

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM136294 (US8853212, DDP-4 Inhibitor 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description A continuous fluorometric assay is employed with the substrate Gly-Pro-AMC, which is cleaved by DPP-4 to release the fluorescent AMC leaving group. T... | US Patent US8853212 (2014) BindingDB Entry DOI: 10.7270/Q2C53JK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50187939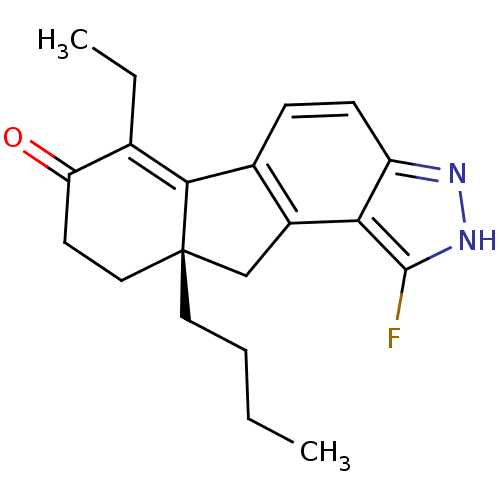 ((S)-9a-butyl-6-ethyl-1-fluoro-8,9,9a,10-tetrahydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERbeta | Bioorg Med Chem Lett 16: 3896-901 (2006) Article DOI: 10.1016/j.bmcl.2006.05.036 BindingDB Entry DOI: 10.7270/Q24T6J01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50187954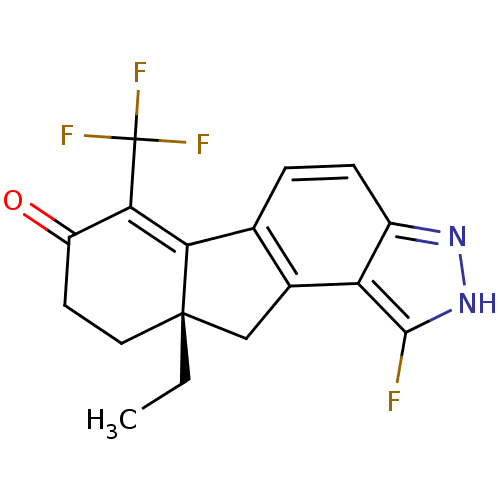 ((S)-9a-ethyl-1-fluoro-6-(trifluoromethyl)-8,9,9a,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERbeta | Bioorg Med Chem Lett 16: 3896-901 (2006) Article DOI: 10.1016/j.bmcl.2006.05.036 BindingDB Entry DOI: 10.7270/Q24T6J01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM136295 (US8853212, DDP-4 Inhibitor 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description A continuous fluorometric assay is employed with the substrate Gly-Pro-AMC, which is cleaved by DPP-4 to release the fluorescent AMC leaving group. T... | US Patent US8853212 (2014) BindingDB Entry DOI: 10.7270/Q2C53JK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM136291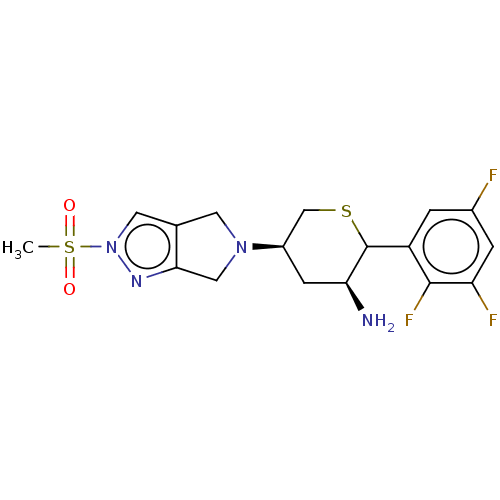 (US8853212, DDP-4 Inhibitor 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description A continuous fluorometric assay is employed with the substrate Gly-Pro-AMC, which is cleaved by DPP-4 to release the fluorescent AMC leaving group. T... | US Patent US8853212 (2014) BindingDB Entry DOI: 10.7270/Q2C53JK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM150239 (US8980929, Example Compound 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description A continuous fluorometric assay is employed with the substrate Gly-Pro-AMC, which is cleaved by DPP-4 to release the fluorescent AMC leaving group. T... | US Patent US8980929 (2015) BindingDB Entry DOI: 10.7270/Q2MK6BMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM136293 (US8853212, DDP-4 Inhibitor 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description A continuous fluorometric assay is employed with the substrate Gly-Pro-AMC, which is cleaved by DPP-4 to release the fluorescent AMC leaving group. T... | US Patent US8853212 (2014) BindingDB Entry DOI: 10.7270/Q2C53JK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM136297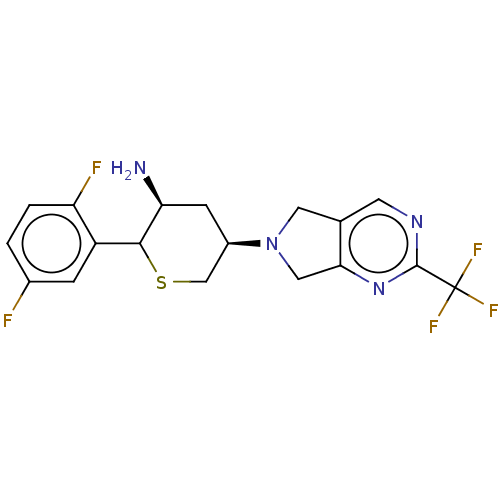 (US8853212, DDP-4 Inhibitor 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description A continuous fluorometric assay is employed with the substrate Gly-Pro-AMC, which is cleaved by DPP-4 to release the fluorescent AMC leaving group. T... | US Patent US8853212 (2014) BindingDB Entry DOI: 10.7270/Q2C53JK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM136298 (US8853212, DDP-4 Inhibitor 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description A continuous fluorometric assay is employed with the substrate Gly-Pro-AMC, which is cleaved by DPP-4 to release the fluorescent AMC leaving group. T... | US Patent US8853212 (2014) BindingDB Entry DOI: 10.7270/Q2C53JK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50187941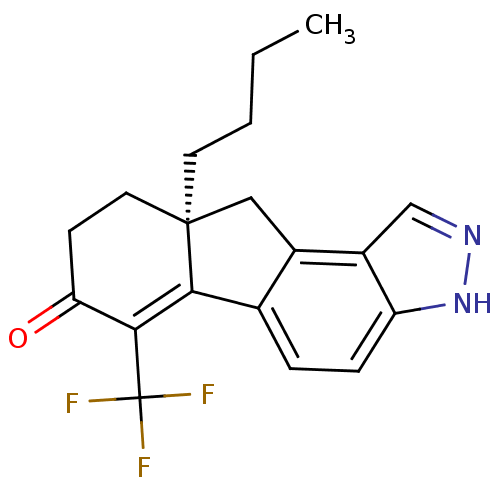 ((S)-9a-butyl-6-(trifluoromethyl)-8,9,9a,10-tetrahy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERbeta | Bioorg Med Chem Lett 16: 3896-901 (2006) Article DOI: 10.1016/j.bmcl.2006.05.036 BindingDB Entry DOI: 10.7270/Q24T6J01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50187956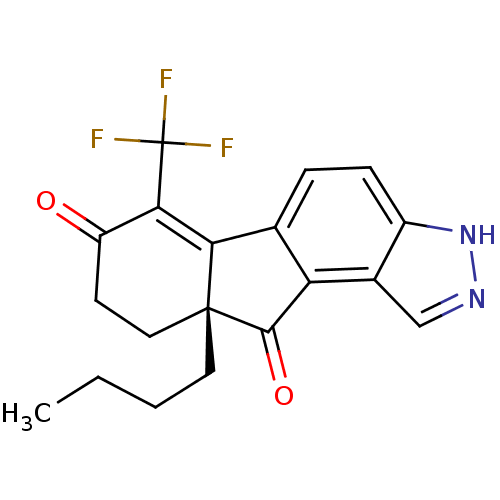 ((R)-9a-butyl-6-(trifluoromethyl)-9,9a-dihydroinden...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERbeta | Bioorg Med Chem Lett 16: 3896-901 (2006) Article DOI: 10.1016/j.bmcl.2006.05.036 BindingDB Entry DOI: 10.7270/Q24T6J01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50187968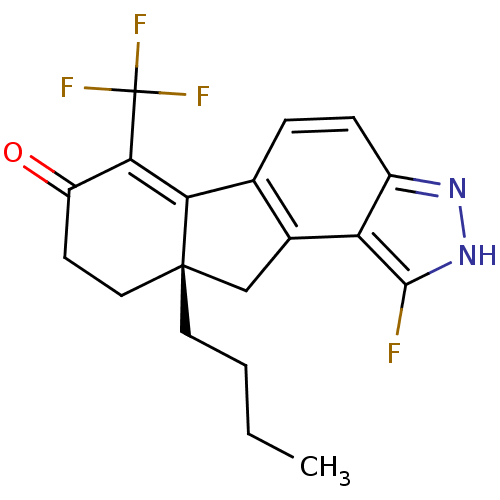 ((S)-9a-butyl-1-fluoro-6-(trifluoromethyl)-8,9,9a,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERbeta | Bioorg Med Chem Lett 16: 3896-901 (2006) Article DOI: 10.1016/j.bmcl.2006.05.036 BindingDB Entry DOI: 10.7270/Q24T6J01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50187973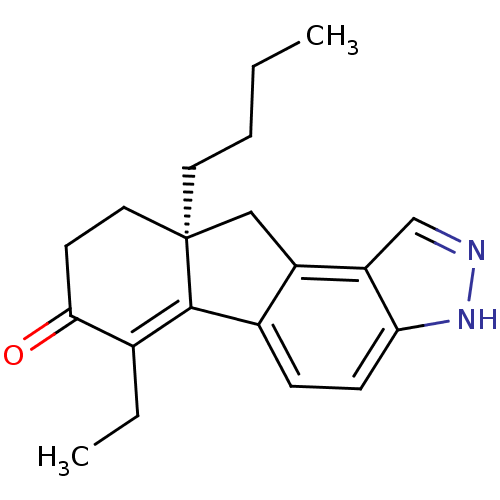 ((S)-9a-butyl-6-ethyl-8,9,9a,10-tetrahydroindeno[2,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERbeta | Bioorg Med Chem Lett 16: 3896-901 (2006) Article DOI: 10.1016/j.bmcl.2006.05.036 BindingDB Entry DOI: 10.7270/Q24T6J01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM136296 (US8853212, DDP-4 Inhibitor 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description A continuous fluorometric assay is employed with the substrate Gly-Pro-AMC, which is cleaved by DPP-4 to release the fluorescent AMC leaving group. T... | US Patent US8853212 (2014) BindingDB Entry DOI: 10.7270/Q2C53JK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50134192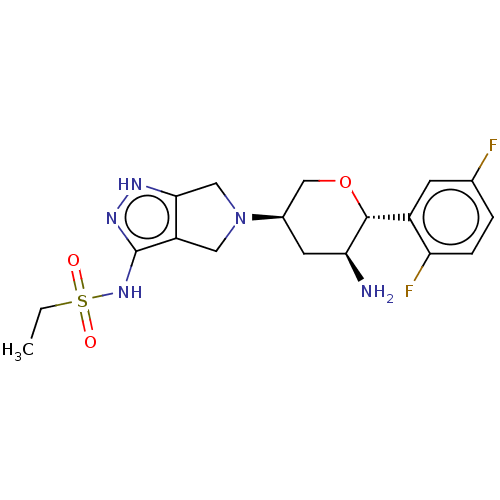 (CHEMBL3734828) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant human DPP4 expressed in Sf9 cells using Gly-Pro-AMC substrate after 30 mins by plate reader analysis | Bioorg Med Chem Lett 25: 5767-71 (2015) Article DOI: 10.1016/j.bmcl.2015.10.070 BindingDB Entry DOI: 10.7270/Q208675S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50187944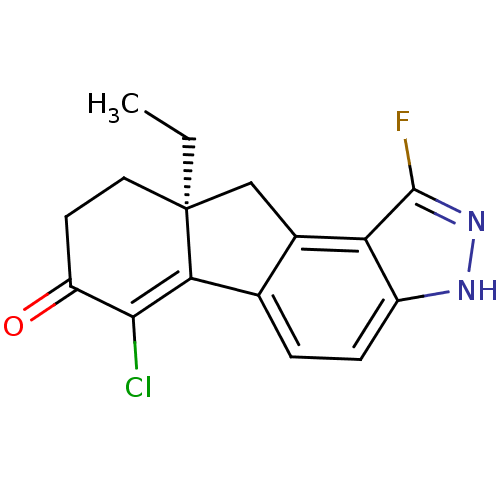 ((S)-6-chloro-9a-ethyl-1-fluoro-8,9,9a,10-tetrahydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERbeta | Bioorg Med Chem Lett 16: 3896-901 (2006) Article DOI: 10.1016/j.bmcl.2006.05.036 BindingDB Entry DOI: 10.7270/Q24T6J01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human recombinant ERbeta by scintillation proximity assay | Bioorg Med Chem Lett 16: 4652-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.103 BindingDB Entry DOI: 10.7270/Q2MP52WG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to ERbeta by scintillation proximity assay | Bioorg Med Chem Lett 16: 3489-94 (2006) Article DOI: 10.1016/j.bmcl.2006.03.098 BindingDB Entry DOI: 10.7270/Q2R2110X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERbeta | Bioorg Med Chem Lett 16: 3896-901 (2006) Article DOI: 10.1016/j.bmcl.2006.05.036 BindingDB Entry DOI: 10.7270/Q24T6J01 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50185859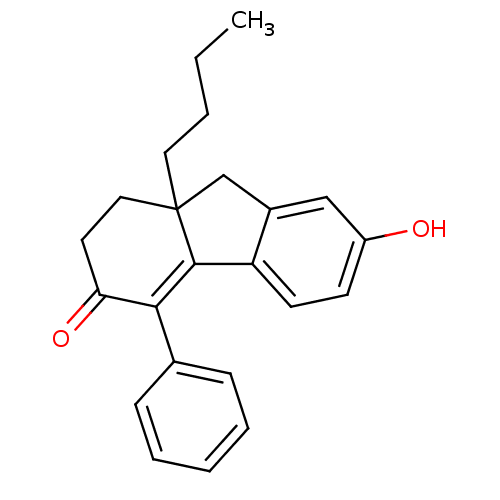 (9a-butyl-7-hydroxy-4-phenyl-1,2,9,9a-tetrahydroflu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to ERbeta by scintillation proximity assay | Bioorg Med Chem Lett 16: 3489-94 (2006) Article DOI: 10.1016/j.bmcl.2006.03.098 BindingDB Entry DOI: 10.7270/Q2R2110X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human recombinant ERalpha by scintillation proximity assay | Bioorg Med Chem Lett 16: 4652-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.103 BindingDB Entry DOI: 10.7270/Q2MP52WG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50134193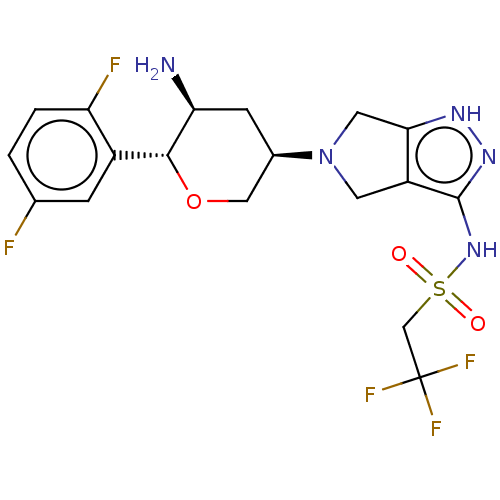 (CHEMBL3735461) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant human DPP4 expressed in Sf9 cells using Gly-Pro-AMC substrate after 30 mins by plate reader analysis | Bioorg Med Chem Lett 25: 5767-71 (2015) Article DOI: 10.1016/j.bmcl.2015.10.070 BindingDB Entry DOI: 10.7270/Q208675S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERalpha | Bioorg Med Chem Lett 16: 3896-901 (2006) Article DOI: 10.1016/j.bmcl.2006.05.036 BindingDB Entry DOI: 10.7270/Q24T6J01 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50185836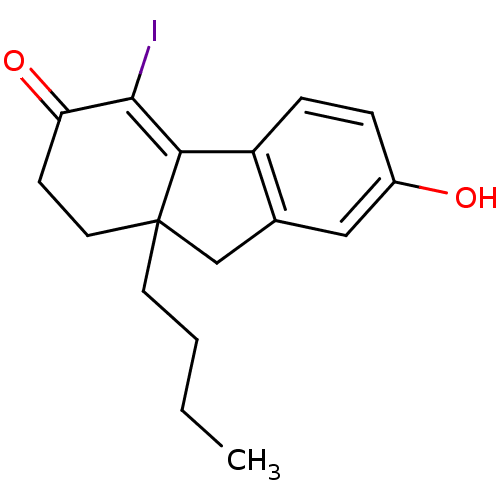 (9a-butyl-7-hydroxy-4-iodo-1,2,9,9a-tetrahydrofluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to ERbeta by scintillation proximity assay | Bioorg Med Chem Lett 16: 3489-94 (2006) Article DOI: 10.1016/j.bmcl.2006.03.098 BindingDB Entry DOI: 10.7270/Q2R2110X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to ERalpha by scintillation proximity assay | Bioorg Med Chem Lett 16: 3489-94 (2006) Article DOI: 10.1016/j.bmcl.2006.03.098 BindingDB Entry DOI: 10.7270/Q2R2110X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50187964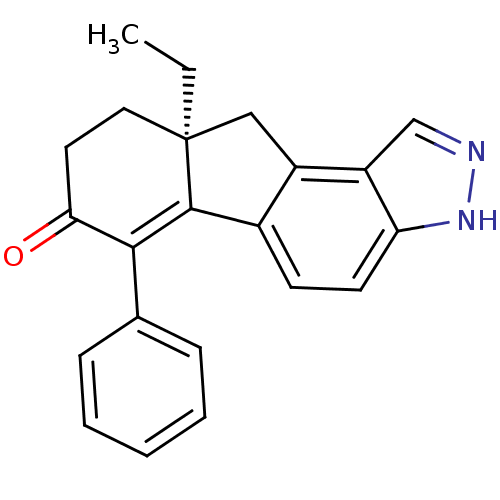 ((S)-9a-ethyl-6-phenyl-8,9,9a,10-tetrahydroindeno[2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERbeta | Bioorg Med Chem Lett 16: 3896-901 (2006) Article DOI: 10.1016/j.bmcl.2006.05.036 BindingDB Entry DOI: 10.7270/Q24T6J01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| PBP2A (Staphylococcus aureus) | BDBM50217404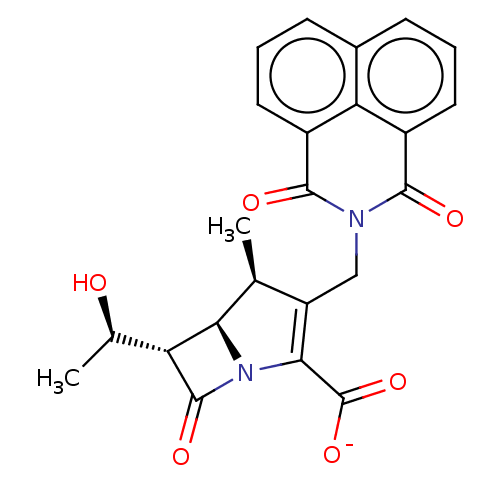 (CHEMBL348278) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was tested for its binding affinity towards Penicillin-binding protein 2a (PBP2a) using [13H]-benzylpenicillin | Bioorg Med Chem Lett 9: 673-8 (1999) BindingDB Entry DOI: 10.7270/Q2TF00JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50187943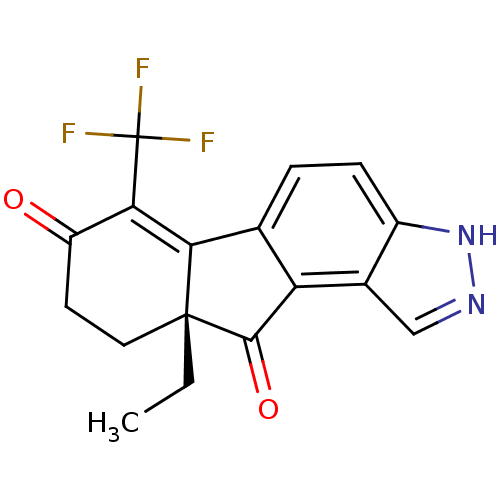 ((R)-9a-ethyl-6-(trifluoromethyl)-9,9a-dihydroinden...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERbeta | Bioorg Med Chem Lett 16: 3896-901 (2006) Article DOI: 10.1016/j.bmcl.2006.05.036 BindingDB Entry DOI: 10.7270/Q24T6J01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50185867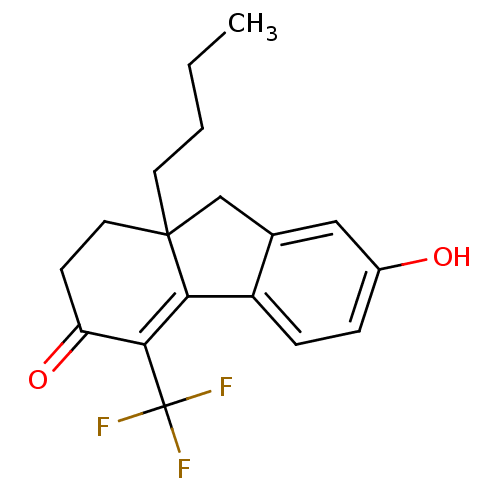 (9a-butyl-7-hydroxy-4-(trifluoromethyl)-1,2,9,9a-te...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to ERbeta by scintillation proximity assay | Bioorg Med Chem Lett 16: 3489-94 (2006) Article DOI: 10.1016/j.bmcl.2006.03.098 BindingDB Entry DOI: 10.7270/Q2R2110X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM136300 (US8853212, DDP-4 Inhibitor 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description A continuous fluorometric assay is employed with the substrate Gly-Pro-AMC, which is cleaved by DPP-4 to release the fluorescent AMC leaving group. T... | US Patent US8853212 (2014) BindingDB Entry DOI: 10.7270/Q2C53JK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50185860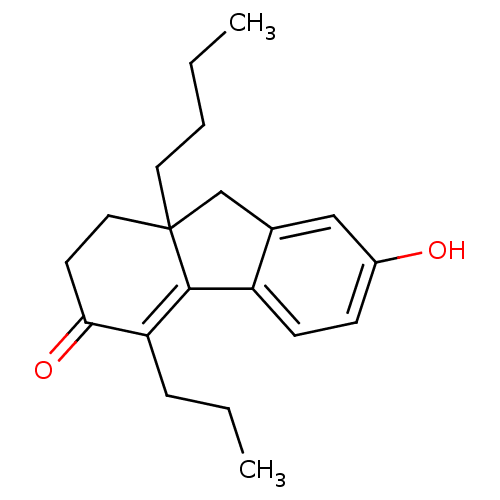 (9a-butyl-7-hydroxy-4-propyl-1,2,9,9a-tetrahydroflu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to ERbeta by scintillation proximity assay | Bioorg Med Chem Lett 16: 3489-94 (2006) Article DOI: 10.1016/j.bmcl.2006.03.098 BindingDB Entry DOI: 10.7270/Q2R2110X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50185849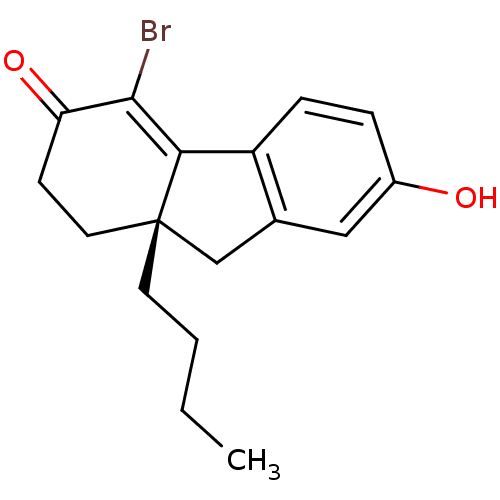 ((9aS)-4-bromo-9a-butyl-7-hydroxy-1,2,9,9a-tetrahyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to ERbeta by scintillation proximity assay | Bioorg Med Chem Lett 16: 3489-94 (2006) Article DOI: 10.1016/j.bmcl.2006.03.098 BindingDB Entry DOI: 10.7270/Q2R2110X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM136292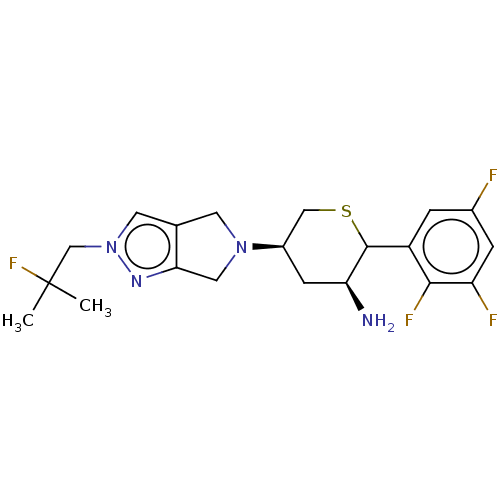 (US8853212, DDP-4 Inhibitor 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description A continuous fluorometric assay is employed with the substrate Gly-Pro-AMC, which is cleaved by DPP-4 to release the fluorescent AMC leaving group. T... | US Patent US8853212 (2014) BindingDB Entry DOI: 10.7270/Q2C53JK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50187959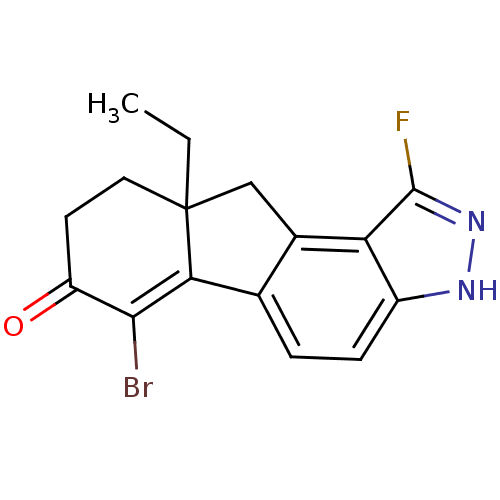 (CHEMBL211870 | rac-6-bromo-9a-ethyl-1-fluoro-8,9,9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERbeta | Bioorg Med Chem Lett 16: 3896-901 (2006) Article DOI: 10.1016/j.bmcl.2006.05.036 BindingDB Entry DOI: 10.7270/Q24T6J01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50134186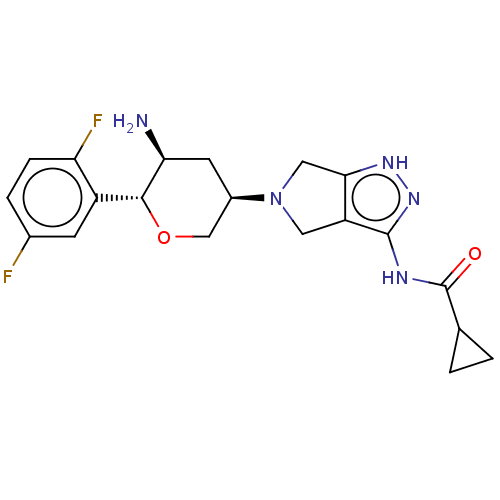 (CHEMBL3734964) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant human DPP4 expressed in Sf9 cells using Gly-Pro-AMC substrate after 30 mins by plate reader analysis | Bioorg Med Chem Lett 25: 5767-71 (2015) Article DOI: 10.1016/j.bmcl.2015.10.070 BindingDB Entry DOI: 10.7270/Q208675S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50187966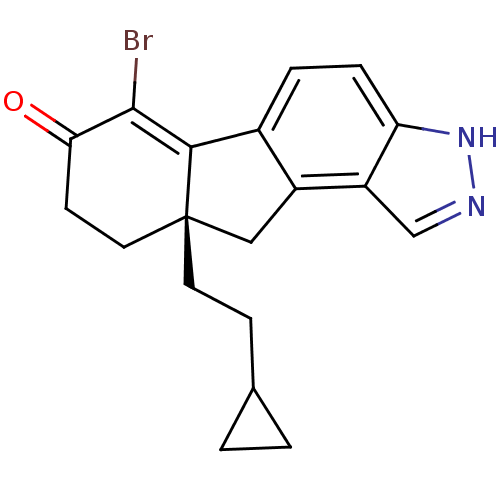 ((S)-6-bromo-9a-(2-cyclopropyl-ethyl)-8,9,9a,10-tet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERbeta | Bioorg Med Chem Lett 16: 3896-901 (2006) Article DOI: 10.1016/j.bmcl.2006.05.036 BindingDB Entry DOI: 10.7270/Q24T6J01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| PBP2A (Staphylococcus aureus) | BDBM50217406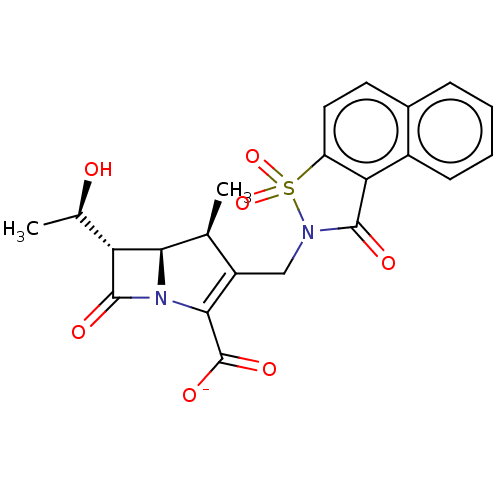 (CHEMBL351921) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was tested for its binding affinity towards Penicillin-binding protein 2a (PBP2a) using [13H]-benzylpenicillin | Bioorg Med Chem Lett 9: 673-8 (1999) BindingDB Entry DOI: 10.7270/Q2TF00JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50185845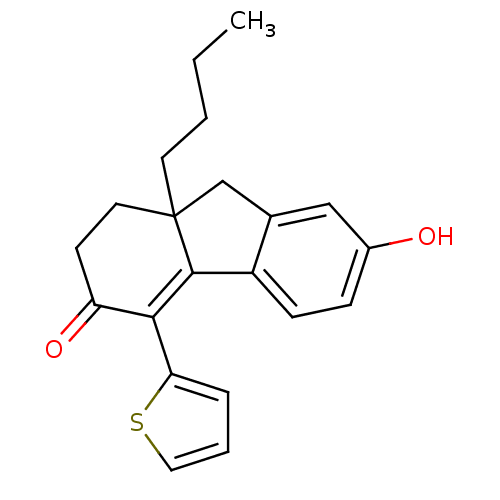 (9a-butyl-7-hydroxy-4-(thiophen-2-yl)-1,2,9,9a-tetr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to ERbeta by scintillation proximity assay | Bioorg Med Chem Lett 16: 3489-94 (2006) Article DOI: 10.1016/j.bmcl.2006.03.098 BindingDB Entry DOI: 10.7270/Q2R2110X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM136299 (US8853212, DDP-4 Inhibitor 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description A continuous fluorometric assay is employed with the substrate Gly-Pro-AMC, which is cleaved by DPP-4 to release the fluorescent AMC leaving group. T... | US Patent US8853212 (2014) BindingDB Entry DOI: 10.7270/Q2C53JK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50134195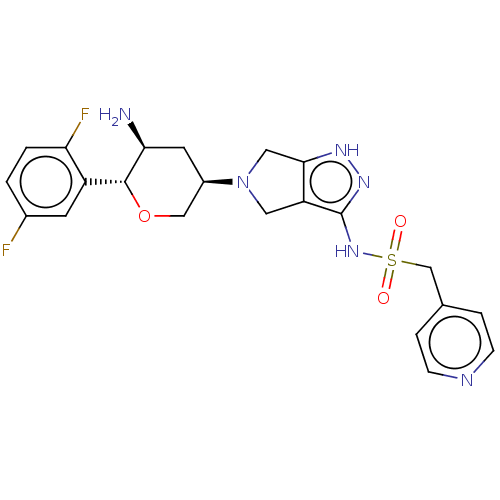 (CHEMBL3735433) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant human DPP4 expressed in Sf9 cells using Gly-Pro-AMC substrate after 30 mins by plate reader analysis | Bioorg Med Chem Lett 25: 5767-71 (2015) Article DOI: 10.1016/j.bmcl.2015.10.070 BindingDB Entry DOI: 10.7270/Q208675S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50134194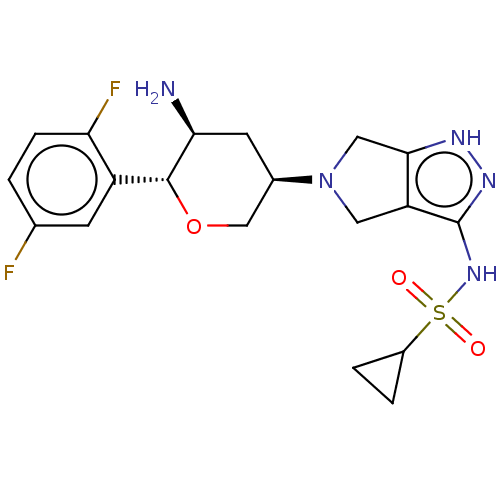 (CHEMBL3734999) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant human DPP4 expressed in Sf9 cells using Gly-Pro-AMC substrate after 30 mins by plate reader analysis | Bioorg Med Chem Lett 25: 5767-71 (2015) Article DOI: 10.1016/j.bmcl.2015.10.070 BindingDB Entry DOI: 10.7270/Q208675S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50185851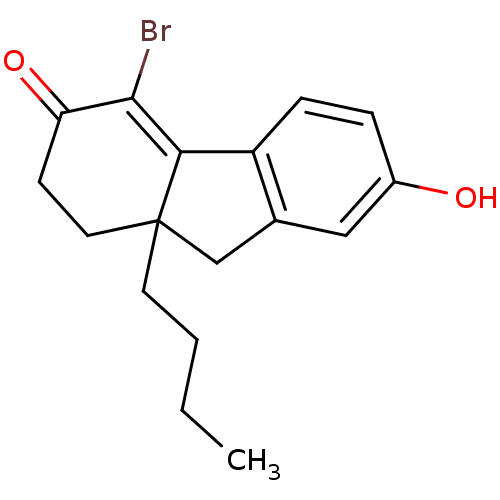 ((R,S)-4-bromo-9a-butyl-7-hydroxy-1,2,9,9a-tetrahyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERbeta | Bioorg Med Chem Lett 16: 3896-901 (2006) Article DOI: 10.1016/j.bmcl.2006.05.036 BindingDB Entry DOI: 10.7270/Q24T6J01 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50189077 ((+/-)-6-bromo-9a-butyl-8,9,9a,10-tetrahydro-3H-1,2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human recombinant ERbeta by scintillation proximity assay | Bioorg Med Chem Lett 16: 4652-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.103 BindingDB Entry DOI: 10.7270/Q2MP52WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50185851 ((R,S)-4-bromo-9a-butyl-7-hydroxy-1,2,9,9a-tetrahyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to ERbeta by scintillation proximity assay | Bioorg Med Chem Lett 16: 3489-94 (2006) Article DOI: 10.1016/j.bmcl.2006.03.098 BindingDB Entry DOI: 10.7270/Q2R2110X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50185859 (9a-butyl-7-hydroxy-4-phenyl-1,2,9,9a-tetrahydroflu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Activity at human ERbeta transfected in HEK293 cells assessed as transactivation of alkaline phosphatase reporter gene | Bioorg Med Chem Lett 16: 3489-94 (2006) Article DOI: 10.1016/j.bmcl.2006.03.098 BindingDB Entry DOI: 10.7270/Q2R2110X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM150234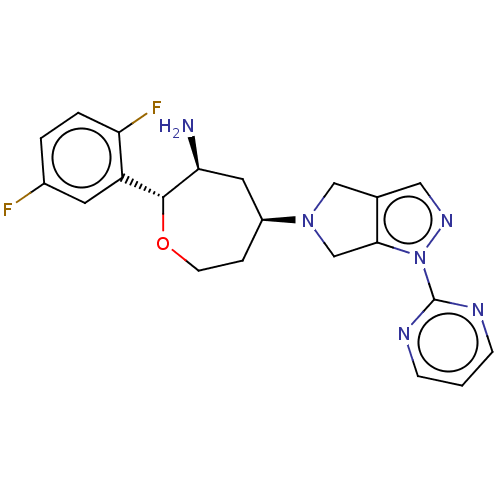 (US8980929, Example Compound 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description A continuous fluorometric assay is employed with the substrate Gly-Pro-AMC, which is cleaved by DPP-4 to release the fluorescent AMC leaving group. T... | US Patent US8980929 (2015) BindingDB Entry DOI: 10.7270/Q2MK6BMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50187947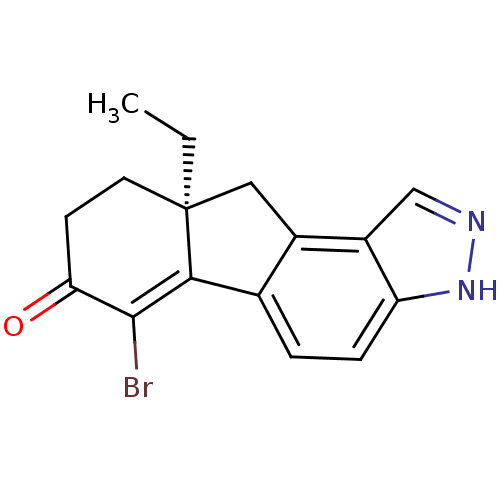 ((S)-6-bromo-9a-ethyl-8,9,9a,10-tetrahydroindeno[2,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERbeta | Bioorg Med Chem Lett 16: 3896-901 (2006) Article DOI: 10.1016/j.bmcl.2006.05.036 BindingDB Entry DOI: 10.7270/Q24T6J01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM136285 (US8853212, DDP-4 Inhibitor 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description A continuous fluorometric assay is employed with the substrate Gly-Pro-AMC, which is cleaved by DPP-4 to release the fluorescent AMC leaving group. T... | US Patent US8853212 (2014) BindingDB Entry DOI: 10.7270/Q2C53JK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM150233 (US8980929, Example Compound 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description A continuous fluorometric assay is employed with the substrate Gly-Pro-AMC, which is cleaved by DPP-4 to release the fluorescent AMC leaving group. T... | US Patent US8980929 (2015) BindingDB Entry DOI: 10.7270/Q2MK6BMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50189069 ((S)(-)-9a-butyl-6-thiophen-2-yl-8,9,9a,10-tetrahyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human recombinant ERbeta by scintillation proximity assay | Bioorg Med Chem Lett 16: 4652-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.103 BindingDB Entry DOI: 10.7270/Q2MP52WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 677 total ) | Next | Last >> |