

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hexokinase-4 (Rattus norvegicus) | BDBM50161668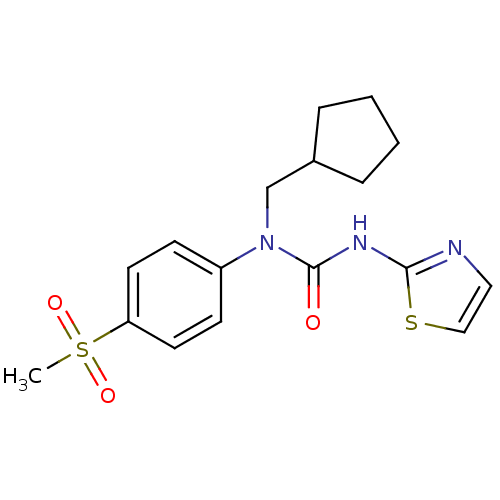 (1-Cyclopentylmethyl-1-(4-methanesulfonyl-phenyl)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 2 (Homo sapiens (Human)) | BDBM50526719 (CHEMBL4537583) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Agonist activity at human TLR2 expressed in HEK-Blue cells co-expressing NF-kappaB-SEAP reporter gene measured after 16 hrs by SEAP reporter gene ass... | J Med Chem 63: 2282-2291 (2020) Article DOI: 10.1021/acs.jmedchem.9b01044 BindingDB Entry DOI: 10.7270/Q2NV9NPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161670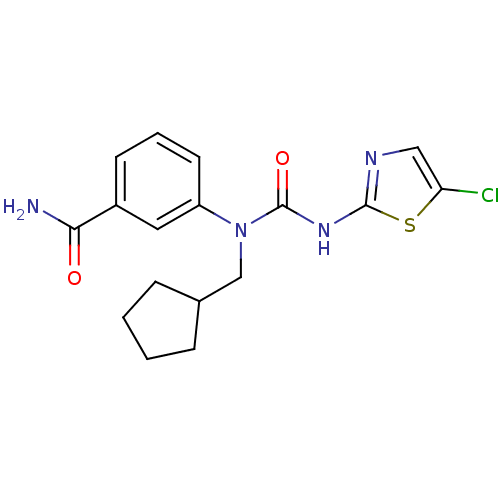 (3-[3-(5-Chloro-thiazol-2-yl)-1-cyclopentylmethyl-u...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161671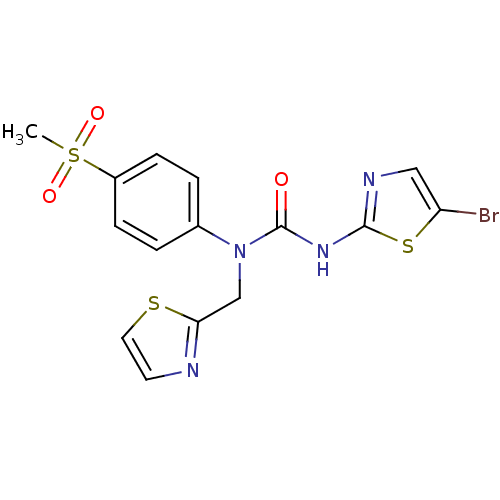 (3-(5-Bromo-thiazol-2-yl)-1-(4-methanesulfonyl-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.41E+4 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161672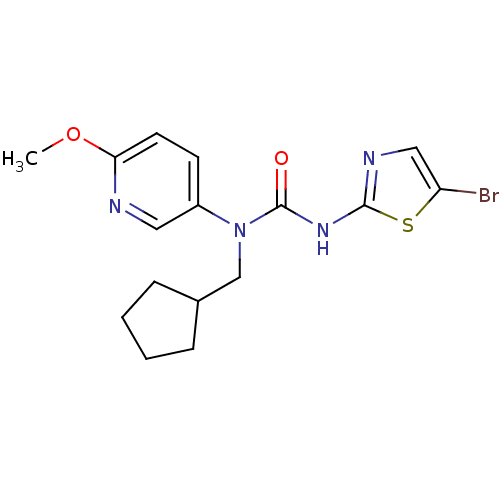 (3-(5-Bromo-thiazol-2-yl)-1-cyclopentylmethyl-1-(6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161673 (1-Cyclopentylmethyl-1-(4-methanesulfonyl-phenyl)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3.42E+4 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161674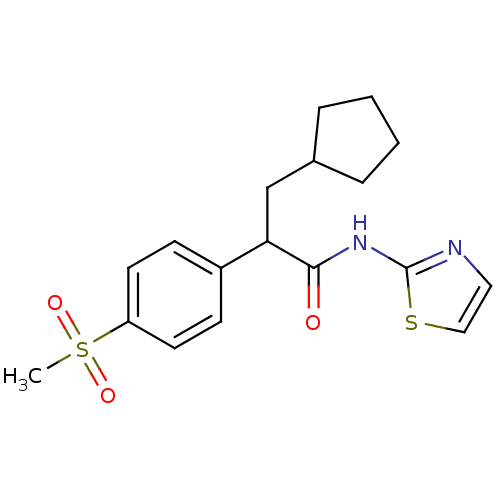 (3-Cyclopentyl-2-(4-methanesulfonyl-phenyl)-N-thiaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 15 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161676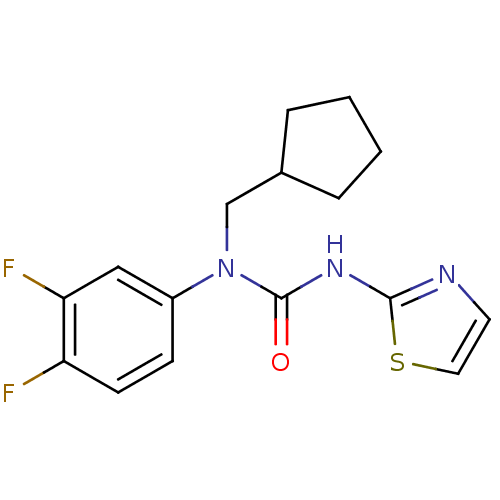 (1-Cyclopentylmethyl-1-(3,4-difluoro-phenyl)-3-thia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.63E+4 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161677 (4-[3-(5-Chloro-thiazol-2-yl)-1-cyclopentylmethyl-u...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161675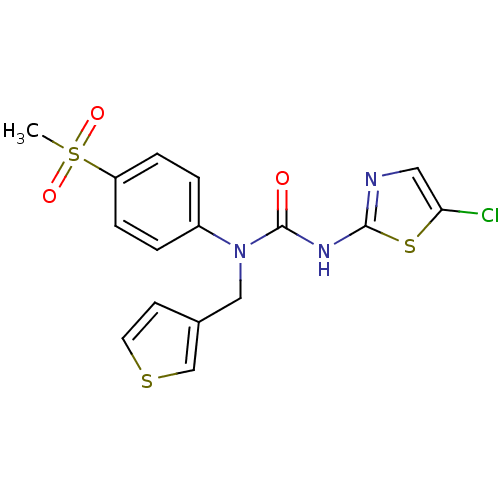 (3-(5-Chloro-thiazol-2-yl)-1-(4-methanesulfonyl-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161680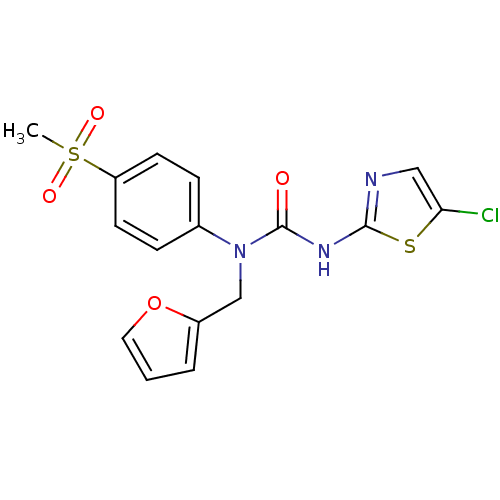 (3-(5-Chloro-thiazol-2-yl)-1-furan-2-ylmethyl-1-(4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.15E+4 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161681 (3-(5-Chloro-thiazol-2-yl)-1-(6-cyano-pyridin-3-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161682 (3-(5-Chloro-thiazol-2-yl)-1-cyclopentylmethyl-1-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161678 (3-(5-Chloro-thiazol-2-yl)-1-cyclopentylmethyl-1-(6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161679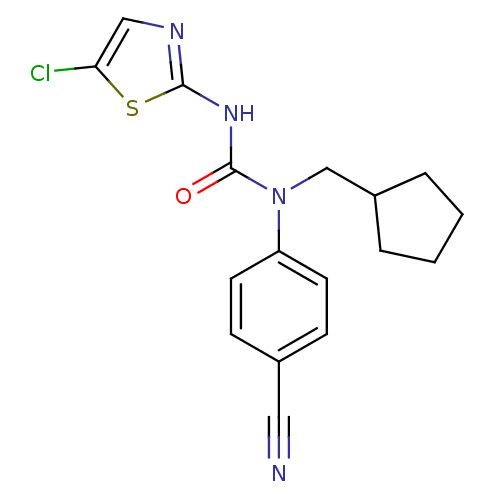 (3-(5-Chloro-thiazol-2-yl)-1-(4-cyano-phenyl)-1-cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161684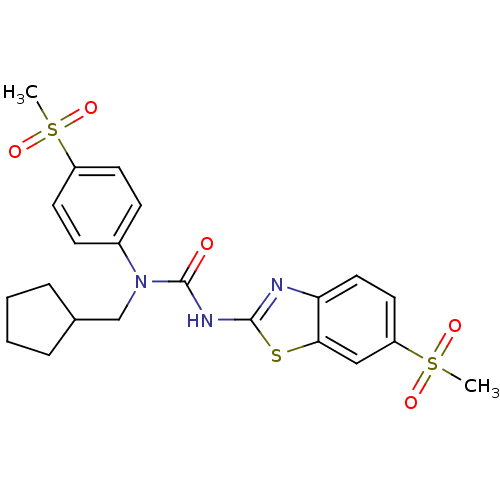 (1-Cyclopentylmethyl-3-(6-methanesulfonyl-benzothia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3.29E+4 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161685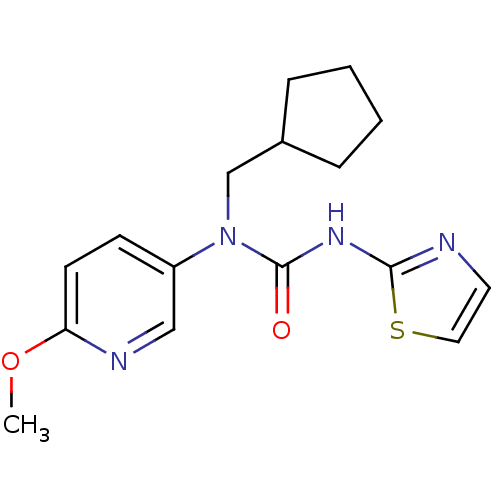 (1-Cyclopentylmethyl-1-(6-methoxy-pyridin-3-yl)-3-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.92E+4 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161683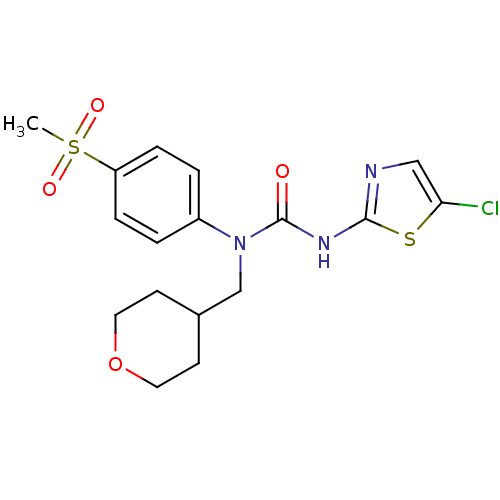 (3-(5-Chloro-thiazol-2-yl)-1-(4-methanesulfonyl-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161674 (3-Cyclopentyl-2-(4-methanesulfonyl-phenyl)-N-thiaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161686 (3-(5-Chloro-thiazol-2-yl)-1-cyclopentylmethyl-1-(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161687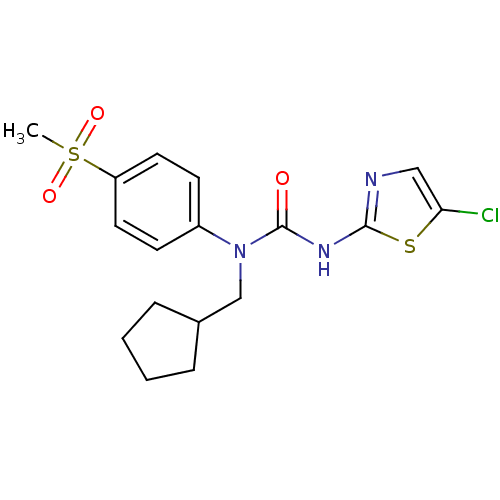 (3-(5-Chloro-thiazol-2-yl)-1-cyclopentylmethyl-1-(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161688 (3-(5-Chloro-thiazol-2-yl)-1-(4-methanesulfonyl-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161689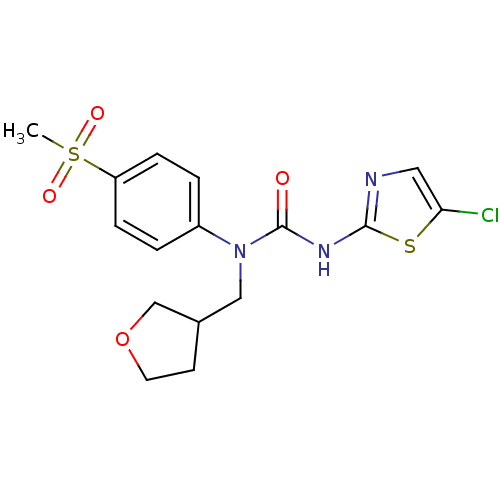 (3-(5-Chloro-thiazol-2-yl)-1-(4-methanesulfonyl-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.19E+4 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161690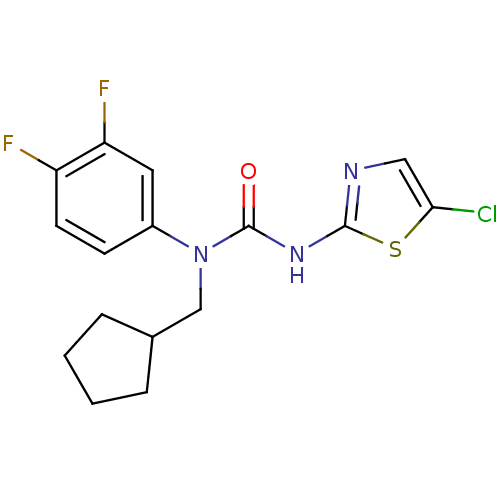 (3-(5-Chloro-thiazol-2-yl)-1-cyclopentylmethyl-1-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161691 (CHEMBL180172 | N-{3-[3-(5-Chloro-thiazol-2-yl)-1-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161694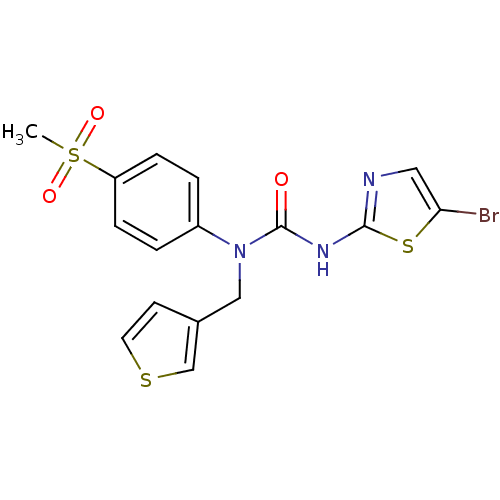 (3-(5-Bromo-thiazol-2-yl)-1-(4-methanesulfonyl-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161693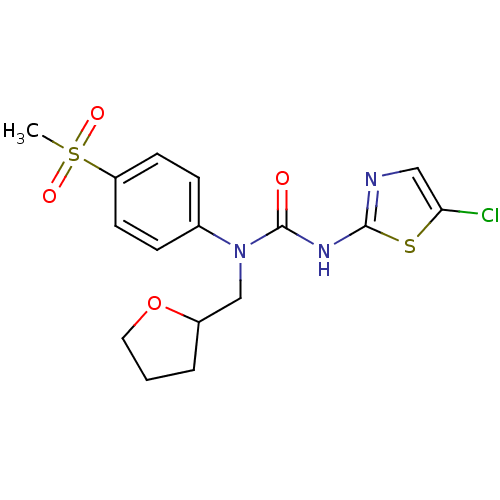 (3-(5-Chloro-thiazol-2-yl)-1-(4-methanesulfonyl-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.16E+4 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161668 (1-Cyclopentylmethyl-1-(4-methanesulfonyl-phenyl)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 15 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161695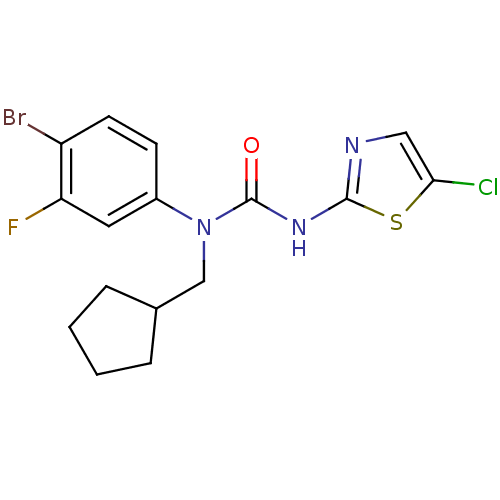 (1-(4-Bromo-3-fluoro-phenyl)-3-(5-chloro-thiazol-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 700 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161692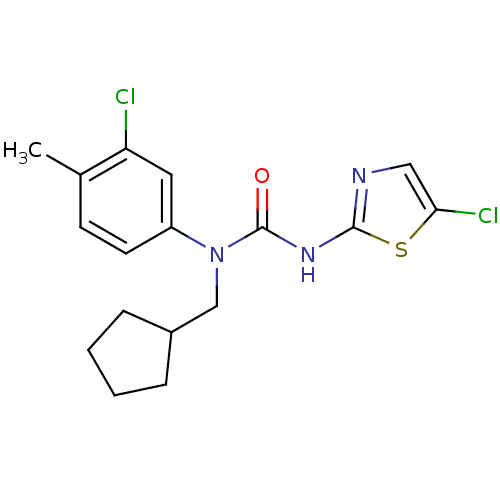 (1-(3-Chloro-4-methyl-phenyl)-3-(5-chloro-thiazol-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 400 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Homo sapiens (Human)) | BDBM50251802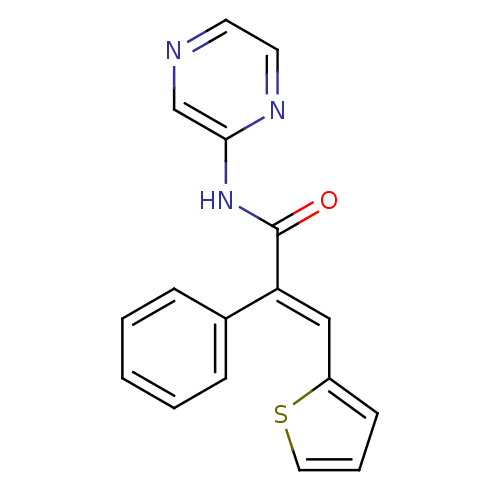 ((E)-2-phenyl-N-(pyrazin-2-yl)-3-(thiophen-2-yl)acr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a |
Oxford OX4 6LT Curated by ChEMBL | Assay Description Activation of GST fused human liver glucokinase assessed as generation of NADPH by G6PDH coupled assay | J Med Chem 51: 4340-5 (2008) Article DOI: 10.1021/jm8003202 BindingDB Entry DOI: 10.7270/Q2R49QKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Homo sapiens (Human)) | BDBM50251450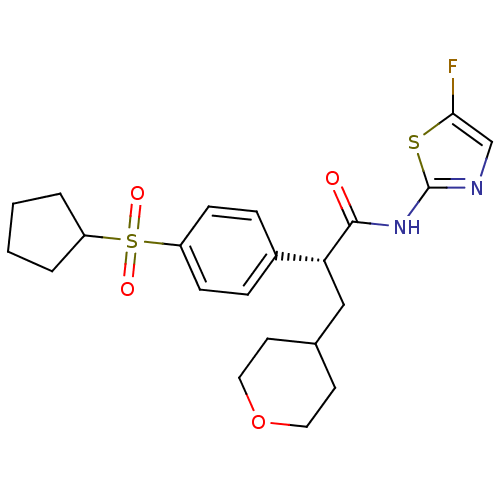 ((R)-2-(4-(cyclopentylsulfonyl)phenyl)-N-(5-fluorot...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
Oxford OX4 6LT Curated by ChEMBL | Assay Description Activation of GST fused human liver glucokinase assessed as generation of NADPH by G6PDH coupled assay | J Med Chem 51: 4340-5 (2008) Article DOI: 10.1021/jm8003202 BindingDB Entry DOI: 10.7270/Q2R49QKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Homo sapiens (Human)) | BDBM50251421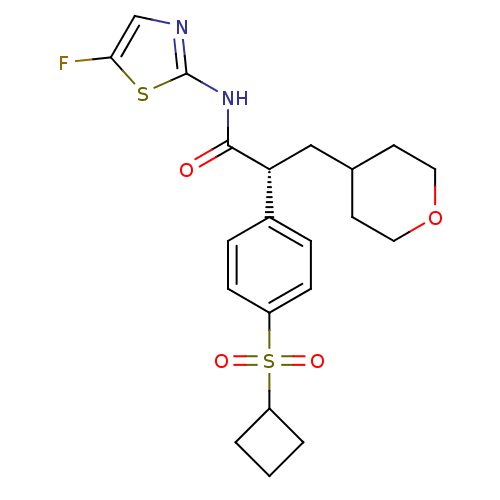 ((R)-2-(4-(cyclobutylsulfonyl)phenyl)-N-(5-fluoroth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 140 | n/a | n/a | n/a | n/a |
Oxford OX4 6LT Curated by ChEMBL | Assay Description Activation of GST fused human liver glucokinase assessed as generation of NADPH by G6PDH coupled assay | J Med Chem 51: 4340-5 (2008) Article DOI: 10.1021/jm8003202 BindingDB Entry DOI: 10.7270/Q2R49QKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Homo sapiens (Human)) | BDBM50251392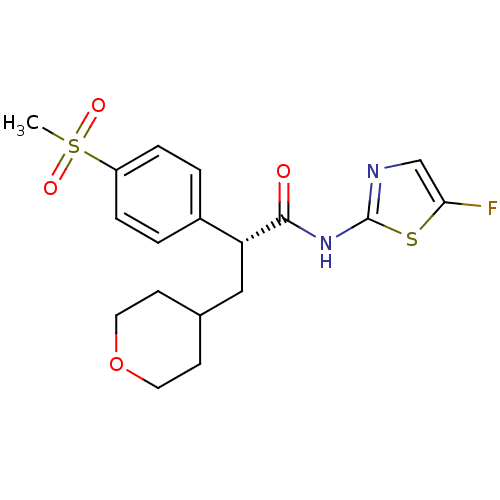 ((R)-N-(5-fluorothiazol-2-yl)-2-(4-(methylsulfonyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.43E+3 | n/a | n/a | n/a | n/a |
Oxford OX4 6LT Curated by ChEMBL | Assay Description Activation of GST fused human liver glucokinase assessed as generation of NADPH by G6PDH coupled assay | J Med Chem 51: 4340-5 (2008) Article DOI: 10.1021/jm8003202 BindingDB Entry DOI: 10.7270/Q2R49QKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Homo sapiens (Human)) | BDBM50251391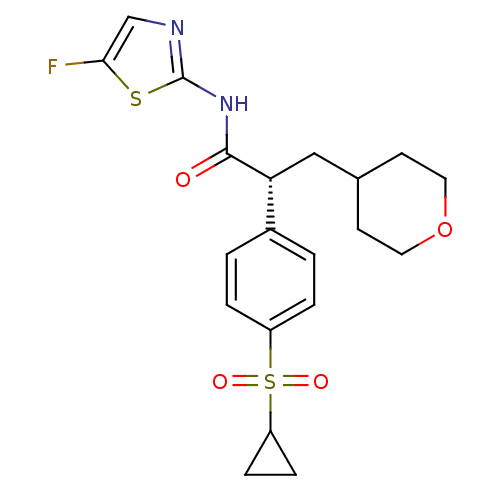 ((2R)-2-(4-Cyclopropanesulfonylphenyl)-N-(5-fluorot...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a |
Oxford OX4 6LT Curated by ChEMBL | Assay Description Activation of GST fused human liver glucokinase assessed as generation of NADPH by G6PDH coupled assay | J Med Chem 51: 4340-5 (2008) Article DOI: 10.1021/jm8003202 BindingDB Entry DOI: 10.7270/Q2R49QKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Homo sapiens (Human)) | BDBM50251390 ((R)-2-(4-(cyclopropylsulfonyl)phenyl)-N-(5-methylt...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 580 | n/a | n/a | n/a | n/a |
Oxford OX4 6LT Curated by ChEMBL | Assay Description Activation of GST fused human liver glucokinase assessed as generation of NADPH by G6PDH coupled assay | J Med Chem 51: 4340-5 (2008) Article DOI: 10.1021/jm8003202 BindingDB Entry DOI: 10.7270/Q2R49QKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Homo sapiens (Human)) | BDBM50251389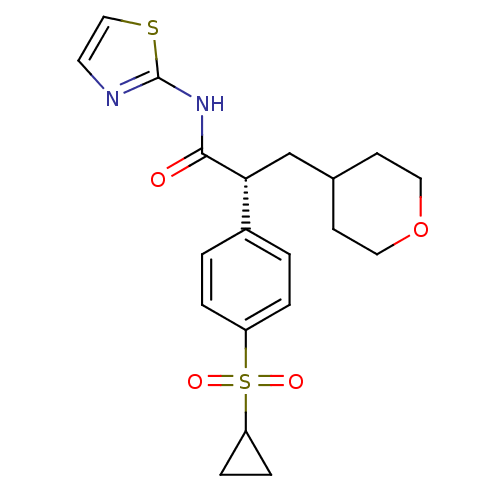 ((R)-2-(4-(cyclopropylsulfonyl)phenyl)-3-(tetrahydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 570 | n/a | n/a | n/a | n/a |
Oxford OX4 6LT Curated by ChEMBL | Assay Description Activation of GST fused human liver glucokinase assessed as generation of NADPH by G6PDH coupled assay | J Med Chem 51: 4340-5 (2008) Article DOI: 10.1021/jm8003202 BindingDB Entry DOI: 10.7270/Q2R49QKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Homo sapiens (Human)) | BDBM50251388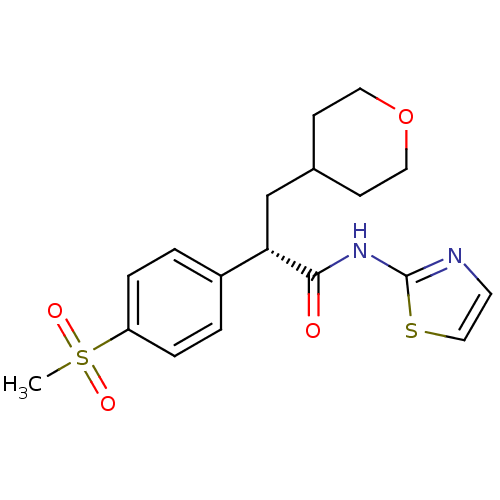 ((S)-2-(4-(methylsulfonyl)phenyl)-3-(tetrahydro-2H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Oxford OX4 6LT Curated by ChEMBL | Assay Description Activation of GST fused human liver glucokinase assessed as generation of NADPH by G6PDH coupled assay | J Med Chem 51: 4340-5 (2008) Article DOI: 10.1021/jm8003202 BindingDB Entry DOI: 10.7270/Q2R49QKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Homo sapiens (Human)) | BDBM50251833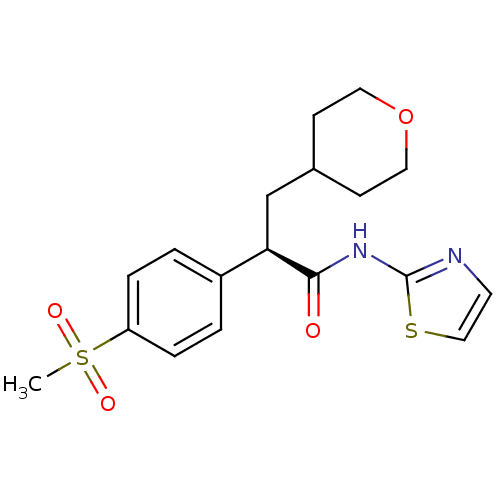 ((R)-2-(4-(methylsulfonyl)phenyl)-3-(tetrahydro-2H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.47E+3 | n/a | n/a | n/a | n/a |
Oxford OX4 6LT Curated by ChEMBL | Assay Description Activation of GST fused human liver glucokinase assessed as generation of NADPH by G6PDH coupled assay | J Med Chem 51: 4340-5 (2008) Article DOI: 10.1021/jm8003202 BindingDB Entry DOI: 10.7270/Q2R49QKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Homo sapiens (Human)) | BDBM50251832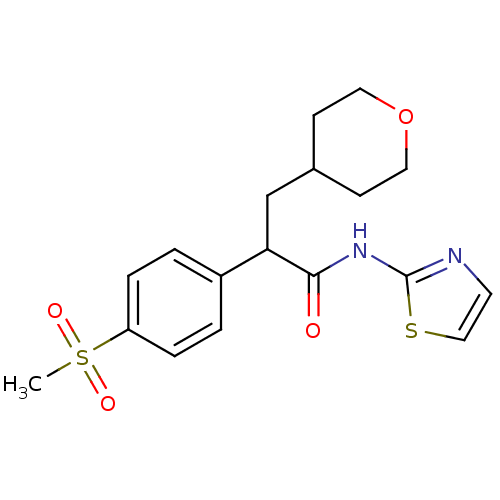 (2-(4-(methylsulfonyl)phenyl)-3-(tetrahydro-2H-pyra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.93E+3 | n/a | n/a | n/a | n/a |
Oxford OX4 6LT Curated by ChEMBL | Assay Description Activation of GST fused human liver glucokinase assessed as generation of NADPH by G6PDH coupled assay | J Med Chem 51: 4340-5 (2008) Article DOI: 10.1021/jm8003202 BindingDB Entry DOI: 10.7270/Q2R49QKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Homo sapiens (Human)) | BDBM50251831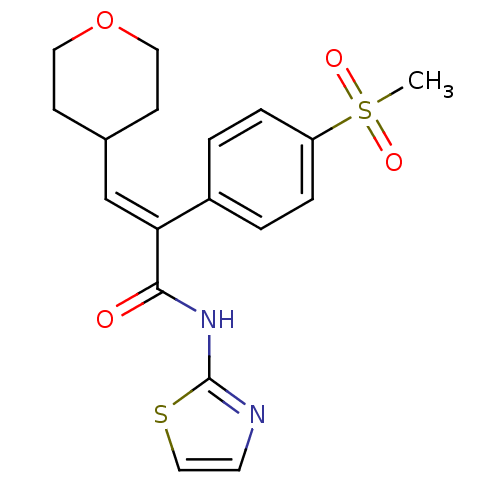 (2-(4-(methylsulfonyl)phenyl)-3-(tetrahydro-2H-pyra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a |
Oxford OX4 6LT Curated by ChEMBL | Assay Description Activation of GST fused human liver glucokinase assessed as generation of NADPH by G6PDH coupled assay | J Med Chem 51: 4340-5 (2008) Article DOI: 10.1021/jm8003202 BindingDB Entry DOI: 10.7270/Q2R49QKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Homo sapiens (Human)) | BDBM50251830 ((Z)-2-(4-(methylsulfonyl)phenyl)-N-(thiazol-2-yl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 560 | n/a | n/a | n/a | n/a |
Oxford OX4 6LT Curated by ChEMBL | Assay Description Activation of GST fused human liver glucokinase assessed as generation of NADPH by G6PDH coupled assay | J Med Chem 51: 4340-5 (2008) Article DOI: 10.1021/jm8003202 BindingDB Entry DOI: 10.7270/Q2R49QKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Homo sapiens (Human)) | BDBM50251829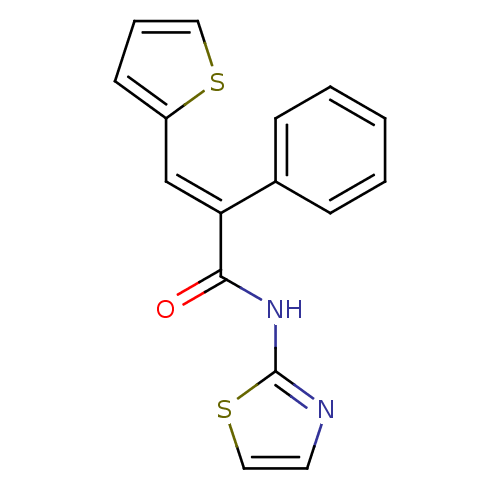 (2-phenyl-N-(thiazol-2-yl)-3-(thiophen-2-yl)acrylam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a |
Oxford OX4 6LT Curated by ChEMBL | Assay Description Activation of GST fused human liver glucokinase assessed as generation of NADPH by G6PDH coupled assay | J Med Chem 51: 4340-5 (2008) Article DOI: 10.1021/jm8003202 BindingDB Entry DOI: 10.7270/Q2R49QKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 2 (Homo sapiens (Human)) | BDBM50526707 (CHEMBL4439863) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 46 | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Agonist activity at human TLR2 expressed in HEK-Blue cells co-expressing NF-kappaB-SEAP reporter gene measured after 16 hrs by SEAP reporter gene ass... | J Med Chem 63: 2282-2291 (2020) Article DOI: 10.1021/acs.jmedchem.9b01044 BindingDB Entry DOI: 10.7270/Q2NV9NPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 2 (Homo sapiens (Human)) | BDBM50526708 (CHEMBL4552043) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.281 | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Agonist activity at human TLR2 expressed in HEK-Blue cells co-expressing NF-kappaB-SEAP reporter gene measured after 16 hrs by SEAP reporter gene ass... | J Med Chem 63: 2282-2291 (2020) Article DOI: 10.1021/acs.jmedchem.9b01044 BindingDB Entry DOI: 10.7270/Q2NV9NPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 2 (Homo sapiens (Human)) | BDBM50526709 (CHEMBL4546202) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.383 | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Agonist activity at human TLR2 expressed in HEK-Blue cells co-expressing NF-kappaB-SEAP reporter gene measured after 16 hrs by SEAP reporter gene ass... | J Med Chem 63: 2282-2291 (2020) Article DOI: 10.1021/acs.jmedchem.9b01044 BindingDB Entry DOI: 10.7270/Q2NV9NPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 2 (Homo sapiens (Human)) | BDBM50526710 (CHEMBL4560927) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.468 | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Agonist activity at human TLR2 expressed in HEK-Blue cells co-expressing NF-kappaB-SEAP reporter gene measured after 16 hrs by SEAP reporter gene ass... | J Med Chem 63: 2282-2291 (2020) Article DOI: 10.1021/acs.jmedchem.9b01044 BindingDB Entry DOI: 10.7270/Q2NV9NPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 2 (Homo sapiens (Human)) | BDBM50526711 (CHEMBL4455047) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 151 | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Agonist activity at human TLR2 expressed in HEK-Blue cells co-expressing NF-kappaB-SEAP reporter gene measured after 16 hrs by SEAP reporter gene ass... | J Med Chem 63: 2282-2291 (2020) Article DOI: 10.1021/acs.jmedchem.9b01044 BindingDB Entry DOI: 10.7270/Q2NV9NPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 2 (Homo sapiens (Human)) | BDBM50526712 (CHEMBL4463804) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 161 | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Agonist activity at human TLR2 expressed in HEK-Blue cells co-expressing NF-kappaB-SEAP reporter gene measured after 16 hrs by SEAP reporter gene ass... | J Med Chem 63: 2282-2291 (2020) Article DOI: 10.1021/acs.jmedchem.9b01044 BindingDB Entry DOI: 10.7270/Q2NV9NPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 2 (Homo sapiens (Human)) | BDBM50526713 (CHEMBL4461230) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.609 | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Agonist activity at human TLR2 expressed in HEK-Blue cells co-expressing NF-kappaB-SEAP reporter gene measured after 16 hrs by SEAP reporter gene ass... | J Med Chem 63: 2282-2291 (2020) Article DOI: 10.1021/acs.jmedchem.9b01044 BindingDB Entry DOI: 10.7270/Q2NV9NPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 56 total ) | Next | Last >> |