 Found 568 hits with Last Name = 'wolf' and Initial = 'c'
Found 568 hits with Last Name = 'wolf' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50045775

((3R,4R)3-[1-(3-Cyclohexyl-3-hydroxy-propyl)-3,4-di...)Show SMILES C[C@H]1CN(CC[C@H](O)C2CCCCC2)CC[C@@]1(C)c1cccc(O)c1 Show InChI InChI=1S/C22H35NO2/c1-17-16-23(13-11-21(25)18-7-4-3-5-8-18)14-12-22(17,2)19-9-6-10-20(24)15-19/h6,9-10,15,17-18,21,24-25H,3-5,7-8,11-14,16H2,1-2H3/t17-,21-,22+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 17: 5349-52 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.008
BindingDB Entry DOI: 10.7270/Q2668CX7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50219919
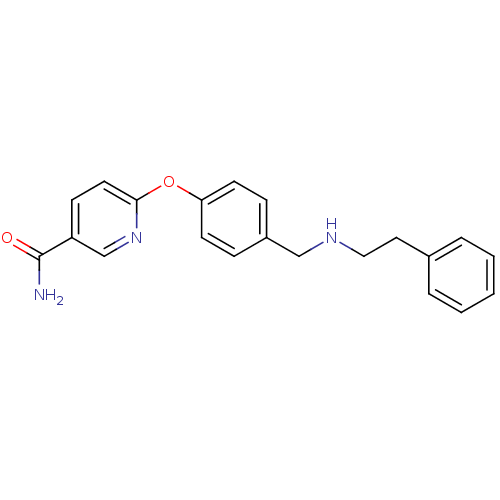
(6-(4-((phenethylamino)methyl)phenoxy)nicotinamide ...)Show InChI InChI=1S/C21H21N3O2/c22-21(25)18-8-11-20(24-15-18)26-19-9-6-17(7-10-19)14-23-13-12-16-4-2-1-3-5-16/h1-11,15,23H,12-14H2,(H2,22,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 17: 5349-52 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.008
BindingDB Entry DOI: 10.7270/Q2668CX7 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183152

(CHEMBL206580 | N-(4-(trifluoromethyl)benzyl)-N-iso...)Show InChI InChI=1S/C17H25F3N2/c1-13(2)11-22(16-7-9-21-10-8-16)12-14-3-5-15(6-4-14)17(18,19)20/h3-6,13,16,21H,7-12H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50219921
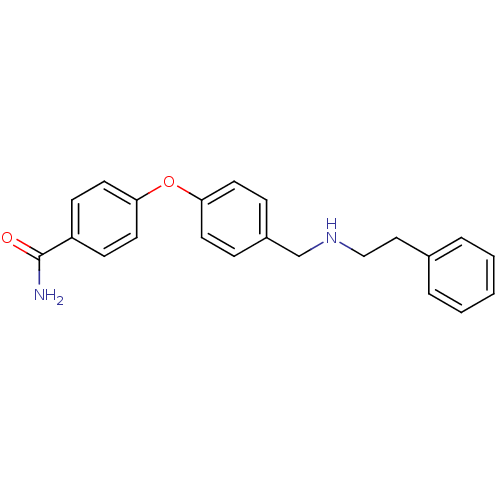
(4-(4-((phenethylamino)methyl)phenoxy)benzamide | C...)Show InChI InChI=1S/C22H22N2O2/c23-22(25)19-8-12-21(13-9-19)26-20-10-6-18(7-11-20)16-24-15-14-17-4-2-1-3-5-17/h1-13,24H,14-16H2,(H2,23,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 17: 5349-52 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.008
BindingDB Entry DOI: 10.7270/Q2668CX7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50045775

((3R,4R)3-[1-(3-Cyclohexyl-3-hydroxy-propyl)-3,4-di...)Show SMILES C[C@H]1CN(CC[C@H](O)C2CCCCC2)CC[C@@]1(C)c1cccc(O)c1 Show InChI InChI=1S/C22H35NO2/c1-17-16-23(13-11-21(25)18-7-4-3-5-8-18)14-12-22(17,2)19-9-6-10-20(24)15-19/h6,9-10,15,17-18,21,24-25H,3-5,7-8,11-14,16H2,1-2H3/t17-,21-,22+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human mu opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay |
Bioorg Med Chem Lett 17: 5349-52 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.008
BindingDB Entry DOI: 10.7270/Q2668CX7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50219919

(6-(4-((phenethylamino)methyl)phenoxy)nicotinamide ...)Show InChI InChI=1S/C21H21N3O2/c22-21(25)18-8-11-20(24-15-18)26-19-9-6-17(7-10-19)14-23-13-12-16-4-2-1-3-5-16/h1-11,15,23H,12-14H2,(H2,22,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human mu opioid receptor expressed in CHO cells in presence of high sodium by SPA |
Bioorg Med Chem Lett 17: 5349-52 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.008
BindingDB Entry DOI: 10.7270/Q2668CX7 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183124
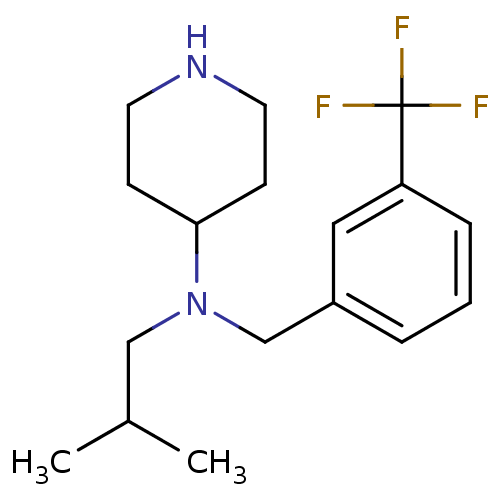
(CHEMBL381373 | N-(3-(trifluoromethyl)benzyl)-N-iso...)Show InChI InChI=1S/C17H25F3N2/c1-13(2)11-22(16-6-8-21-9-7-16)12-14-4-3-5-15(10-14)17(18,19)20/h3-5,10,13,16,21H,6-9,11-12H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183122

(4-((isobutyl(piperidin-4-yl)amino)methyl)benzonitr...)Show InChI InChI=1S/C17H25N3/c1-14(2)12-20(17-7-9-19-10-8-17)13-16-5-3-15(11-18)4-6-16/h3-6,14,17,19H,7-10,12-13H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50219921

(4-(4-((phenethylamino)methyl)phenoxy)benzamide | C...)Show InChI InChI=1S/C22H22N2O2/c23-22(25)19-8-12-21(13-9-19)26-20-10-6-18(7-11-20)16-24-15-14-17-4-2-1-3-5-17/h1-13,24H,14-16H2,(H2,23,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human mu opioid receptor expressed in CHO cells in presence of high sodium by SPA |
Bioorg Med Chem Lett 17: 5349-52 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.008
BindingDB Entry DOI: 10.7270/Q2668CX7 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183121
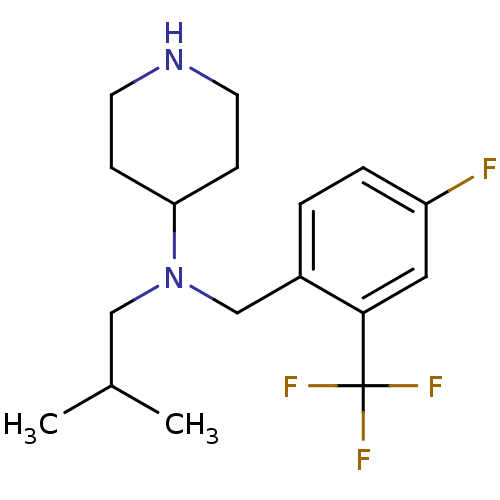
(CHEMBL441358 | N-(4-fluoro-2-(trifluoromethyl)benz...)Show InChI InChI=1S/C17H24F4N2/c1-12(2)10-23(15-5-7-22-8-6-15)11-13-3-4-14(18)9-16(13)17(19,20)21/h3-4,9,12,15,22H,5-8,10-11H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50045775

((3R,4R)3-[1-(3-Cyclohexyl-3-hydroxy-propyl)-3,4-di...)Show SMILES C[C@H]1CN(CC[C@H](O)C2CCCCC2)CC[C@@]1(C)c1cccc(O)c1 Show InChI InChI=1S/C22H35NO2/c1-17-16-23(13-11-21(25)18-7-4-3-5-8-18)14-12-22(17,2)19-9-6-10-20(24)15-19/h6,9-10,15,17-18,21,24-25H,3-5,7-8,11-14,16H2,1-2H3/t17-,21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human kappa opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay |
Bioorg Med Chem Lett 17: 5349-52 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.008
BindingDB Entry DOI: 10.7270/Q2668CX7 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183126

(CHEMBL207374 | N-(2,4-dimethylbenzyl)-N-isobutylpi...)Show InChI InChI=1S/C18H30N2/c1-14(2)12-20(18-7-9-19-10-8-18)13-17-6-5-15(3)11-16(17)4/h5-6,11,14,18-19H,7-10,12-13H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183177

(4-((4-fluoro-2-(trifluoromethyl)benzyl)(piperidin-...)Show InChI InChI=1S/C17H21F4N3/c18-14-4-3-13(16(11-14)17(19,20)21)12-24(10-2-1-7-22)15-5-8-23-9-6-15/h3-4,11,15,23H,1-2,5-6,8-10,12H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183154

(CHEMBL207067 | CHEMBL207214 | N-(4-fluoro-2-(trifl...)Show InChI InChI=1S/C17H22F4N2/c18-14-4-3-13(16(9-14)17(19,20)21)11-23(10-12-1-2-12)15-5-7-22-8-6-15/h3-4,9,12,15,22H,1-2,5-8,10-11H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183141

(3-((4-fluoro-2-(trifluoromethyl)benzyl)(piperidin-...)Show InChI InChI=1S/C16H19F4N3/c17-13-3-2-12(15(10-13)16(18,19)20)11-23(9-1-6-21)14-4-7-22-8-5-14/h2-3,10,14,22H,1,4-5,7-9,11H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(GUINEA PIG) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 39: 346-51 (1991)
BindingDB Entry DOI: 10.7270/Q2222S8S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50219918
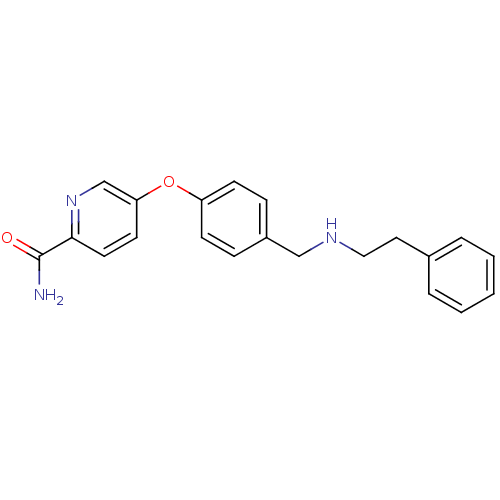
(5-(4-((phenethylamino)methyl)phenoxy)picolinamide ...)Show InChI InChI=1S/C21H21N3O2/c22-21(25)20-11-10-19(15-24-20)26-18-8-6-17(7-9-18)14-23-13-12-16-4-2-1-3-5-16/h1-11,15,23H,12-14H2,(H2,22,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 17: 5349-52 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.008
BindingDB Entry DOI: 10.7270/Q2668CX7 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183137
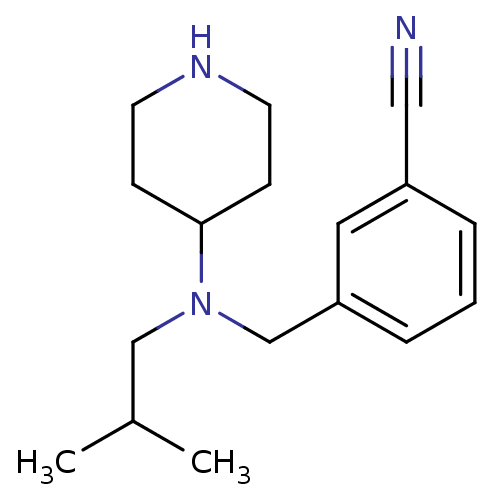
(1-(2-cyclohexyl-4-methylpentyl)-3-ethynylbenzene f...)Show InChI InChI=1S/C17H25N3/c1-14(2)12-20(17-6-8-19-9-7-17)13-16-5-3-4-15(10-16)11-18/h3-5,10,14,17,19H,6-9,12-13H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183136
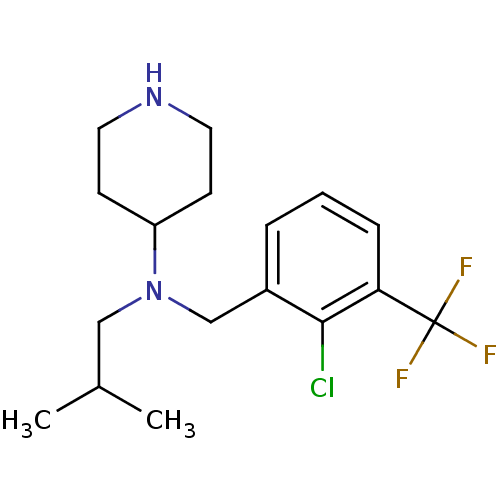
(CHEMBL208533 | N-(2-chloro-3-(trifluoromethyl)benz...)Show InChI InChI=1S/C17H24ClF3N2/c1-12(2)10-23(14-6-8-22-9-7-14)11-13-4-3-5-15(16(13)18)17(19,20)21/h3-5,12,14,22H,6-11H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183135
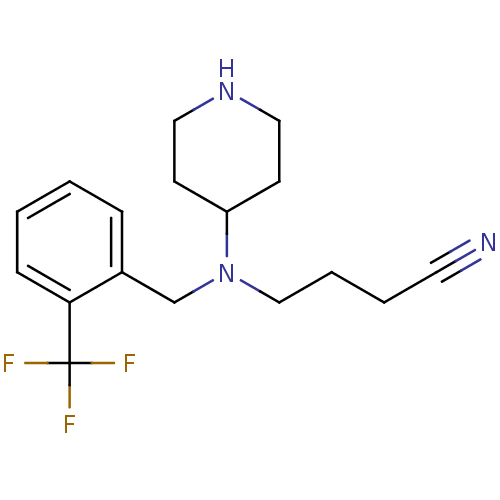
(4-((2-(trifluoromethyl)benzyl)(piperidin-4-yl)amin...)Show InChI InChI=1S/C17H22F3N3/c18-17(19,20)16-6-2-1-5-14(16)13-23(12-4-3-9-21)15-7-10-22-11-8-15/h1-2,5-6,15,22H,3-4,7-8,10-13H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM60212

((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O Show InChI InChI=1S/C20H23NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,15,18,22,24H,1-2,5-10H2/t15-,18+,19+,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 17: 5349-52 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.008
BindingDB Entry DOI: 10.7270/Q2668CX7 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183173
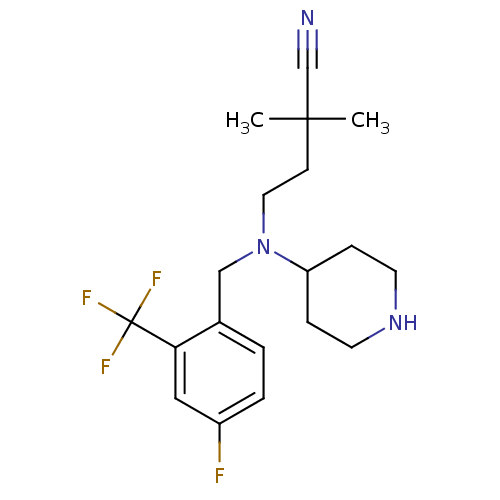
(4-((4-fluoro-2-(trifluoromethyl)benzyl)(piperidin-...)Show SMILES CC(C)(CCN(Cc1ccc(F)cc1C(F)(F)F)C1CCNCC1)C#N Show InChI InChI=1S/C19H25F4N3/c1-18(2,13-24)7-10-26(16-5-8-25-9-6-16)12-14-3-4-15(20)11-17(14)19(21,22)23/h3-4,11,16,25H,5-10,12H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183154

(CHEMBL207067 | CHEMBL207214 | N-(4-fluoro-2-(trifl...)Show InChI InChI=1S/C17H22F4N2/c18-14-4-3-13(16(9-14)17(19,20)21)11-23(10-12-1-2-12)15-5-7-22-8-6-15/h3-4,9,12,15,22H,1-2,5-8,10-11H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183134
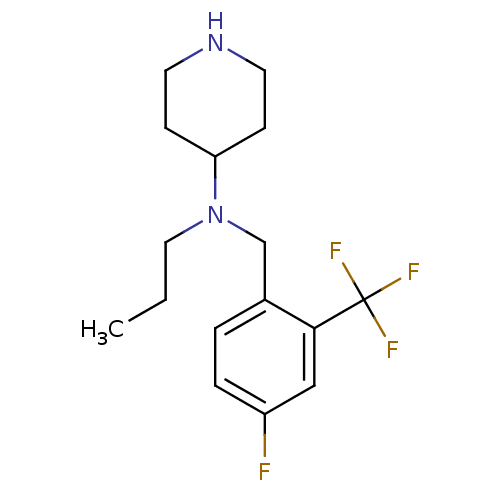
(CHEMBL207854 | N-(4-fluoro-2-(trifluoromethyl)benz...)Show InChI InChI=1S/C16H22F4N2/c1-2-9-22(14-5-7-21-8-6-14)11-12-3-4-13(17)10-15(12)16(18,19)20/h3-4,10,14,21H,2,5-9,11H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM60212

((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O Show InChI InChI=1S/C20H23NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,15,18,22,24H,1-2,5-10H2/t15-,18+,19+,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human mu opioid receptor expressed in CHO cells in presence of high sodium by SPA |
Bioorg Med Chem Lett 17: 5349-52 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.008
BindingDB Entry DOI: 10.7270/Q2668CX7 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50219921

(4-(4-((phenethylamino)methyl)phenoxy)benzamide | C...)Show InChI InChI=1S/C22H22N2O2/c23-22(25)19-8-12-21(13-9-19)26-20-10-6-18(7-11-20)16-24-15-14-17-4-2-1-3-5-17/h1-13,24H,14-16H2,(H2,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human delta opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay |
Bioorg Med Chem Lett 17: 5349-52 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.008
BindingDB Entry DOI: 10.7270/Q2668CX7 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183176
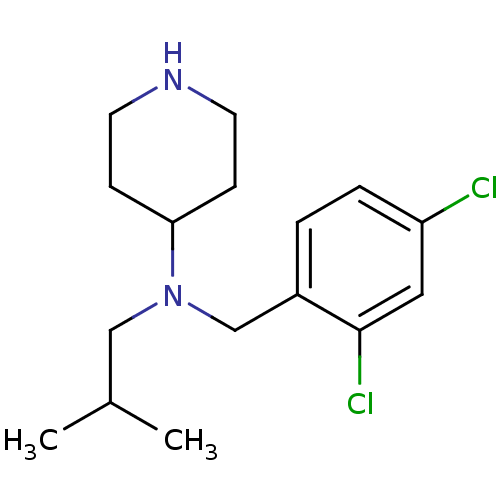
(CHEMBL207860 | N-(2,4-dichlorobenzyl)-N-isobutylpi...)Show InChI InChI=1S/C16H24Cl2N2/c1-12(2)10-20(15-5-7-19-8-6-15)11-13-3-4-14(17)9-16(13)18/h3-4,9,12,15,19H,5-8,10-11H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50219919

(6-(4-((phenethylamino)methyl)phenoxy)nicotinamide ...)Show InChI InChI=1S/C21H21N3O2/c22-21(25)18-8-11-20(24-15-18)26-19-9-6-17(7-10-19)14-23-13-12-16-4-2-1-3-5-16/h1-11,15,23H,12-14H2,(H2,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human delta opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay |
Bioorg Med Chem Lett 17: 5349-52 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.008
BindingDB Entry DOI: 10.7270/Q2668CX7 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183166
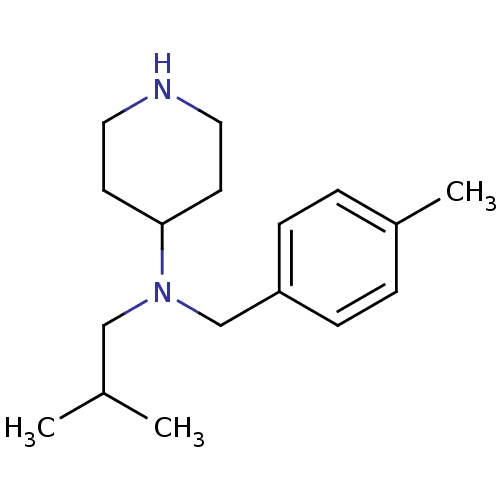
(CHEMBL380191 | isobutyl-(4-methyl-benzyl)-piperidi...)Show InChI InChI=1S/C17H28N2/c1-14(2)12-19(17-8-10-18-11-9-17)13-16-6-4-15(3)5-7-16/h4-7,14,17-18H,8-13H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183147
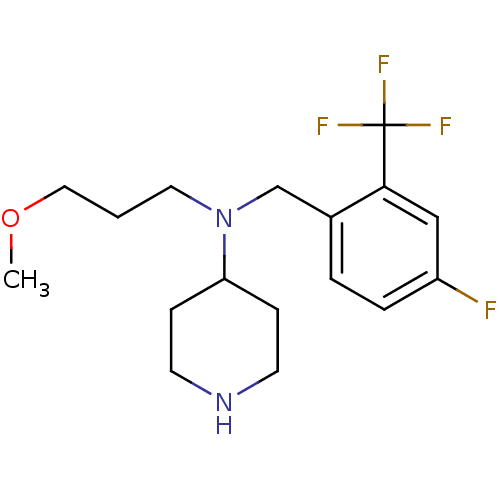
(CHEMBL207755 | N-(4-fluoro-2-(trifluoromethyl)benz...)Show InChI InChI=1S/C17H24F4N2O/c1-24-10-2-9-23(15-5-7-22-8-6-15)12-13-3-4-14(18)11-16(13)17(19,20)21/h3-4,11,15,22H,2,5-10,12H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183165

(CHEMBL438059 | N-(2-(methylthio)benzyl)-N-isobutyl...)Show InChI InChI=1S/C17H28N2S/c1-14(2)12-19(16-8-10-18-11-9-16)13-15-6-4-5-7-17(15)20-3/h4-7,14,16,18H,8-13H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183160

(2-((isobutyl(piperidin-4-yl)amino)methyl)benzonitr...)Show InChI InChI=1S/C17H25N3/c1-14(2)12-20(17-7-9-19-10-8-17)13-16-6-4-3-5-15(16)11-18/h3-6,14,17,19H,7-10,12-13H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50219921

(4-(4-((phenethylamino)methyl)phenoxy)benzamide | C...)Show InChI InChI=1S/C22H22N2O2/c23-22(25)19-8-12-21(13-9-19)26-20-10-6-18(7-11-20)16-24-15-14-17-4-2-1-3-5-17/h1-13,24H,14-16H2,(H2,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human kappa opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay |
Bioorg Med Chem Lett 17: 5349-52 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.008
BindingDB Entry DOI: 10.7270/Q2668CX7 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50219919

(6-(4-((phenethylamino)methyl)phenoxy)nicotinamide ...)Show InChI InChI=1S/C21H21N3O2/c22-21(25)18-8-11-20(24-15-18)26-19-9-6-17(7-10-19)14-23-13-12-16-4-2-1-3-5-16/h1-11,15,23H,12-14H2,(H2,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human kappa opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay |
Bioorg Med Chem Lett 17: 5349-52 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.008
BindingDB Entry DOI: 10.7270/Q2668CX7 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50045775

((3R,4R)3-[1-(3-Cyclohexyl-3-hydroxy-propyl)-3,4-di...)Show SMILES C[C@H]1CN(CC[C@H](O)C2CCCCC2)CC[C@@]1(C)c1cccc(O)c1 Show InChI InChI=1S/C22H35NO2/c1-17-16-23(13-11-21(25)18-7-4-3-5-8-18)14-12-22(17,2)19-9-6-10-20(24)15-19/h6,9-10,15,17-18,21,24-25H,3-5,7-8,11-14,16H2,1-2H3/t17-,21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human delta opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay |
Bioorg Med Chem Lett 17: 5349-52 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.008
BindingDB Entry DOI: 10.7270/Q2668CX7 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50219919

(6-(4-((phenethylamino)methyl)phenoxy)nicotinamide ...)Show InChI InChI=1S/C21H21N3O2/c22-21(25)18-8-11-20(24-15-18)26-19-9-6-17(7-10-19)14-23-13-12-16-4-2-1-3-5-16/h1-11,15,23H,12-14H2,(H2,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]bremazocine from human delta opioid receptor expressed in CHO cells in presence of high sodium by SPA |
Bioorg Med Chem Lett 17: 5349-52 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.008
BindingDB Entry DOI: 10.7270/Q2668CX7 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183146
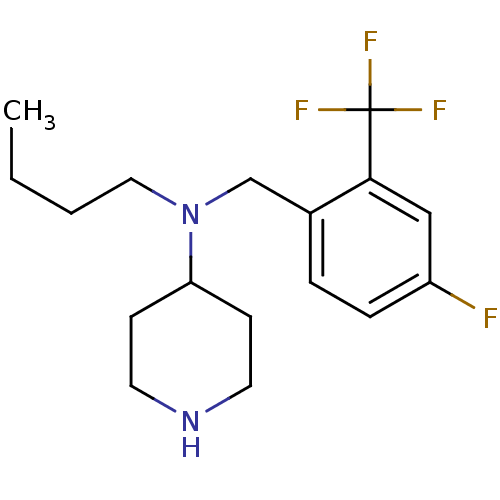
(CHEMBL426316 | N-(4-fluoro-2-(trifluoromethyl)benz...)Show InChI InChI=1S/C17H24F4N2/c1-2-3-10-23(15-6-8-22-9-7-15)12-13-4-5-14(18)11-16(13)17(19,20)21/h4-5,11,15,22H,2-3,6-10,12H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183138
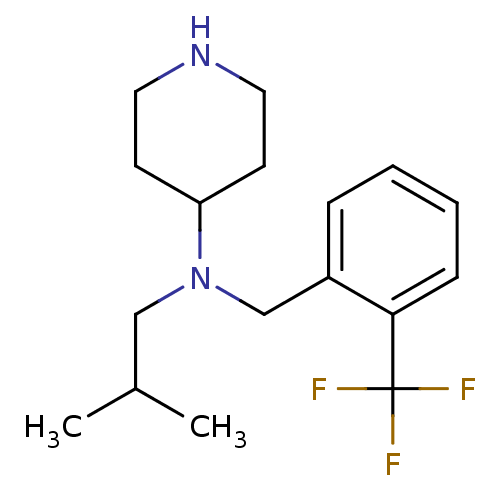
(CHEMBL380890 | N-(2-(trifluoromethyl)benzyl)-N-iso...)Show InChI InChI=1S/C17H25F3N2/c1-13(2)11-22(15-7-9-21-10-8-15)12-14-5-3-4-6-16(14)17(18,19)20/h3-6,13,15,21H,7-12H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183144
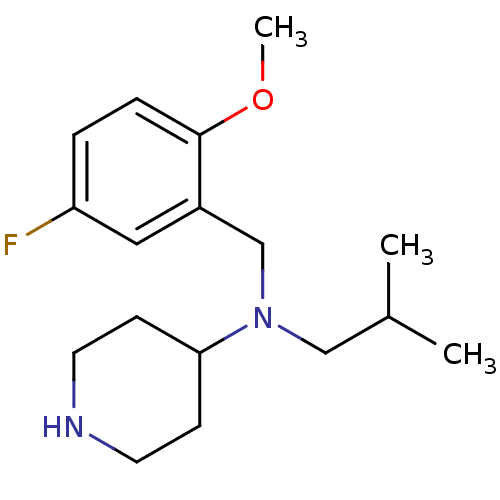
(CHEMBL207810 | N-(5-fluoro-2-methoxybenzyl)-N-isob...)Show InChI InChI=1S/C17H27FN2O/c1-13(2)11-20(16-6-8-19-9-7-16)12-14-10-15(18)4-5-17(14)21-3/h4-5,10,13,16,19H,6-9,11-12H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50183151
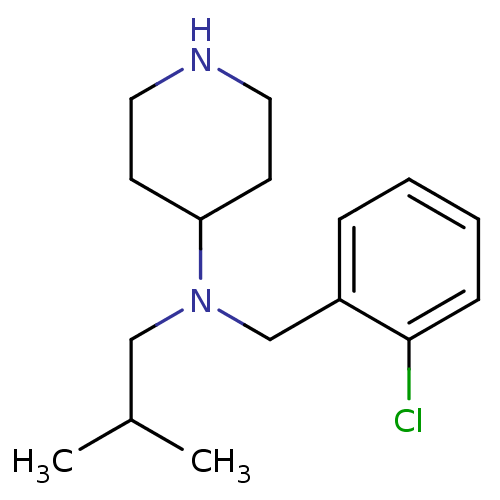
(CHEMBL208251 | N-(2-chlorobenzyl)-N-isobutylpiperi...)Show InChI InChI=1S/C16H25ClN2/c1-13(2)11-19(15-7-9-18-10-8-15)12-14-5-3-4-6-16(14)17/h3-6,13,15,18H,7-12H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]nisoxetine from NET |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183129

(CHEMBL377882 | N-(2-(trifluoromethyl)benzyl)-N-(cy...)Show InChI InChI=1S/C17H23F3N2/c18-17(19,20)16-4-2-1-3-14(16)12-22(11-13-5-6-13)15-7-9-21-10-8-15/h1-4,13,15,21H,5-12H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183127
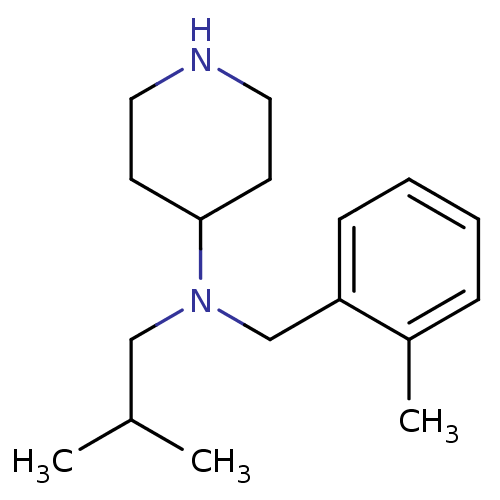
(CHEMBL208067 | isobutyl-(2-methyl-benzyl)-piperidi...)Show InChI InChI=1S/C17H28N2/c1-14(2)12-19(17-8-10-18-11-9-17)13-16-7-5-4-6-15(16)3/h4-7,14,17-18H,8-13H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183133
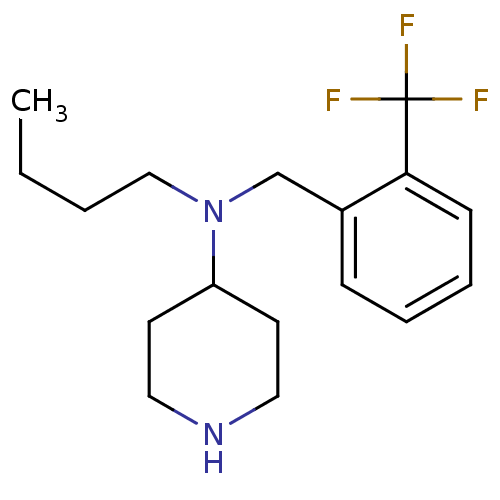
(CHEMBL207023 | N-(2-(trifluoromethyl)benzyl)-N-but...)Show InChI InChI=1S/C17H25F3N2/c1-2-3-12-22(15-8-10-21-11-9-15)13-14-6-4-5-7-16(14)17(18,19)20/h4-7,15,21H,2-3,8-13H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183151

(CHEMBL208251 | N-(2-chlorobenzyl)-N-isobutylpiperi...)Show InChI InChI=1S/C16H25ClN2/c1-13(2)11-19(15-7-9-18-10-8-15)12-14-5-3-4-6-16(14)17/h3-6,13,15,18H,7-12H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50183121

(CHEMBL441358 | N-(4-fluoro-2-(trifluoromethyl)benz...)Show InChI InChI=1S/C17H24F4N2/c1-12(2)10-23(15-5-7-22-8-6-15)11-13-3-4-14(18)9-16(13)17(19,20)21/h3-4,9,12,15,22H,5-8,10-11H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]nisoxetine from NET |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50219918

(5-(4-((phenethylamino)methyl)phenoxy)picolinamide ...)Show InChI InChI=1S/C21H21N3O2/c22-21(25)20-11-10-19(15-24-20)26-18-8-6-17(7-9-18)14-23-13-12-16-4-2-1-3-5-16/h1-11,15,23H,12-14H2,(H2,22,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human mu opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay |
Bioorg Med Chem Lett 17: 5349-52 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.008
BindingDB Entry DOI: 10.7270/Q2668CX7 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183128
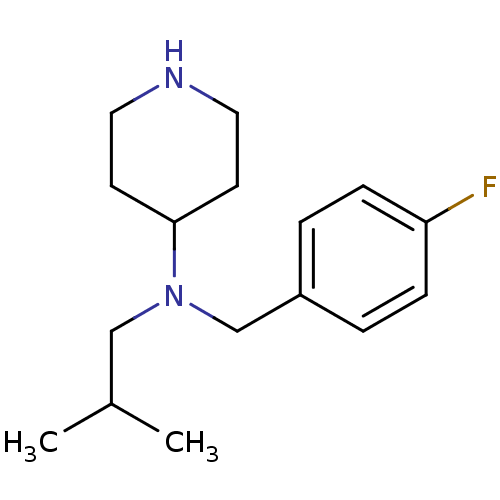
(CHEMBL207719 | N-(4-fluorobenzyl)-N-isobutylpiperi...)Show InChI InChI=1S/C16H25FN2/c1-13(2)11-19(16-7-9-18-10-8-16)12-14-3-5-15(17)6-4-14/h3-6,13,16,18H,7-12H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50219921

(4-(4-((phenethylamino)methyl)phenoxy)benzamide | C...)Show InChI InChI=1S/C22H22N2O2/c23-22(25)19-8-12-21(13-9-19)26-20-10-6-18(7-11-20)16-24-15-14-17-4-2-1-3-5-17/h1-13,24H,14-16H2,(H2,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]bremazocine from human delta opioid receptor expressed in CHO cells in presence of high sodium by SPA |
Bioorg Med Chem Lett 17: 5349-52 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.008
BindingDB Entry DOI: 10.7270/Q2668CX7 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50183176

(CHEMBL207860 | N-(2,4-dichlorobenzyl)-N-isobutylpi...)Show InChI InChI=1S/C16H24Cl2N2/c1-12(2)10-20(15-5-7-19-8-6-15)11-13-3-4-14(17)9-16(13)18/h3-4,9,12,15,19H,5-8,10-11H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]nisoxetine from NET |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183164

(CHEMBL208535 | N-(2,5-dichlorobenzyl)-N-isobutylpi...)Show InChI InChI=1S/C16H24Cl2N2/c1-12(2)10-20(15-5-7-19-8-6-15)11-13-9-14(17)3-4-16(13)18/h3-4,9,12,15,19H,5-8,10-11H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data