

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM412656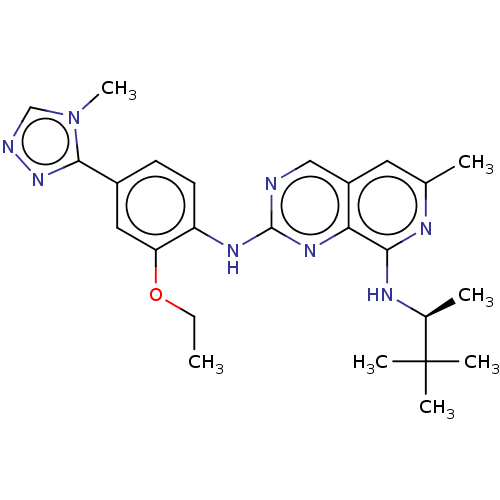 (US10399974, Example 54) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241208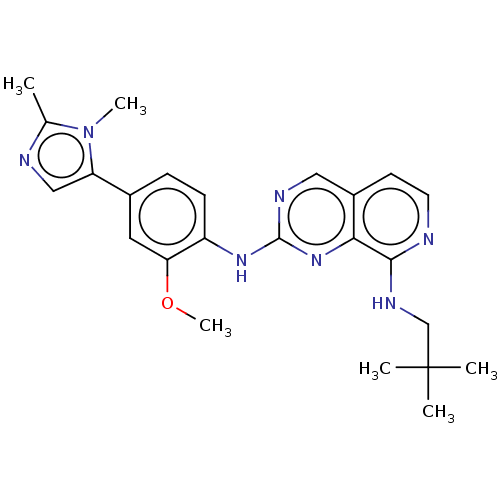 (US11046688, Example 50 | US9409907, 50) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50464039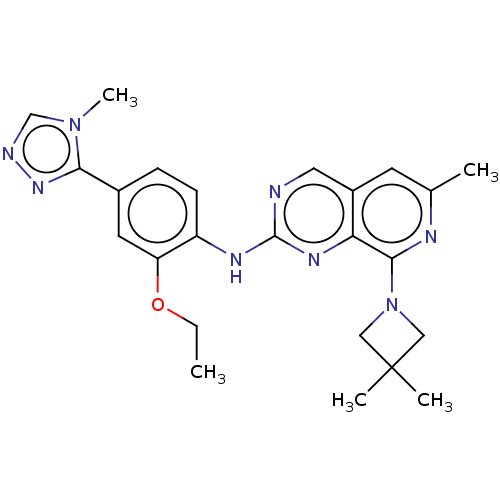 (CHEMBL4245639) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241333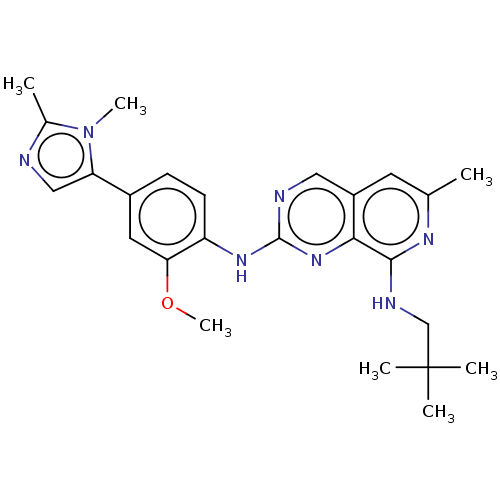 (US10479788, Example 177 | US11046688, Example 177 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM412611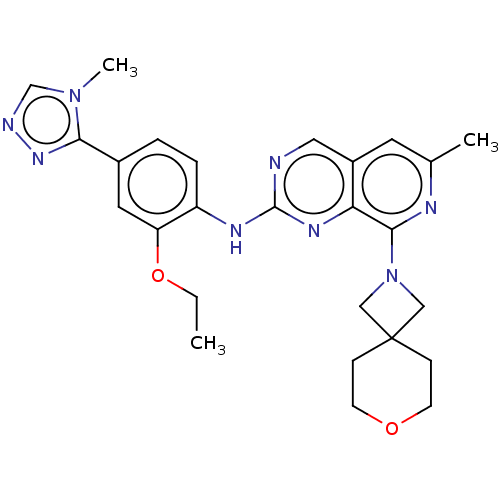 (N-(2-ethoxy-4-(4-methyl-4H-1,2,4-triazol-3-yl)phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241338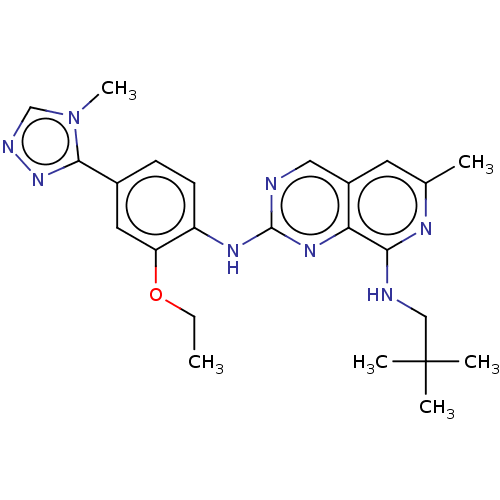 (US10479788, Example 182 | US11046688, Example 182 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50464037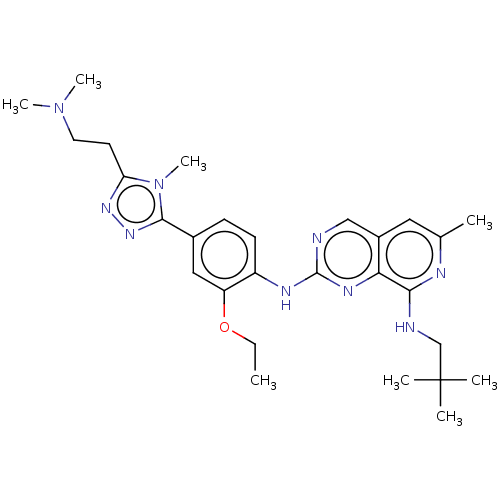 (CHEMBL4240502) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM412614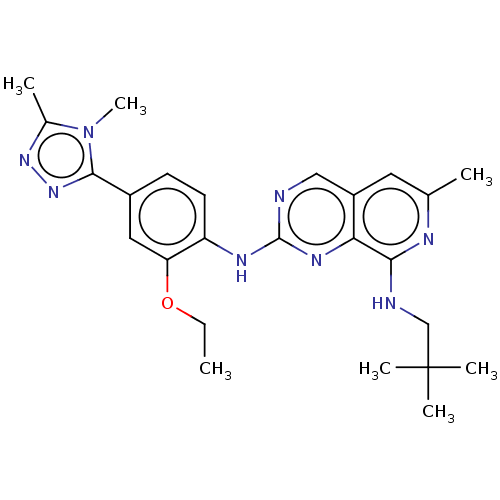 (N2-(4-(4,5-dimethyl-4H-1,2,4-triazol-3-yl)-2-ethox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241335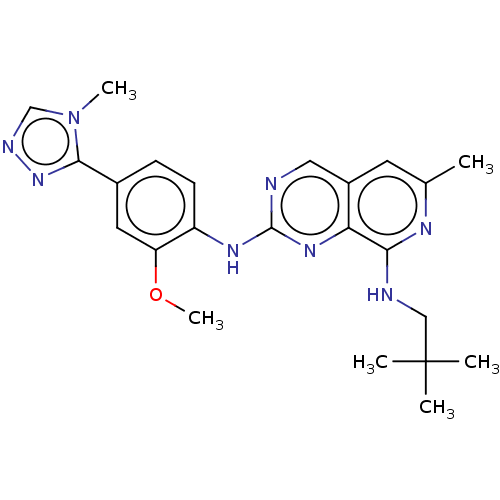 (US9409907, 179) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50464040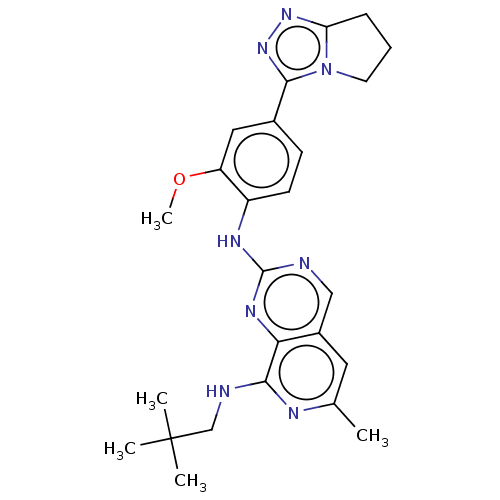 (CHEMBL4251352) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241235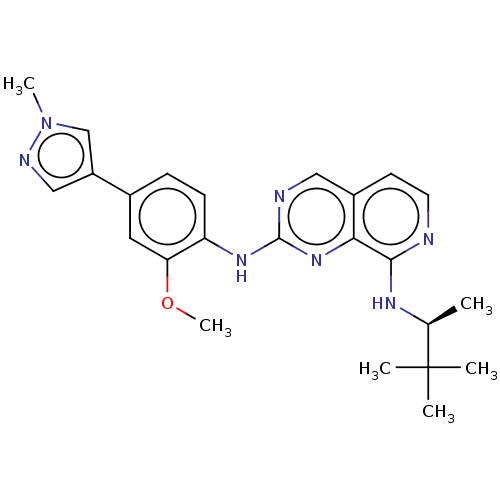 (US10479788, Example 77 | US11046688, Example 77 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of human full length MPS1 expressed in recombinant baculovirus infected Sf9 insect cells using 5FAM-DHTGFLTEYVATRCONH2 as substrate after ... | J Med Chem 59: 3671-88 (2016) Article DOI: 10.1021/acs.jmedchem.5b01811 BindingDB Entry DOI: 10.7270/Q2DZ0B6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM412610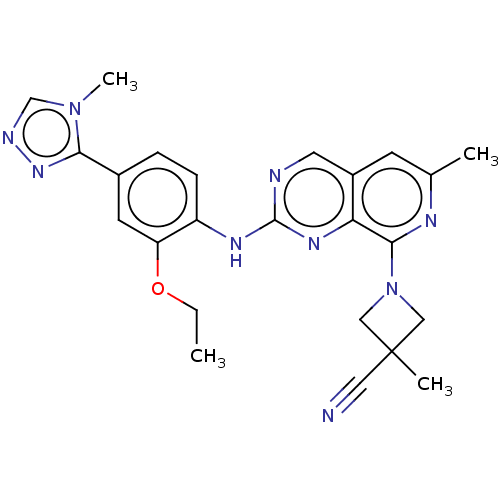 (1-(2-((2-ethoxy-4-(4-methyl-4H-1,2,4-triazol-3-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104371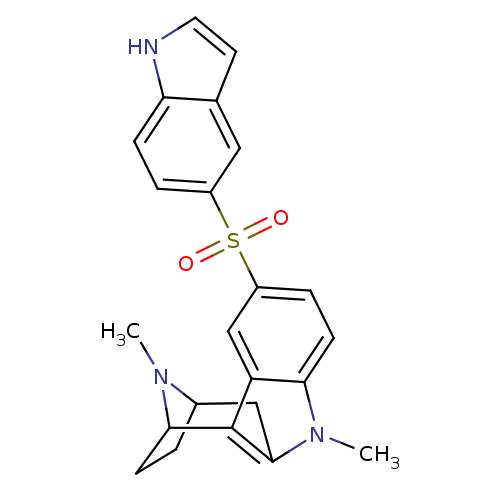 (US8575186, 134/183/184 | US8575186, 199 | US857518...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104331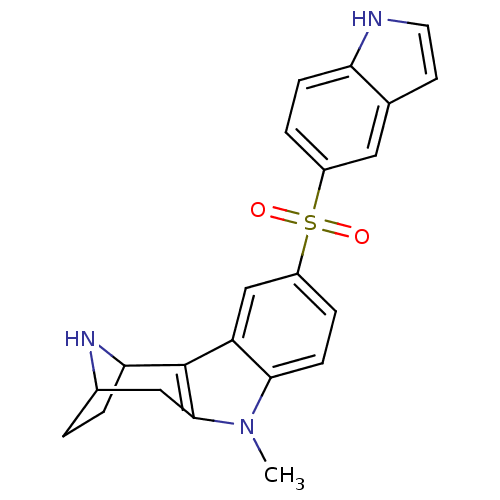 (US8575186, 177 | US8575186, 178 | US8575186, 94/16...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104408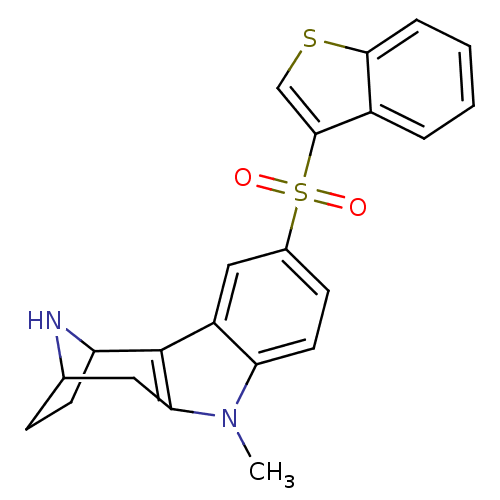 (US8575186, 171/172 | US8575186, 188) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104378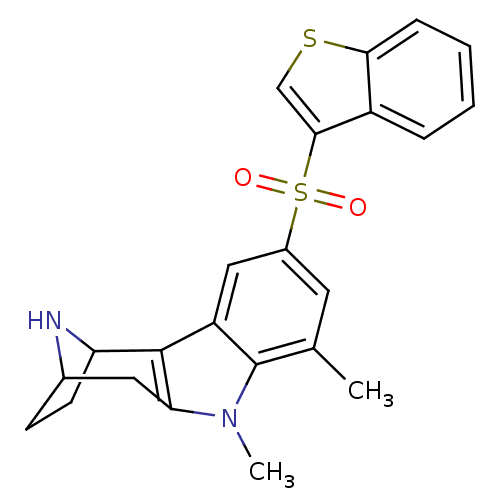 (US8575186, 141) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104382 (US8575186, 145) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104467 (US8575186, 230/231 | US8575186, 247) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50364980 (CHEMBL1950775) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT6 serotonin receptor by scintillation proximity assay | Bioorg Med Chem Lett 22: 1494-8 (2012) Article DOI: 10.1016/j.bmcl.2012.01.022 BindingDB Entry DOI: 10.7270/Q2RB753Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50364980 (CHEMBL1950775) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT6 serotonin receptor by scintillation proximity assay | Bioorg Med Chem Lett 22: 1494-8 (2012) Article DOI: 10.1016/j.bmcl.2012.01.022 BindingDB Entry DOI: 10.7270/Q2RB753Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104331 (US8575186, 177 | US8575186, 178 | US8575186, 94/16...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104361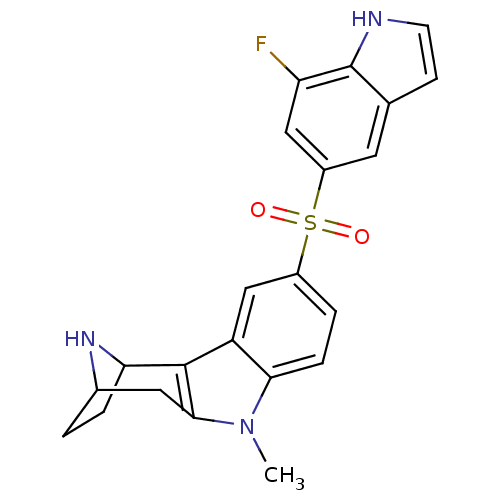 (US8575186, 124) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50364981 (CHEMBL1950776) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT6 serotonin receptor by scintillation proximity assay | Bioorg Med Chem Lett 22: 1494-8 (2012) Article DOI: 10.1016/j.bmcl.2012.01.022 BindingDB Entry DOI: 10.7270/Q2RB753Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104371 (US8575186, 134/183/184 | US8575186, 199 | US857518...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104477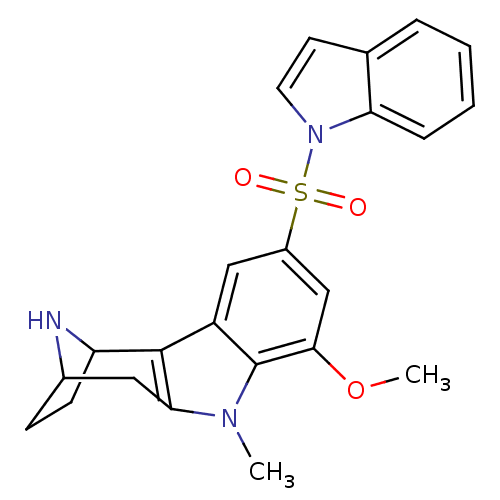 (US8575186, 240/241 | US8575186, 257) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50364981 (CHEMBL1950776) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT6 serotonin receptor by scintillation proximity assay | Bioorg Med Chem Lett 22: 1494-8 (2012) Article DOI: 10.1016/j.bmcl.2012.01.022 BindingDB Entry DOI: 10.7270/Q2RB753Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50364981 (CHEMBL1950776) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT6 serotonin receptor by scintillation proximity assay | Bioorg Med Chem Lett 22: 1494-8 (2012) Article DOI: 10.1016/j.bmcl.2012.01.022 BindingDB Entry DOI: 10.7270/Q2RB753Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104408 (US8575186, 171/172 | US8575186, 188) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50364980 (CHEMBL1950775) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT6 serotonin receptor by scintillation proximity assay | Bioorg Med Chem Lett 22: 1494-8 (2012) Article DOI: 10.1016/j.bmcl.2012.01.022 BindingDB Entry DOI: 10.7270/Q2RB753Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104331 (US8575186, 177 | US8575186, 178 | US8575186, 94/16...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50464038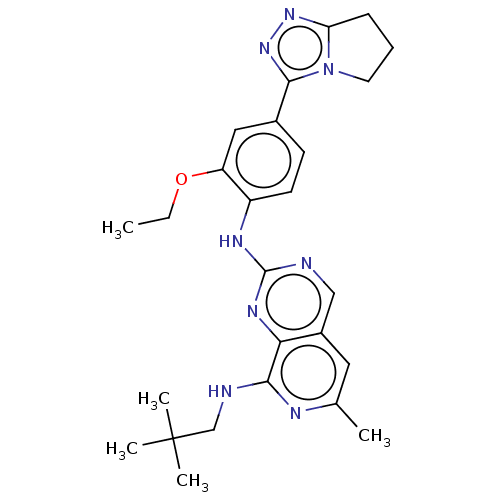 (CHEMBL4250961) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104402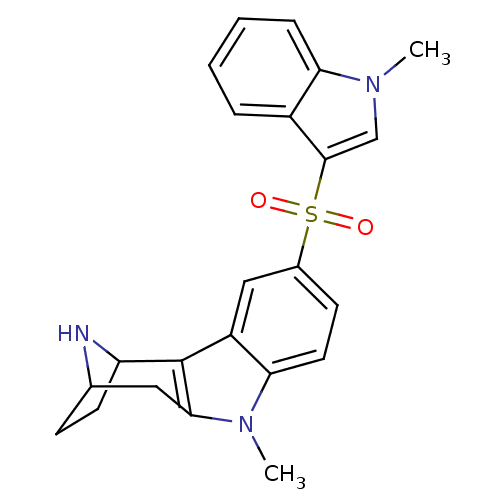 (US8575186, 165/166 | US8575186, 182) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104321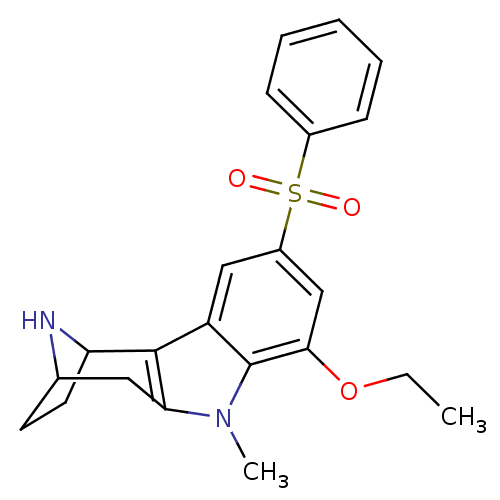 (US8575186, 250 | US8575186, 251 | US8575186, 84/23...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104371 (US8575186, 134/183/184 | US8575186, 199 | US857518...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104475 (US8575186, 238/239 | US8575186, 255) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241226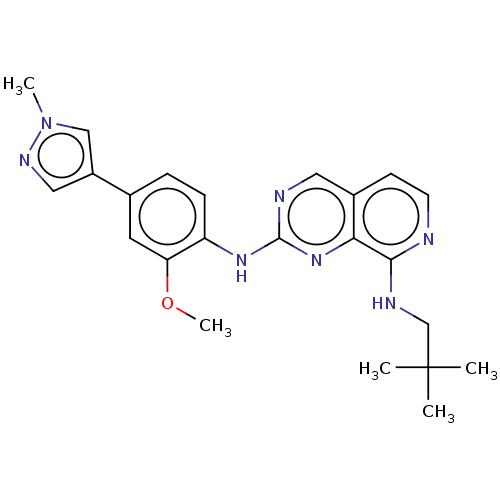 (US10479788, Example 68 | US11046688, Example 68 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104454 (US8575186, 217/218 | US8575186, 234) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104376 (US8575186, 139) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104360 (US8575186, 123) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104302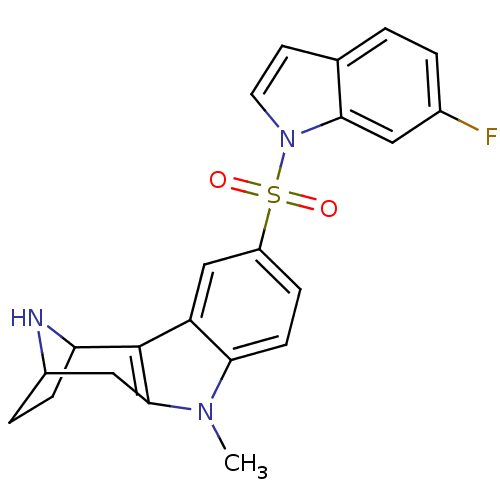 (US8575186, 203 | US8575186, 204 | US8575186, 65/18...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241226 (US10479788, Example 68 | US11046688, Example 68 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of human full length MPS1 expressed in recombinant baculovirus infected Sf9 insect cells using 5FAM-DHTGFLTEYVATRCONH2 as substrate after ... | J Med Chem 59: 3671-88 (2016) Article DOI: 10.1021/acs.jmedchem.5b01811 BindingDB Entry DOI: 10.7270/Q2DZ0B6T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104430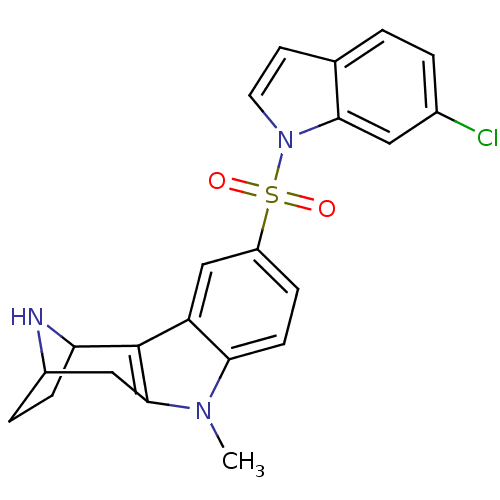 (US8575186, 193/194 | US8575186, 210) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104456 (US8575186, 219/220 | US8575186, 236) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104446 (US8575186, 209/215/216 | US8575186, 231 | US857518...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104462 (US8575186, 225/226 | US8575186, 242) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104377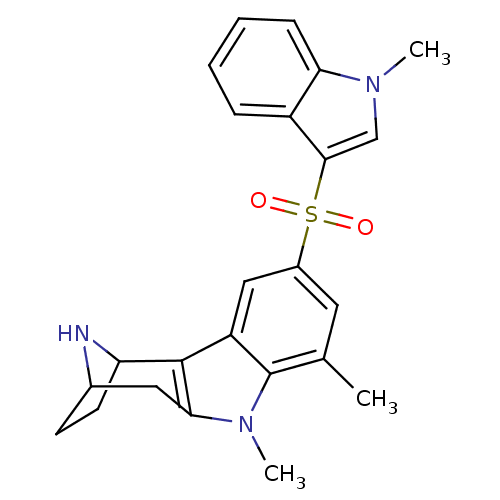 (US8575186, 140) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104458 (US8575186, 221/222 | US8575186, 238) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104320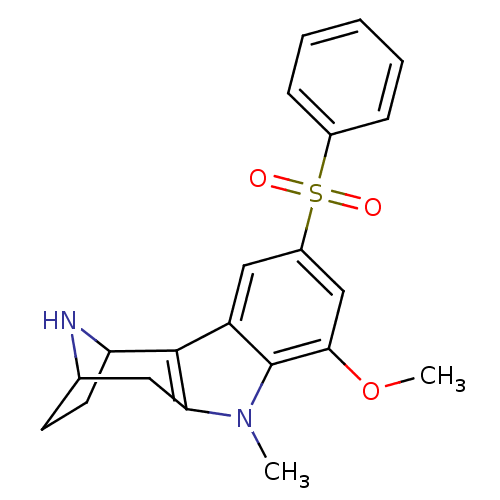 (US8575186, 228 | US8575186, 229 | US8575186, 83/21...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104479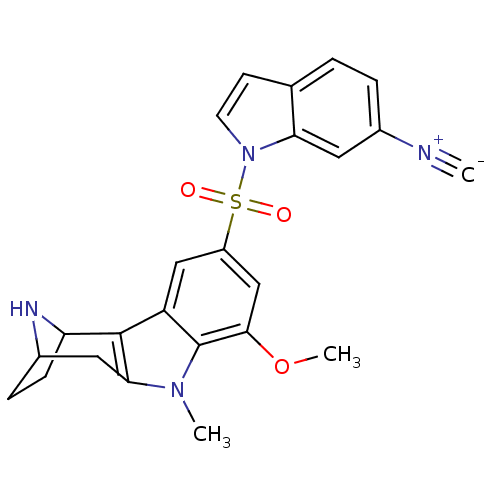 (US8575186, 242/243 | US8575186, 259) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104302 (US8575186, 203 | US8575186, 204 | US8575186, 65/18...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2081 total ) | Next | Last >> |