homo sapiens (Human)) | BDBM19264

((4E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dih...)Show InChI InChI=1S/C17H20O6/c1-9(5-7-13(18)19)4-6-11-15(20)14-12(8-23-17(14)21)10(2)16(11)22-3/h4,20H,5-8H2,1-3H3,(H,18,19)/b9-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmasset Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inosine-5'-monophosphate dehydrogenase 1 (IMPDH type I) |
J Med Chem 45: 703-12 (2002)
BindingDB Entry DOI: 10.7270/Q29S1RS4 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50474152

(CHEBI:6956 | CHEMBL299031)Show SMILES [H][C@@]1(CC)CN2CCc3c([nH]c4cccc(OC)c34)[C@]2([H])C[C@]1([H])C(=C/OC)\C(=O)OC Show InChI InChI=1S/C23H30N2O4/c1-5-14-12-25-10-9-15-21-18(7-6-8-20(21)28-3)24-22(15)19(25)11-16(14)17(13-27-2)23(26)29-4/h6-8,13-14,16,19,24H,5,9-12H2,1-4H3/b17-13+/t14-,16+,19+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University
Curated by ChEMBL
| Assay Description
Binding affinity using guinea pig brain membrane preparations, towards Opioid receptor delta 1 using [3H]DPDPE as radioligand |
J Med Chem 45: 1949-56 (2002)
Article DOI: 10.1021/jm010576e
BindingDB Entry DOI: 10.7270/Q2WS8X0T |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50474149
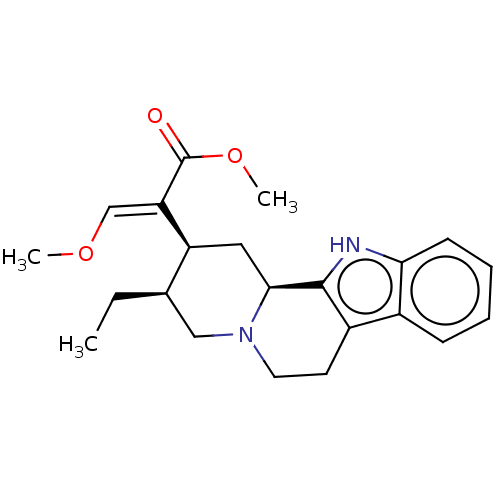
(CHEBI:70073 | Corynanrheidine)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)CN1CCc1c2[nH]c2ccccc12)C(=C/OC)\C(=O)OC |r| Show InChI InChI=1S/C22H28N2O3/c1-4-14-12-24-10-9-16-15-7-5-6-8-19(15)23-21(16)20(24)11-17(14)18(13-26-2)22(25)27-3/h5-8,13-14,17,20,23H,4,9-12H2,1-3H3/b18-13+/t14-,17+,20+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University
Curated by ChEMBL
| Assay Description
Binding affinity using guinea pig brain membrane preparations, towards Opioid receptor mu 1 using [3H]- DAMGO as radioligand |
J Med Chem 45: 1949-56 (2002)
Article DOI: 10.1021/jm010576e
BindingDB Entry DOI: 10.7270/Q2WS8X0T |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50474150

(CHEMBL58362)Show SMILES [H][C@@]12C[C@]([H])(C(=C/OC)\C(=O)OC)[C@]([H])(CC)CN1CC[C@]21Nc2cccc(OC)c2C1=O Show InChI InChI=1S/C23H30N2O5/c1-5-14-12-25-10-9-23(19(25)11-15(14)16(13-28-2)22(27)30-4)21(26)20-17(24-23)7-6-8-18(20)29-3/h6-8,13-15,19,24H,5,9-12H2,1-4H3/b16-13+/t14-,15+,19+,23+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University
Curated by ChEMBL
| Assay Description
Binding affinity using guinea pig brain membrane preparations, towards Opioid receptor kappa 1 using [3H]- U-69,593 as radioligand |
J Med Chem 45: 1949-56 (2002)
Article DOI: 10.1021/jm010576e
BindingDB Entry DOI: 10.7270/Q2WS8X0T |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 1
(Homo sapiens (Human)) | BDBM50367389

(CHEMBL605602)Show SMILES NC(=O)c1csc(n1)C1O[C@H](COP(O)(=O)CP(O)(=O)OC[C@@H]2OC([C@@H](O)[C@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H27N7O13P2S/c21-16-10-18(24-4-23-16)27(5-25-10)20-14(31)12(29)9(40-20)2-38-42(35,36)6-41(33,34)37-1-8-11(28)13(30)15(39-8)19-26-7(3-43-19)17(22)32/h3-5,8-9,11-15,20,28-31H,1-2,6H2,(H2,22,32)(H,33,34)(H,35,36)(H2,21,23,24)/t8-,9+,11-,12+,13-,14+,15?,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmasset Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inosine-5'-monophosphate dehydrogenase 1 (IMPDH type I) |
J Med Chem 45: 703-12 (2002)
BindingDB Entry DOI: 10.7270/Q29S1RS4 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50367389

(CHEMBL605602)Show SMILES NC(=O)c1csc(n1)C1O[C@H](COP(O)(=O)CP(O)(=O)OC[C@@H]2OC([C@@H](O)[C@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H27N7O13P2S/c21-16-10-18(24-4-23-16)27(5-25-10)20-14(31)12(29)9(40-20)2-38-42(35,36)6-41(33,34)37-1-8-11(28)13(30)15(39-8)19-26-7(3-43-19)17(22)32/h3-5,8-9,11-15,20,28-31H,1-2,6H2,(H2,22,32)(H,33,34)(H,35,36)(H2,21,23,24)/t8-,9+,11-,12+,13-,14+,15?,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmasset Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inosine-5'-monophosphate dehydrogenase 2 (IMPDH type II) |
J Med Chem 45: 703-12 (2002)
BindingDB Entry DOI: 10.7270/Q29S1RS4 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50367389

(CHEMBL605602)Show SMILES NC(=O)c1csc(n1)C1O[C@H](COP(O)(=O)CP(O)(=O)OC[C@@H]2OC([C@@H](O)[C@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H27N7O13P2S/c21-16-10-18(24-4-23-16)27(5-25-10)20-14(31)12(29)9(40-20)2-38-42(35,36)6-41(33,34)37-1-8-11(28)13(30)15(39-8)19-26-7(3-43-19)17(22)32/h3-5,8-9,11-15,20,28-31H,1-2,6H2,(H2,22,32)(H,33,34)(H,35,36)(H2,21,23,24)/t8-,9+,11-,12+,13-,14+,15?,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmasset Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inosine-5'-monophosphate dehydrogenase 2 (IMPDH type II) |
J Med Chem 45: 703-12 (2002)
BindingDB Entry DOI: 10.7270/Q29S1RS4 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 1
(Homo sapiens (Human)) | BDBM50367389

(CHEMBL605602)Show SMILES NC(=O)c1csc(n1)C1O[C@H](COP(O)(=O)CP(O)(=O)OC[C@@H]2OC([C@@H](O)[C@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H27N7O13P2S/c21-16-10-18(24-4-23-16)27(5-25-10)20-14(31)12(29)9(40-20)2-38-42(35,36)6-41(33,34)37-1-8-11(28)13(30)15(39-8)19-26-7(3-43-19)17(22)32/h3-5,8-9,11-15,20,28-31H,1-2,6H2,(H2,22,32)(H,33,34)(H,35,36)(H2,21,23,24)/t8-,9+,11-,12+,13-,14+,15?,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmasset Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inosine-5'-monophosphate dehydrogenase 1 (IMPDH type I) |
J Med Chem 45: 703-12 (2002)
BindingDB Entry DOI: 10.7270/Q29S1RS4 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50474153

(CHEMBL61630)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)CN1CC[C@@]1(O)C2=Nc2cccc(OC)c12)C(=C/OC)\C(=O)OC |t:15| Show InChI InChI=1S/C23H30N2O5/c1-5-14-12-25-10-9-23(27)20-17(7-6-8-19(20)29-3)24-21(23)18(25)11-15(14)16(13-28-2)22(26)30-4/h6-8,13-15,18,27H,5,9-12H2,1-4H3/b16-13+/t14-,15+,18+,23+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University
Curated by ChEMBL
| Assay Description
Binding affinity using guinea pig brain membrane preparations, towards Opioid receptor kappa 1 using [3H]- U-69,593 as radioligand |
J Med Chem 45: 1949-56 (2002)
Article DOI: 10.1021/jm010576e
BindingDB Entry DOI: 10.7270/Q2WS8X0T |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50474153

(CHEMBL61630)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)CN1CC[C@@]1(O)C2=Nc2cccc(OC)c12)C(=C/OC)\C(=O)OC |t:15| Show InChI InChI=1S/C23H30N2O5/c1-5-14-12-25-10-9-23(27)20-17(7-6-8-19(20)29-3)24-21(23)18(25)11-15(14)16(13-28-2)22(26)30-4/h6-8,13-15,18,27H,5,9-12H2,1-4H3/b16-13+/t14-,15+,18+,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University
Curated by ChEMBL
| Assay Description
Binding affinity using guinea pig brain membrane preparations, towards Opioid receptor delta 1 using [3H]DPDPE as radioligand |
J Med Chem 45: 1949-56 (2002)
Article DOI: 10.1021/jm010576e
BindingDB Entry DOI: 10.7270/Q2WS8X0T |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50370187
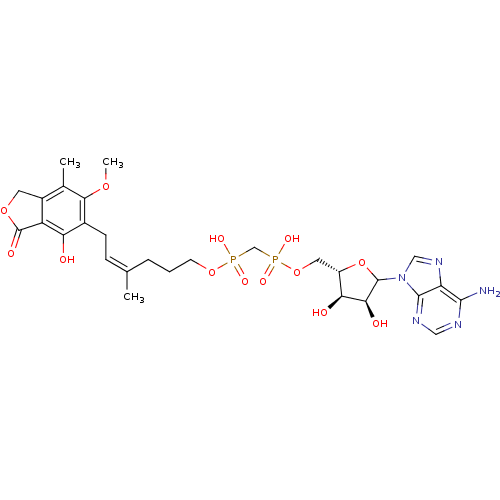
(CHEMBL608195)Show SMILES COc1c(C)c2COC(=O)c2c(O)c1C\C=C(\C)CCCOP(O)(=O)CP(O)(=O)OC[C@@H]1OC([C@@H](O)[C@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C28H37N5O13P2/c1-14(6-7-16-21(34)19-17(9-43-28(19)37)15(2)24(16)42-3)5-4-8-44-47(38,39)13-48(40,41)45-10-18-22(35)23(36)27(46-18)33-12-32-20-25(29)30-11-31-26(20)33/h6,11-12,18,22-23,27,34-36H,4-5,7-10,13H2,1-3H3,(H,38,39)(H,40,41)(H2,29,30,31)/b14-6-/t18-,22-,23-,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmasset Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inosine-5'-monophosphate dehydrogenase 2 (IMPDH type II) |
J Med Chem 45: 703-12 (2002)
BindingDB Entry DOI: 10.7270/Q29S1RS4 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50370187

(CHEMBL608195)Show SMILES COc1c(C)c2COC(=O)c2c(O)c1C\C=C(\C)CCCOP(O)(=O)CP(O)(=O)OC[C@@H]1OC([C@@H](O)[C@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C28H37N5O13P2/c1-14(6-7-16-21(34)19-17(9-43-28(19)37)15(2)24(16)42-3)5-4-8-44-47(38,39)13-48(40,41)45-10-18-22(35)23(36)27(46-18)33-12-32-20-25(29)30-11-31-26(20)33/h6,11-12,18,22-23,27,34-36H,4-5,7-10,13H2,1-3H3,(H,38,39)(H,40,41)(H2,29,30,31)/b14-6-/t18-,22-,23-,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmasset Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inosine-5'-monophosphate dehydrogenase 2 (IMPDH type II) |
J Med Chem 45: 703-12 (2002)
BindingDB Entry DOI: 10.7270/Q29S1RS4 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 1
(Homo sapiens (Human)) | BDBM50369298

(CHEMBL605601)Show SMILES NC(=O)c1csc(n1)C1O[C@H](COP(O)(=O)C(F)(F)P(O)(=O)OC[C@@H]2OC([C@@H](O)[C@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H25F2N7O13P2S/c21-20(22,43(35,36)39-1-7-10(30)12(32)14(41-7)18-28-6(3-45-18)16(24)34)44(37,38)40-2-8-11(31)13(33)19(42-8)29-5-27-9-15(23)25-4-26-17(9)29/h3-5,7-8,10-14,19,30-33H,1-2H2,(H2,24,34)(H,35,36)(H,37,38)(H2,23,25,26)/t7-,8+,10-,11+,12-,13+,14?,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmasset Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inosine-5'-monophosphate dehydrogenase 1 (IMPDH type I) |
J Med Chem 45: 703-12 (2002)
BindingDB Entry DOI: 10.7270/Q29S1RS4 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50369419

(CHEMBL610421)Show SMILES COc1c(C)c2COC(=O)c2c(O)c1C\C=C(/C)CCCOP(O)(=O)CP(O)(=O)OC[C@@H]1OC([C@@H](O)[C@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C28H37N5O13P2/c1-14(6-7-16-21(34)19-17(9-43-28(19)37)15(2)24(16)42-3)5-4-8-44-47(38,39)13-48(40,41)45-10-18-22(35)23(36)27(46-18)33-12-32-20-25(29)30-11-31-26(20)33/h6,11-12,18,22-23,27,34-36H,4-5,7-10,13H2,1-3H3,(H,38,39)(H,40,41)(H2,29,30,31)/b14-6+/t18-,22-,23-,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Codon Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inosine-5'-monophosphate dehydrogenase 2 (IMPDH type II isoform) |
J Med Chem 41: 618-22 (1998)
Article DOI: 10.1021/jm970705k
BindingDB Entry DOI: 10.7270/Q20C4WGZ |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50369298

(CHEMBL605601)Show SMILES NC(=O)c1csc(n1)C1O[C@H](COP(O)(=O)C(F)(F)P(O)(=O)OC[C@@H]2OC([C@@H](O)[C@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H25F2N7O13P2S/c21-20(22,43(35,36)39-1-7-10(30)12(32)14(41-7)18-28-6(3-45-18)16(24)34)44(37,38)40-2-8-11(31)13(33)19(42-8)29-5-27-9-15(23)25-4-26-17(9)29/h3-5,7-8,10-14,19,30-33H,1-2H2,(H2,24,34)(H,35,36)(H,37,38)(H2,23,25,26)/t7-,8+,10-,11+,12-,13+,14?,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmasset Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inosine-5'-monophosphate dehydrogenase 2 (IMPDH type II) |
J Med Chem 45: 703-12 (2002)
BindingDB Entry DOI: 10.7270/Q29S1RS4 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 1
(Homo sapiens (Human)) | BDBM50369298

(CHEMBL605601)Show SMILES NC(=O)c1csc(n1)C1O[C@H](COP(O)(=O)C(F)(F)P(O)(=O)OC[C@@H]2OC([C@@H](O)[C@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H25F2N7O13P2S/c21-20(22,43(35,36)39-1-7-10(30)12(32)14(41-7)18-28-6(3-45-18)16(24)34)44(37,38)40-2-8-11(31)13(33)19(42-8)29-5-27-9-15(23)25-4-26-17(9)29/h3-5,7-8,10-14,19,30-33H,1-2H2,(H2,24,34)(H,35,36)(H,37,38)(H2,23,25,26)/t7-,8+,10-,11+,12-,13+,14?,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmasset Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inosine-5'-monophosphate dehydrogenase 1 (IMPDH type I) |
J Med Chem 45: 703-12 (2002)
BindingDB Entry DOI: 10.7270/Q29S1RS4 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50369298

(CHEMBL605601)Show SMILES NC(=O)c1csc(n1)C1O[C@H](COP(O)(=O)C(F)(F)P(O)(=O)OC[C@@H]2OC([C@@H](O)[C@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H25F2N7O13P2S/c21-20(22,43(35,36)39-1-7-10(30)12(32)14(41-7)18-28-6(3-45-18)16(24)34)44(37,38)40-2-8-11(31)13(33)19(42-8)29-5-27-9-15(23)25-4-26-17(9)29/h3-5,7-8,10-14,19,30-33H,1-2H2,(H2,24,34)(H,35,36)(H,37,38)(H2,23,25,26)/t7-,8+,10-,11+,12-,13+,14?,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmasset Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inosine-5'-monophosphate dehydrogenase 2 (IMPDH type II) |
J Med Chem 45: 703-12 (2002)
BindingDB Entry DOI: 10.7270/Q29S1RS4 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 1
(Homo sapiens (Human)) | BDBM50370187

(CHEMBL608195)Show SMILES COc1c(C)c2COC(=O)c2c(O)c1C\C=C(\C)CCCOP(O)(=O)CP(O)(=O)OC[C@@H]1OC([C@@H](O)[C@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C28H37N5O13P2/c1-14(6-7-16-21(34)19-17(9-43-28(19)37)15(2)24(16)42-3)5-4-8-44-47(38,39)13-48(40,41)45-10-18-22(35)23(36)27(46-18)33-12-32-20-25(29)30-11-31-26(20)33/h6,11-12,18,22-23,27,34-36H,4-5,7-10,13H2,1-3H3,(H,38,39)(H,40,41)(H2,29,30,31)/b14-6-/t18-,22-,23-,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmasset Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inosine-5'-monophosphate dehydrogenase 1 (IMPDH type I) |
J Med Chem 45: 703-12 (2002)
BindingDB Entry DOI: 10.7270/Q29S1RS4 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 1
(Homo sapiens (Human)) | BDBM50370187

(CHEMBL608195)Show SMILES COc1c(C)c2COC(=O)c2c(O)c1C\C=C(\C)CCCOP(O)(=O)CP(O)(=O)OC[C@@H]1OC([C@@H](O)[C@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C28H37N5O13P2/c1-14(6-7-16-21(34)19-17(9-43-28(19)37)15(2)24(16)42-3)5-4-8-44-47(38,39)13-48(40,41)45-10-18-22(35)23(36)27(46-18)33-12-32-20-25(29)30-11-31-26(20)33/h6,11-12,18,22-23,27,34-36H,4-5,7-10,13H2,1-3H3,(H,38,39)(H,40,41)(H2,29,30,31)/b14-6-/t18-,22-,23-,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmasset Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inosine-5'-monophosphate dehydrogenase 1 (IMPDH type I) |
J Med Chem 45: 703-12 (2002)
BindingDB Entry DOI: 10.7270/Q29S1RS4 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50108912

(CHEMBL162782 | {Hydroxy-[2-(4-hydroxy-6-methoxy-7-...)Show SMILES COc1c(C)c2COC(=O)c2c(O)c1CCOP(O)(=O)CP(O)(O)=O Show InChI InChI=1S/C13H18O10P2/c1-7-9-5-22-13(15)10(9)11(14)8(12(7)21-2)3-4-23-25(19,20)6-24(16,17)18/h14H,3-6H2,1-2H3,(H,19,20)(H2,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmasset Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inosine-5'-monophosphate dehydrogenase 2 (IMPDH type II) |
J Med Chem 45: 703-12 (2002)
BindingDB Entry DOI: 10.7270/Q29S1RS4 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50108912

(CHEMBL162782 | {Hydroxy-[2-(4-hydroxy-6-methoxy-7-...)Show SMILES COc1c(C)c2COC(=O)c2c(O)c1CCOP(O)(=O)CP(O)(O)=O Show InChI InChI=1S/C13H18O10P2/c1-7-9-5-22-13(15)10(9)11(14)8(12(7)21-2)3-4-23-25(19,20)6-24(16,17)18/h14H,3-6H2,1-2H3,(H,19,20)(H2,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmasset Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inosine-5'-monophosphate dehydrogenase 2 (IMPDH type II) |
J Med Chem 45: 703-12 (2002)
BindingDB Entry DOI: 10.7270/Q29S1RS4 |
More data for this
Ligand-Target Pair | |
Alcohol dehydrogenase 1A
(Homo sapiens (Human)) | BDBM50192456
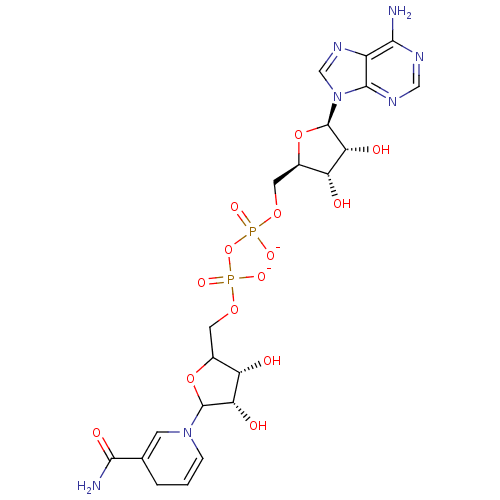
([(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihyd...)Show SMILES NC(=O)C1=CN(C=CC1)C1OC(COP([O-])(=O)OP([O-])(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@H](O)[C@@H]1O |c:6,t:3| Show InChI InChI=1S/C21H29N7O14P2/c22-17-12-19(25-7-24-17)28(8-26-12)21-16(32)14(30)11(41-21)6-39-44(36,37)42-43(34,35)38-5-10-13(29)15(31)20(40-10)27-3-1-2-9(4-27)18(23)33/h1,3-4,7-8,10-11,13-16,20-21,29-32H,2,5-6H2,(H2,23,33)(H,34,35)(H,36,37)(H2,22,24,25)/p-2/t10?,11-,13+,14-,15+,16-,20?,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester Medical Center
Curated by ChEMBL
| Assay Description
Inhibition constant against mammalian liver alcohol dehydrogenase (ADH) |
J Med Chem 37: 392-9 (1994)
BindingDB Entry DOI: 10.7270/Q2RN38H5 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 417 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University
Curated by ChEMBL
| Assay Description
Binding affinity using guinea pig brain membrane preparations, towards Opioid receptor delta 1 using [3H]DPDPE as radioligand |
J Med Chem 45: 1949-56 (2002)
Article DOI: 10.1021/jm010576e
BindingDB Entry DOI: 10.7270/Q2WS8X0T |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 468 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University
Curated by ChEMBL
| Assay Description
Binding affinity using guinea pig brain membrane preparations, towards Opioid receptor kappa 1 using [3H]- U-69,593 as radioligand |
J Med Chem 45: 1949-56 (2002)
Article DOI: 10.1021/jm010576e
BindingDB Entry DOI: 10.7270/Q2WS8X0T |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50108910
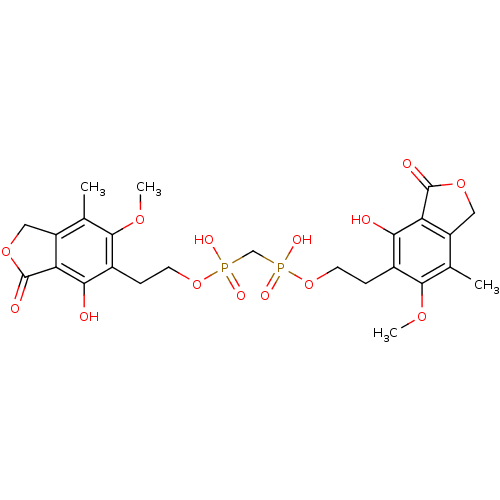
(CHEMBL348359 | {Hydroxy-[2-(4-hydroxy-6-methoxy-7-...)Show SMILES COc1c(C)c2COC(=O)c2c(O)c1CCOP(O)(=O)CP(O)(=O)OCCc1c(O)c2C(=O)OCc2c(C)c1OC Show InChI InChI=1S/C25H30O14P2/c1-12-16-9-36-24(28)18(16)20(26)14(22(12)34-3)5-7-38-40(30,31)11-41(32,33)39-8-6-15-21(27)19-17(10-37-25(19)29)13(2)23(15)35-4/h26-27H,5-11H2,1-4H3,(H,30,31)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmasset Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inosine-5'-monophosphate dehydrogenase 2 (IMPDH type II) |
J Med Chem 45: 703-12 (2002)
BindingDB Entry DOI: 10.7270/Q29S1RS4 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50108910

(CHEMBL348359 | {Hydroxy-[2-(4-hydroxy-6-methoxy-7-...)Show SMILES COc1c(C)c2COC(=O)c2c(O)c1CCOP(O)(=O)CP(O)(=O)OCCc1c(O)c2C(=O)OCc2c(C)c1OC Show InChI InChI=1S/C25H30O14P2/c1-12-16-9-36-24(28)18(16)20(26)14(22(12)34-3)5-7-38-40(30,31)11-41(32,33)39-8-6-15-21(27)19-17(10-37-25(19)29)13(2)23(15)35-4/h26-27H,5-11H2,1-4H3,(H,30,31)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmasset Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inosine-5'-monophosphate dehydrogenase 2 (IMPDH type II) |
J Med Chem 45: 703-12 (2002)
BindingDB Entry DOI: 10.7270/Q29S1RS4 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 1
(Homo sapiens (Human)) | BDBM50370188
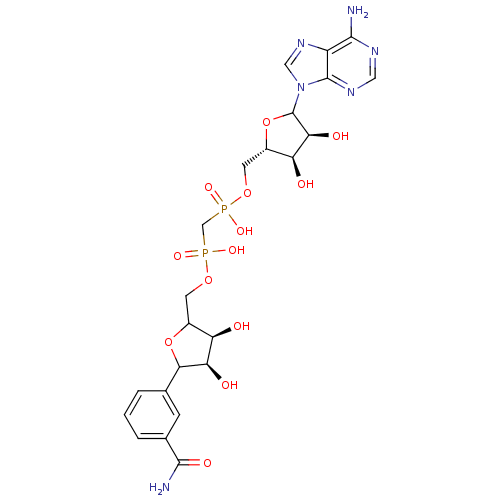
(CHEMBL608194)Show SMILES NC(=O)c1cccc(c1)C1OC(COP(O)(=O)CP(O)(=O)OC[C@@H]2OC([C@@H](O)[C@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C23H30N6O13P2/c24-20-14-22(27-7-26-20)29(8-28-14)23-18(33)16(31)13(42-23)6-40-44(37,38)9-43(35,36)39-5-12-15(30)17(32)19(41-12)10-2-1-3-11(4-10)21(25)34/h1-4,7-8,12-13,15-19,23,30-33H,5-6,9H2,(H2,25,34)(H,35,36)(H,37,38)(H2,24,26,27)/t12?,13-,15+,16-,17+,18-,19?,23?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmasset Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inosine-5'-monophosphate dehydrogenase 1 (IMPDH type I) |
J Med Chem 45: 703-12 (2002)
BindingDB Entry DOI: 10.7270/Q29S1RS4 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 1
(Homo sapiens (Human)) | BDBM50370188

(CHEMBL608194)Show SMILES NC(=O)c1cccc(c1)C1OC(COP(O)(=O)CP(O)(=O)OC[C@@H]2OC([C@@H](O)[C@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C23H30N6O13P2/c24-20-14-22(27-7-26-20)29(8-28-14)23-18(33)16(31)13(42-23)6-40-44(37,38)9-43(35,36)39-5-12-15(30)17(32)19(41-12)10-2-1-3-11(4-10)21(25)34/h1-4,7-8,12-13,15-19,23,30-33H,5-6,9H2,(H2,25,34)(H,35,36)(H,37,38)(H2,24,26,27)/t12?,13-,15+,16-,17+,18-,19?,23?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmasset Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inosine-5'-monophosphate dehydrogenase 1 (IMPDH type I) |
J Med Chem 45: 703-12 (2002)
BindingDB Entry DOI: 10.7270/Q29S1RS4 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 1
(Homo sapiens (Human)) | BDBM50108912

(CHEMBL162782 | {Hydroxy-[2-(4-hydroxy-6-methoxy-7-...)Show SMILES COc1c(C)c2COC(=O)c2c(O)c1CCOP(O)(=O)CP(O)(O)=O Show InChI InChI=1S/C13H18O10P2/c1-7-9-5-22-13(15)10(9)11(14)8(12(7)21-2)3-4-23-25(19,20)6-24(16,17)18/h14H,3-6H2,1-2H3,(H,19,20)(H2,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmasset Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inosine-5'-monophosphate dehydrogenase 1 (IMPDH type I) |
J Med Chem 45: 703-12 (2002)
BindingDB Entry DOI: 10.7270/Q29S1RS4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data