 Found 8415 hits with Last Name = 'wright' and Initial = 'n'
Found 8415 hits with Last Name = 'wright' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343152
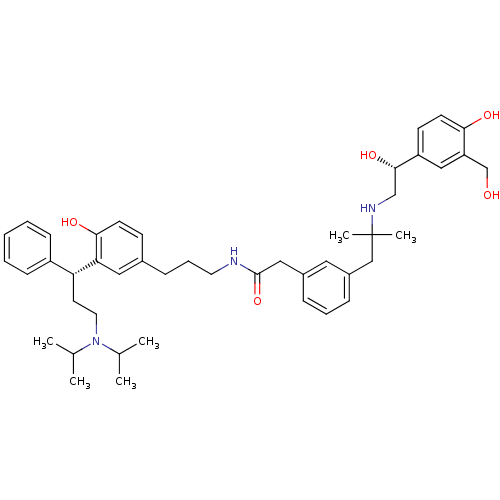
(CHEMBL1773196 | N-(3-(3-((R)-3-(diisopropylamino)-...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCCNC(=O)Cc2cccc(CC(C)(C)NC[C@H](O)c3ccc(O)c(CO)c3)c2)ccc1O)C(C)C |r| Show InChI InChI=1S/C45H61N3O5/c1-31(2)48(32(3)4)23-21-39(36-15-8-7-9-16-36)40-25-33(17-19-42(40)51)14-11-22-46-44(53)26-34-12-10-13-35(24-34)28-45(5,6)47-29-43(52)37-18-20-41(50)38(27-37)30-49/h7-10,12-13,15-20,24-25,27,31-32,39,43,47,49-52H,11,14,21-23,26,28-30H2,1-6H3,(H,46,53)/t39-,43+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50128835
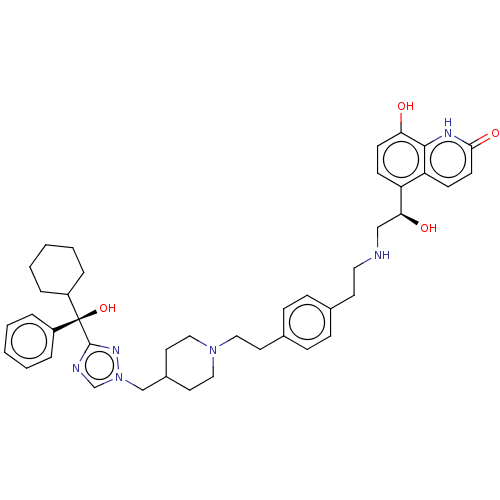
(CHEMBL3629360)Show SMILES O[C@@H](CNCCc1ccc(CCN2CCC(Cn3cnc(n3)[C@@](O)(C3CCCCC3)c3ccccc3)CC2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C42H52N6O4/c49-37-17-15-35(36-16-18-39(51)45-40(36)37)38(50)27-43-23-19-30-11-13-31(14-12-30)20-24-47-25-21-32(22-26-47)28-48-29-44-41(46-48)42(52,33-7-3-1-4-8-33)34-9-5-2-6-10-34/h1,3-4,7-8,11-18,29,32,34,38,43,49-50,52H,2,5-6,9-10,19-28H2,(H,45,51)/t38-,42-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
Curated by ChEMBL
| Assay Description
Antagonist activity at muscarinic M2 receptor (unknown origin) |
Bioorg Med Chem Lett 25: 5121-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.008
BindingDB Entry DOI: 10.7270/Q2JQ12V7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50128835

(CHEMBL3629360)Show SMILES O[C@@H](CNCCc1ccc(CCN2CCC(Cn3cnc(n3)[C@@](O)(C3CCCCC3)c3ccccc3)CC2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C42H52N6O4/c49-37-17-15-35(36-16-18-39(51)45-40(36)37)38(50)27-43-23-19-30-11-13-31(14-12-30)20-24-47-25-21-32(22-26-47)28-48-29-44-41(46-48)42(52,33-7-3-1-4-8-33)34-9-5-2-6-10-34/h1,3-4,7-8,11-18,29,32,34,38,43,49-50,52H,2,5-6,9-10,19-28H2,(H,45,51)/t38-,42-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
Curated by ChEMBL
| Assay Description
Antagonist activity at muscarinic M1 receptor (unknown origin) |
Bioorg Med Chem Lett 25: 5121-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.008
BindingDB Entry DOI: 10.7270/Q2JQ12V7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343153
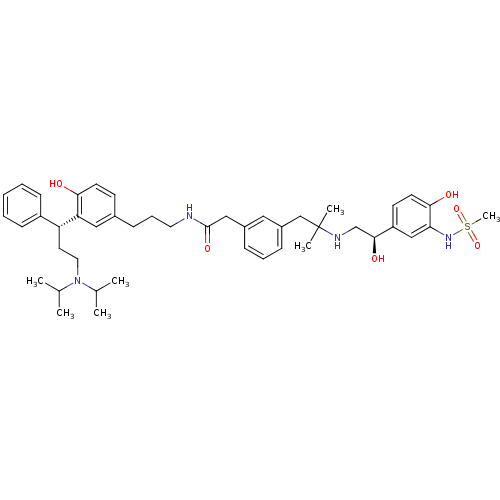
(CHEMBL1773197 | N-(3-(3-((R)-3-(diisopropylamino)-...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCCNC(=O)Cc2cccc(CC(C)(C)NC[C@H](O)c3ccc(O)c(NS(C)(=O)=O)c3)c2)ccc1O)C(C)C |r| Show InChI InChI=1S/C45H62N4O6S/c1-31(2)49(32(3)4)24-22-38(36-16-9-8-10-17-36)39-26-33(18-20-41(39)50)15-12-23-46-44(53)27-34-13-11-14-35(25-34)29-45(5,6)47-30-43(52)37-19-21-42(51)40(28-37)48-56(7,54)55/h8-11,13-14,16-21,25-26,28,31-32,38,43,47-48,50-52H,12,15,22-24,27,29-30H2,1-7H3,(H,46,53)/t38-,43+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.276 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50128835

(CHEMBL3629360)Show SMILES O[C@@H](CNCCc1ccc(CCN2CCC(Cn3cnc(n3)[C@@](O)(C3CCCCC3)c3ccccc3)CC2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C42H52N6O4/c49-37-17-15-35(36-16-18-39(51)45-40(36)37)38(50)27-43-23-19-30-11-13-31(14-12-30)20-24-47-25-21-32(22-26-47)28-48-29-44-41(46-48)42(52,33-7-3-1-4-8-33)34-9-5-2-6-10-34/h1,3-4,7-8,11-18,29,32,34,38,43,49-50,52H,2,5-6,9-10,19-28H2,(H,45,51)/t38-,42-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl scopolamine from human cloned muscarinic M3 receptor by dilution method |
Bioorg Med Chem Lett 25: 5121-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.008
BindingDB Entry DOI: 10.7270/Q2JQ12V7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343161
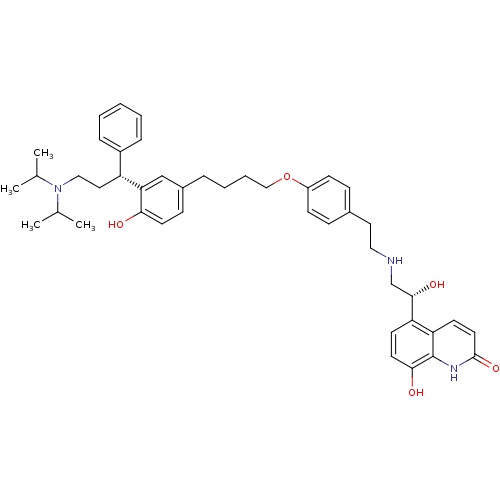
(5-((R)-2-(4-(4-(3-((R)-3-(diisopropylamino)-1-phen...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCCCOc2ccc(CCNC[C@H](O)c3ccc(O)c4[nH]c(=O)ccc34)cc2)ccc1O)C(C)C |r| Show InChI InChI=1S/C44H55N3O5/c1-30(2)47(31(3)4)26-24-36(34-11-6-5-7-12-34)39-28-33(15-20-40(39)48)10-8-9-27-52-35-16-13-32(14-17-35)23-25-45-29-42(50)37-18-21-41(49)44-38(37)19-22-43(51)46-44/h5-7,11-22,28,30-31,36,42,45,48-50H,8-10,23-27,29H2,1-4H3,(H,46,51)/t36-,42+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.305 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343161

(5-((R)-2-(4-(4-(3-((R)-3-(diisopropylamino)-1-phen...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCCCOc2ccc(CCNC[C@H](O)c3ccc(O)c4[nH]c(=O)ccc34)cc2)ccc1O)C(C)C |r| Show InChI InChI=1S/C44H55N3O5/c1-30(2)47(31(3)4)26-24-36(34-11-6-5-7-12-34)39-28-33(15-20-40(39)48)10-8-9-27-52-35-16-13-32(14-17-35)23-25-45-29-42(50)37-18-21-41(49)44-38(37)19-22-43(51)46-44/h5-7,11-22,28,30-31,36,42,45,48-50H,8-10,23-27,29H2,1-4H3,(H,46,51)/t36-,42+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl scopolamine from human cloned muscarinic M3 receptor by dilution method |
Bioorg Med Chem Lett 25: 5121-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.008
BindingDB Entry DOI: 10.7270/Q2JQ12V7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50128837
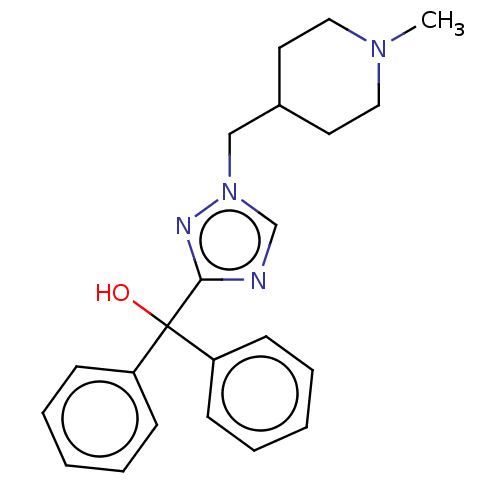
(CHEMBL3629354)Show SMILES CN1CCC(Cn2cnc(n2)C(O)(c2ccccc2)c2ccccc2)CC1 Show InChI InChI=1S/C22H26N4O/c1-25-14-12-18(13-15-25)16-26-17-23-21(24-26)22(27,19-8-4-2-5-9-19)20-10-6-3-7-11-20/h2-11,17-18,27H,12-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl scopolamine from human cloned muscarinic M3 receptor by dilution method |
Bioorg Med Chem Lett 25: 5121-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.008
BindingDB Entry DOI: 10.7270/Q2JQ12V7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50128835

(CHEMBL3629360)Show SMILES O[C@@H](CNCCc1ccc(CCN2CCC(Cn3cnc(n3)[C@@](O)(C3CCCCC3)c3ccccc3)CC2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C42H52N6O4/c49-37-17-15-35(36-16-18-39(51)45-40(36)37)38(50)27-43-23-19-30-11-13-31(14-12-30)20-24-47-25-21-32(22-26-47)28-48-29-44-41(46-48)42(52,33-7-3-1-4-8-33)34-9-5-2-6-10-34/h1,3-4,7-8,11-18,29,32,34,38,43,49-50,52H,2,5-6,9-10,19-28H2,(H,45,51)/t38-,42-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
Curated by ChEMBL
| Assay Description
Antagonist activity at muscarinic M4 receptor (unknown origin) |
Bioorg Med Chem Lett 25: 5121-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.008
BindingDB Entry DOI: 10.7270/Q2JQ12V7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343154
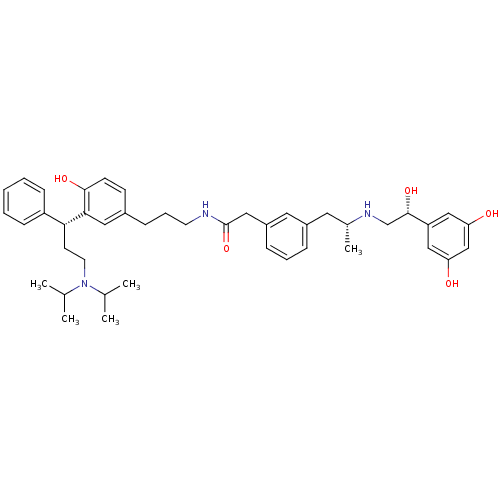
(2-(3-((R)-2-((R)-2-(3,5-dihydroxyphenyl)-2-hydroxy...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCCNC(=O)Cc2cccc(C[C@@H](C)NC[C@H](O)c3cc(O)cc(O)c3)c2)ccc1O)C(C)C |r| Show InChI InChI=1S/C43H57N3O5/c1-29(2)46(30(3)4)20-18-39(35-14-7-6-8-15-35)40-23-32(16-17-41(40)49)13-10-19-44-43(51)24-34-12-9-11-33(22-34)21-31(5)45-28-42(50)36-25-37(47)27-38(48)26-36/h6-9,11-12,14-17,22-23,25-27,29-31,39,42,45,47-50H,10,13,18-21,24,28H2,1-5H3,(H,44,51)/t31-,39-,42+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.397 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343157
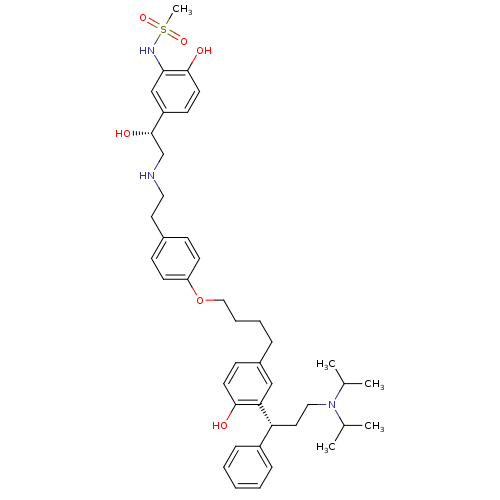
(CHEMBL1773264 | N-(5-((R)-2-(4-(4-(3-((R)-3-(diiso...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCCCOc2ccc(CCNC[C@H](O)c3ccc(O)c(NS(C)(=O)=O)c3)cc2)ccc1O)C(C)C |r| Show InChI InChI=1S/C42H57N3O6S/c1-30(2)45(31(3)4)25-23-37(34-12-7-6-8-13-34)38-27-33(16-20-40(38)46)11-9-10-26-51-36-18-14-32(15-19-36)22-24-43-29-42(48)35-17-21-41(47)39(28-35)44-52(5,49)50/h6-8,12-21,27-28,30-31,37,42-44,46-48H,9-11,22-26,29H2,1-5H3/t37-,42+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.634 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50128830

(CHEMBL3629353)Show SMILES O[C@@H](CNCCCCCCCCCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccc(O)c(Cl)c1)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C38H47ClN4O6/c39-31-24-26(12-15-33(31)44)28-10-6-7-11-32(28)41-38(48)49-27-18-22-43(23-19-27)21-9-5-3-1-2-4-8-20-40-25-35(46)29-13-16-34(45)37-30(29)14-17-36(47)42-37/h6-7,10-17,24,27,35,40,44-46H,1-5,8-9,18-23,25H2,(H,41,48)(H,42,47)/t35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl scopolamine from human cloned muscarinic M3 receptor by dilution method |
Bioorg Med Chem Lett 25: 5121-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.008
BindingDB Entry DOI: 10.7270/Q2JQ12V7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343158
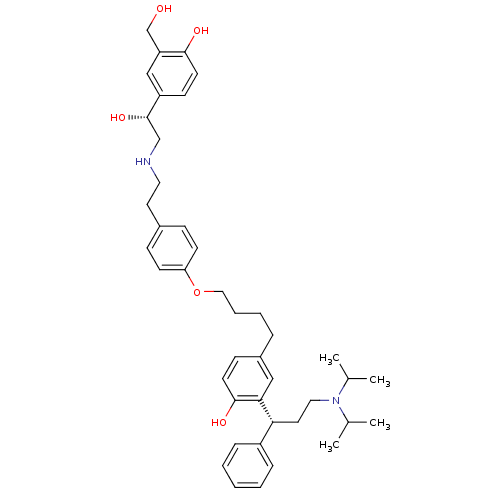
(2-((R)-3-(diisopropylamino)-1-phenylpropyl)-4-(4-(...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCCCOc2ccc(CCNC[C@H](O)c3ccc(O)c(CO)c3)cc2)ccc1O)C(C)C |r| Show InChI InChI=1S/C42H56N2O5/c1-30(2)44(31(3)4)24-22-38(34-11-6-5-7-12-34)39-26-33(15-19-41(39)47)10-8-9-25-49-37-17-13-32(14-18-37)21-23-43-28-42(48)35-16-20-40(46)36(27-35)29-45/h5-7,11-20,26-27,30-31,38,42-43,45-48H,8-10,21-25,28-29H2,1-4H3/t38-,42+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.765 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343160
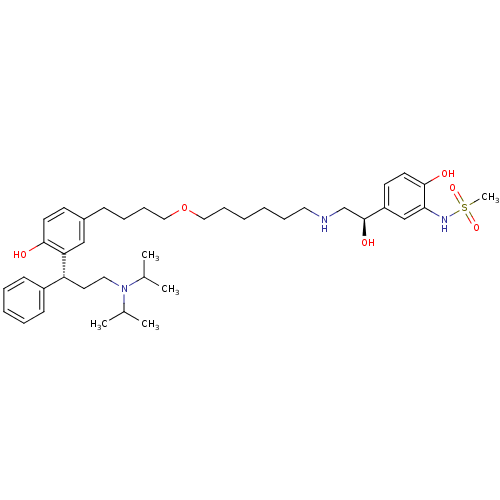
(CHEMBL1773266 | N-(5-((R)-2-(6-(4-(3-((R)-3-(diiso...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCCCOCCCCCCNC[C@H](O)c2ccc(O)c(NS(C)(=O)=O)c2)ccc1O)C(C)C |r| Show InChI InChI=1S/C40H61N3O6S/c1-30(2)43(31(3)4)24-22-35(33-16-9-8-10-17-33)36-27-32(18-20-38(36)44)15-11-14-26-49-25-13-7-6-12-23-41-29-40(46)34-19-21-39(45)37(28-34)42-50(5,47)48/h8-10,16-21,27-28,30-31,35,40-42,44-46H,6-7,11-15,22-26,29H2,1-5H3/t35-,40+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50128836
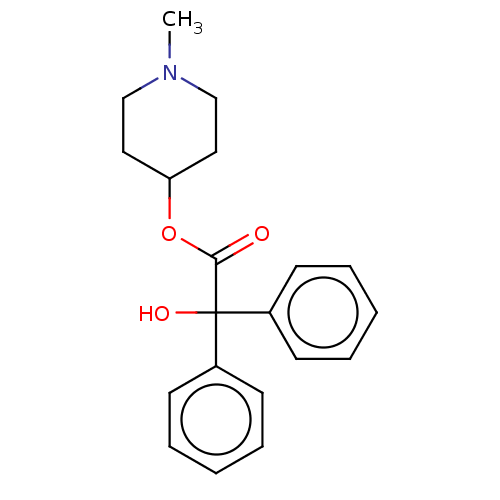
(CHEMBL143228)Show InChI InChI=1S/C20H23NO3/c1-21-14-12-18(13-15-21)24-19(22)20(23,16-8-4-2-5-9-16)17-10-6-3-7-11-17/h2-11,18,23H,12-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl scopolamine from human cloned muscarinic M3 receptor by dilution method |
Bioorg Med Chem Lett 25: 5121-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.008
BindingDB Entry DOI: 10.7270/Q2JQ12V7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM50128835

(CHEMBL3629360)Show SMILES O[C@@H](CNCCc1ccc(CCN2CCC(Cn3cnc(n3)[C@@](O)(C3CCCCC3)c3ccccc3)CC2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C42H52N6O4/c49-37-17-15-35(36-16-18-39(51)45-40(36)37)38(50)27-43-23-19-30-11-13-31(14-12-30)20-24-47-25-21-32(22-26-47)28-48-29-44-41(46-48)42(52,33-7-3-1-4-8-33)34-9-5-2-6-10-34/h1,3-4,7-8,11-18,29,32,34,38,43,49-50,52H,2,5-6,9-10,19-28H2,(H,45,51)/t38-,42-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
Curated by ChEMBL
| Assay Description
Antagonist activity at muscarinic M5 receptor (unknown origin) |
Bioorg Med Chem Lett 25: 5121-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.008
BindingDB Entry DOI: 10.7270/Q2JQ12V7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50128838
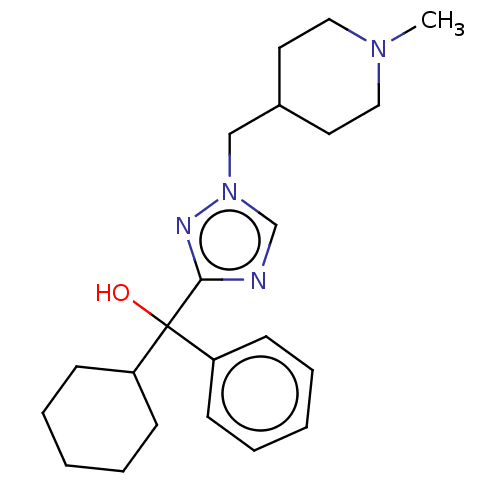
(CHEMBL3629355)Show SMILES CN1CCC(Cn2cnc(n2)C(O)(C2CCCCC2)c2ccccc2)CC1 Show InChI InChI=1S/C22H32N4O/c1-25-14-12-18(13-15-25)16-26-17-23-21(24-26)22(27,19-8-4-2-5-9-19)20-10-6-3-7-11-20/h2,4-5,8-9,17-18,20,27H,3,6-7,10-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl scopolamine from human cloned muscarinic M3 receptor by dilution method |
Bioorg Med Chem Lett 25: 5121-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.008
BindingDB Entry DOI: 10.7270/Q2JQ12V7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343156
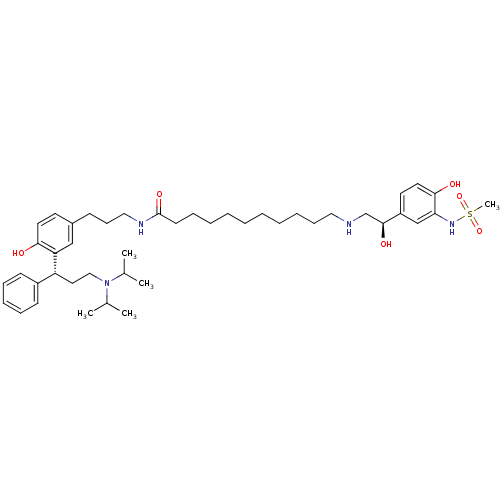
(CHEMBL1773263 | N-(3-(3-((R)-3-(diisopropylamino)-...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCCNC(=O)CCCCCCCCCCNC[C@H](O)c2ccc(O)c(NS(C)(=O)=O)c2)ccc1O)C(C)C |r| Show InChI InChI=1S/C44H68N4O6S/c1-33(2)48(34(3)4)29-26-38(36-19-13-12-14-20-36)39-30-35(22-24-41(39)49)18-17-28-46-44(52)21-15-10-8-6-7-9-11-16-27-45-32-43(51)37-23-25-42(50)40(31-37)47-55(5,53)54/h12-14,19-20,22-25,30-31,33-34,38,43,45,47,49-51H,6-11,15-18,21,26-29,32H2,1-5H3,(H,46,52)/t38-,43+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM82070

(CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
In vitro Binding affinity towards alpha-3 (PC12) nAChR was determined |
Bioorg Med Chem Lett 11: 319-21 (2001)
BindingDB Entry DOI: 10.7270/Q2028S36 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-7
(Mus musculus) | BDBM50096713
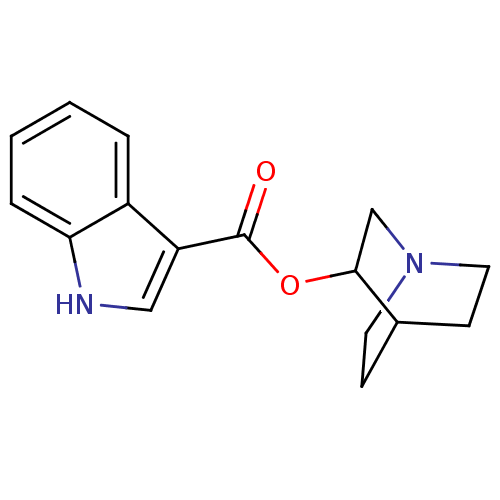
(1H-Indole-3-carboxylic acid 1-aza-bicyclo[2.2.2]oc...)Show SMILES O=C(OC1CN2CCC1CC2)c1c[nH]c2ccccc12 |THB:2:3:9.10:7.6,(10.78,-2.61,;10.78,-4.18,;12.1,-4.96,;13.37,-6.22,;12.58,-7.47,;14.26,-6.58,;15.87,-7.55,;16.69,-6.38,;15.12,-5.43,;15.11,-4.33,;14.26,-5.17,;9.44,-4.93,;9.44,-6.45,;8.09,-7.22,;6.74,-6.45,;5.4,-7.22,;4.07,-6.45,;4.07,-4.94,;5.4,-4.15,;6.74,-4.9,)| Show InChI InChI=1S/C16H18N2O2/c19-16(13-9-17-14-4-2-1-3-12(13)14)20-15-10-18-7-5-11(15)6-8-18/h1-4,9,11,15,17H,5-8,10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
In vitro Binding affinity towards alpha-7 nAChR was determined |
Bioorg Med Chem Lett 11: 319-21 (2001)
BindingDB Entry DOI: 10.7270/Q2028S36 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50165008

((+)-(R)-2-(alpha-(2-(Diisopropylamino)ethyl)benzyl...)Show InChI InChI=1S/C22H31NO/c1-16(2)23(17(3)4)14-13-20(19-9-7-6-8-10-19)21-15-18(5)11-12-22(21)24/h6-12,15-17,20,24H,13-14H2,1-5H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50128834
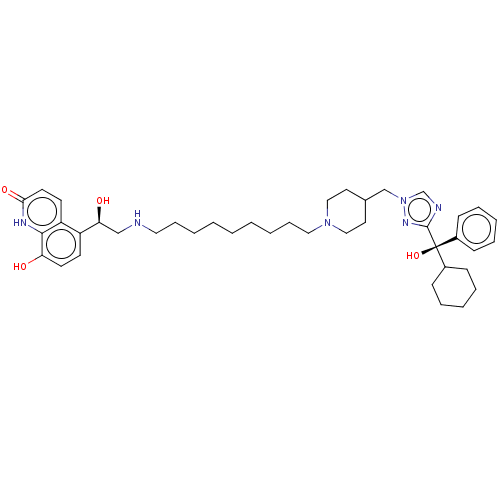
(CHEMBL3629359)Show SMILES O[C@@H](CNCCCCCCCCCN1CCC(Cn2cnc(n2)[C@@](O)(C2CCCCC2)c2ccccc2)CC1)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C41H58N6O4/c48-36-20-18-34(35-19-21-38(50)44-39(35)36)37(49)28-42-24-12-4-2-1-3-5-13-25-46-26-22-31(23-27-46)29-47-30-43-40(45-47)41(51,32-14-8-6-9-15-32)33-16-10-7-11-17-33/h6,8-9,14-15,18-21,30-31,33,37,42,48-49,51H,1-5,7,10-13,16-17,22-29H2,(H,44,50)/t37-,41-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl scopolamine from human cloned muscarinic M3 receptor by dilution method |
Bioorg Med Chem Lett 25: 5121-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.008
BindingDB Entry DOI: 10.7270/Q2JQ12V7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50128831
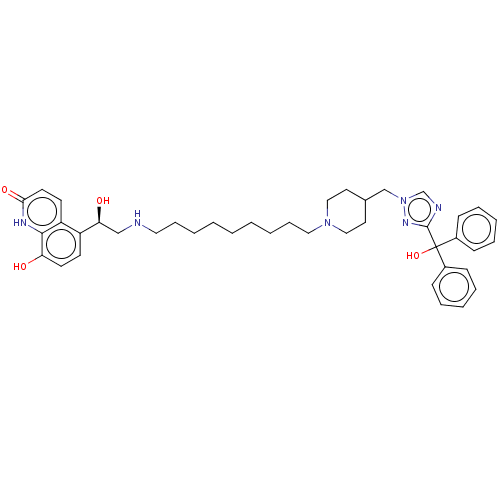
(CHEMBL3629356)Show SMILES O[C@@H](CNCCCCCCCCCN1CCC(Cn2cnc(n2)C(O)(c2ccccc2)c2ccccc2)CC1)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C41H52N6O4/c48-36-20-18-34(35-19-21-38(50)44-39(35)36)37(49)28-42-24-12-4-2-1-3-5-13-25-46-26-22-31(23-27-46)29-47-30-43-40(45-47)41(51,32-14-8-6-9-15-32)33-16-10-7-11-17-33/h6-11,14-21,30-31,37,42,48-49,51H,1-5,12-13,22-29H2,(H,44,50)/t37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl scopolamine from human cloned muscarinic M3 receptor by dilution method |
Bioorg Med Chem Lett 25: 5121-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.008
BindingDB Entry DOI: 10.7270/Q2JQ12V7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343159

(4-((R)-2-(4-(4-(3-((R)-3-(diisopropylamino)-1-phen...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCCCOc2ccc(CCNC[C@H](O)c3ccc(O)c(O)c3)cc2)ccc1O)C(C)C |r| Show InChI InChI=1S/C41H54N2O5/c1-29(2)43(30(3)4)24-22-36(33-11-6-5-7-12-33)37-26-32(15-19-38(37)44)10-8-9-25-48-35-17-13-31(14-18-35)21-23-42-28-41(47)34-16-20-39(45)40(46)27-34/h5-7,11-20,26-27,29-30,36,41-42,44-47H,8-10,21-25,28H2,1-4H3/t36-,41+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343155
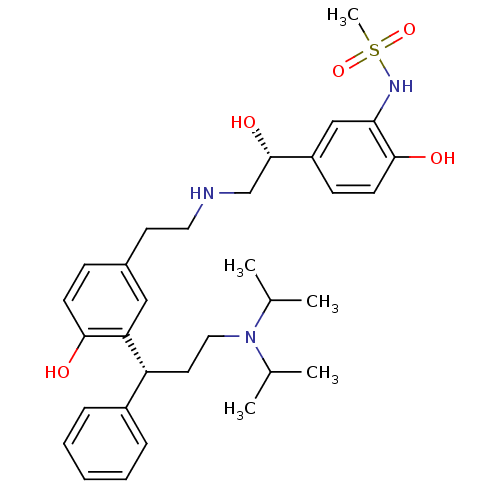
(CHEMBL1773262 | N-(5-((R)-2-(3-((R)-3-(diisopropyl...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCNC[C@H](O)c2ccc(O)c(NS(C)(=O)=O)c2)ccc1O)C(C)C |r| Show InChI InChI=1S/C32H45N3O5S/c1-22(2)35(23(3)4)18-16-27(25-9-7-6-8-10-25)28-19-24(11-13-30(28)36)15-17-33-21-32(38)26-12-14-31(37)29(20-26)34-41(5,39)40/h6-14,19-20,22-23,27,32-34,36-38H,15-18,21H2,1-5H3/t27-,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Mus musculus) | BDBM50096712

(3-(1H-Indole-3-carbonyloxy)-8,8-dimethyl-8-azonia-...)Show SMILES C[N+]1(C)C2CCC1CC(C2)OC(=O)c1c[nH]c2ccccc12 |TLB:10:8:1:4.5| Show InChI InChI=1S/C18H22N2O2/c1-20(2)12-7-8-13(20)10-14(9-12)22-18(21)16-11-19-17-6-4-3-5-15(16)17/h3-6,11-14H,7-10H2,1-2H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
In vitro Binding affinity towards alpha-7 nAChR was determined |
Bioorg Med Chem Lett 11: 319-21 (2001)
BindingDB Entry DOI: 10.7270/Q2028S36 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Mus musculus) | BDBM50108392

((3-ENDO)-8-METHYL-8-AZABICYCLO[3.2.1]OCT-3-YL 1H-I...)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)c1c[nH]c2ccccc12 |r,THB:9:7:4.3:1| Show InChI InChI=1S/C17H20N2O2/c1-19-11-6-7-12(19)9-13(8-11)21-17(20)15-10-18-16-5-3-2-4-14(15)16/h2-5,10-13,18H,6-9H2,1H3/t11-,12+,13+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
In vitro Binding affinity towards alpha-7 nAChR was determined |
Bioorg Med Chem Lett 11: 319-21 (2001)
BindingDB Entry DOI: 10.7270/Q2028S36 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50128833
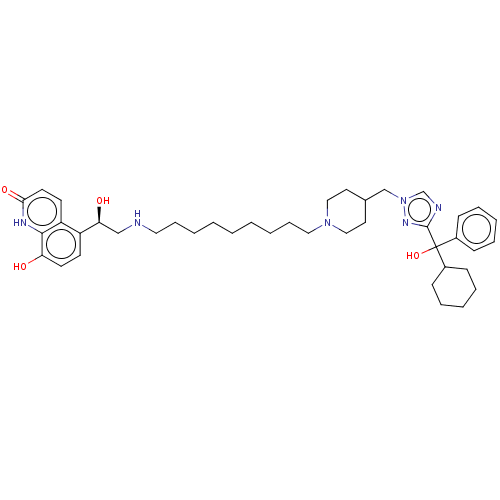
(CHEMBL3629358)Show SMILES O[C@@H](CNCCCCCCCCCN1CCC(Cn2cnc(n2)C(O)(C2CCCCC2)c2ccccc2)CC1)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C41H58N6O4/c48-36-20-18-34(35-19-21-38(50)44-39(35)36)37(49)28-42-24-12-4-2-1-3-5-13-25-46-26-22-31(23-27-46)29-47-30-43-40(45-47)41(51,32-14-8-6-9-15-32)33-16-10-7-11-17-33/h6,8-9,14-15,18-21,30-31,33,37,42,48-49,51H,1-5,7,10-13,16-17,22-29H2,(H,44,50)/t37-,41?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl scopolamine from human cloned muscarinic M3 receptor by dilution method |
Bioorg Med Chem Lett 25: 5121-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.008
BindingDB Entry DOI: 10.7270/Q2JQ12V7 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Mus musculus) | BDBM50096711
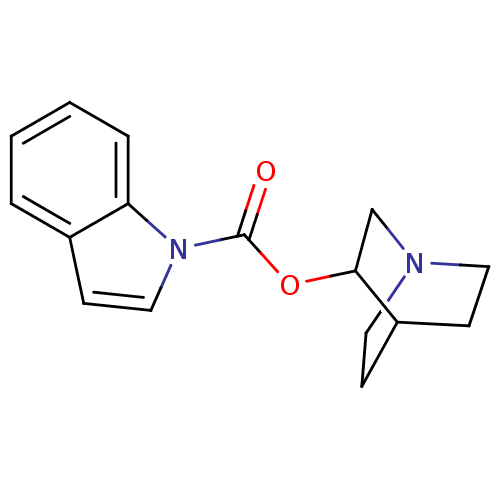
(CHEMBL320963 | Indole-1-carboxylic acid 1-aza-bicy...)Show SMILES O=C(OC1CN2CCC1CC2)n1ccc2ccccc12 |THB:2:3:9.10:7.6,(7.39,-10.08,;8.5,-8.89,;9.81,-9.66,;11.07,-10.94,;10.28,-12.18,;11.95,-11.29,;13.58,-12.25,;14.4,-11.1,;12.81,-10.15,;12.81,-9.03,;11.95,-9.89,;8.08,-7.22,;9.43,-6.44,;9.43,-4.92,;6.74,-4.9,;5.39,-4.15,;4.07,-4.93,;4.07,-6.44,;5.39,-7.22,;6.74,-6.44,)| Show InChI InChI=1S/C16H18N2O2/c19-16(18-10-7-12-3-1-2-4-14(12)18)20-15-11-17-8-5-13(15)6-9-17/h1-4,7,10,13,15H,5-6,8-9,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
In vitro Binding affinity towards alpha-7 nAChR was determined |
Bioorg Med Chem Lett 11: 319-21 (2001)
BindingDB Entry DOI: 10.7270/Q2028S36 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50128832
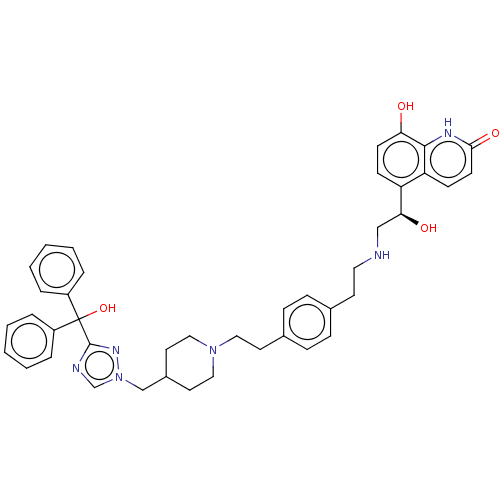
(CHEMBL3629357)Show SMILES O[C@@H](CNCCc1ccc(CCN2CCC(Cn3cnc(n3)C(O)(c3ccccc3)c3ccccc3)CC2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C42H46N6O4/c49-37-17-15-35(36-16-18-39(51)45-40(36)37)38(50)27-43-23-19-30-11-13-31(14-12-30)20-24-47-25-21-32(22-26-47)28-48-29-44-41(46-48)42(52,33-7-3-1-4-8-33)34-9-5-2-6-10-34/h1-18,29,32,38,43,49-50,52H,19-28H2,(H,45,51)/t38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl scopolamine from human cloned muscarinic M3 receptor by dilution method |
Bioorg Med Chem Lett 25: 5121-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.008
BindingDB Entry DOI: 10.7270/Q2JQ12V7 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3
(Homo sapiens (Human)) | BDBM82070

(CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3/t10-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
In vitro Binding affinity towards alpha-3 (PC12) nAChR was determined |
Bioorg Med Chem Lett 11: 319-21 (2001)
BindingDB Entry DOI: 10.7270/Q2028S36 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Mus musculus) | BDBM82070

(CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3/t10-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
In vitro Binding affinity towards alpha-7 nAChR was determined |
Bioorg Med Chem Lett 11: 319-21 (2001)
BindingDB Entry DOI: 10.7270/Q2028S36 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-3
(Homo sapiens (Human)) | BDBM50096712

(3-(1H-Indole-3-carbonyloxy)-8,8-dimethyl-8-azonia-...)Show SMILES C[N+]1(C)C2CCC1CC(C2)OC(=O)c1c[nH]c2ccccc12 |TLB:10:8:1:4.5| Show InChI InChI=1S/C18H22N2O2/c1-20(2)12-7-8-13(20)10-14(9-12)22-18(21)16-11-19-17-6-4-3-5-15(16)17/h3-6,11-14H,7-10H2,1-2H3/p+1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
In vitro Binding affinity towards alpha-3 (PC12) nAChR was determined |
Bioorg Med Chem Lett 11: 319-21 (2001)
BindingDB Entry DOI: 10.7270/Q2028S36 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50096713

(1H-Indole-3-carboxylic acid 1-aza-bicyclo[2.2.2]oc...)Show SMILES O=C(OC1CN2CCC1CC2)c1c[nH]c2ccccc12 |THB:2:3:9.10:7.6,(10.78,-2.61,;10.78,-4.18,;12.1,-4.96,;13.37,-6.22,;12.58,-7.47,;14.26,-6.58,;15.87,-7.55,;16.69,-6.38,;15.12,-5.43,;15.11,-4.33,;14.26,-5.17,;9.44,-4.93,;9.44,-6.45,;8.09,-7.22,;6.74,-6.45,;5.4,-7.22,;4.07,-6.45,;4.07,-4.94,;5.4,-4.15,;6.74,-4.9,)| Show InChI InChI=1S/C16H18N2O2/c19-16(13-9-17-14-4-2-1-3-12(13)14)20-15-10-18-7-5-11(15)6-8-18/h1-4,9,11,15,17H,5-8,10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
In vitro Binding affinity towards alpha-1-beta-1-gamma-delta nAChR was determined |
Bioorg Med Chem Lett 11: 319-21 (2001)
BindingDB Entry DOI: 10.7270/Q2028S36 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Mus musculus) | BDBM85330

(CAS_68647 | NSC_68647 | ONDANSETRON | Ondansetron ...)Show InChI InChI=1S/C18H19N3O/c1-12-19-9-10-21(12)11-13-7-8-16-17(18(13)22)14-5-3-4-6-15(14)20(16)2/h3-6,9-10,13H,7-8,11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
In vitro Binding affinity towards alpha-7 nAChR was determined |
Bioorg Med Chem Lett 11: 319-21 (2001)
BindingDB Entry DOI: 10.7270/Q2028S36 |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha/beta/delta/gamma
(Homo sapiens (Human)) | BDBM50096712

(3-(1H-Indole-3-carbonyloxy)-8,8-dimethyl-8-azonia-...)Show SMILES C[N+]1(C)C2CCC1CC(C2)OC(=O)c1c[nH]c2ccccc12 |TLB:10:8:1:4.5| Show InChI InChI=1S/C18H22N2O2/c1-20(2)12-7-8-13(20)10-14(9-12)22-18(21)16-11-19-17-6-4-3-5-15(16)17/h3-6,11-14H,7-10H2,1-2H3/p+1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
In vitro Binding affinity towards Nicotinic acetylcholine receptor alpha4-beta2 was determined |
Bioorg Med Chem Lett 11: 319-21 (2001)
BindingDB Entry DOI: 10.7270/Q2028S36 |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha/beta/delta/gamma
(Homo sapiens (Human)) | BDBM82070

(CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3/t10-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
In vitro Binding affinity towards Nicotinic acetylcholine receptor alpha4-beta2 was determined |
Bioorg Med Chem Lett 11: 319-21 (2001)
BindingDB Entry DOI: 10.7270/Q2028S36 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50096712

(3-(1H-Indole-3-carbonyloxy)-8,8-dimethyl-8-azonia-...)Show SMILES C[N+]1(C)C2CCC1CC(C2)OC(=O)c1c[nH]c2ccccc12 |TLB:10:8:1:4.5| Show InChI InChI=1S/C18H22N2O2/c1-20(2)12-7-8-13(20)10-14(9-12)22-18(21)16-11-19-17-6-4-3-5-15(16)17/h3-6,11-14H,7-10H2,1-2H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
In vitro Binding affinity towards Nicotinic acetylcholine receptor alpha4-beta2 was determined |
Bioorg Med Chem Lett 11: 319-21 (2001)
BindingDB Entry DOI: 10.7270/Q2028S36 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Mus musculus) | BDBM50000483
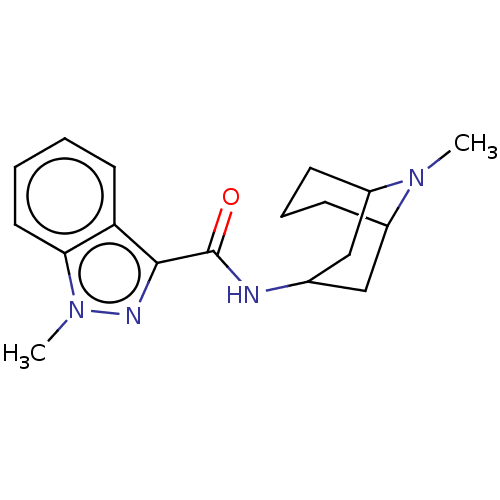
((BRL 43694)1-Methyl-1H-indazole-3-carboxylic acid ...)Show SMILES CN1C2CCCC1CC(C2)NC(=O)c1nn(C)c2ccccc12 |THB:10:8:1:3.5.4| Show InChI InChI=1S/C18H24N4O/c1-21-13-6-5-7-14(21)11-12(10-13)19-18(23)17-15-8-3-4-9-16(15)22(2)20-17/h3-4,8-9,12-14H,5-7,10-11H2,1-2H3,(H,19,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
In vitro Binding affinity towards alpha-7 nAChR was determined |
Bioorg Med Chem Lett 11: 319-21 (2001)
BindingDB Entry DOI: 10.7270/Q2028S36 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50096711

(CHEMBL320963 | Indole-1-carboxylic acid 1-aza-bicy...)Show SMILES O=C(OC1CN2CCC1CC2)n1ccc2ccccc12 |THB:2:3:9.10:7.6,(7.39,-10.08,;8.5,-8.89,;9.81,-9.66,;11.07,-10.94,;10.28,-12.18,;11.95,-11.29,;13.58,-12.25,;14.4,-11.1,;12.81,-10.15,;12.81,-9.03,;11.95,-9.89,;8.08,-7.22,;9.43,-6.44,;9.43,-4.92,;6.74,-4.9,;5.39,-4.15,;4.07,-4.93,;4.07,-6.44,;5.39,-7.22,;6.74,-6.44,)| Show InChI InChI=1S/C16H18N2O2/c19-16(18-10-7-12-3-1-2-4-14(12)18)20-15-11-17-8-5-13(15)6-9-17/h1-4,7,10,13,15H,5-6,8-9,11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
In vitro Binding affinity towards alpha-1-beta-1-gamma-delta nAChR was determined |
Bioorg Med Chem Lett 11: 319-21 (2001)
BindingDB Entry DOI: 10.7270/Q2028S36 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3
(Homo sapiens (Human)) | BDBM50108392

((3-ENDO)-8-METHYL-8-AZABICYCLO[3.2.1]OCT-3-YL 1H-I...)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)c1c[nH]c2ccccc12 |r,THB:9:7:4.3:1| Show InChI InChI=1S/C17H20N2O2/c1-19-11-6-7-12(19)9-13(8-11)21-17(20)15-10-18-16-5-3-2-4-14(15)16/h2-5,10-13,18H,6-9H2,1H3/t11-,12+,13+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
In vitro Binding affinity towards alpha-3 (PC12) nAChR was determined |
Bioorg Med Chem Lett 11: 319-21 (2001)
BindingDB Entry DOI: 10.7270/Q2028S36 |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha/beta/delta/gamma
(Homo sapiens (Human)) | BDBM50108392

((3-ENDO)-8-METHYL-8-AZABICYCLO[3.2.1]OCT-3-YL 1H-I...)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)c1c[nH]c2ccccc12 |r,THB:9:7:4.3:1| Show InChI InChI=1S/C17H20N2O2/c1-19-11-6-7-12(19)9-13(8-11)21-17(20)15-10-18-16-5-3-2-4-14(15)16/h2-5,10-13,18H,6-9H2,1H3/t11-,12+,13+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
In vitro Binding affinity towards alpha-1-beta-1-gamma delta nAChR was determined |
Bioorg Med Chem Lett 11: 319-21 (2001)
BindingDB Entry DOI: 10.7270/Q2028S36 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50000483

((BRL 43694)1-Methyl-1H-indazole-3-carboxylic acid ...)Show SMILES CN1C2CCCC1CC(C2)NC(=O)c1nn(C)c2ccccc12 |THB:10:8:1:3.5.4| Show InChI InChI=1S/C18H24N4O/c1-21-13-6-5-7-14(21)11-12(10-13)19-18(23)17-15-8-3-4-9-16(15)22(2)20-17/h3-4,8-9,12-14H,5-7,10-11H2,1-2H3,(H,19,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
In vitro Binding affinity towards Nicotinic acetylcholine receptor alpha4-beta2 was determined |
Bioorg Med Chem Lett 11: 319-21 (2001)
BindingDB Entry DOI: 10.7270/Q2028S36 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM85330

(CAS_68647 | NSC_68647 | ONDANSETRON | Ondansetron ...)Show InChI InChI=1S/C18H19N3O/c1-12-19-9-10-21(12)11-13-7-8-16-17(18(13)22)14-5-3-4-6-15(14)20(16)2/h3-6,9-10,13H,7-8,11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
In vitro Binding affinity towards Nicotinic acetylcholine receptor alpha4-beta2 was determined |
Bioorg Med Chem Lett 11: 319-21 (2001)
BindingDB Entry DOI: 10.7270/Q2028S36 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50108392

((3-ENDO)-8-METHYL-8-AZABICYCLO[3.2.1]OCT-3-YL 1H-I...)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)c1c[nH]c2ccccc12 |r,THB:9:7:4.3:1| Show InChI InChI=1S/C17H20N2O2/c1-19-11-6-7-12(19)9-13(8-11)21-17(20)15-10-18-16-5-3-2-4-14(15)16/h2-5,10-13,18H,6-9H2,1H3/t11-,12+,13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
In vitro Binding affinity towards 5-hydroxytryptamine 3 receptor was determined |
Bioorg Med Chem Lett 11: 319-21 (2001)
BindingDB Entry DOI: 10.7270/Q2028S36 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3
(Homo sapiens (Human)) | BDBM50000483

((BRL 43694)1-Methyl-1H-indazole-3-carboxylic acid ...)Show SMILES CN1C2CCCC1CC(C2)NC(=O)c1nn(C)c2ccccc12 |THB:10:8:1:3.5.4| Show InChI InChI=1S/C18H24N4O/c1-21-13-6-5-7-14(21)11-12(10-13)19-18(23)17-15-8-3-4-9-16(15)22(2)20-17/h3-4,8-9,12-14H,5-7,10-11H2,1-2H3,(H,19,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 6.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
In vitro Binding affinity towards 5-hydroxytryptamine 3 receptor was determined |
Bioorg Med Chem Lett 11: 319-21 (2001)
BindingDB Entry DOI: 10.7270/Q2028S36 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50316213
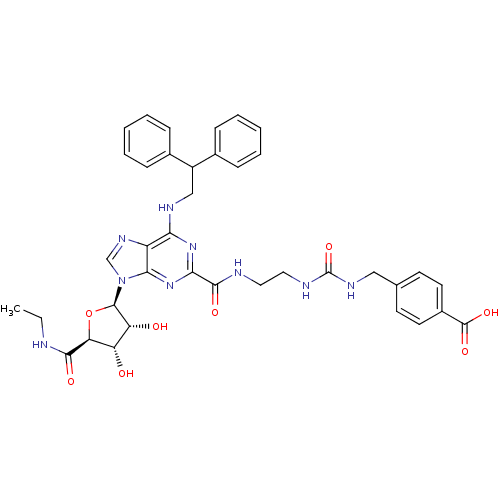
(4-((3-(2-(6-(2,2-diphenylethylamino)-9-((2R,3R,4S,...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC(c3ccccc3)c3ccccc3)nc(nc12)C(=O)NCCNC(=O)NCc1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C38H41N9O8/c1-2-39-34(50)30-28(48)29(49)36(55-30)47-21-44-27-31(42-20-26(23-9-5-3-6-10-23)24-11-7-4-8-12-24)45-32(46-33(27)47)35(51)40-17-18-41-38(54)43-19-22-13-15-25(16-14-22)37(52)53/h3-16,21,26,28-30,36,48-49H,2,17-20H2,1H3,(H,39,50)(H,40,51)(H,52,53)(H2,41,43,54)(H,42,45,46)/t28-,29+,30-,36+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Agonist activity at adenosine A2A receptor in fMLP-stimulated human neutrophils assessed as inhibition of superoxide production by colorimetric analy... |
Bioorg Med Chem Lett 19: 4471-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.027
BindingDB Entry DOI: 10.7270/Q2ST7Q0C |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM592293
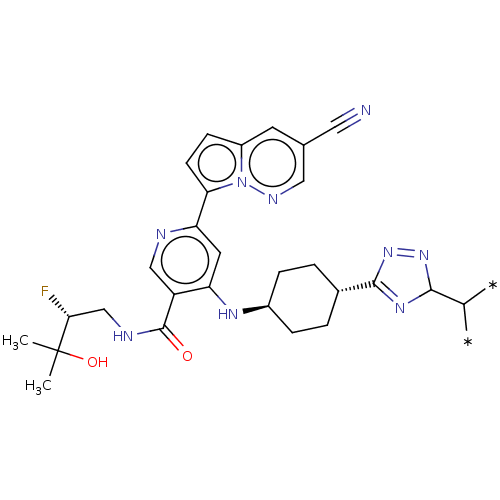
(6-(3-cyanopyrrolo[1,2- b]pyridazin-7-yl)-4-(((1r,...)Show SMILES CC(C)(O)[C@H](F)CNC(=O)c1cnc(cc1N[C@H]1CC[C@@H](CC1)C1=NC(N=N1)C(-*)-*)-c1ccc2cc(cnn12)C#N |r,wU:4.4,17.17,wD:20.24,$;;;;;;;;;;;;;;;;;;;;;;;;;;;;;star_e;star_e;;;;;;;;;;;$,c:28,t:25,(-9.79,-1.54,;-8.46,-.77,;-8.46,.77,;-9.79,,;-7.12,-1.54,;-7.12,-3.08,;-5.79,-.77,;-4.46,-1.54,;-3.12,-.77,;-3.12,.77,;-1.79,-1.54,;-1.79,-3.08,;-.45,-3.85,;.88,-3.08,;.88,-1.54,;-.45,-.77,;-.45,.77,;.88,1.54,;.88,3.08,;2.21,3.85,;3.55,3.08,;3.55,1.54,;2.21,.77,;4.88,3.85,;6.29,3.22,;7.32,4.37,;6.55,5.7,;5.04,5.38,;8.85,4.21,;9.79,5.43,;9.45,2.79,;2.21,-3.85,;2.37,-5.38,;3.88,-5.7,;4.65,-4.37,;6.16,-4.05,;6.63,-2.58,;5.6,-1.44,;4.1,-1.76,;3.62,-3.22,;8.14,-2.26,;9.65,-1.94,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.639 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The enzyme and peptide solution were incubated with compound for 15 minutes at room temp before the reaction was initiated by the addition of ATP. Th... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q25X2DTZ |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM420298

(CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...)Show SMILES COc1cccc2[nH]c(cc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C(=O)CO Show InChI InChI=1S/C24H32N4O6/c1-13(2)9-18(23(32)27-17(20(30)12-29)10-14-7-8-25-22(14)31)28-24(33)19-11-15-16(26-19)5-4-6-21(15)34-3/h4-6,11,13-14,17-18,26,29H,7-10,12H2,1-3H3,(H,25,31)(H,27,32)(H,28,33)/t14-,17-,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PDB
UniChem
| | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM592291
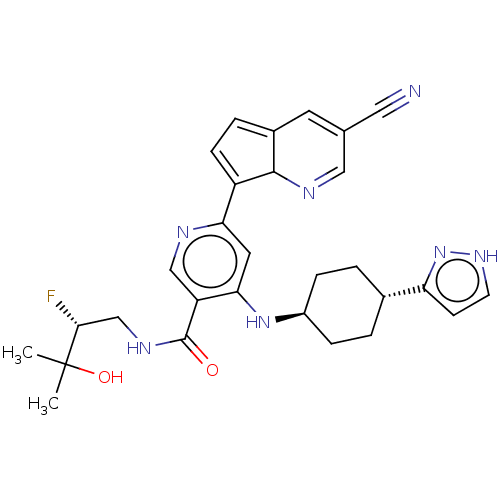
(4-(((1r,4R)-4-(1H-pyrazol-3- yl)cyclohexyl)amino)...)Show SMILES CC(C)(O)[C@H](F)CNC(=O)c1cnc(cc1N[C@H]1CC[C@@H](CC1)c1cc[nH]n1)C1=CC=C2C=C(C=NC12)C#N |r,wU:4.4,17.17,wD:20.24,c:35,37,t:31,33,(-9.72,-1.54,;-8.38,-.77,;-8.38,.77,;-9.72,,;-7.05,-1.54,;-7.05,-3.08,;-5.72,-.77,;-4.38,-1.54,;-3.05,-.77,;-3.05,.77,;-1.72,-1.54,;-1.72,-3.08,;-.38,-3.85,;.95,-3.08,;.95,-1.54,;-.38,-.77,;-.38,.77,;.95,1.54,;.95,3.08,;2.29,3.85,;3.62,3.08,;3.62,1.54,;2.29,.77,;4.95,3.85,;5.11,5.38,;6.62,5.7,;7.39,4.37,;6.36,3.22,;2.29,-3.85,;2.45,-5.38,;3.95,-5.7,;4.72,-4.37,;6.23,-4.05,;6.71,-2.58,;5.67,-1.44,;4.17,-1.76,;3.69,-3.22,;8.21,-2.26,;9.72,-1.94,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.822 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The enzyme and peptide solution were incubated with compound for 15 minutes at room temp before the reaction was initiated by the addition of ATP. Th... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q25X2DTZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data