 Found 3099 hits with Last Name = 'winum' and Initial = 'jy'
Found 3099 hits with Last Name = 'winum' and Initial = 'jy' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50153970

(4-(4,6-Diethoxy-[1,3,5]triazin-2-ylamino)-benzenes...)Show InChI InChI=1S/C13H17N5O4S/c1-3-21-12-16-11(17-13(18-12)22-4-2)15-9-5-7-10(8-6-9)23(14,19)20/h5-8H,3-4H2,1-2H3,(H2,14,19,20)(H,15,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase IX expressed in Escherichia coli BL21 |
Bioorg Med Chem Lett 14: 5427-33 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.087
BindingDB Entry DOI: 10.7270/Q2XD12DQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50153971
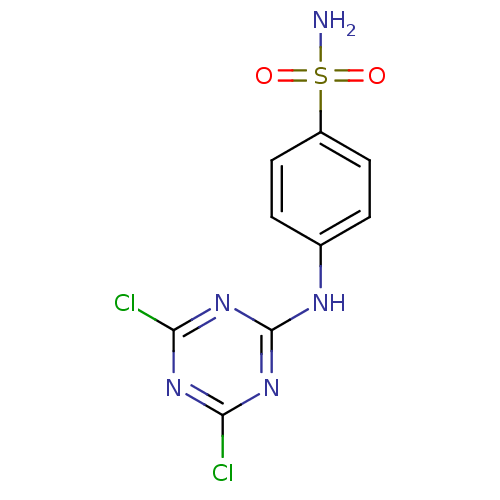
(4-(4,6-Dichloro-[1,3,5]triazin-2-ylamino)-benzenes...)Show InChI InChI=1S/C9H7Cl2N5O2S/c10-7-14-8(11)16-9(15-7)13-5-1-3-6(4-2-5)19(12,17)18/h1-4H,(H2,12,17,18)(H,13,14,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase IX expressed in Escherichia coli BL21 |
Bioorg Med Chem Lett 14: 5427-33 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.087
BindingDB Entry DOI: 10.7270/Q2XD12DQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM11625
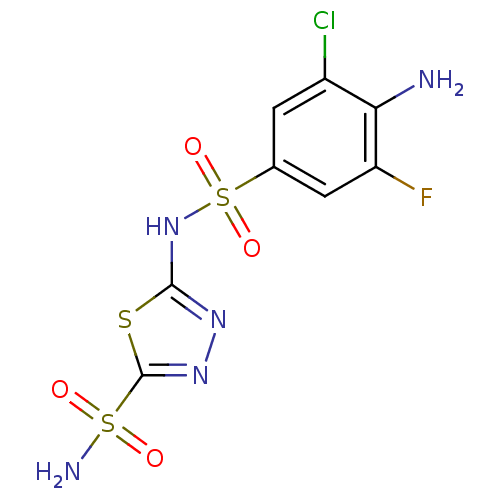
(2-N-(4-amino-3-chloro-5-fluorobenzene)-1,3,4-thiad...)Show SMILES Nc1c(F)cc(cc1Cl)S(=O)(=O)Nc1nnc(s1)S(N)(=O)=O Show InChI InChI=1S/C8H7ClFN5O4S3/c9-4-1-3(2-5(10)6(4)11)22(18,19)15-7-13-14-8(20-7)21(12,16)17/h1-2H,11H2,(H,13,15)(H2,12,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universit£ Montpellier II
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase II by spectrophotometry at pH 7.5 |
Bioorg Med Chem 19: 1172-8 (2011)
Article DOI: 10.1016/j.bmc.2010.12.048
BindingDB Entry DOI: 10.7270/Q2C53M44 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50153964

(4-((4,6-diethoxy-1,3,5-triazin-2-ylamino)methyl)be...)Show InChI InChI=1S/C14H19N5O4S/c1-3-22-13-17-12(18-14(19-13)23-4-2)16-9-10-5-7-11(8-6-10)24(15,20)21/h5-8H,3-4,9H2,1-2H3,(H2,15,20,21)(H,16,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase IX expressed in Escherichia coli BL21 |
Bioorg Med Chem Lett 14: 5427-33 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.087
BindingDB Entry DOI: 10.7270/Q2XD12DQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50395391
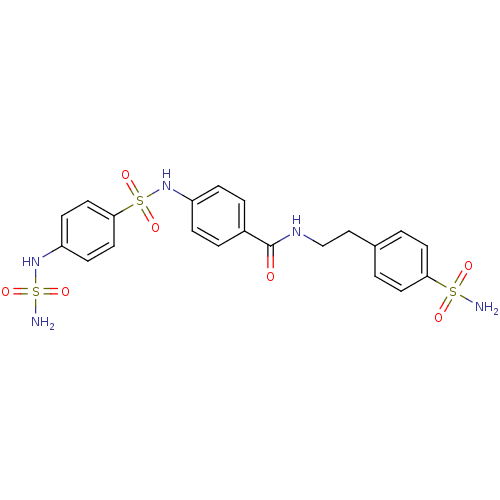
(CHEMBL2164737)Show SMILES NS(=O)(=O)Nc1ccc(cc1)S(=O)(=O)Nc1ccc(cc1)C(=O)NCCc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C21H23N5O7S3/c22-34(28,29)19-9-1-15(2-10-19)13-14-24-21(27)16-3-5-17(6-4-16)25-35(30,31)20-11-7-18(8-12-20)26-36(23,32)33/h1-12,25-26H,13-14H2,(H,24,27)(H2,22,28,29)(H2,23,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Biostrutture e Bioimmagini-CNR
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA9 catalytic domain preincubated for 15 mins by stopped-flow CO2 hydration assay |
J Med Chem 55: 6776-83 (2012)
Article DOI: 10.1021/jm300818k
BindingDB Entry DOI: 10.7270/Q2736S2H |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50395391

(CHEMBL2164737)Show SMILES NS(=O)(=O)Nc1ccc(cc1)S(=O)(=O)Nc1ccc(cc1)C(=O)NCCc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C21H23N5O7S3/c22-34(28,29)19-9-1-15(2-10-19)13-14-24-21(27)16-3-5-17(6-4-16)25-35(30,31)20-11-7-18(8-12-20)26-36(23,32)33/h1-12,25-26H,13-14H2,(H,24,27)(H2,22,28,29)(H2,23,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Biostrutture e Bioimmagini-CNR
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA12 catalytic domain preincubated for 15 mins by stopped-flow CO2 hydration assay |
J Med Chem 55: 6776-83 (2012)
Article DOI: 10.1021/jm300818k
BindingDB Entry DOI: 10.7270/Q2736S2H |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50155543
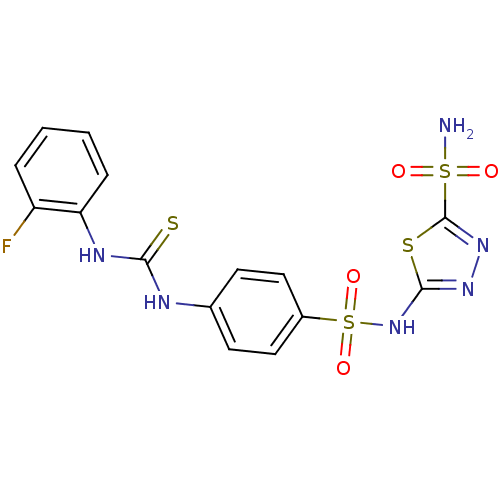
(5-{4-[3-(2-Fluoro-phenyl)-thioureido]-benzenesulfo...)Show SMILES NS(=O)(=O)c1nnc(NS(=O)(=O)c2ccc(NC(=S)Nc3ccccc3F)cc2)s1 Show InChI InChI=1S/C15H13FN6O4S4/c16-11-3-1-2-4-12(11)19-13(27)18-9-5-7-10(8-6-9)30(25,26)22-14-20-21-15(28-14)29(17,23)24/h1-8H,(H,20,22)(H2,17,23,24)(H2,18,19,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Binding affinity towards human cloned carbonic anhydrase II |
Bioorg Med Chem Lett 14: 5775-80 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.062
BindingDB Entry DOI: 10.7270/Q2154HT4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 6
(Homo sapiens (Human)) | BDBM50137675
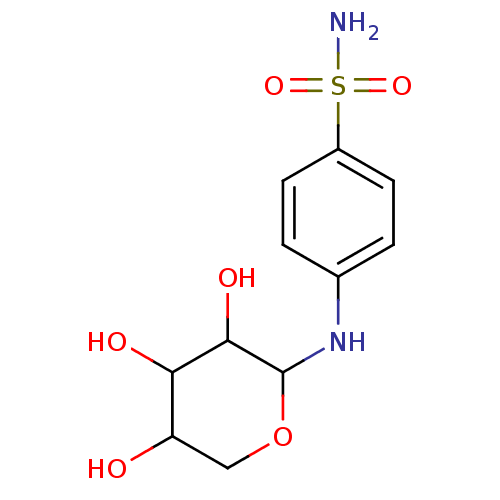
(4-(3,4,5-Trihydroxy-tetrahydro-pyran-2-ylamino)-be...)Show InChI InChI=1S/C11H16N2O6S/c12-20(17,18)7-3-1-6(2-4-7)13-11-10(16)9(15)8(14)5-19-11/h1-4,8-11,13-16H,5H2,(H2,12,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.560 | -51.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 20 |
Institut des Biomolécules Max Mousseron (IBMM)
| Assay Description
Inhibition assay using carbonic anhydrases with an SX.18MV-R, a stopped-flow instrument from Applied Photophysics was used for assaying the CA cataly... |
Chem Biol Drug Des 74: 636-9 (2009)
Article DOI: 10.1111/j.1747-0285.2009.00902.x
BindingDB Entry DOI: 10.7270/Q2VX0F1H |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50155547
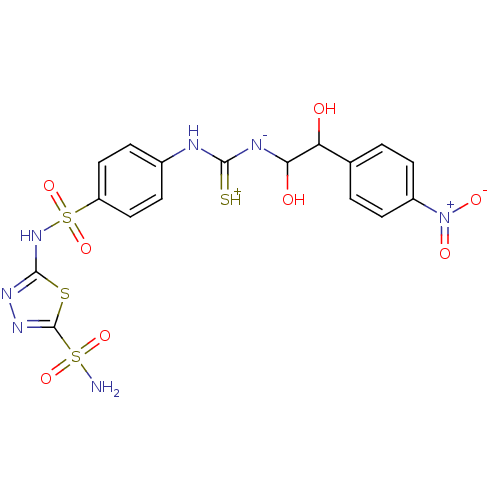
(5-(4-{3-[1,2-Dihydroxy-2-(4-nitro-phenyl)-ethyl]-t...)Show SMILES NS(=O)(=O)c1nnc(NS(=O)(=O)c2ccc(NC(=[SH+])[N-]C(O)C(O)c3ccc(cc3)[N+]([O-])=O)cc2)s1 Show InChI InChI=1S/C17H17N7O8S4/c18-35(29,30)17-22-21-16(34-17)23-36(31,32)12-7-3-10(4-8-12)19-15(33)20-14(26)13(25)9-1-5-11(6-2-9)24(27)28/h1-8,13-14,25-26H,(H5,18,19,20,21,23,29,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Binding affinity towards human cloned carbonic anhydrase II |
Bioorg Med Chem Lett 14: 5775-80 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.062
BindingDB Entry DOI: 10.7270/Q2154HT4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50155546
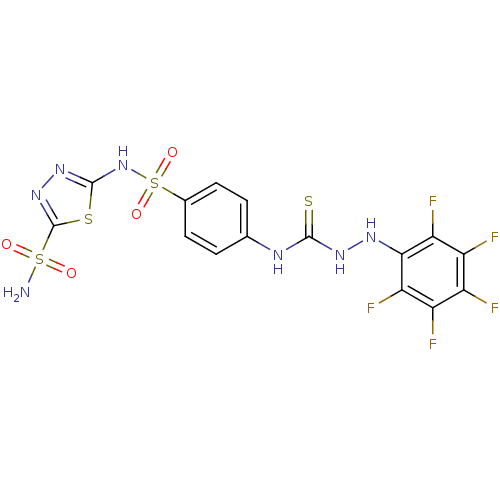
(CHEMBL445650 | N-[4-({[5-(aminosulfonyl)-1,3,4-thi...)Show SMILES NS(=O)(=O)c1nnc(NS(=O)(=O)c2ccc(NC(=S)NNc3c(F)c(F)c(F)c(F)c3F)cc2)s1 Show InChI InChI=1S/C15H10F5N7O4S4/c16-7-8(17)10(19)12(11(20)9(7)18)23-24-13(32)22-5-1-3-6(4-2-5)35(30,31)27-14-25-26-15(33-14)34(21,28)29/h1-4,23H,(H,25,27)(H2,21,28,29)(H2,22,24,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Binding affinity towards human cloned carbonic anhydrase II |
Bioorg Med Chem Lett 14: 5775-80 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.062
BindingDB Entry DOI: 10.7270/Q2154HT4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50155547

(5-(4-{3-[1,2-Dihydroxy-2-(4-nitro-phenyl)-ethyl]-t...)Show SMILES NS(=O)(=O)c1nnc(NS(=O)(=O)c2ccc(NC(=[SH+])[N-]C(O)C(O)c3ccc(cc3)[N+]([O-])=O)cc2)s1 Show InChI InChI=1S/C17H17N7O8S4/c18-35(29,30)17-22-21-16(34-17)23-36(31,32)12-7-3-10(4-8-12)19-15(33)20-14(26)13(25)9-1-5-11(6-2-9)24(27)28/h1-8,13-14,25-26H,(H5,18,19,20,21,23,29,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Binding affinity towards human cloned carbonic anhydrase I |
Bioorg Med Chem Lett 14: 5775-80 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.062
BindingDB Entry DOI: 10.7270/Q2154HT4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50155545
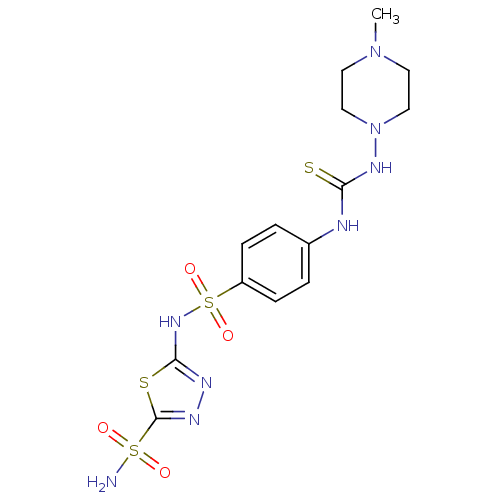
(5-{4-[3-(4-Methyl-piperazin-1-yl)-thioureido]-benz...)Show SMILES CN1CCN(CC1)NC(=S)Nc1ccc(cc1)S(=O)(=O)Nc1nnc(s1)S(N)(=O)=O Show InChI InChI=1S/C14H20N8O4S4/c1-21-6-8-22(9-7-21)19-12(27)16-10-2-4-11(5-3-10)30(25,26)20-13-17-18-14(28-13)29(15,23)24/h2-5H,6-9H2,1H3,(H,17,20)(H2,15,23,24)(H2,16,19,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Binding affinity towards human cloned carbonic anhydrase I |
Bioorg Med Chem Lett 14: 5775-80 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.062
BindingDB Entry DOI: 10.7270/Q2154HT4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50395391

(CHEMBL2164737)Show SMILES NS(=O)(=O)Nc1ccc(cc1)S(=O)(=O)Nc1ccc(cc1)C(=O)NCCc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C21H23N5O7S3/c22-34(28,29)19-9-1-15(2-10-19)13-14-24-21(27)16-3-5-17(6-4-16)25-35(30,31)20-11-7-18(8-12-20)26-36(23,32)33/h1-12,25-26H,13-14H2,(H,24,27)(H2,22,28,29)(H2,23,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Biostrutture e Bioimmagini-CNR
Curated by ChEMBL
| Assay Description
Inhibition of full length human CA2 cytosolic isoform preincubated for 15 mins by stopped-flow CO2 hydration assay |
J Med Chem 55: 6776-83 (2012)
Article DOI: 10.1021/jm300818k
BindingDB Entry DOI: 10.7270/Q2736S2H |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50155542
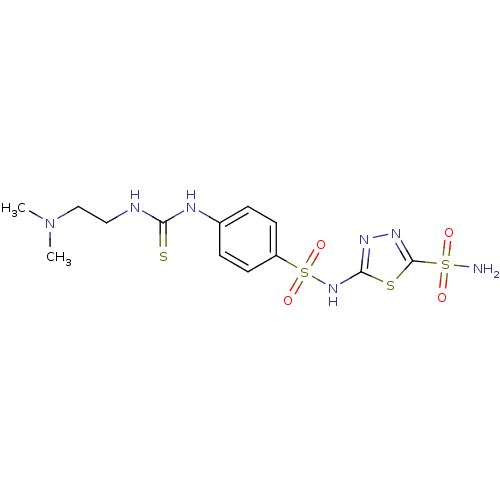
(5-{4-[3-(2-Dimethylamino-ethyl)-thioureido]-benzen...)Show SMILES CN(C)CCNC(=S)Nc1ccc(cc1)S(=O)(=O)Nc1nnc(s1)S(N)(=O)=O Show InChI InChI=1S/C13H19N7O4S4/c1-20(2)8-7-15-11(25)16-9-3-5-10(6-4-9)28(23,24)19-12-17-18-13(26-12)27(14,21)22/h3-6H,7-8H2,1-2H3,(H,17,19)(H2,14,21,22)(H2,15,16,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Binding affinity towards human cloned carbonic anhydrase I |
Bioorg Med Chem Lett 14: 5775-80 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.062
BindingDB Entry DOI: 10.7270/Q2154HT4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 6
(Homo sapiens (Human)) | BDBM50137677
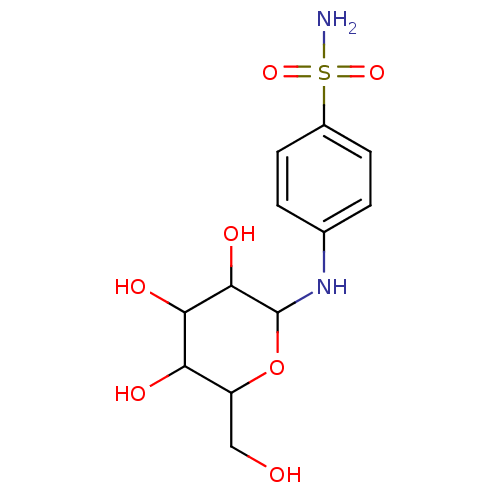
(4-(3,4,5-Trihydroxy-6-hydroxymethyl-tetrahydro-pyr...)Show InChI InChI=1S/C12H18N2O7S/c13-22(19,20)7-3-1-6(2-4-7)14-12-11(18)10(17)9(16)8(5-15)21-12/h1-4,8-12,14-18H,5H2,(H2,13,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.640 | -51.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 20 |
Institut des Biomolécules Max Mousseron (IBMM)
| Assay Description
Inhibition assay using carbonic anhydrases with an SX.18MV-R, a stopped-flow instrument from Applied Photophysics was used for assaying the CA cataly... |
Chem Biol Drug Des 74: 636-9 (2009)
Article DOI: 10.1111/j.1747-0285.2009.00902.x
BindingDB Entry DOI: 10.7270/Q2VX0F1H |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM33280
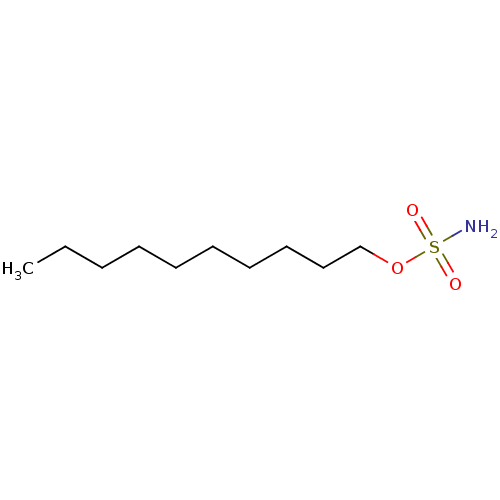
(CHEMBL153094 | aliphatic sulfamate, 2)Show InChI InChI=1S/C10H23NO3S/c1-2-3-4-5-6-7-8-9-10-14-15(11,12)13/h2-10H2,1H3,(H2,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.700 | -51.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 23 |
CNR
| Assay Description
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... |
J Med Chem 52: 5990-8 (2009)
Article DOI: 10.1021/jm900641r
BindingDB Entry DOI: 10.7270/Q2GF0RVR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50155546

(CHEMBL445650 | N-[4-({[5-(aminosulfonyl)-1,3,4-thi...)Show SMILES NS(=O)(=O)c1nnc(NS(=O)(=O)c2ccc(NC(=S)NNc3c(F)c(F)c(F)c(F)c3F)cc2)s1 Show InChI InChI=1S/C15H10F5N7O4S4/c16-7-8(17)10(19)12(11(20)9(7)18)23-24-13(32)22-5-1-3-6(4-2-5)35(30,31)27-14-25-26-15(33-14)34(21,28)29/h1-4,23H,(H,25,27)(H2,21,28,29)(H2,22,24,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Binding affinity towards human cloned carbonic anhydrase I |
Bioorg Med Chem Lett 14: 5775-80 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.062
BindingDB Entry DOI: 10.7270/Q2154HT4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM33280

(CHEMBL153094 | aliphatic sulfamate, 2)Show InChI InChI=1S/C10H23NO3S/c1-2-3-4-5-6-7-8-9-10-14-15(11,12)13/h2-10H2,1H3,(H2,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Montpellier II
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II. |
J Med Chem 46: 5471-7 (2003)
Article DOI: 10.1021/jm030911u
BindingDB Entry DOI: 10.7270/Q2KP81KV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50155545

(5-{4-[3-(4-Methyl-piperazin-1-yl)-thioureido]-benz...)Show SMILES CN1CCN(CC1)NC(=S)Nc1ccc(cc1)S(=O)(=O)Nc1nnc(s1)S(N)(=O)=O Show InChI InChI=1S/C14H20N8O4S4/c1-21-6-8-22(9-7-21)19-12(27)16-10-2-4-11(5-3-10)30(25,26)20-13-17-18-14(28-13)29(15,23)24/h2-5H,6-9H2,1H3,(H,17,20)(H2,15,23,24)(H2,16,19,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Binding affinity towards human cloned carbonic anhydrase II |
Bioorg Med Chem Lett 14: 5775-80 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.062
BindingDB Entry DOI: 10.7270/Q2154HT4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50155543

(5-{4-[3-(2-Fluoro-phenyl)-thioureido]-benzenesulfo...)Show SMILES NS(=O)(=O)c1nnc(NS(=O)(=O)c2ccc(NC(=S)Nc3ccccc3F)cc2)s1 Show InChI InChI=1S/C15H13FN6O4S4/c16-11-3-1-2-4-12(11)19-13(27)18-9-5-7-10(8-6-9)30(25,26)22-14-20-21-15(28-14)29(17,23)24/h1-8H,(H,20,22)(H2,17,23,24)(H2,18,19,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Binding affinity towards human cloned carbonic anhydrase I |
Bioorg Med Chem Lett 14: 5775-80 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.062
BindingDB Entry DOI: 10.7270/Q2154HT4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50228328
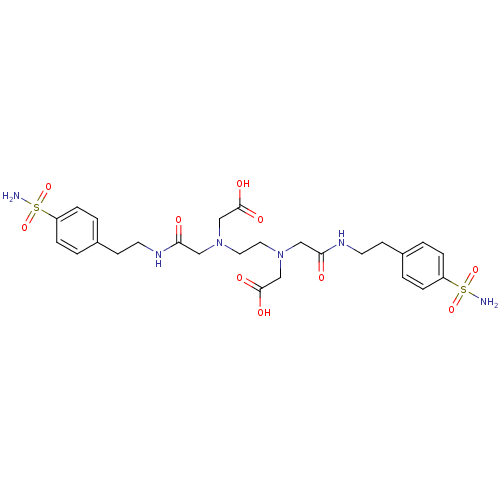
(([2-(Carboxymethyl-{[2-(4-sulfamoyl-phenyl)-ethylc...)Show SMILES NS(=O)(=O)c1ccc(CCNC(=O)CN(CCN(CC(O)=O)CC(=O)NCCc2ccc(cc2)S(N)(=O)=O)CC(O)=O)cc1 Show InChI InChI=1S/C26H36N6O10S2/c27-43(39,40)21-5-1-19(2-6-21)9-11-29-23(33)15-31(17-25(35)36)13-14-32(18-26(37)38)16-24(34)30-12-10-20-3-7-22(8-4-20)44(28,41)42/h1-8H,9-18H2,(H,29,33)(H,30,34)(H,35,36)(H,37,38)(H2,27,39,40)(H2,28,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Nationale Sup£rieure de Chimie de Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 by CO2 hydration stopped flow assay |
Bioorg Med Chem Lett 18: 836-41 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.025
BindingDB Entry DOI: 10.7270/Q2JH3N16 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 6
(Homo sapiens (Human)) | BDBM11638
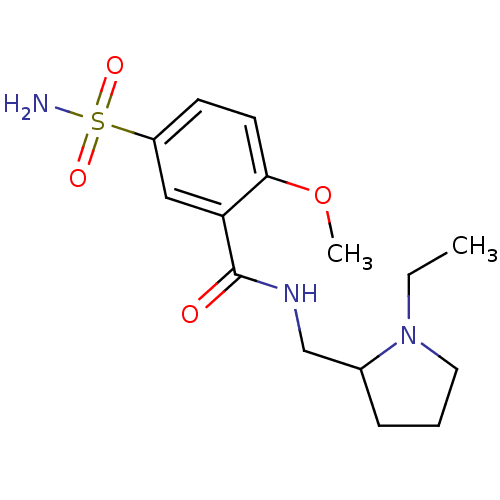
(CHEMBL26 | Compound 7 | N-[(1-ethylpyrrolidin-2-yl...)Show InChI InChI=1S/C15H23N3O4S/c1-3-18-8-4-5-11(18)10-17-15(19)13-9-12(23(16,20)21)6-7-14(13)22-2/h6-7,9,11H,3-5,8,10H2,1-2H3,(H,17,19)(H2,16,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | -51.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 20 |
Institut des Biomolécules Max Mousseron (IBMM)
| Assay Description
Inhibition assay using carbonic anhydrases with an SX.18MV-R, a stopped-flow instrument from Applied Photophysics was used for assaying the CA cataly... |
Chem Biol Drug Des 74: 636-9 (2009)
Article DOI: 10.1111/j.1747-0285.2009.00902.x
BindingDB Entry DOI: 10.7270/Q2VX0F1H |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50155548

(5-{4-[3-(2-Morpholin-4-yl-ethyl)-thioureido]-benze...)Show SMILES NS(=O)(=O)c1nnc(NS(=O)(=O)c2ccc(NC(=S)NCCN3CCOCC3)cc2)s1 Show InChI InChI=1S/C15H21N7O5S4/c16-30(23,24)15-20-19-14(29-15)21-31(25,26)12-3-1-11(2-4-12)18-13(28)17-5-6-22-7-9-27-10-8-22/h1-4H,5-10H2,(H,19,21)(H2,16,23,24)(H2,17,18,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Binding affinity towards human cloned carbonic anhydrase II |
Bioorg Med Chem Lett 14: 5775-80 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.062
BindingDB Entry DOI: 10.7270/Q2154HT4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM10882
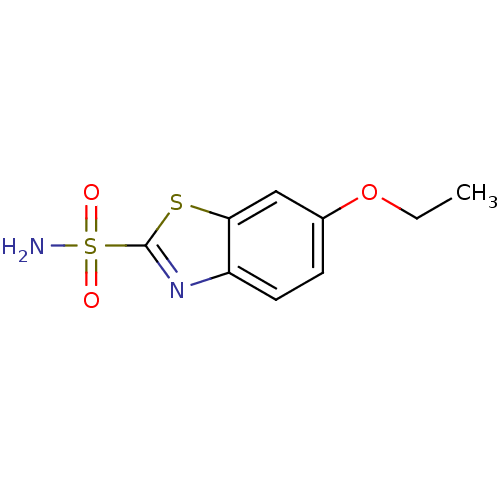
(6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...)Show InChI InChI=1S/C9H10N2O3S2/c1-2-14-6-3-4-7-8(5-6)15-9(11-7)16(10,12)13/h3-5H,2H2,1H3,(H2,10,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Biostrutture e Bioimmagini-CNR
Curated by ChEMBL
| Assay Description
Inhibition of full length human carbonic anhydrase 7 preincubated for 15 mins by CO2 hydration stopped-flow assay |
Bioorg Med Chem Lett 20: 3601-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.114
BindingDB Entry DOI: 10.7270/Q2NV9K7Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM10882

(6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...)Show InChI InChI=1S/C9H10N2O3S2/c1-2-14-6-3-4-7-8(5-6)15-9(11-7)16(10,12)13/h3-5H,2H2,1H3,(H2,10,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Montpellier II
| Assay Description
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... |
J Med Chem 49: 7024-31 (2006)
Article DOI: 10.1021/jm060807n
BindingDB Entry DOI: 10.7270/Q24T6GKT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50228326
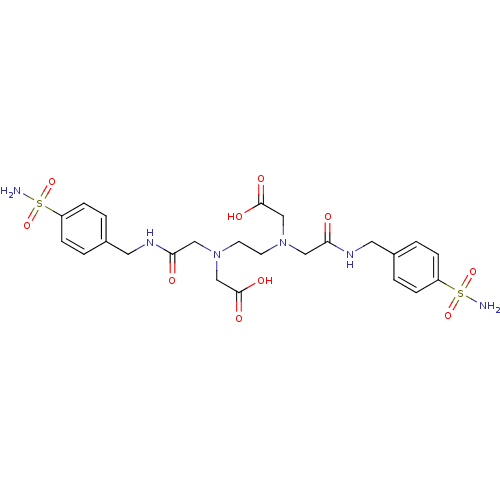
(CHEMBL1795055 | CHEMBL285436 | {(2-{Carboxymethyl-...)Show SMILES NS(=O)(=O)c1ccc(CNC(=O)CN(CCN(CC(O)=O)CC(=O)NCc2ccc(cc2)S(N)(=O)=O)CC(O)=O)cc1 Show InChI InChI=1S/C24H32N6O10S2/c25-41(37,38)19-5-1-17(2-6-19)11-27-21(31)13-29(15-23(33)34)9-10-30(16-24(35)36)14-22(32)28-12-18-3-7-20(8-4-18)42(26,39)40/h1-8H,9-16H2,(H,27,31)(H,28,32)(H,33,34)(H,35,36)(H2,25,37,38)(H2,26,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Nationale Sup£rieure de Chimie de Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 by CO2 hydration stopped flow assay |
Bioorg Med Chem Lett 18: 836-41 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.025
BindingDB Entry DOI: 10.7270/Q2JH3N16 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM10887
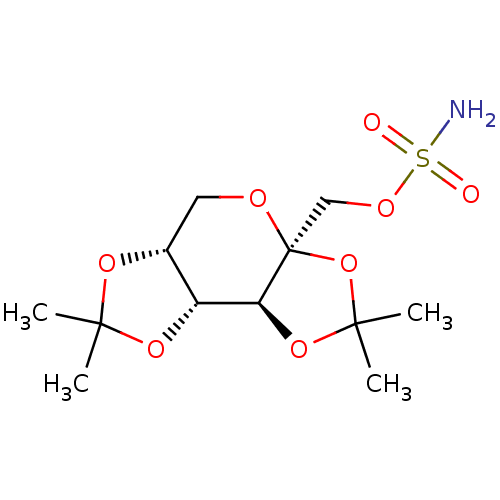
(Sulfamate 7 | Topiramate (TPM) | US11535599, Examp...)Show SMILES CC1(C)O[C@@H]2CO[C@@]3(COS(N)(=O)=O)OC(C)(C)O[C@H]3[C@@H]2O1 |r| Show InChI InChI=1S/C12H21NO8S/c1-10(2)18-7-5-16-12(6-17-22(13,14)15)9(8(7)19-10)20-11(3,4)21-12/h7-9H,5-6H2,1-4H3,(H2,13,14,15)/t7-,8-,9+,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Nationale Sup£rieure de Chimie de Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA7 after 15 mins by CO2 hydration stopped flow assay |
Bioorg Med Chem Lett 17: 2685-91 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.008
BindingDB Entry DOI: 10.7270/Q2VM4D3D |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM10887

(Sulfamate 7 | Topiramate (TPM) | US11535599, Examp...)Show SMILES CC1(C)O[C@@H]2CO[C@@]3(COS(N)(=O)=O)OC(C)(C)O[C@H]3[C@@H]2O1 |r| Show InChI InChI=1S/C12H21NO8S/c1-10(2)18-7-5-16-12(6-17-22(13,14)15)9(8(7)19-10)20-11(3,4)21-12/h7-9H,5-6H2,1-4H3,(H2,13,14,15)/t7-,8-,9+,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Montpellier II
| Assay Description
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... |
J Med Chem 49: 7024-31 (2006)
Article DOI: 10.1021/jm060807n
BindingDB Entry DOI: 10.7270/Q24T6GKT |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50155542

(5-{4-[3-(2-Dimethylamino-ethyl)-thioureido]-benzen...)Show SMILES CN(C)CCNC(=S)Nc1ccc(cc1)S(=O)(=O)Nc1nnc(s1)S(N)(=O)=O Show InChI InChI=1S/C13H19N7O4S4/c1-20(2)8-7-15-11(25)16-9-3-5-10(6-4-9)28(23,24)19-12-17-18-13(26-12)27(14,21)22/h3-6H,7-8H2,1-2H3,(H,17,19)(H2,14,21,22)(H2,15,16,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Binding affinity towards human cloned carbonic anhydrase II |
Bioorg Med Chem Lett 14: 5775-80 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.062
BindingDB Entry DOI: 10.7270/Q2154HT4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM10887

(Sulfamate 7 | Topiramate (TPM) | US11535599, Examp...)Show SMILES CC1(C)O[C@@H]2CO[C@@]3(COS(N)(=O)=O)OC(C)(C)O[C@H]3[C@@H]2O1 |r| Show InChI InChI=1S/C12H21NO8S/c1-10(2)18-7-5-16-12(6-17-22(13,14)15)9(8(7)19-10)20-11(3,4)21-12/h7-9H,5-6H2,1-4H3,(H2,13,14,15)/t7-,8-,9+,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Biostrutture e Bioimmagini-CNR
Curated by ChEMBL
| Assay Description
Inhibition of full length human carbonic anhydrase 7 preincubated for 15 mins by CO2 hydration stopped-flow assay |
Bioorg Med Chem Lett 20: 3601-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.114
BindingDB Entry DOI: 10.7270/Q2NV9K7Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50155541

(Aminobenzolamide derivative | CHEMBL435094)Show SMILES NS(=O)(=O)c1nnc(NS(=O)(=O)c2ccc(NC(=S)NCCCN3CCN(CCCNC(=S)Nc4ccc(cc4)S(=O)(=O)Nc4nnc(s4)S(N)(=O)=O)CC3)cc2)s1 Show InChI InChI=1S/C28H38N14O8S8/c29-55(43,44)27-37-35-25(53-27)39-57(47,48)21-7-3-19(4-8-21)33-23(51)31-11-1-13-41-15-17-42(18-16-41)14-2-12-32-24(52)34-20-5-9-22(10-6-20)58(49,50)40-26-36-38-28(54-26)56(30,45)46/h3-10H,1-2,11-18H2,(H,35,39)(H,36,40)(H2,29,43,44)(H2,30,45,46)(H2,31,33,51)(H2,32,34,52) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Binding affinity towards human cloned carbonic anhydrase II |
Bioorg Med Chem Lett 14: 5775-80 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.062
BindingDB Entry DOI: 10.7270/Q2154HT4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 6
(Homo sapiens (Human)) | BDBM82123
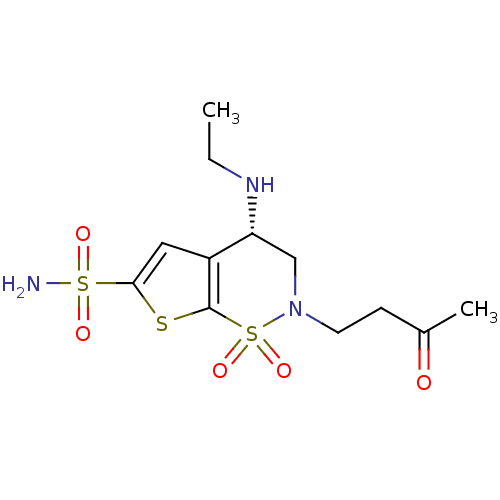
(Brinzolamide, BRZ)Show SMILES CCN[C@@H]1CN(CCC(C)=O)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C12H19N3O5S3/c1-3-14-10-7-15(5-4-8(2)16)23(19,20)12-9(10)6-11(21-12)22(13,17)18/h6,10,14H,3-5,7H2,1-2H3,(H2,13,17,18)/t10-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | -50.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 20 |
Institut des Biomolécules Max Mousseron (IBMM)
| Assay Description
Inhibition assay using carbonic anhydrases with an SX.18MV-R, a stopped-flow instrument from Applied Photophysics was used for assaying the CA cataly... |
Chem Biol Drug Des 74: 636-9 (2009)
Article DOI: 10.1111/j.1747-0285.2009.00902.x
BindingDB Entry DOI: 10.7270/Q2VX0F1H |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50155540

(5-{4-[3-(2-Piperazin-1-yl-ethyl)-thioureido]-benze...)Show SMILES NS(=O)(=O)c1nnc(NS(=O)(=O)c2ccc(NC(=S)NCCN3CCNCC3)cc2)s1 Show InChI InChI=1S/C15H22N8O4S4/c16-30(24,25)15-21-20-14(29-15)22-31(26,27)12-3-1-11(2-4-12)19-13(28)18-7-10-23-8-5-17-6-9-23/h1-4,17H,5-10H2,(H,20,22)(H2,16,24,25)(H2,18,19,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Binding affinity towards human cloned carbonic anhydrase II |
Bioorg Med Chem Lett 14: 5775-80 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.062
BindingDB Entry DOI: 10.7270/Q2154HT4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50155544
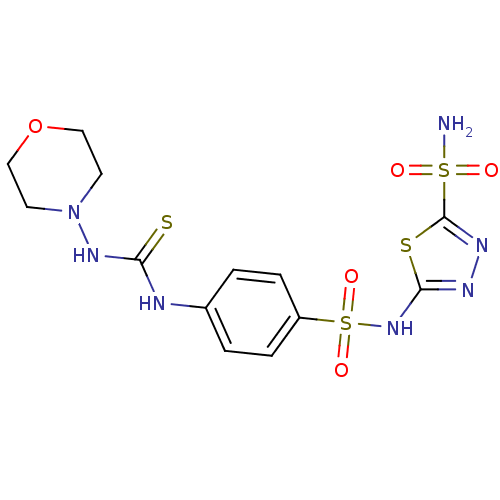
(5-[4-(3-Morpholin-4-yl-thioureido)-benzenesulfonyl...)Show SMILES NS(=O)(=O)c1nnc(NS(=O)(=O)c2ccc(NC(=S)NN3CCOCC3)cc2)s1 Show InChI InChI=1S/C13H17N7O5S4/c14-28(21,22)13-17-16-12(27-13)19-29(23,24)10-3-1-9(2-4-10)15-11(26)18-20-5-7-25-8-6-20/h1-4H,5-8H2,(H,16,19)(H2,14,21,22)(H2,15,18,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Binding affinity towards human cloned carbonic anhydrase II |
Bioorg Med Chem Lett 14: 5775-80 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.062
BindingDB Entry DOI: 10.7270/Q2154HT4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50112638
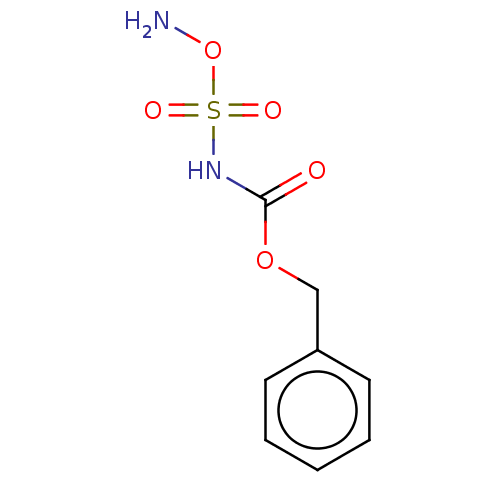
(CHEMBL3609431)Show InChI InChI=1S/C8H10N2O5S/c9-15-16(12,13)10-8(11)14-6-7-4-2-1-3-5-7/h1-5H,6,9H2,(H,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut des Biomol£cules Max Mousseron (IBMM) UMR 5247 CNRS-ENSCM-Universit£ de Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of full length human carbonic anhydrase-2 by stopped-flow CO2 hydration assay |
ACS Med Chem Lett 6: 819-21 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00175
BindingDB Entry DOI: 10.7270/Q2NZ89D9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50387125
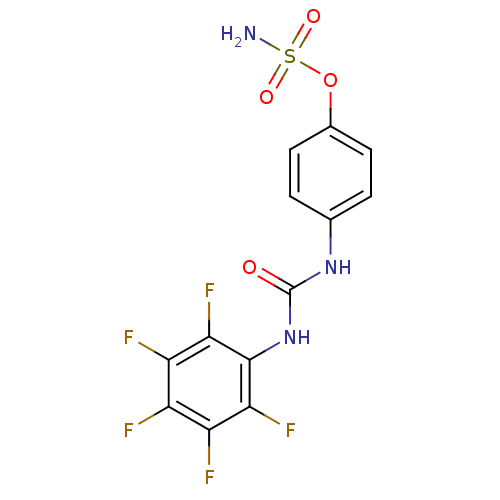
(4-ureidophenyl sulfamate ring derivative 3j | CHEM...)Show SMILES NS(=O)(=O)Oc1ccc(NC(=O)Nc2c(F)c(F)c(F)c(F)c2F)cc1 Show InChI InChI=1S/C13H8F5N3O4S/c14-7-8(15)10(17)12(11(18)9(7)16)21-13(22)20-5-1-3-6(4-2-5)25-26(19,23)24/h1-4H,(H2,19,23,24)(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant transmembrane CA12 preincubated for 15 mins by stopped-flow CO2 hydration method |
J Med Chem 55: 5591-600 (2012)
Article DOI: 10.1021/jm300529u
BindingDB Entry DOI: 10.7270/Q23R0TZM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50387125

(4-ureidophenyl sulfamate ring derivative 3j | CHEM...)Show SMILES NS(=O)(=O)Oc1ccc(NC(=O)Nc2c(F)c(F)c(F)c(F)c2F)cc1 Show InChI InChI=1S/C13H8F5N3O4S/c14-7-8(15)10(17)12(11(18)9(7)16)21-13(22)20-5-1-3-6(4-2-5)25-26(19,23)24/h1-4H,(H2,19,23,24)(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Nationale Sup£rieure de Chimie de Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 12 preincubated for 15 mins measured for 10 to 100 sec using phenol red indicator-based stopped flow assay |
Bioorg Med Chem Lett 22: 4681-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.083
BindingDB Entry DOI: 10.7270/Q20866C2 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167353

(4-[2-(4,6-Diamino-[1,3,5]triazin-2-ylamino)-ethyl]...)Show InChI InChI=1S/C11H15N7O2S/c12-9-16-10(13)18-11(17-9)15-6-5-7-1-3-8(4-2-7)21(14,19)20/h1-4H,5-6H2,(H2,14,19,20)(H5,12,13,15,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3102-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.056
BindingDB Entry DOI: 10.7270/Q2NP256X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167355
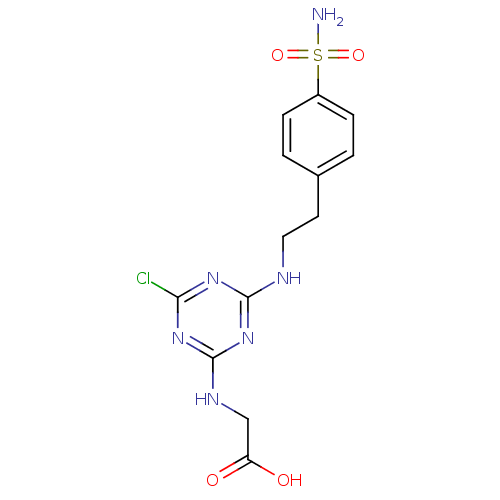
(CHEMBL189526 | {4-Chloro-6-[2-(4-sulfamoyl-phenyl)...)Show SMILES NS(=O)(=O)c1ccc(CCNc2nc(Cl)nc(NCC(O)=O)n2)cc1 Show InChI InChI=1S/C13H15ClN6O4S/c14-11-18-12(20-13(19-11)17-7-10(21)22)16-6-5-8-1-3-9(4-2-8)25(15,23)24/h1-4H,5-7H2,(H,21,22)(H2,15,23,24)(H2,16,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3102-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.056
BindingDB Entry DOI: 10.7270/Q2NP256X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50136078

(CHEMBL436261 | Sulfamic acid phenethyl ester)Show InChI InChI=1S/C8H11NO3S/c9-13(10,11)12-7-6-8-4-2-1-3-5-8/h1-5H,6-7H2,(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Montpellier II
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II. |
J Med Chem 46: 5471-7 (2003)
Article DOI: 10.1021/jm030911u
BindingDB Entry DOI: 10.7270/Q2KP81KV |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50098102
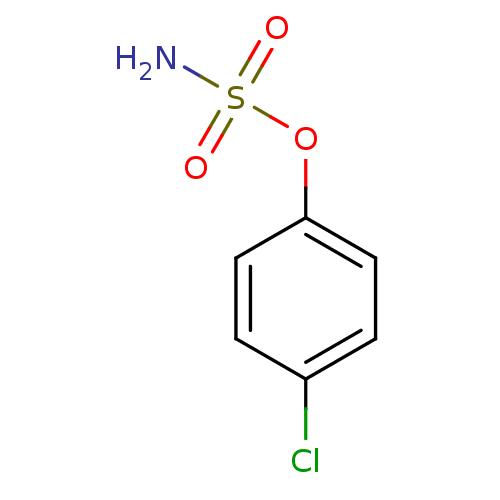
(CHEMBL23350 | Sulfamic acid 4-chloro-phenyl ester)Show InChI InChI=1S/C6H6ClNO3S/c7-5-1-3-6(4-2-5)11-12(8,9)10/h1-4H,(H2,8,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier II
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase II |
J Med Chem 46: 2197-204 (2003)
Article DOI: 10.1021/jm021124k
BindingDB Entry DOI: 10.7270/Q28P617T |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50098102

(CHEMBL23350 | Sulfamic acid 4-chloro-phenyl ester)Show InChI InChI=1S/C6H6ClNO3S/c7-5-1-3-6(4-2-5)11-12(8,9)10/h1-4H,(H2,8,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universit£ Montpellier II
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase II by spectrophotometry at pH 7.5 |
Bioorg Med Chem 19: 1172-8 (2011)
Article DOI: 10.1016/j.bmc.2010.12.048
BindingDB Entry DOI: 10.7270/Q2C53M44 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50153969
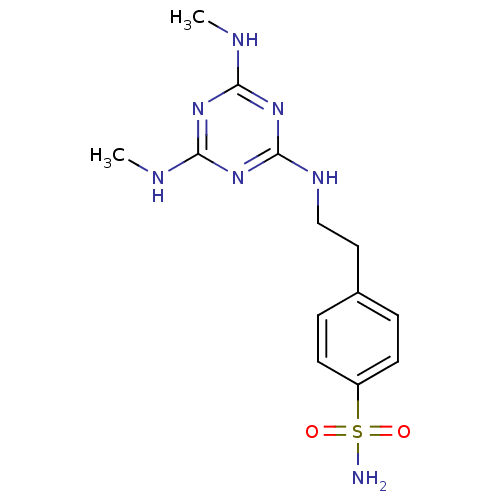
(4-[2-(4,6-Bis-methylamino-[1,3,5]triazin-2-ylamino...)Show InChI InChI=1S/C13H19N7O2S/c1-15-11-18-12(16-2)20-13(19-11)17-8-7-9-3-5-10(6-4-9)23(14,21)22/h3-6H,7-8H2,1-2H3,(H2,14,21,22)(H3,15,16,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase IX expressed in Escherichia coli BL21 |
Bioorg Med Chem Lett 14: 5427-33 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.087
BindingDB Entry DOI: 10.7270/Q2XD12DQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167337
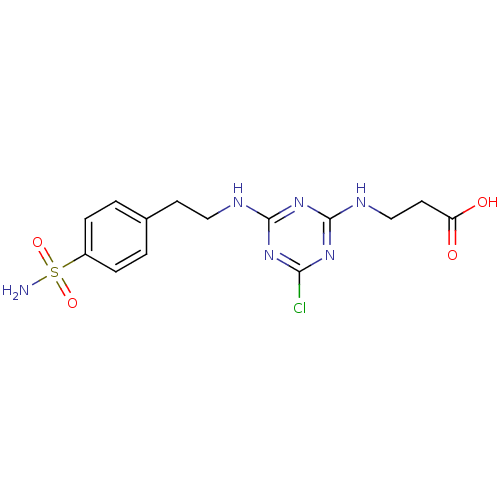
(3-{4-Chloro-6-[2-(4-sulfamoyl-phenyl)-ethylamino]-...)Show SMILES NS(=O)(=O)c1ccc(CCNc2nc(Cl)nc(NCCC(O)=O)n2)cc1 Show InChI InChI=1S/C14H17ClN6O4S/c15-12-19-13(21-14(20-12)18-8-6-11(22)23)17-7-5-9-1-3-10(4-2-9)26(16,24)25/h1-4H,5-8H2,(H,22,23)(H2,16,24,25)(H2,17,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3102-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.056
BindingDB Entry DOI: 10.7270/Q2NP256X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167361
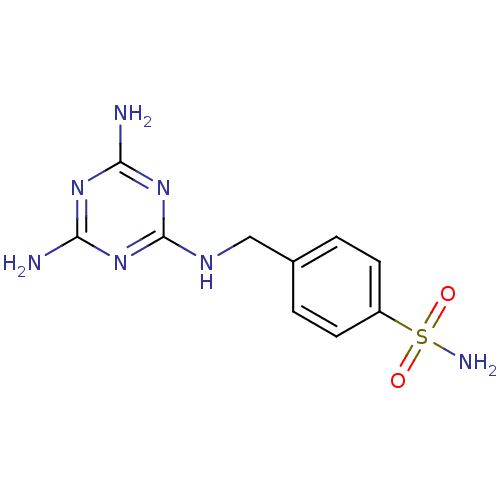
(4-[(4,6-Diamino-[1,3,5]triazin-2-ylamino)-methyl]-...)Show InChI InChI=1S/C10H13N7O2S/c11-8-15-9(12)17-10(16-8)14-5-6-1-3-7(4-2-6)20(13,18)19/h1-4H,5H2,(H2,13,18,19)(H5,11,12,14,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3102-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.056
BindingDB Entry DOI: 10.7270/Q2NP256X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50098106
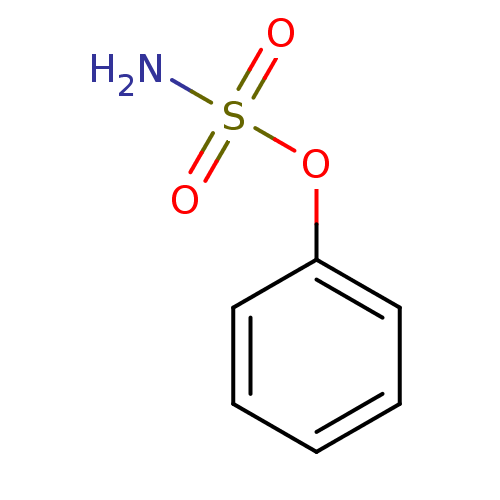
(CHEMBL24259 | PHENYLSULFAMATE | Sulfamic acid phen...)Show InChI InChI=1S/C6H7NO3S/c7-11(8,9)10-6-4-2-1-3-5-6/h1-5H,(H2,7,8,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier II
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase II |
J Med Chem 46: 2197-204 (2003)
Article DOI: 10.1021/jm021124k
BindingDB Entry DOI: 10.7270/Q28P617T |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167350

(4-[(4,6-Bis-dimethylamino-[1,3,5]triazin-2-ylamino...)Show SMILES CN(C)c1nc(NCc2ccc(cc2)S(N)(=O)=O)nc(n1)N(C)C Show InChI InChI=1S/C14H21N7O2S/c1-20(2)13-17-12(18-14(19-13)21(3)4)16-9-10-5-7-11(8-6-10)24(15,22)23/h5-8H,9H2,1-4H3,(H2,15,22,23)(H,16,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3102-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.056
BindingDB Entry DOI: 10.7270/Q2NP256X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50153976
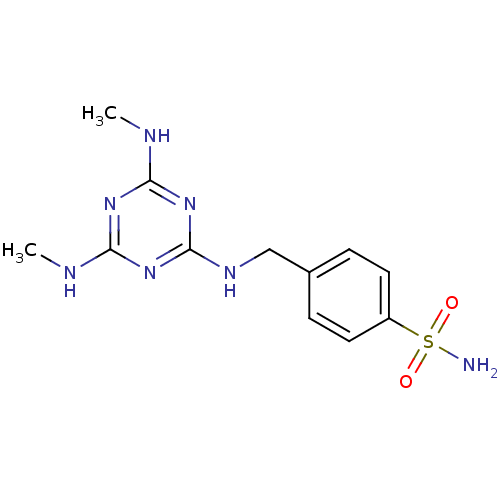
(4-[(4,6-Bis-methylamino-[1,3,5]triazin-2-ylamino)-...)Show InChI InChI=1S/C12H17N7O2S/c1-14-10-17-11(15-2)19-12(18-10)16-7-8-3-5-9(6-4-8)22(13,20)21/h3-6H,7H2,1-2H3,(H2,13,20,21)(H3,14,15,16,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase IX expressed in Escherichia coli BL21 |
Bioorg Med Chem Lett 14: 5427-33 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.087
BindingDB Entry DOI: 10.7270/Q2XD12DQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167341
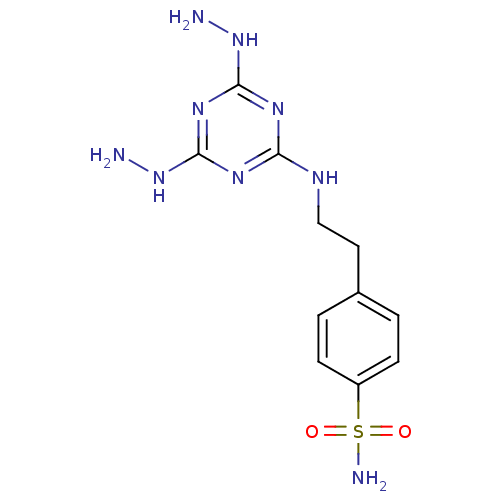
(4-[2-(4,6-Dihydrazino-[1,3,5]triazin-2-ylamino)-et...)Show InChI InChI=1S/C11H17N9O2S/c12-19-10-16-9(17-11(18-10)20-13)15-6-5-7-1-3-8(4-2-7)23(14,21)22/h1-4H,5-6,12-13H2,(H2,14,21,22)(H3,15,16,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3102-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.056
BindingDB Entry DOI: 10.7270/Q2NP256X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167357
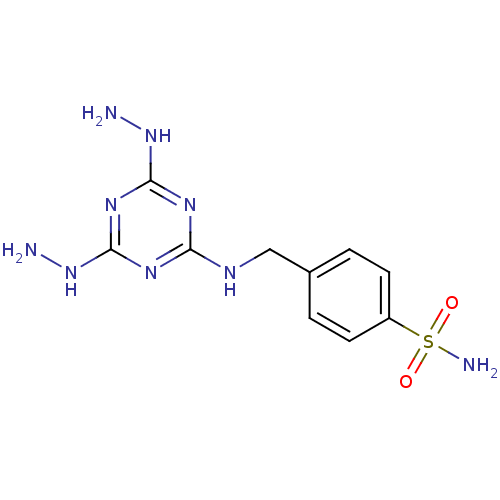
(4-[(4,6-Dihydrazino-[1,3,5]triazin-2-ylamino)-meth...)Show InChI InChI=1S/C10H15N9O2S/c11-18-9-15-8(16-10(17-9)19-12)14-5-6-1-3-7(4-2-6)22(13,20)21/h1-4H,5,11-12H2,(H2,13,20,21)(H3,14,15,16,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3102-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.056
BindingDB Entry DOI: 10.7270/Q2NP256X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data