

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50017021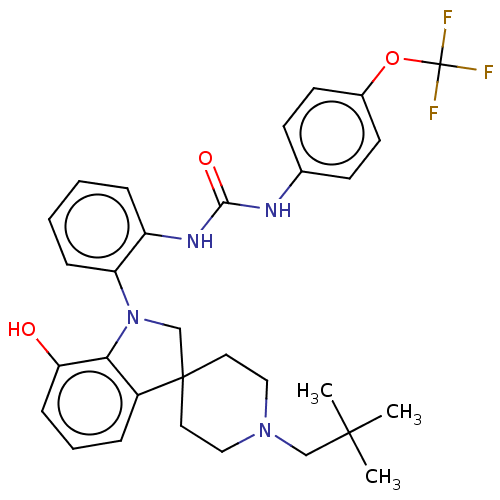 (CHEMBL3287047 | US9428504, 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to P2Y1 receptor in human platelets | Bioorg Med Chem Lett 24: 1294-8 (2014) Article DOI: 10.1016/j.bmcl.2014.01.066 BindingDB Entry DOI: 10.7270/Q20G3MQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50017084 (CHEMBL3287050 | US9428504, 61) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at P2Y1 receptor in human platelets by FLIPR assay in presence of 2-methylthio-ADP | Bioorg Med Chem Lett 24: 1294-8 (2014) Article DOI: 10.1016/j.bmcl.2014.01.066 BindingDB Entry DOI: 10.7270/Q20G3MQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50017090 (CHEMBL3287044 | US9428504, 59) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at P2Y1 receptor in human platelets by FLIPR assay in presence of 2-methylthio-ADP | Bioorg Med Chem Lett 24: 1294-8 (2014) Article DOI: 10.1016/j.bmcl.2014.01.066 BindingDB Entry DOI: 10.7270/Q20G3MQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50017089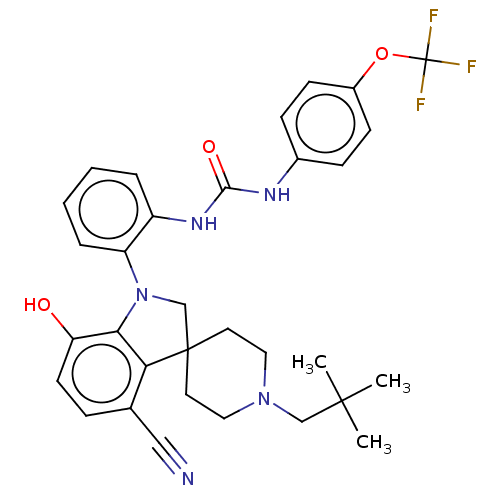 (CHEMBL3287043 | US9428504, 8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at P2Y1 receptor in human platelets by FLIPR assay in presence of 2-methylthio-ADP | Bioorg Med Chem Lett 24: 1294-8 (2014) Article DOI: 10.1016/j.bmcl.2014.01.066 BindingDB Entry DOI: 10.7270/Q20G3MQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50017083 (CHEMBL3287049 | US9428504, 39) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at P2Y1 receptor in human platelets by FLIPR assay in presence of 2-methylthio-ADP | Bioorg Med Chem Lett 24: 1294-8 (2014) Article DOI: 10.1016/j.bmcl.2014.01.066 BindingDB Entry DOI: 10.7270/Q20G3MQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50017085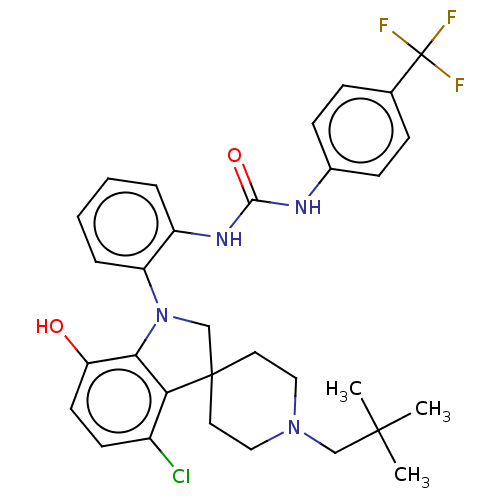 (CHEMBL3287051 | US9428504, 60) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at P2Y1 receptor in human platelets by FLIPR assay in presence of 2-methylthio-ADP | Bioorg Med Chem Lett 24: 1294-8 (2014) Article DOI: 10.1016/j.bmcl.2014.01.066 BindingDB Entry DOI: 10.7270/Q20G3MQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50015276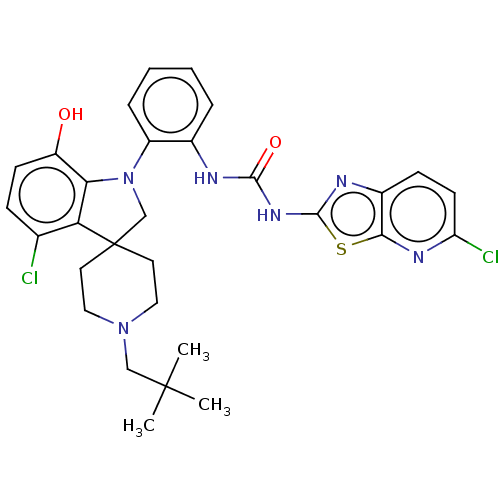 (CHEMBL3263056 | US9428504, 166 | US9428504, 167) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at P2Y1 receptor in human platelets by FLIPR assay in presence of 2-methylthio-ADP | Bioorg Med Chem Lett 24: 1294-8 (2014) Article DOI: 10.1016/j.bmcl.2014.01.066 BindingDB Entry DOI: 10.7270/Q20G3MQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50017088 (CHEMBL3287042 | US9428504, 89) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at P2Y1 receptor in human platelets by FLIPR assay in presence of 2-methylthio-ADP | Bioorg Med Chem Lett 24: 1294-8 (2014) Article DOI: 10.1016/j.bmcl.2014.01.066 BindingDB Entry DOI: 10.7270/Q20G3MQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50017087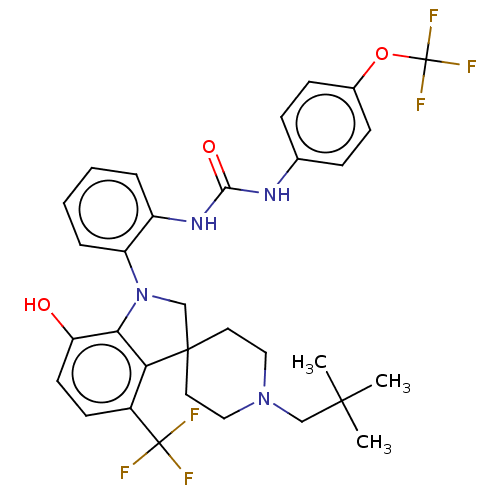 (CHEMBL3287041 | US9428504, 10) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at P2Y1 receptor in human platelets by FLIPR assay in presence of 2-methylthio-ADP | Bioorg Med Chem Lett 24: 1294-8 (2014) Article DOI: 10.1016/j.bmcl.2014.01.066 BindingDB Entry DOI: 10.7270/Q20G3MQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50017086 (CHEMBL3287052 | US9428504, 79) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at P2Y1 receptor in human platelets by FLIPR assay in presence of 2-methylthio-ADP | Bioorg Med Chem Lett 24: 1294-8 (2014) Article DOI: 10.1016/j.bmcl.2014.01.066 BindingDB Entry DOI: 10.7270/Q20G3MQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50017075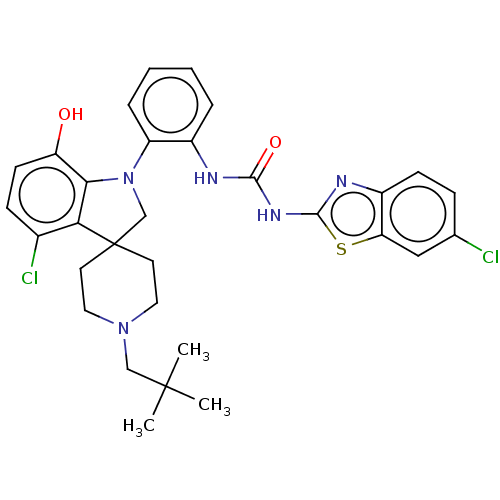 (CHEMBL3287040 | US9428504, 38) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at P2Y1 receptor in human platelets by FLIPR assay in presence of 2-methylthio-ADP | Bioorg Med Chem Lett 24: 1294-8 (2014) Article DOI: 10.1016/j.bmcl.2014.01.066 BindingDB Entry DOI: 10.7270/Q20G3MQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50017021 (CHEMBL3287047 | US9428504, 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at P2Y1 receptor in human platelets by FLIPR assay in presence of 2-methylthio-ADP | Bioorg Med Chem Lett 24: 1294-8 (2014) Article DOI: 10.1016/j.bmcl.2014.01.066 BindingDB Entry DOI: 10.7270/Q20G3MQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50017091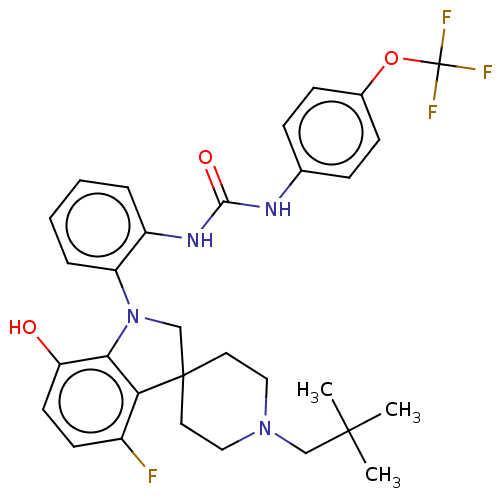 (CHEMBL3287045 | US9428504, 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at P2Y1 receptor in human platelets by FLIPR assay in presence of 2-methylthio-ADP | Bioorg Med Chem Lett 24: 1294-8 (2014) Article DOI: 10.1016/j.bmcl.2014.01.066 BindingDB Entry DOI: 10.7270/Q20G3MQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM9047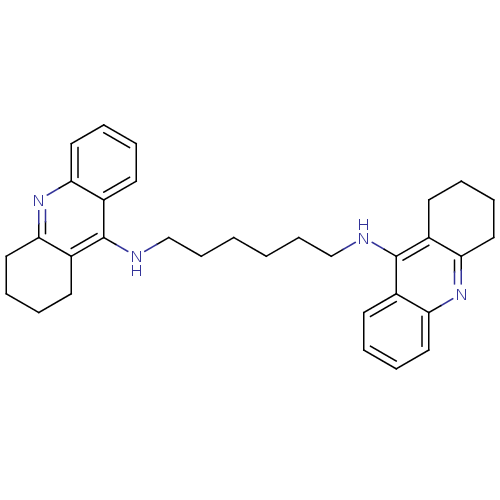 (Bis-THA inhibitor 5 | CHEMBL73800 | Hexylene-Linke...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hong Kong University of Science and Technology | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | Bioorg Med Chem 7: 351-7 (1999) Article DOI: 10.1016/s0968-0896(98)00213-2 BindingDB Entry DOI: 10.7270/Q2T43R9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50017123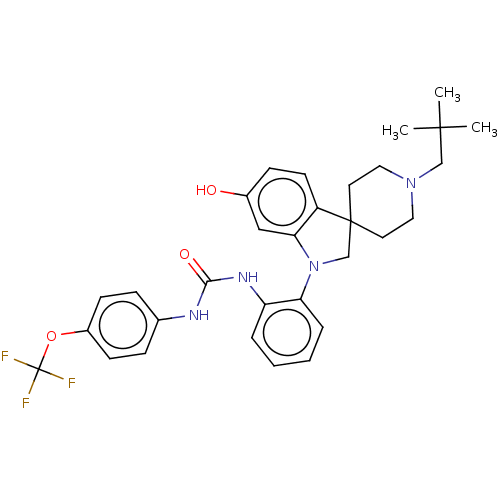 (CHEMBL3287046) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at P2Y1 receptor in human platelets by FLIPR assay in presence of 2-methylthio-ADP | Bioorg Med Chem Lett 24: 1294-8 (2014) Article DOI: 10.1016/j.bmcl.2014.01.066 BindingDB Entry DOI: 10.7270/Q20G3MQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8962 (Bis-THA inhibitor 4 | CHEMBL179732 | N-[5-(1,2,3,4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hong Kong University of Science and Technology | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | Bioorg Med Chem 7: 351-7 (1999) Article DOI: 10.1016/s0968-0896(98)00213-2 BindingDB Entry DOI: 10.7270/Q2T43R9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10469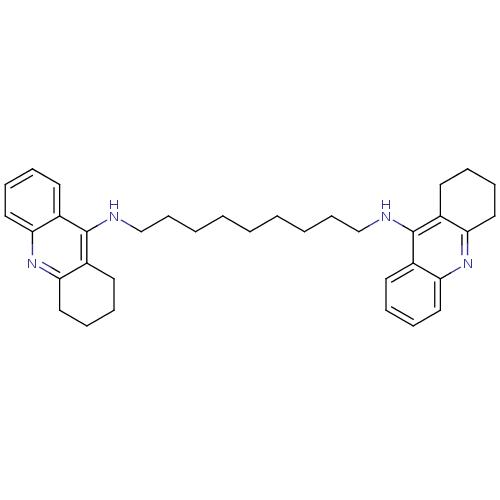 (Bis-THA inhibitor 1c | Bis-THA inhibitor 8 | CHEMB...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hong Kong University of Science and Technology | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | Bioorg Med Chem 7: 351-7 (1999) Article DOI: 10.1016/s0968-0896(98)00213-2 BindingDB Entry DOI: 10.7270/Q2T43R9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10470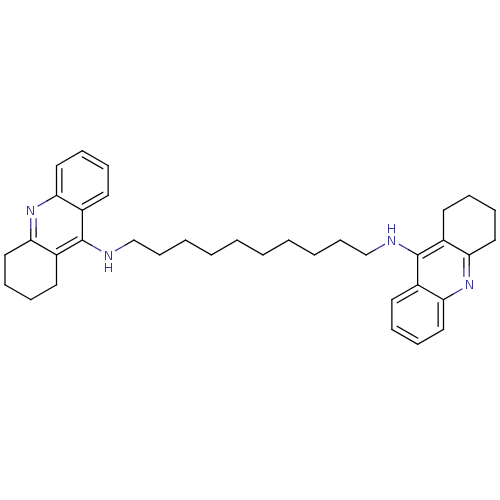 (Bis-THA inhibitor 1d | Bis-THA inhibitor 9 | CHEMB...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hong Kong University of Science and Technology | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | Bioorg Med Chem 7: 351-7 (1999) Article DOI: 10.1016/s0968-0896(98)00213-2 BindingDB Entry DOI: 10.7270/Q2T43R9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hong Kong University of Science and Technology | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | Bioorg Med Chem 7: 351-7 (1999) Article DOI: 10.1016/s0968-0896(98)00213-2 BindingDB Entry DOI: 10.7270/Q2T43R9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM10478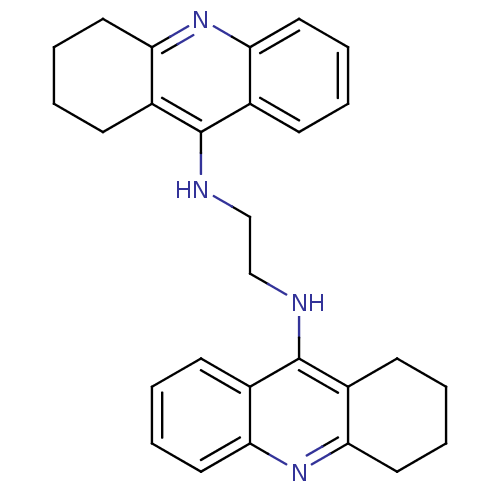 (A2A.2HCl | Bis-THA inhibitor 1 | CHEMBL213377 | N-...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 102 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hong Kong University of Science and Technology | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | Bioorg Med Chem 7: 351-7 (1999) Article DOI: 10.1016/s0968-0896(98)00213-2 BindingDB Entry DOI: 10.7270/Q2T43R9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM9047 (Bis-THA inhibitor 5 | CHEMBL73800 | Hexylene-Linke...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 119 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hong Kong University of Science and Technology | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | Bioorg Med Chem 7: 351-7 (1999) Article DOI: 10.1016/s0968-0896(98)00213-2 BindingDB Entry DOI: 10.7270/Q2T43R9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50017019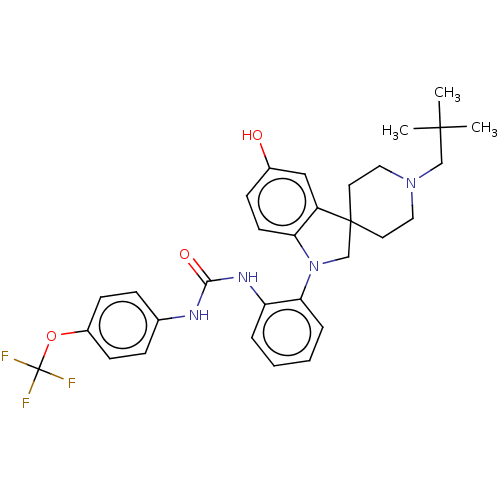 (CHEMBL3287039) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >150 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at P2Y1 receptor in human platelets by FLIPR assay in presence of 2-methylthio-ADP | Bioorg Med Chem Lett 24: 1294-8 (2014) Article DOI: 10.1016/j.bmcl.2014.01.066 BindingDB Entry DOI: 10.7270/Q20G3MQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50017020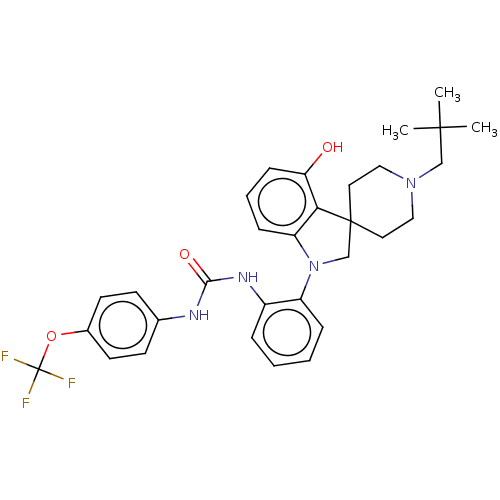 (CHEMBL3287048) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >150 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at P2Y1 receptor in human platelets by FLIPR assay in presence of 2-methylthio-ADP | Bioorg Med Chem Lett 24: 1294-8 (2014) Article DOI: 10.1016/j.bmcl.2014.01.066 BindingDB Entry DOI: 10.7270/Q20G3MQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM10479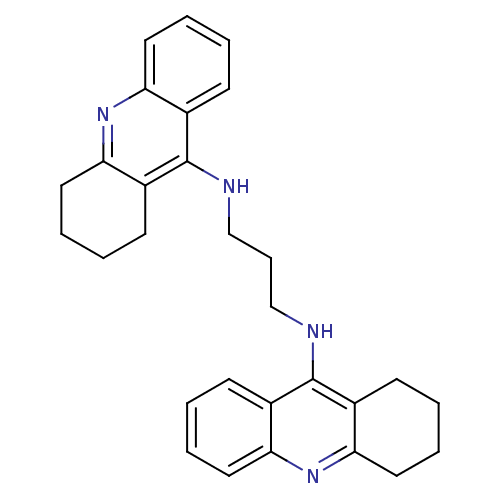 (A3A.2HCl | Bis-THA inhibitor 2 | CHEMBL378006 | N-...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 152 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hong Kong University of Science and Technology | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | Bioorg Med Chem 7: 351-7 (1999) Article DOI: 10.1016/s0968-0896(98)00213-2 BindingDB Entry DOI: 10.7270/Q2T43R9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM10469 (Bis-THA inhibitor 1c | Bis-THA inhibitor 8 | CHEMB...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 155 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hong Kong University of Science and Technology | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | Bioorg Med Chem 7: 351-7 (1999) Article DOI: 10.1016/s0968-0896(98)00213-2 BindingDB Entry DOI: 10.7270/Q2T43R9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10480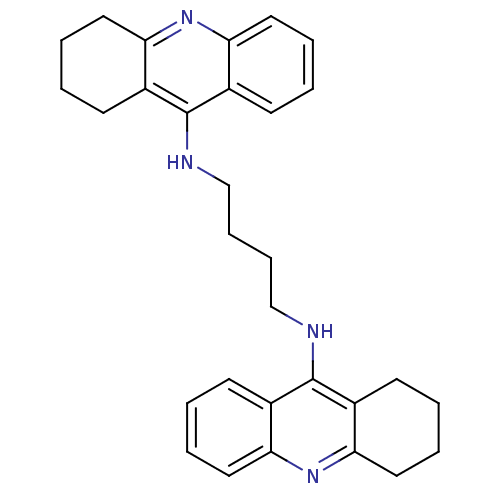 (A4A.2HCl | Bis-THA inhibitor 3 | CHEMBL211313 | N-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 157 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hong Kong University of Science and Technology | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | Bioorg Med Chem 7: 351-7 (1999) Article DOI: 10.1016/s0968-0896(98)00213-2 BindingDB Entry DOI: 10.7270/Q2T43R9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM10470 (Bis-THA inhibitor 1d | Bis-THA inhibitor 9 | CHEMB...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 167 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hong Kong University of Science and Technology | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | Bioorg Med Chem 7: 351-7 (1999) Article DOI: 10.1016/s0968-0896(98)00213-2 BindingDB Entry DOI: 10.7270/Q2T43R9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (Homo sapiens (Human)) | BDBM50015276 (CHEMBL3263056 | US9428504, 166 | US9428504, 167) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at P2X1 receptor (unknown origin) by FLIPR assay | Bioorg Med Chem Lett 24: 1294-8 (2014) Article DOI: 10.1016/j.bmcl.2014.01.066 BindingDB Entry DOI: 10.7270/Q20G3MQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 223 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hong Kong University of Science and Technology | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | Bioorg Med Chem 7: 351-7 (1999) Article DOI: 10.1016/s0968-0896(98)00213-2 BindingDB Entry DOI: 10.7270/Q2T43R9Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM10480 (A4A.2HCl | Bis-THA inhibitor 3 | CHEMBL211313 | N-...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 252 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hong Kong University of Science and Technology | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | Bioorg Med Chem 7: 351-7 (1999) Article DOI: 10.1016/s0968-0896(98)00213-2 BindingDB Entry DOI: 10.7270/Q2T43R9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10479 (A3A.2HCl | Bis-THA inhibitor 2 | CHEMBL378006 | N-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 254 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hong Kong University of Science and Technology | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | Bioorg Med Chem 7: 351-7 (1999) Article DOI: 10.1016/s0968-0896(98)00213-2 BindingDB Entry DOI: 10.7270/Q2T43R9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM8962 (Bis-THA inhibitor 4 | CHEMBL179732 | N-[5-(1,2,3,4...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 329 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hong Kong University of Science and Technology | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | Bioorg Med Chem 7: 351-7 (1999) Article DOI: 10.1016/s0968-0896(98)00213-2 BindingDB Entry DOI: 10.7270/Q2T43R9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10478 (A2A.2HCl | Bis-THA inhibitor 1 | CHEMBL213377 | N-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 711 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hong Kong University of Science and Technology | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | Bioorg Med Chem 7: 351-7 (1999) Article DOI: 10.1016/s0968-0896(98)00213-2 BindingDB Entry DOI: 10.7270/Q2T43R9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50496099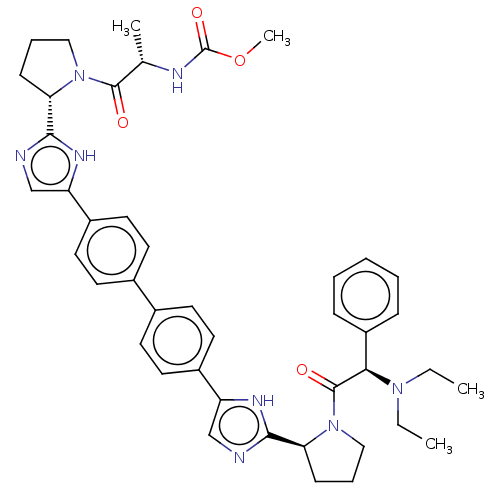 (CHEMBL3121151) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human ERG by whole cell patch clamp assay | J Med Chem 57: 2013-32 (2014) Article DOI: 10.1021/jm401836p BindingDB Entry DOI: 10.7270/Q28918V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50015276 (CHEMBL3263056 | US9428504, 166 | US9428504, 167) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at P2Y2 receptor (unknown origin) | Bioorg Med Chem Lett 24: 1294-8 (2014) Article DOI: 10.1016/j.bmcl.2014.01.066 BindingDB Entry DOI: 10.7270/Q20G3MQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50015276 (CHEMBL3263056 | US9428504, 166 | US9428504, 167) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor (unknown origin) | Bioorg Med Chem Lett 24: 1294-8 (2014) Article DOI: 10.1016/j.bmcl.2014.01.066 BindingDB Entry DOI: 10.7270/Q20G3MQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50387084 (BMS-790052 | DACLATASVIR) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human ERG by whole cell patch clamp assay | J Med Chem 57: 2013-32 (2014) Article DOI: 10.1021/jm401836p BindingDB Entry DOI: 10.7270/Q28918V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2/3 (Homo sapiens (Human)) | BDBM50130610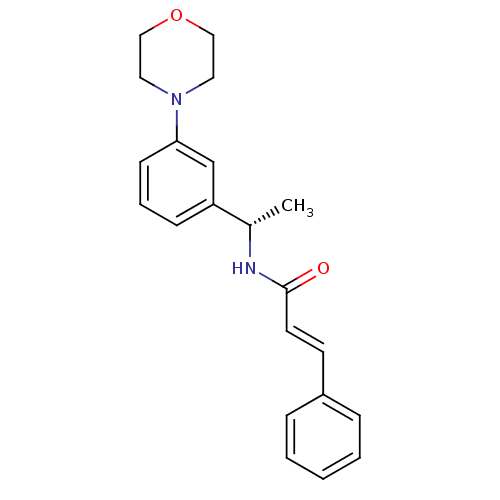 ((E)-N-[(S)-1-(3-Morpholin-4-yl-phenyl)-ethyl]-3-ph...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 690 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Effect on resting membrane potential in SH-SY5Y human neuroblastoma cells expressing native KCNQ channels | J Med Chem 46: 3197-200 (2003) Article DOI: 10.1021/jm034073f BindingDB Entry DOI: 10.7270/Q2D21X11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Mus musculus) | BDBM50130610 ((E)-N-[(S)-1-(3-Morpholin-4-yl-phenyl)-ethyl]-3-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Induced current in Xenopus laevis oocytes expressing cloned mKCNQ2 channels | J Med Chem 46: 3197-200 (2003) Article DOI: 10.1021/jm034073f BindingDB Entry DOI: 10.7270/Q2D21X11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||