

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50146629 (CHEMBL330040 | N-Hydroxy-2-[(4-nitro-benzyl)-(4-tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of human matrix metalloprotease-1 | J Med Chem 47: 2761-7 (2004) Article DOI: 10.1021/jm031061l BindingDB Entry DOI: 10.7270/Q2ZG6T0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50088118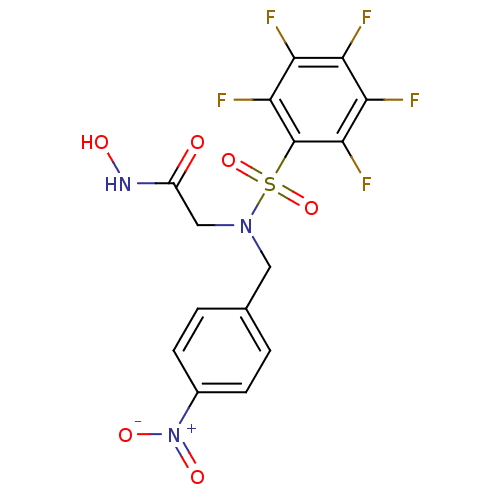 (2-(N-(4-nitrobenzyl)-2,3,4,5,6-pentafluorophenylsu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of human matrix metalloprotease-1 | J Med Chem 47: 2761-7 (2004) Article DOI: 10.1021/jm031061l BindingDB Entry DOI: 10.7270/Q2ZG6T0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50082556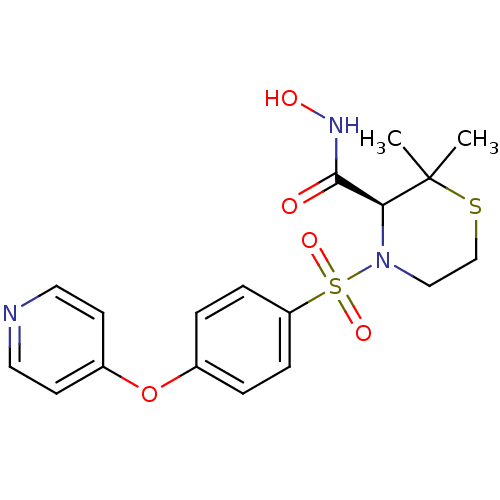 ((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of human matrix metalloprotease-1 | J Med Chem 47: 2761-7 (2004) Article DOI: 10.1021/jm031061l BindingDB Entry DOI: 10.7270/Q2ZG6T0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM8465 ((2R)-N-hydroxy-2-[(4-methoxybenzene)(pyridin-3-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of human matrix metalloprotease-1 | J Med Chem 47: 2761-7 (2004) Article DOI: 10.1021/jm031061l BindingDB Entry DOI: 10.7270/Q2ZG6T0B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50146636 (2-{4-[4-(4-CHLORO-PHENOXY)-BENZENESULFONYL]-TETRAH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of human matrix metalloprotease-1 | J Med Chem 47: 2761-7 (2004) Article DOI: 10.1021/jm031061l BindingDB Entry DOI: 10.7270/Q2ZG6T0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50146633 (1-[4-(4-Imidazol-1-yl-phenoxy)-piperidine-1-sulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of human matrix metalloprotease-1 | J Med Chem 47: 2761-7 (2004) Article DOI: 10.1021/jm031061l BindingDB Entry DOI: 10.7270/Q2ZG6T0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50146635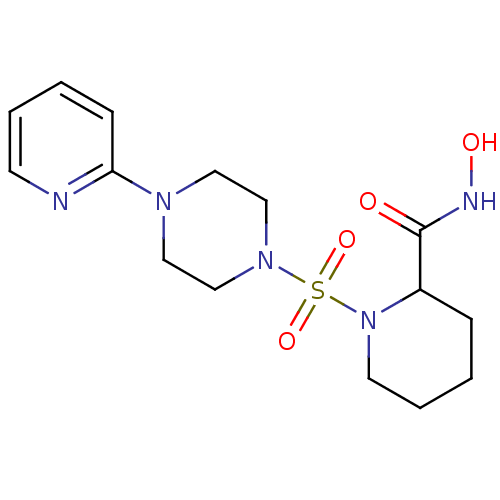 (1-(4-Pyridin-2-yl-piperazine-1-sulfonyl)-piperidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 423 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of human matrix metalloprotease-1 | J Med Chem 47: 2761-7 (2004) Article DOI: 10.1021/jm031061l BindingDB Entry DOI: 10.7270/Q2ZG6T0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (HAMSTER) | BDBM50366377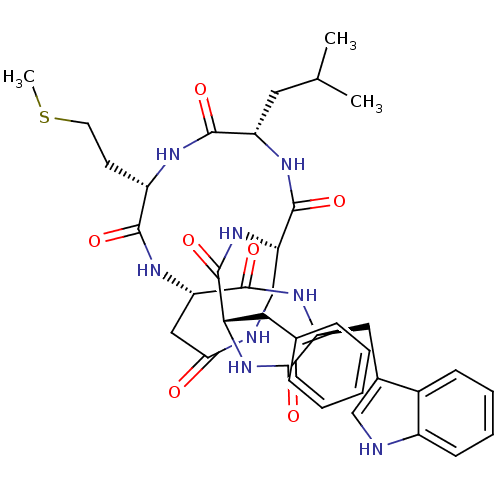 (CHEMBL334721 | MEN-10627) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for binding affinity of compound against [125I]NKA binding to neurokinin-2 (NK-2) receptor in hamster urinary bladder | Bioorg Med Chem Lett 7: 31-36 (1997) Article DOI: 10.1016/S0960-894X(96)00570-7 BindingDB Entry DOI: 10.7270/Q2KD1ZDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50146634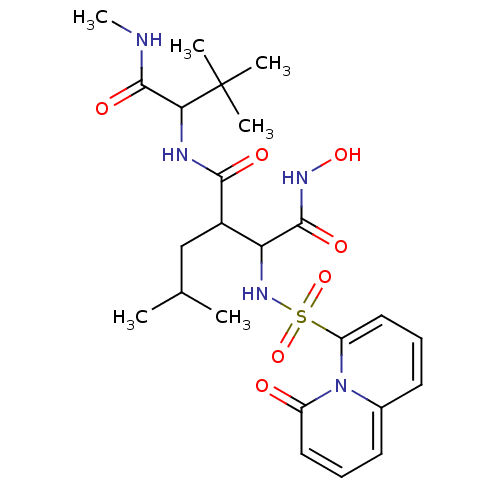 (CHEMBL328903 | N*1*-(2,2-Dimethyl-1-methylcarbamoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of human matrix metalloprotease-1 | J Med Chem 47: 2761-7 (2004) Article DOI: 10.1021/jm031061l BindingDB Entry DOI: 10.7270/Q2ZG6T0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 [321-609] (Homo sapiens (Human)) | BDBM304740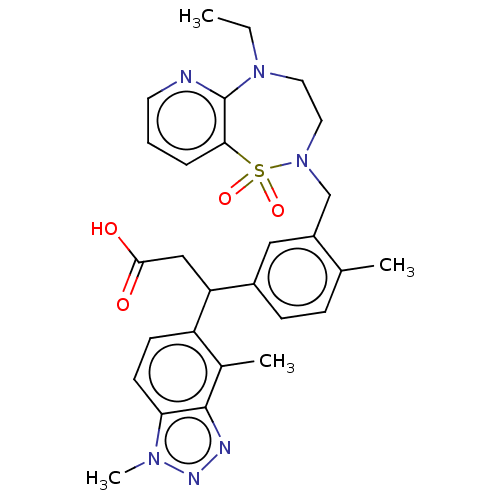 (3-(1,4-dimethyl-1H-benzo[d][1,2,3]triazol-5-yl)-3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Astex Therapeutics, Ltd US Patent | Assay Description 100 nL of 100× compound dose response curves (serial 3-fold dilutions) in DMSO are stamped using an Echo liquid handling system (Labcyte) into 384-we... | US Patent US10144731 (2018) BindingDB Entry DOI: 10.7270/Q25X2C17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 [321-609] (Homo sapiens (Human)) | BDBM304737 (3-(3-((4-ethyl-1,1-dioxido-3,4-dihydro-2H-benzo[b]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Astex Therapeutics, Ltd US Patent | Assay Description 100 nL of 100× compound dose response curves (serial 3-fold dilutions) in DMSO are stamped using an Echo liquid handling system (Labcyte) into 384-we... | US Patent US10144731 (2018) BindingDB Entry DOI: 10.7270/Q25X2C17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 [321-609] (Homo sapiens (Human)) | BDBM304738 (3-(3-((4-ethyl-1,1-dioxido-3,4-dihydro-2H-pyrido[2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Astex Therapeutics, Ltd US Patent | Assay Description 100 nL of 100× compound dose response curves (serial 3-fold dilutions) in DMSO are stamped using an Echo liquid handling system (Labcyte) into 384-we... | US Patent US10144731 (2018) BindingDB Entry DOI: 10.7270/Q25X2C17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 [321-609] (Homo sapiens (Human)) | BDBM304736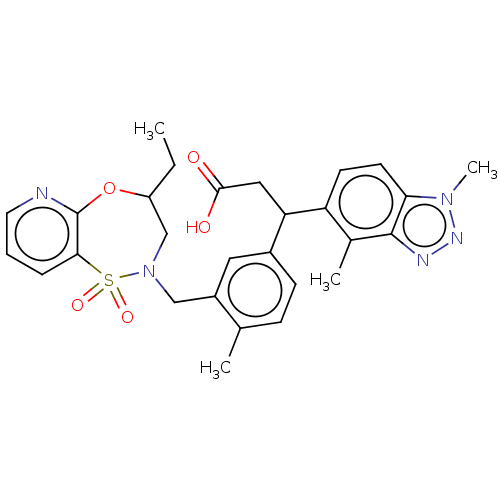 (3-(1,4-dimethyl-1H-benzo[d][1,2,3]triazol-5-yl)-3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Astex Therapeutics, Ltd US Patent | Assay Description 100 nL of 100× compound dose response curves (serial 3-fold dilutions) in DMSO are stamped using an Echo liquid handling system (Labcyte) into 384-we... | US Patent US10144731 (2018) BindingDB Entry DOI: 10.7270/Q25X2C17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 [321-609] (Homo sapiens (Human)) | BDBM304734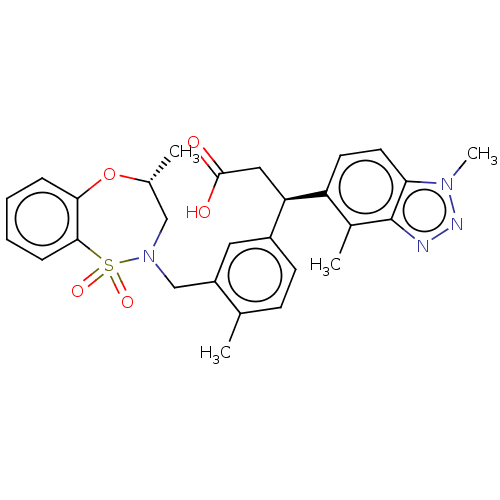 ((R)-3-(1,4-dimethyl-1H-benzo[d][1,2,3]triazol-5-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Astex Therapeutics, Ltd US Patent | Assay Description 100 nL of 100× compound dose response curves (serial 3-fold dilutions) in DMSO are stamped using an Echo liquid handling system (Labcyte) into 384-we... | US Patent US10144731 (2018) BindingDB Entry DOI: 10.7270/Q25X2C17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 [321-609] (Homo sapiens (Human)) | BDBM304732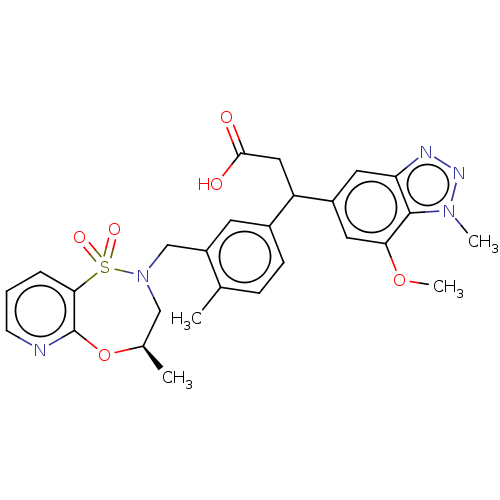 (3-(7-methoxy-1-methyl-1H-benzo[d][1,2,3]triazol-5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Astex Therapeutics, Ltd US Patent | Assay Description 100 nL of 100× compound dose response curves (serial 3-fold dilutions) in DMSO are stamped using an Echo liquid handling system (Labcyte) into 384-we... | US Patent US10144731 (2018) BindingDB Entry DOI: 10.7270/Q25X2C17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 [321-609] (Homo sapiens (Human)) | BDBM304730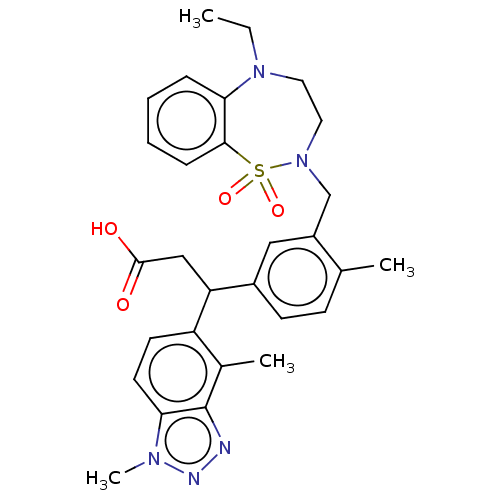 (3-(1,4-dimethyl-1H-benzo[d][1,2,3]triazol-5-yl)-3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Astex Therapeutics, Ltd US Patent | Assay Description 100 nL of 100× compound dose response curves (serial 3-fold dilutions) in DMSO are stamped using an Echo liquid handling system (Labcyte) into 384-we... | US Patent US10144731 (2018) BindingDB Entry DOI: 10.7270/Q25X2C17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 [321-609] (Homo sapiens (Human)) | BDBM304727 (3-(7-methoxy-1-methyl-1H-benzo[d][1,2,3]triazol-5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Astex Therapeutics, Ltd US Patent | Assay Description 100 nL of 100× compound dose response curves (serial 3-fold dilutions) in DMSO are stamped using an Echo liquid handling system (Labcyte) into 384-we... | US Patent US10144731 (2018) BindingDB Entry DOI: 10.7270/Q25X2C17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 [321-609] (Homo sapiens (Human)) | BDBM304725 (3-(1,4-dimethyl-1H-benzo[d][1,2,3]triazol-5-yl)-3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Astex Therapeutics, Ltd US Patent | Assay Description 100 nL of 100× compound dose response curves (serial 3-fold dilutions) in DMSO are stamped using an Echo liquid handling system (Labcyte) into 384-we... | US Patent US10144731 (2018) BindingDB Entry DOI: 10.7270/Q25X2C17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50146639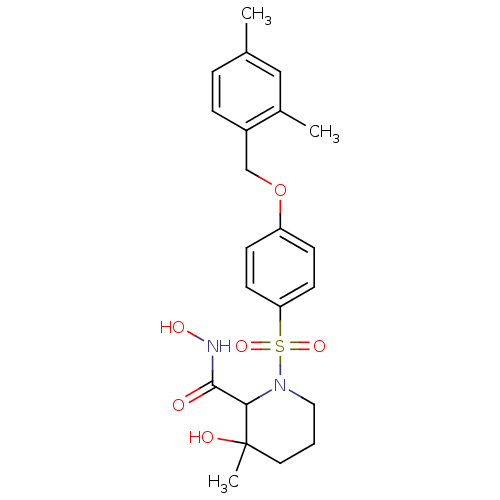 (1-[4-(2,4-Dimethyl-benzyloxy)-benzenesulfonyl]-3-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of human matrix metalloprotease-1 | J Med Chem 47: 2761-7 (2004) Article DOI: 10.1021/jm031061l BindingDB Entry DOI: 10.7270/Q2ZG6T0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 [321-609] (Homo sapiens (Human)) | BDBM304887 (3-(3-(((R)-4-ethyl-1,1-dioxido-3,4-dihydro-2H-benz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Astex Therapeutics, Ltd US Patent | Assay Description 100 nL of 100× compound dose response curves (serial 3-fold dilutions) in DMSO are stamped using an Echo liquid handling system (Labcyte) into 384-we... | US Patent US10144731 (2018) BindingDB Entry DOI: 10.7270/Q25X2C17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 [321-609] (Homo sapiens (Human)) | BDBM304755 (3-(3-(((R)-4-ethyl-1,1-dioxido-3,4-dihydro-2H-pyri...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Astex Therapeutics, Ltd US Patent | Assay Description 100 nL of 100× compound dose response curves (serial 3-fold dilutions) in DMSO are stamped using an Echo liquid handling system (Labcyte) into 384-we... | US Patent US10144731 (2018) BindingDB Entry DOI: 10.7270/Q25X2C17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 (Homo sapiens (Human)) | BDBM304727 (3-(7-methoxy-1-methyl-1H-benzo[d][1,2,3]triazol-5-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of 5'-TAMRA-labelled NRF2 peptide from human N-terminal His6-tagged KEAP1 Kelch domain (321 to 609 residues) expressed in baculovirus in... | J Med Chem 62: 4683-4702 (2019) Article DOI: 10.1021/acs.jmedchem.9b00279 BindingDB Entry DOI: 10.7270/Q2PK0KKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 (Homo sapiens (Human)) | BDBM304721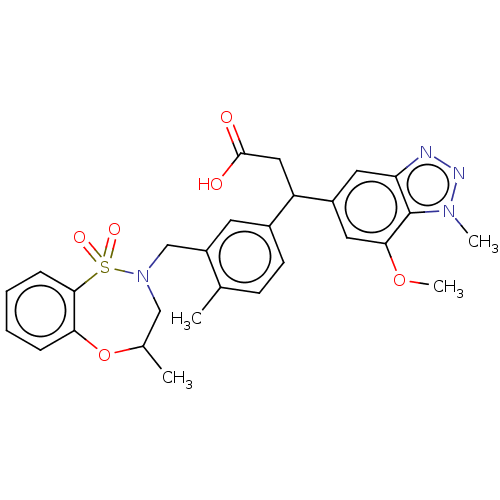 (3-(7-methoxy-1-methyl-1H-benzo[d][1,2,3]triazol-5-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of 5'-TAMRA-labelled NRF2 peptide from human N-terminal His6-tagged KEAP1 Kelch domain (321 to 609 residues) expressed in baculovirus in... | J Med Chem 62: 4683-4702 (2019) Article DOI: 10.1021/acs.jmedchem.9b00279 BindingDB Entry DOI: 10.7270/Q2PK0KKB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50071839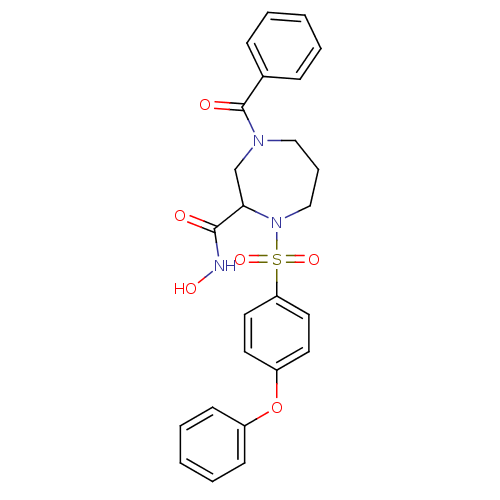 (4-Benzoyl-1-(4-phenoxy-benzenesulfonyl)-[1,4]diaze...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of human matrix metalloprotease-1 | J Med Chem 47: 2761-7 (2004) Article DOI: 10.1021/jm031061l BindingDB Entry DOI: 10.7270/Q2ZG6T0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 (Homo sapiens (Human)) | BDBM304748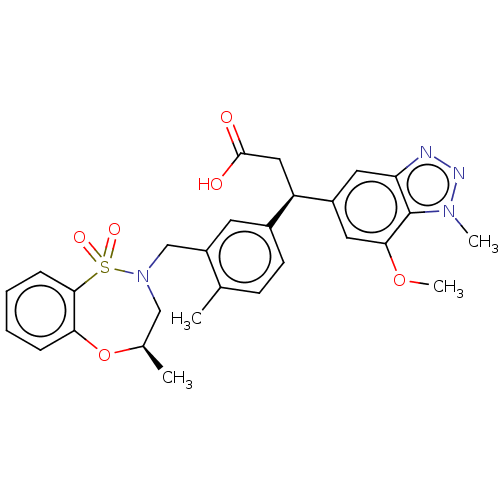 (BDBM304749 | US10144731, Example 34a) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of 5'-TAMRA-labelled NRF2 peptide from human N-terminal His6-tagged KEAP1 Kelch domain (321 to 609 residues) expressed in baculovirus in... | J Med Chem 62: 4683-4702 (2019) Article DOI: 10.1021/acs.jmedchem.9b00279 BindingDB Entry DOI: 10.7270/Q2PK0KKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM8465 ((2R)-N-hydroxy-2-[(4-methoxybenzene)(pyridin-3-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of human matrix metalloprotease-1 | J Med Chem 47: 2761-7 (2004) Article DOI: 10.1021/jm031061l BindingDB Entry DOI: 10.7270/Q2ZG6T0B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Phosphatidylinositol 5-phosphate 4-kinase type-2 beta (Homo sapiens (Human)) | BDBM50615191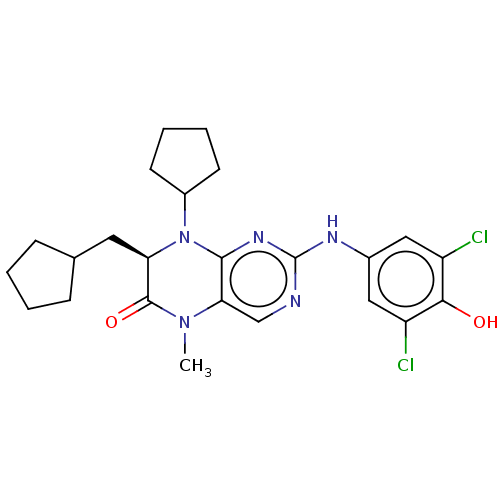 (CHEMBL5271804) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PDB UniChem | PDB | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM183589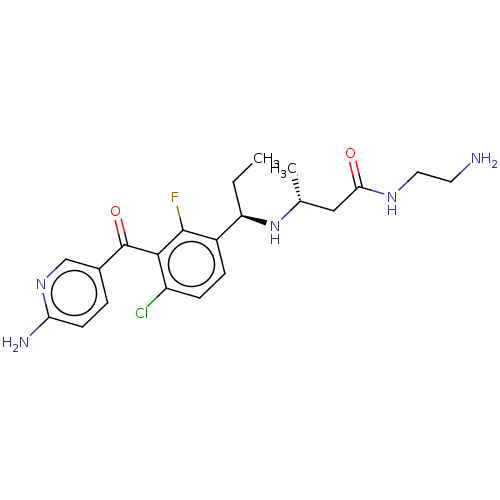 (US9145354, 241) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | 25 |
ASTEX THERAPEUTICS LIMITED US Patent | Assay Description The HCV NS3 protease functions have been extensively studied and are considered as potential targets for antiviral therapy: see for example the many ... | US Patent US9145354 (2015) BindingDB Entry DOI: 10.7270/Q2VH5MMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 (Homo sapiens (Human)) | BDBM304414 (3-(7-methoxy-1-methyl-1H-benzo[d][1,2,3]triazol-5-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of 5'-TAMRA-labelled NRF2 peptide from human N-terminal His6-tagged KEAP1 Kelch domain (321 to 609 residues) expressed in baculovirus in... | J Med Chem 62: 4683-4702 (2019) Article DOI: 10.1021/acs.jmedchem.9b00279 BindingDB Entry DOI: 10.7270/Q2PK0KKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM183547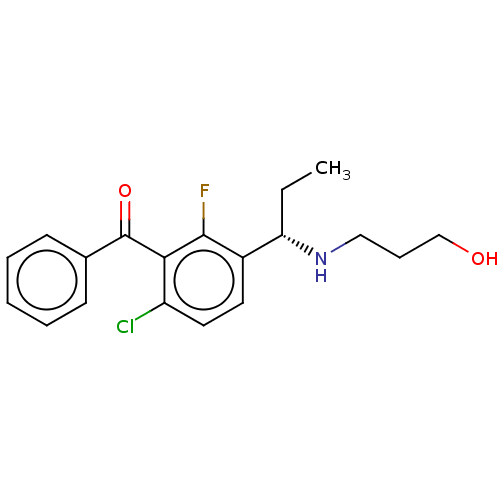 (US9145354, 182) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | 25 |
ASTEX THERAPEUTICS LIMITED US Patent | Assay Description The HCV NS3 protease functions have been extensively studied and are considered as potential targets for antiviral therapy: see for example the many ... | US Patent US9145354 (2015) BindingDB Entry DOI: 10.7270/Q2VH5MMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50146631 ((S)-2-[(S)-2-((R)-2-Hydroxycarbamoylmethyl-4-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of human matrix metalloprotease-1 | J Med Chem 47: 2761-7 (2004) Article DOI: 10.1021/jm031061l BindingDB Entry DOI: 10.7270/Q2ZG6T0B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha (Homo sapiens) | BDBM50615191 (CHEMBL5271804) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PDB UniChem | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 (Homo sapiens (Human)) | BDBM50601802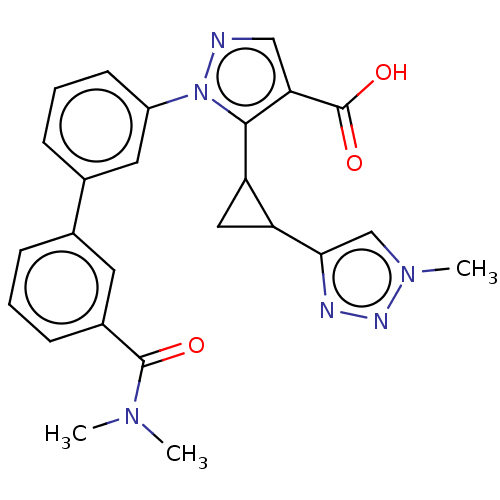 (CHEMBL5203783) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01351 BindingDB Entry DOI: 10.7270/Q2JS9VJF | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kelch-like ECH-associated protein 1 (Homo sapiens (Human)) | BDBM304728 (3-(7-methoxy-1-methyl-1H-benzo[d][1,2,3]triazol-5-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of 5'-TAMRA-labelled NRF2 peptide from human N-terminal His6-tagged KEAP1 Kelch domain (321 to 609 residues) expressed in baculovirus in... | J Med Chem 62: 4683-4702 (2019) Article DOI: 10.1021/acs.jmedchem.9b00279 BindingDB Entry DOI: 10.7270/Q2PK0KKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 (Homo sapiens (Human)) | BDBM50601811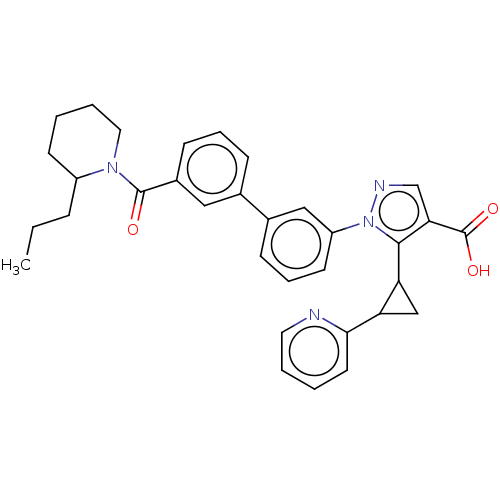 (CHEMBL5189627) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01351 BindingDB Entry DOI: 10.7270/Q2JS9VJF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM183530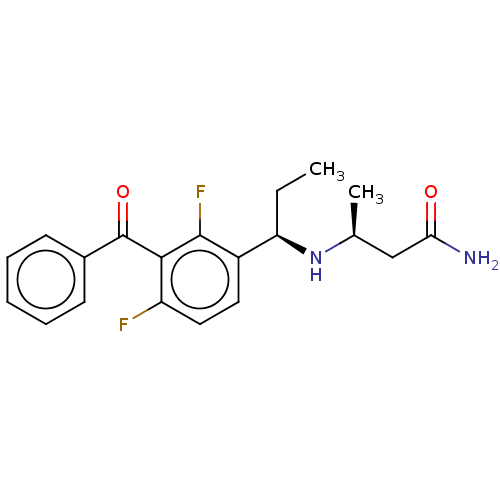 (US9145354, 157) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | 25 |
ASTEX THERAPEUTICS LIMITED US Patent | Assay Description The HCV NS3 protease functions have been extensively studied and are considered as potential targets for antiviral therapy: see for example the many ... | US Patent US9145354 (2015) BindingDB Entry DOI: 10.7270/Q2VH5MMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 (Homo sapiens (Human)) | BDBM304768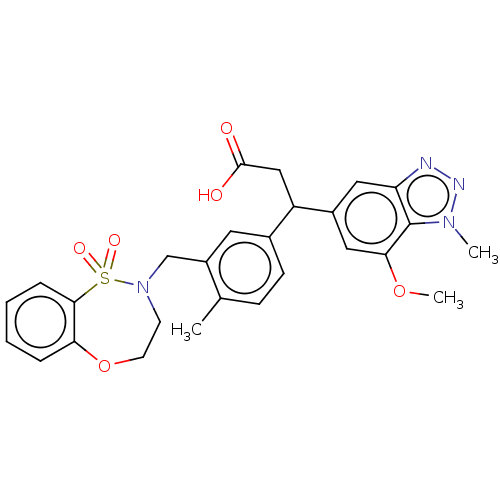 (3-(3-((1,1-dioxido-3,4-dihydro-2H-benzo[b][1,4,5]o...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of 5'-TAMRA-labelled NRF2 peptide from human N-terminal His6-tagged KEAP1 Kelch domain (321 to 609 residues) expressed in baculovirus in... | J Med Chem 62: 4683-4702 (2019) Article DOI: 10.1021/acs.jmedchem.9b00279 BindingDB Entry DOI: 10.7270/Q2PK0KKB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM183490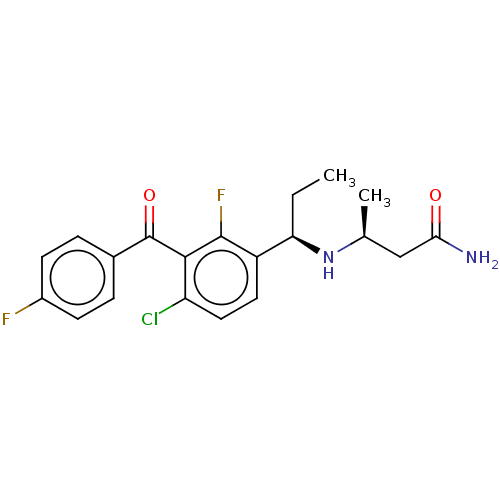 (US9145354, 97) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | 25 |
ASTEX THERAPEUTICS LIMITED US Patent | Assay Description The HCV NS3 protease functions have been extensively studied and are considered as potential targets for antiviral therapy: see for example the many ... | US Patent US9145354 (2015) BindingDB Entry DOI: 10.7270/Q2VH5MMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM183527 (US9145354, 152) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | 25 |
ASTEX THERAPEUTICS LIMITED US Patent | Assay Description The HCV NS3 protease functions have been extensively studied and are considered as potential targets for antiviral therapy: see for example the many ... | US Patent US9145354 (2015) BindingDB Entry DOI: 10.7270/Q2VH5MMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM183469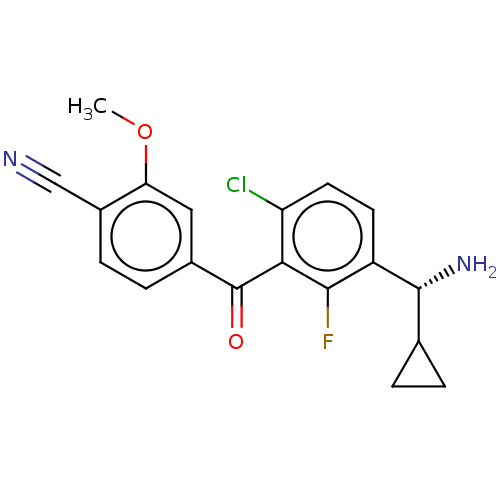 (US9145354, 69) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | 25 |
ASTEX THERAPEUTICS LIMITED US Patent | Assay Description The HCV NS3 protease functions have been extensively studied and are considered as potential targets for antiviral therapy: see for example the many ... | US Patent US9145354 (2015) BindingDB Entry DOI: 10.7270/Q2VH5MMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 [321-609] (Homo sapiens (Human)) | BDBM304946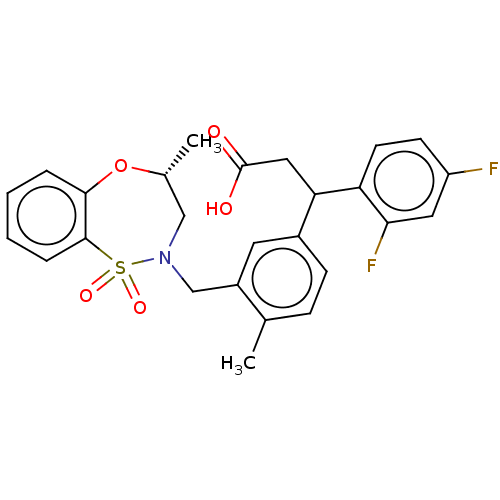 (3-(2,4-Difluorophenyl)-3-(4-methyl-3-(((R)-4-methy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Astex Therapeutics, Ltd US Patent | Assay Description 100 nL of 100× compound dose response curves (serial 3-fold dilutions) in DMSO are stamped using an Echo liquid handling system (Labcyte) into 384-we... | US Patent US10144731 (2018) BindingDB Entry DOI: 10.7270/Q25X2C17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 [321-609] (Homo sapiens (Human)) | BDBM304731 (3-(7-methoxy-1-methyl-1H-benzo[d][1,2,3]triazol-5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Astex Therapeutics, Ltd US Patent | Assay Description 100 nL of 100× compound dose response curves (serial 3-fold dilutions) in DMSO are stamped using an Echo liquid handling system (Labcyte) into 384-we... | US Patent US10144731 (2018) BindingDB Entry DOI: 10.7270/Q25X2C17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 [321-609] (Homo sapiens (Human)) | BDBM304974 (3-(1,4-dimethyl-1H-benzo[d][1,2,3]triazol-5-yl)-3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Astex Therapeutics, Ltd US Patent | Assay Description 100 nL of 100× compound dose response curves (serial 3-fold dilutions) in DMSO are stamped using an Echo liquid handling system (Labcyte) into 384-we... | US Patent US10144731 (2018) BindingDB Entry DOI: 10.7270/Q25X2C17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 [321-609] (Homo sapiens (Human)) | BDBM304785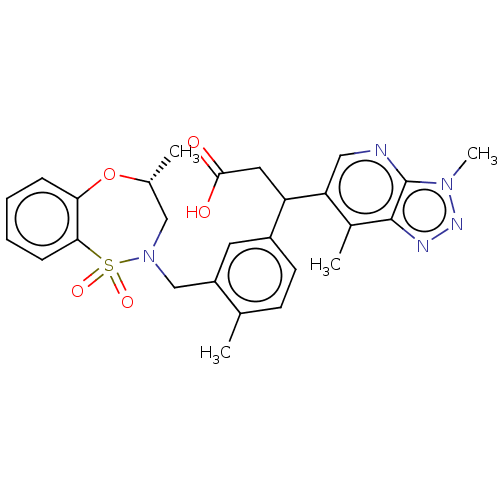 (3-(3,7-dimethyl-3H-[1,2,3]triazolo[4,5-b]pyridin-6...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Astex Therapeutics, Ltd US Patent | Assay Description 100 nL of 100× compound dose response curves (serial 3-fold dilutions) in DMSO are stamped using an Echo liquid handling system (Labcyte) into 384-we... | US Patent US10144731 (2018) BindingDB Entry DOI: 10.7270/Q25X2C17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 [321-609] (Homo sapiens (Human)) | BDBM304787 (3-(3-((4-ethyl-1,1-dioxido-3,4-dihydro-2H-benzo[b]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Astex Therapeutics, Ltd US Patent | Assay Description 100 nL of 100× compound dose response curves (serial 3-fold dilutions) in DMSO are stamped using an Echo liquid handling system (Labcyte) into 384-we... | US Patent US10144731 (2018) BindingDB Entry DOI: 10.7270/Q25X2C17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 [321-609] (Homo sapiens (Human)) | BDBM304789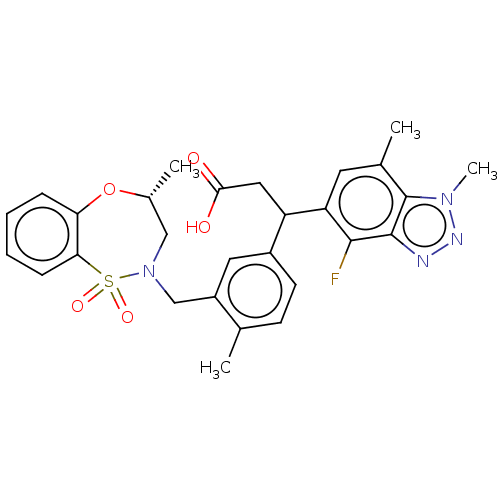 (3-(4-fluoro-1,7-dimethyl-1H-benzo[d][1,2,3]triazol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Astex Therapeutics, Ltd US Patent | Assay Description 100 nL of 100× compound dose response curves (serial 3-fold dilutions) in DMSO are stamped using an Echo liquid handling system (Labcyte) into 384-we... | US Patent US10144731 (2018) BindingDB Entry DOI: 10.7270/Q25X2C17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 [321-609] (Homo sapiens (Human)) | BDBM304820 (2-methyl-3-(1-methyl-1H-benzo[d][1,2,3]triazol-5-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Astex Therapeutics, Ltd US Patent | Assay Description 100 nL of 100× compound dose response curves (serial 3-fold dilutions) in DMSO are stamped using an Echo liquid handling system (Labcyte) into 384-we... | US Patent US10144731 (2018) BindingDB Entry DOI: 10.7270/Q25X2C17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 [321-609] (Homo sapiens (Human)) | BDBM304825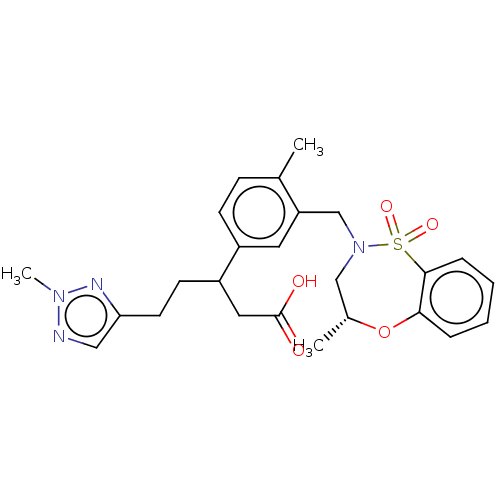 (5-(2-Methyl-2H-1,2,3-triazol-4-yl)-3-(4-methyl-3-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Astex Therapeutics, Ltd US Patent | Assay Description 100 nL of 100× compound dose response curves (serial 3-fold dilutions) in DMSO are stamped using an Echo liquid handling system (Labcyte) into 384-we... | US Patent US10144731 (2018) BindingDB Entry DOI: 10.7270/Q25X2C17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 [321-609] (Homo sapiens (Human)) | BDBM304829 (3-(1,4-Dimethyl-1H-benzo[d][1,2,3]triazol-5-yl)-3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Astex Therapeutics, Ltd US Patent | Assay Description 100 nL of 100× compound dose response curves (serial 3-fold dilutions) in DMSO are stamped using an Echo liquid handling system (Labcyte) into 384-we... | US Patent US10144731 (2018) BindingDB Entry DOI: 10.7270/Q25X2C17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 [321-609] (Homo sapiens (Human)) | BDBM304833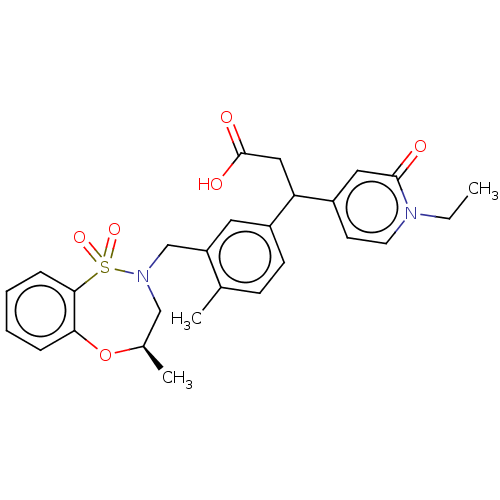 (3-(1-Ethyl-2-oxo-1,2-dihydropyridin-4-yl)-3-(4-met...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Astex Therapeutics, Ltd US Patent | Assay Description 100 nL of 100× compound dose response curves (serial 3-fold dilutions) in DMSO are stamped using an Echo liquid handling system (Labcyte) into 384-we... | US Patent US10144731 (2018) BindingDB Entry DOI: 10.7270/Q25X2C17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 996 total ) | Next | Last >> |