 Found 215 hits with Last Name = 'wu' and Initial = 'li'
Found 215 hits with Last Name = 'wu' and Initial = 'li' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50184807

((R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-me...)Show SMILES C[C@@H](O)COc1cn2ncnc(Oc3ccc4[nH]c(C)cc4c3F)c2c1C Show InChI InChI=1S/C19H19FN4O3/c1-10-6-13-14(23-10)4-5-15(17(13)20)27-19-18-12(3)16(26-8-11(2)25)7-24(18)22-9-21-19/h4-7,9,11,23,25H,8H2,1-3H3/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against VEGFR2 |
J Med Chem 49: 2143-6 (2006)
Article DOI: 10.1021/jm051106d
BindingDB Entry DOI: 10.7270/Q2WW7H7N |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50184807

((R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-me...)Show SMILES C[C@@H](O)COc1cn2ncnc(Oc3ccc4[nH]c(C)cc4c3F)c2c1C Show InChI InChI=1S/C19H19FN4O3/c1-10-6-13-14(23-10)4-5-15(17(13)20)27-19-18-12(3)16(26-8-11(2)25)7-24(18)22-9-21-19/h4-7,9,11,23,25H,8H2,1-3H3/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against VEGFR2 |
J Med Chem 49: 2143-6 (2006)
Article DOI: 10.1021/jm051106d
BindingDB Entry DOI: 10.7270/Q2WW7H7N |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Mus musculus) | BDBM50184807

((R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-me...)Show SMILES C[C@@H](O)COc1cn2ncnc(Oc3ccc4[nH]c(C)cc4c3F)c2c1C Show InChI InChI=1S/C19H19FN4O3/c1-10-6-13-14(23-10)4-5-15(17(13)20)27-19-18-12(3)16(26-8-11(2)25)7-24(18)22-9-21-19/h4-7,9,11,23,25H,8H2,1-3H3/t11-/m1/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against Flk1 |
J Med Chem 49: 2143-6 (2006)
Article DOI: 10.1021/jm051106d
BindingDB Entry DOI: 10.7270/Q2WW7H7N |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM50184807

((R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-me...)Show SMILES C[C@@H](O)COc1cn2ncnc(Oc3ccc4[nH]c(C)cc4c3F)c2c1C Show InChI InChI=1S/C19H19FN4O3/c1-10-6-13-14(23-10)4-5-15(17(13)20)27-19-18-12(3)16(26-8-11(2)25)7-24(18)22-9-21-19/h4-7,9,11,23,25H,8H2,1-3H3/t11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against VEGFR1 |
J Med Chem 49: 2143-6 (2006)
Article DOI: 10.1021/jm051106d
BindingDB Entry DOI: 10.7270/Q2WW7H7N |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM13320

(1-Methyl-1H-imidazole-4-sulfonic Acid [6-Cyano-1-(...)Show SMILES CC(=C)CN(C1CN(Cc2cncn2C)c2ccc(cc2C1)C#N)S(=O)(=O)c1cn(C)cn1 Show InChI InChI=1S/C23H27N7O2S/c1-17(2)11-30(33(31,32)23-14-27(3)16-26-23)20-8-19-7-18(9-24)5-6-22(19)29(12-20)13-21-10-25-15-28(21)4/h5-7,10,14-16,20H,1,8,11-13H2,2-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM13319
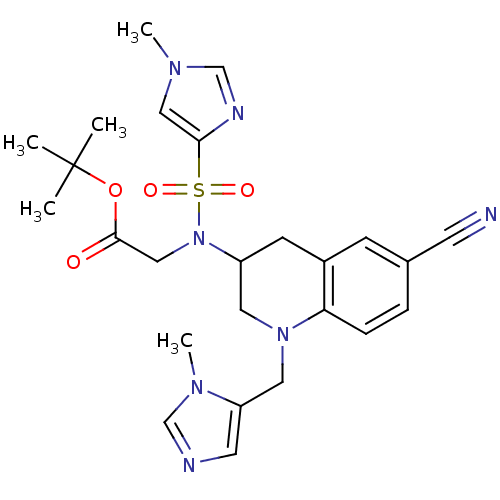
(BMS-386914 | CHEMBL183536 | [[6-Cyano-1-(3-methyl-...)Show SMILES Cn1cnc(c1)S(=O)(=O)N(CC(=O)OC(C)(C)C)C1CN(Cc2cncn2C)c2ccc(cc2C1)C#N Show InChI InChI=1S/C25H31N7O4S/c1-25(2,3)36-24(33)15-32(37(34,35)23-14-29(4)17-28-23)20-9-19-8-18(10-26)6-7-22(19)31(12-20)13-21-11-27-16-30(21)5/h6-8,11,14,16-17,20H,9,12-13,15H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50164021
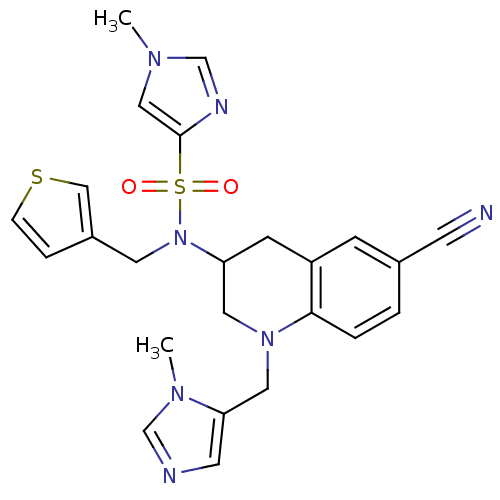
(1-Methyl-1H-imidazole-4-sulfonic acid [6-cyano-1-(...)Show SMILES Cn1cnc(c1)S(=O)(=O)N(Cc1ccsc1)C1CN(Cc2cncn2C)c2ccc(cc2C1)C#N Show InChI InChI=1S/C24H25N7O2S2/c1-28-14-24(27-17-28)35(32,33)31(11-19-5-6-34-15-19)21-8-20-7-18(9-25)3-4-23(20)30(12-21)13-22-10-26-16-29(22)2/h3-7,10,14-17,21H,8,11-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50164027

(1-Methyl-1H-pyrazole-4-sulfonic acid [6-cyano-1-(3...)Show SMILES Cn1cc(cn1)S(=O)(=O)N(Cc1ccsc1)C1CN(Cc2cncn2C)c2ccc(cc2C1)C#N Show InChI InChI=1S/C24H25N7O2S2/c1-28-17-26-10-22(28)14-30-13-21(8-20-7-18(9-25)3-4-24(20)30)31(12-19-5-6-34-16-19)35(32,33)23-11-27-29(2)15-23/h3-7,10-11,15-17,21H,8,12-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50164025
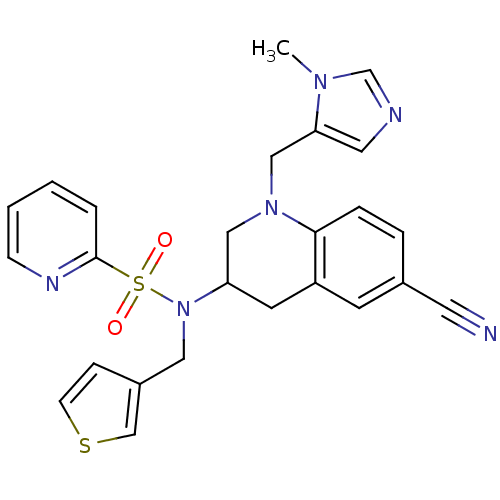
(BMS-316810 | CHEMBL360330 | Pyridine-2-sulfonic ac...)Show SMILES Cn1cncc1CN1CC(Cc2cc(ccc12)C#N)N(Cc1ccsc1)S(=O)(=O)c1ccccn1 Show InChI InChI=1S/C25H24N6O2S2/c1-29-18-27-13-23(29)16-30-15-22(11-21-10-19(12-26)5-6-24(21)30)31(14-20-7-9-34-17-20)35(32,33)25-4-2-3-8-28-25/h2-10,13,17-18,22H,11,14-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50164003
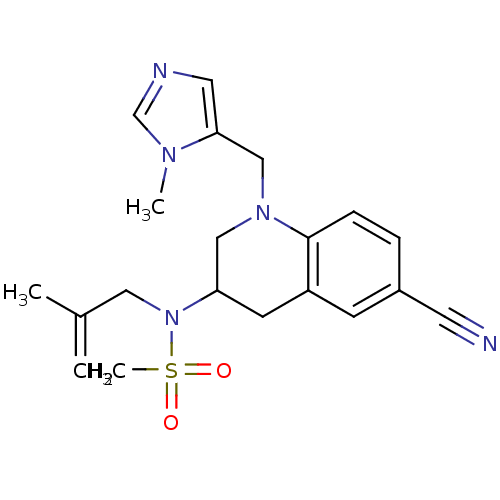
(CHEMBL183773 | N-[6-Cyano-1-(3-methyl-3H-imidazol-...)Show SMILES CC(=C)CN(C1CN(Cc2cncn2C)c2ccc(cc2C1)C#N)S(C)(=O)=O Show InChI InChI=1S/C20H25N5O2S/c1-15(2)11-25(28(4,26)27)18-8-17-7-16(9-21)5-6-20(17)24(12-18)13-19-10-22-14-23(19)3/h5-7,10,14,18H,1,8,11-13H2,2-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50164017
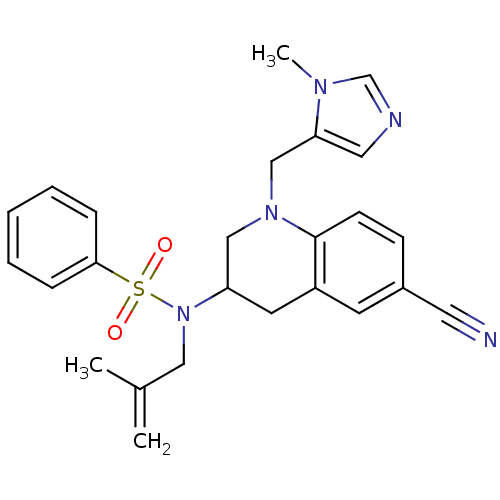
(CHEMBL183544 | N-[6-Cyano-1-(3-methyl-3H-imidazol-...)Show SMILES CC(=C)CN(C1CN(Cc2cncn2C)c2ccc(cc2C1)C#N)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C25H27N5O2S/c1-19(2)15-30(33(31,32)24-7-5-4-6-8-24)22-12-21-11-20(13-26)9-10-25(21)29(16-22)17-23-14-27-18-28(23)3/h4-11,14,18,22H,1,12,15-17H2,2-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50164023
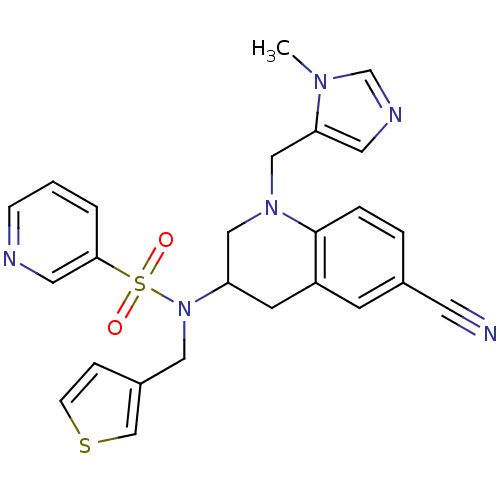
(CHEMBL180307 | Pyridine-3-sulfonic acid [6-cyano-1...)Show SMILES Cn1cncc1CN1CC(Cc2cc(ccc12)C#N)N(Cc1ccsc1)S(=O)(=O)c1cccnc1 Show InChI InChI=1S/C25H24N6O2S2/c1-29-18-28-12-23(29)16-30-15-22(10-21-9-19(11-26)4-5-25(21)30)31(14-20-6-8-34-17-20)35(32,33)24-3-2-7-27-13-24/h2-9,12-13,17-18,22H,10,14-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50164009
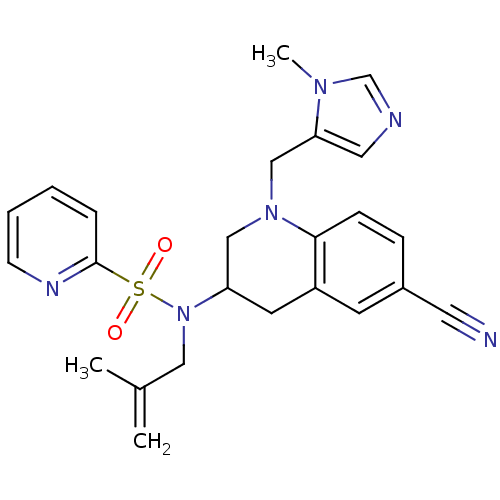
(CHEMBL182813 | Pyridine-2-sulfonic acid [6-cyano-1...)Show SMILES CC(=C)CN(C1CN(Cc2cncn2C)c2ccc(cc2C1)C#N)S(=O)(=O)c1ccccn1 Show InChI InChI=1S/C24H26N6O2S/c1-18(2)14-30(33(31,32)24-6-4-5-9-27-24)21-11-20-10-19(12-25)7-8-23(20)29(15-21)16-22-13-26-17-28(22)3/h4-10,13,17,21H,1,11,14-16H2,2-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50164013
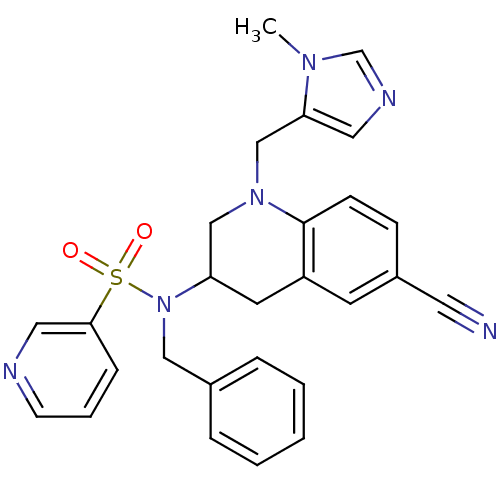
(CHEMBL439846 | Pyridine-3-sulfonic acid benzyl-[6-...)Show SMILES Cn1cncc1CN1CC(Cc2cc(ccc12)C#N)N(Cc1ccccc1)S(=O)(=O)c1cccnc1 Show InChI InChI=1S/C27H26N6O2S/c1-31-20-30-15-25(31)19-32-18-24(13-23-12-22(14-28)9-10-27(23)32)33(17-21-6-3-2-4-7-21)36(34,35)26-8-5-11-29-16-26/h2-12,15-16,20,24H,13,17-19H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50370480
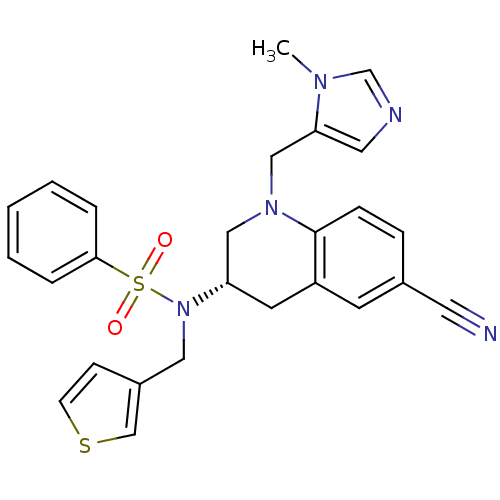
(CHEMBL1169541)Show SMILES Cn1cncc1CN1C[C@H](Cc2cc(ccc12)C#N)N(Cc1ccsc1)S(=O)(=O)c1ccccc1 |r| Show InChI InChI=1S/C26H25N5O2S2/c1-29-19-28-14-24(29)17-30-16-23(12-22-11-20(13-27)7-8-26(22)30)31(15-21-9-10-34-18-21)35(32,33)25-5-3-2-4-6-25/h2-11,14,18-19,23H,12,15-17H2,1H3/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM13323
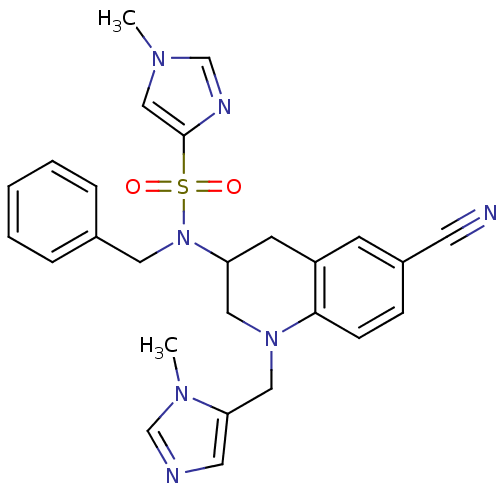
(1-Methyl-1H-imidazole-4-sulfonic Acid Benzyl-[6-cy...)Show SMILES Cn1cnc(c1)S(=O)(=O)N(Cc1ccccc1)C1CN(Cc2cncn2C)c2ccc(cc2C1)C#N Show InChI InChI=1S/C26H27N7O2S/c1-30-17-26(29-19-30)36(34,35)33(14-20-6-4-3-5-7-20)23-11-22-10-21(12-27)8-9-25(22)32(15-23)16-24-13-28-18-31(24)2/h3-10,13,17-19,23H,11,14-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50164012
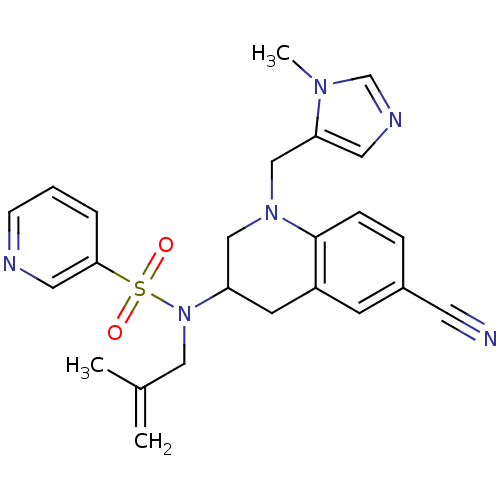
(CHEMBL183217 | Pyridine-3-sulfonic acid [6-cyano-1...)Show SMILES CC(=C)CN(C1CN(Cc2cncn2C)c2ccc(cc2C1)C#N)S(=O)(=O)c1cccnc1 Show InChI InChI=1S/C24H26N6O2S/c1-18(2)14-30(33(31,32)23-5-4-8-26-13-23)21-10-20-9-19(11-25)6-7-24(20)29(15-21)16-22-12-27-17-28(22)3/h4-9,12-13,17,21H,1,10,14-16H2,2-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50164016

(CHEMBL179598 | [[6-Cyano-1-(3-methyl-3H-imidazol-4...)Show SMILES Cn1cncc1CN1CC(Cc2cc(ccc12)C#N)N(CC(=O)OC(C)(C)C)S(=O)(=O)c1ccccn1 Show InChI InChI=1S/C26H30N6O4S/c1-26(2,3)36-25(33)17-32(37(34,35)24-7-5-6-10-29-24)21-12-20-11-19(13-27)8-9-23(20)31(15-21)16-22-14-28-18-30(22)4/h5-11,14,18,21H,12,15-17H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50164026
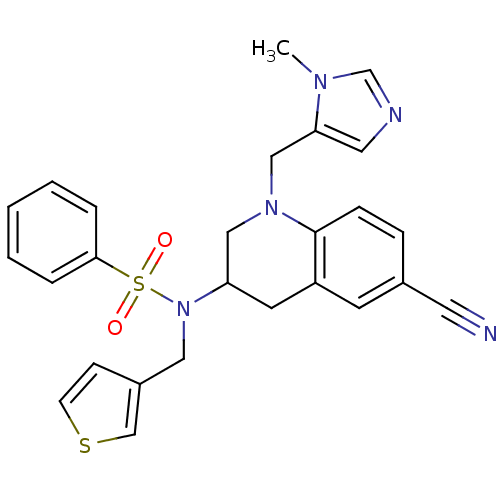
(CHEMBL360917 | N-[6-Cyano-1-(3-methyl-3H-imidazol-...)Show SMILES Cn1cncc1CN1CC(Cc2cc(ccc12)C#N)N(Cc1ccsc1)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C26H25N5O2S2/c1-29-19-28-14-24(29)17-30-16-23(12-22-11-20(13-27)7-8-26(22)30)31(15-21-9-10-34-18-21)35(32,33)25-5-3-2-4-6-25/h2-11,14,18-19,23H,12,15-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50164022
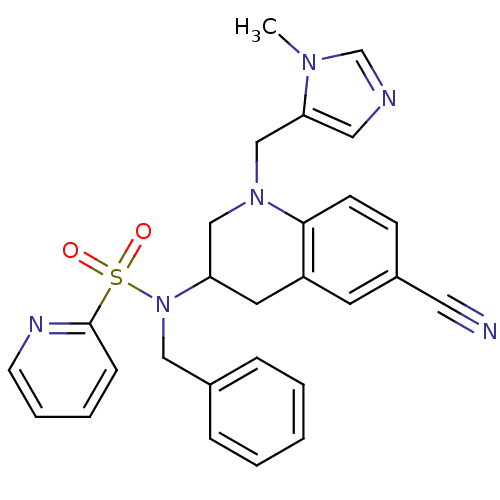
(CHEMBL180411 | Pyridine-2-sulfonic acid benzyl-[6-...)Show SMILES Cn1cncc1CN1CC(Cc2cc(ccc12)C#N)N(Cc1ccccc1)S(=O)(=O)c1ccccn1 Show InChI InChI=1S/C27H26N6O2S/c1-31-20-29-16-25(31)19-32-18-24(14-23-13-22(15-28)10-11-26(23)32)33(17-21-7-3-2-4-8-21)36(34,35)27-9-5-6-12-30-27/h2-13,16,20,24H,14,17-19H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50370479
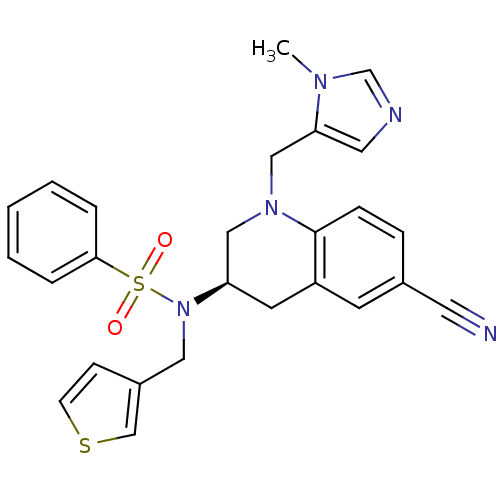
(CHEMBL1201849)Show SMILES Cn1cncc1CN1C[C@@H](Cc2cc(ccc12)C#N)N(Cc1ccsc1)S(=O)(=O)c1ccccc1 |r| Show InChI InChI=1S/C26H25N5O2S2/c1-29-19-28-14-24(29)17-30-16-23(12-22-11-20(13-27)7-8-26(22)30)31(15-21-9-10-34-18-21)35(32,33)25-5-3-2-4-6-25/h2-11,14,18-19,23H,12,15-17H2,1H3/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50164019
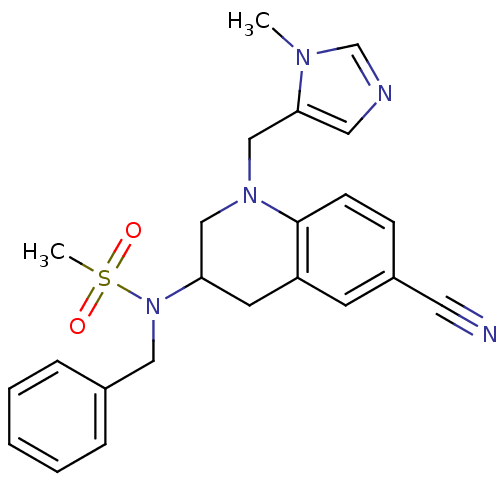
(CHEMBL360557 | N-Benzyl-N-[6-cyano-1-(3-methyl-3H-...)Show SMILES Cn1cncc1CN1CC(Cc2cc(ccc12)C#N)N(Cc1ccccc1)S(C)(=O)=O Show InChI InChI=1S/C23H25N5O2S/c1-26-17-25-13-22(26)16-27-15-21(11-20-10-19(12-24)8-9-23(20)27)28(31(2,29)30)14-18-6-4-3-5-7-18/h3-10,13,17,21H,11,14-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50164020
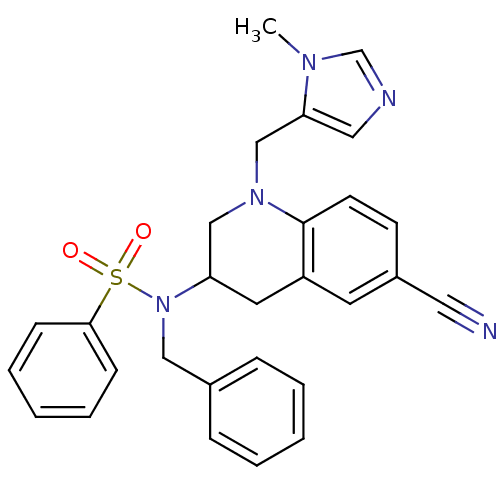
(CHEMBL180710 | N-Benzyl-N-[6-cyano-1-(3-methyl-3H-...)Show SMILES Cn1cncc1CN1CC(Cc2cc(ccc12)C#N)N(Cc1ccccc1)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C28H27N5O2S/c1-31-21-30-17-26(31)20-32-19-25(15-24-14-23(16-29)12-13-28(24)32)33(18-22-8-4-2-5-9-22)36(34,35)27-10-6-3-7-11-27/h2-14,17,21,25H,15,18-20H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50164011
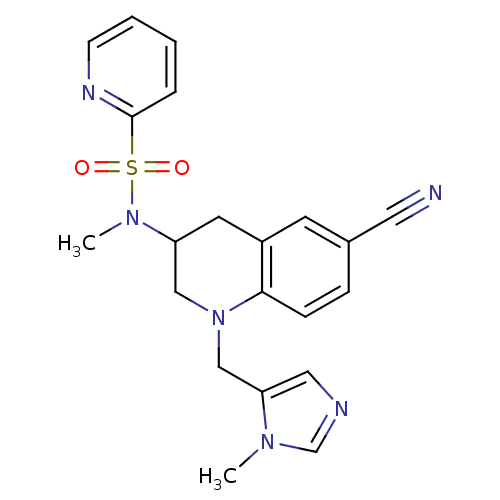
(CHEMBL183481 | Pyridine-2-sulfonic acid [6-cyano-1...)Show SMILES CN(C1CN(Cc2cncn2C)c2ccc(cc2C1)C#N)S(=O)(=O)c1ccccn1 Show InChI InChI=1S/C21H22N6O2S/c1-25-15-23-12-19(25)14-27-13-18(10-17-9-16(11-22)6-7-20(17)27)26(2)30(28,29)21-5-3-4-8-24-21/h3-9,12,15,18H,10,13-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50408442
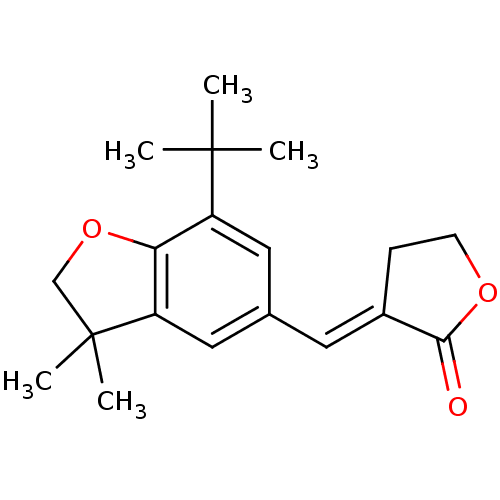
(CHEMBL2112567)Show InChI InChI=1S/C19H24O3/c1-18(2,3)14-9-12(8-13-6-7-21-17(13)20)10-15-16(14)22-11-19(15,4)5/h8-10H,6-7,11H2,1-5H3/b13-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 41: 3515-29 (1998)
Article DOI: 10.1021/jm9802416
BindingDB Entry DOI: 10.7270/Q2V98763 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50164024
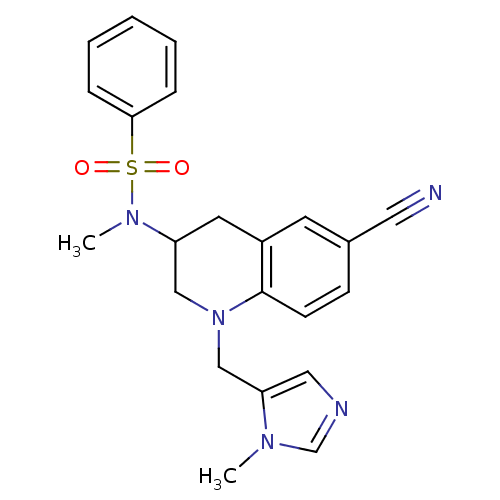
(CHEMBL183804 | N-[6-Cyano-1-(3-methyl-3H-imidazol-...)Show SMILES CN(C1CN(Cc2cncn2C)c2ccc(cc2C1)C#N)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C22H23N5O2S/c1-25-16-24-13-20(25)15-27-14-19(11-18-10-17(12-23)8-9-22(18)27)26(2)30(28,29)21-6-4-3-5-7-21/h3-10,13,16,19H,11,14-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50164004
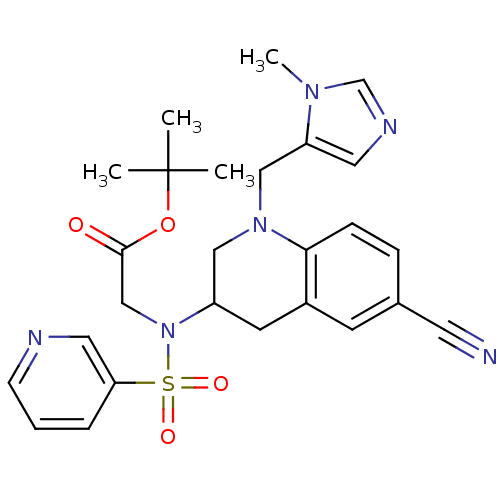
(CHEMBL183590 | [[6-Cyano-1-(3-methyl-3H-imidazol-4...)Show SMILES Cn1cncc1CN1CC(Cc2cc(ccc12)C#N)N(CC(=O)OC(C)(C)C)S(=O)(=O)c1cccnc1 Show InChI InChI=1S/C26H30N6O4S/c1-26(2,3)36-25(33)17-32(37(34,35)23-6-5-9-28-14-23)21-11-20-10-19(12-27)7-8-24(20)31(15-21)16-22-13-29-18-30(22)4/h5-10,13-14,18,21H,11,15-17H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50164010
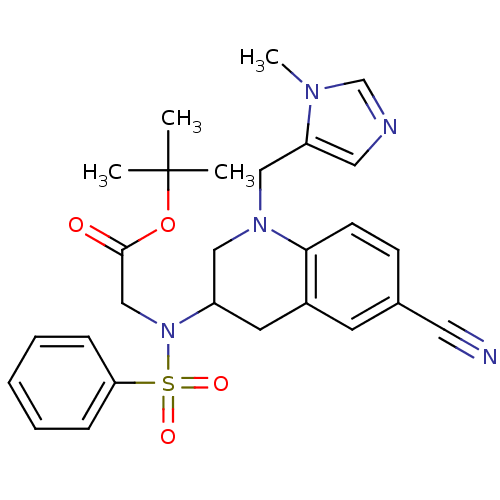
(CHEMBL182351 | {Benzenesulfonyl-[6-cyano-1-(3-meth...)Show SMILES Cn1cncc1CN1CC(Cc2cc(ccc12)C#N)N(CC(=O)OC(C)(C)C)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C27H31N5O4S/c1-27(2,3)36-26(33)18-32(37(34,35)24-8-6-5-7-9-24)22-13-21-12-20(14-28)10-11-25(21)31(16-22)17-23-15-29-19-30(23)4/h5-12,15,19,22H,13,16-18H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50164018
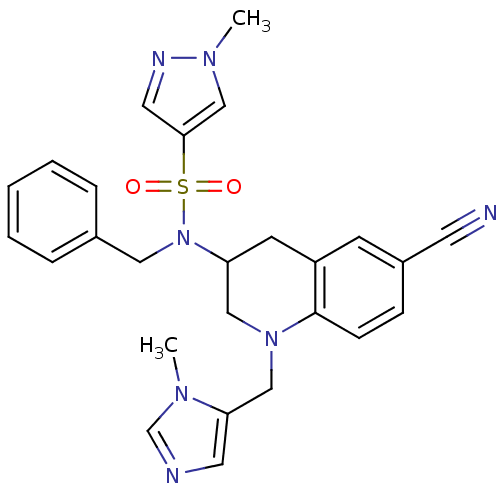
(1-Methyl-1H-pyrazole-4-sulfonic acid benzyl-[6-cya...)Show SMILES Cn1cc(cn1)S(=O)(=O)N(Cc1ccccc1)C1CN(Cc2cncn2C)c2ccc(cc2C1)C#N Show InChI InChI=1S/C26H27N7O2S/c1-30-19-28-13-24(30)17-32-16-23(11-22-10-21(12-27)8-9-26(22)32)33(15-20-6-4-3-5-7-20)36(34,35)25-14-29-31(2)18-25/h3-10,13-14,18-19,23H,11,15-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50063777
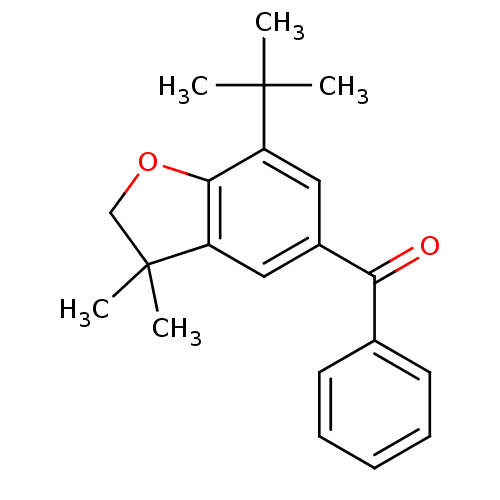
((7-tert-Butyl-3,3-dimethyl-2,3-dihydro-benzofuran-...)Show InChI InChI=1S/C21H24O2/c1-20(2,3)16-11-15(18(22)14-9-7-6-8-10-14)12-17-19(16)23-13-21(17,4)5/h6-12H,13H2,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Concentration (in microM) to inhibit 50% of Prostaglandin G/H synthase 2 (COX-2) and is expressed in IC50. |
J Med Chem 41: 1112-23 (1998)
Article DOI: 10.1021/jm970679q
BindingDB Entry DOI: 10.7270/Q2K936N4 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50164014
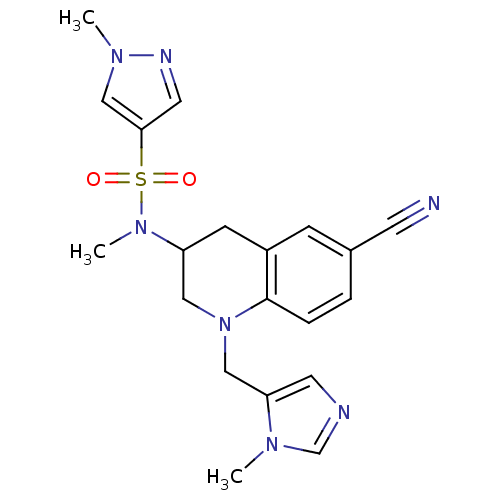
(1-Methyl-1H-pyrazole-4-sulfonic acid [6-cyano-1-(3...)Show SMILES CN(C1CN(Cc2cncn2C)c2ccc(cc2C1)C#N)S(=O)(=O)c1cnn(C)c1 Show InChI InChI=1S/C20H23N7O2S/c1-24-14-22-9-18(24)12-27-11-17(7-16-6-15(8-21)4-5-20(16)27)26(3)30(28,29)19-10-23-25(2)13-19/h4-6,9-10,13-14,17H,7,11-12H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50164003

(CHEMBL183773 | N-[6-Cyano-1-(3-methyl-3H-imidazol-...)Show SMILES CC(=C)CN(C1CN(Cc2cncn2C)c2ccc(cc2C1)C#N)S(C)(=O)=O Show InChI InChI=1S/C20H25N5O2S/c1-15(2)11-25(28(4,26)27)18-8-17-7-16(9-21)5-6-20(17)24(12-18)13-19-10-22-14-23(19)3/h5-7,10,14,18H,1,8,11-13H2,2-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of cellular reversion in H-ras transformed Rat-1 cells |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50164008
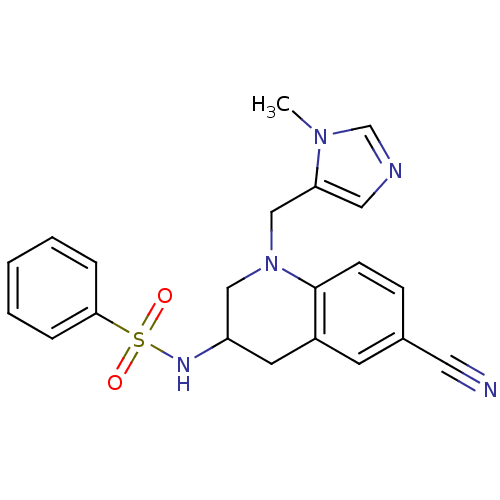
(CHEMBL362816 | N-[6-Cyano-1-(3-methyl-3H-imidazol-...)Show SMILES Cn1cncc1CN1CC(Cc2cc(ccc12)C#N)NS(=O)(=O)c1ccccc1 Show InChI InChI=1S/C21H21N5O2S/c1-25-15-23-12-19(25)14-26-13-18(10-17-9-16(11-22)7-8-21(17)26)24-29(27,28)20-5-3-2-4-6-20/h2-9,12,15,18,24H,10,13-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50164016

(CHEMBL179598 | [[6-Cyano-1-(3-methyl-3H-imidazol-4...)Show SMILES Cn1cncc1CN1CC(Cc2cc(ccc12)C#N)N(CC(=O)OC(C)(C)C)S(=O)(=O)c1ccccn1 Show InChI InChI=1S/C26H30N6O4S/c1-26(2,3)36-25(33)17-32(37(34,35)24-7-5-6-10-29-24)21-12-20-11-19(13-27)8-9-23(20)31(15-21)16-22-14-28-18-30(22)4/h5-11,14,18,21H,12,15-17H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of cellular reversion in H-ras transformed Rat-1 cells |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50164025

(BMS-316810 | CHEMBL360330 | Pyridine-2-sulfonic ac...)Show SMILES Cn1cncc1CN1CC(Cc2cc(ccc12)C#N)N(Cc1ccsc1)S(=O)(=O)c1ccccn1 Show InChI InChI=1S/C25H24N6O2S2/c1-29-18-27-13-23(29)16-30-15-22(11-21-10-19(12-26)5-6-24(21)30)31(14-20-7-9-34-17-20)35(32,33)25-4-2-3-8-28-25/h2-10,13,17-18,22H,11,14-16H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of cellular reversion in H-ras transformed Rat-1 cells |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50066572
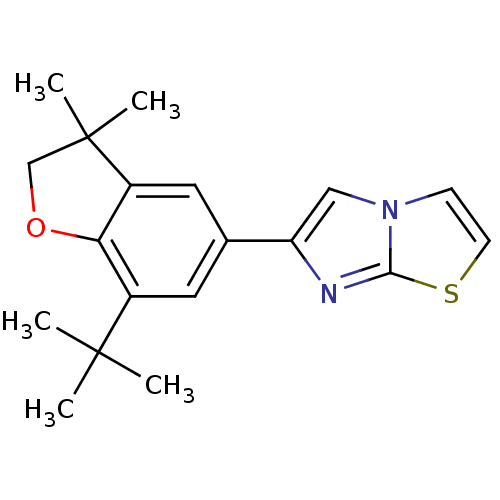
(6-(7-tert-Butyl-3,3-dimethyl-2,3-dihydro-benzofura...)Show InChI InChI=1S/C19H22N2OS/c1-18(2,3)13-8-12(9-14-16(13)22-11-19(14,4)5)15-10-21-6-7-23-17(21)20-15/h6-10H,11H2,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 41: 3515-29 (1998)
Article DOI: 10.1021/jm9802416
BindingDB Entry DOI: 10.7270/Q2V98763 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50063780
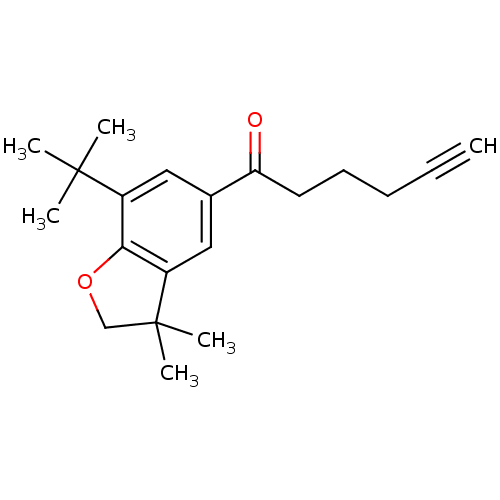
(1-(7-tert-Butyl-3,3-dimethyl-2,3-dihydro-benzofura...)Show InChI InChI=1S/C20H26O2/c1-7-8-9-10-17(21)14-11-15(19(2,3)4)18-16(12-14)20(5,6)13-22-18/h1,11-12H,8-10,13H2,2-6H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 41: 1124-37 (1998)
Article DOI: 10.1021/jm970680p
BindingDB Entry DOI: 10.7270/Q2FJ2FXD |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50063780

(1-(7-tert-Butyl-3,3-dimethyl-2,3-dihydro-benzofura...)Show InChI InChI=1S/C20H26O2/c1-7-8-9-10-17(21)14-11-15(19(2,3)4)18-16(12-14)20(5,6)13-22-18/h1,11-12H,8-10,13H2,2-6H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Concentration (in microM) to inhibit 50% of Prostaglandin G/H synthase 2 (COX-2) and is expressed in IC50. |
J Med Chem 41: 1112-23 (1998)
Article DOI: 10.1021/jm970679q
BindingDB Entry DOI: 10.7270/Q2K936N4 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50184818
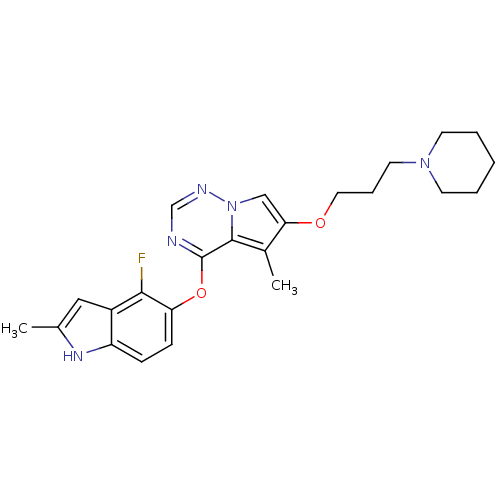
(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-methyl-6-...)Show SMILES Cc1cc2c(F)c(Oc3ncnn4cc(OCCCN5CCCCC5)c(C)c34)ccc2[nH]1 Show InChI InChI=1S/C24H28FN5O2/c1-16-13-18-19(28-16)7-8-20(22(18)25)32-24-23-17(2)21(14-30(23)27-15-26-24)31-12-6-11-29-9-4-3-5-10-29/h7-8,13-15,28H,3-6,9-12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against VEGFR2 |
J Med Chem 49: 2143-6 (2006)
Article DOI: 10.1021/jm051106d
BindingDB Entry DOI: 10.7270/Q2WW7H7N |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50184822
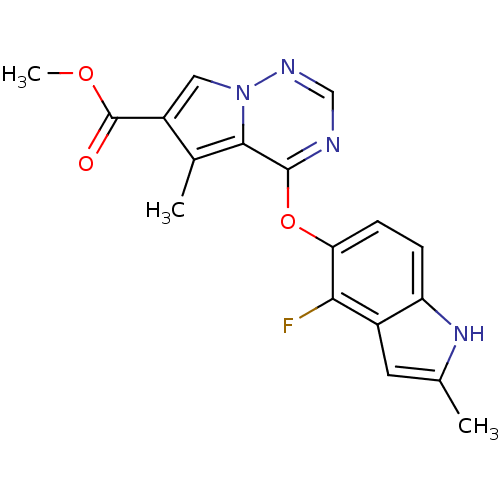
(CHEMBL383671 | methyl 4-(4-fluoro-2-methyl-1H-indo...)Show SMILES COC(=O)c1cn2ncnc(Oc3ccc4[nH]c(C)cc4c3F)c2c1C Show InChI InChI=1S/C18H15FN4O3/c1-9-6-11-13(22-9)4-5-14(15(11)19)26-17-16-10(2)12(18(24)25-3)7-23(16)21-8-20-17/h4-8,22H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against VEGFR2 |
J Med Chem 49: 2143-6 (2006)
Article DOI: 10.1021/jm051106d
BindingDB Entry DOI: 10.7270/Q2WW7H7N |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50164015
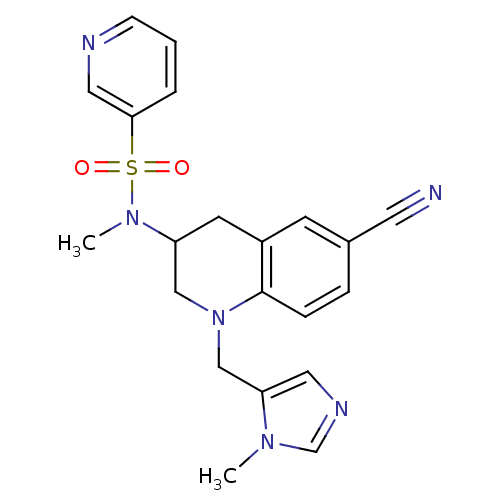
(CHEMBL183091 | Pyridine-3-sulfonic acid [6-cyano-1...)Show SMILES CN(C1CN(Cc2cncn2C)c2ccc(cc2C1)C#N)S(=O)(=O)c1cccnc1 Show InChI InChI=1S/C21H22N6O2S/c1-25-15-24-11-19(25)14-27-13-18(9-17-8-16(10-22)5-6-21(17)27)26(2)30(28,29)20-4-3-7-23-12-20/h3-8,11-12,15,18H,9,13-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50184812

(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-methyl-6-...)Show SMILES CN1CCC(CCOc2cn3ncnc(Oc4ccc5[nH]c(C)cc5c4F)c3c2C)CC1 Show InChI InChI=1S/C24H28FN5O2/c1-15-12-18-19(28-15)4-5-20(22(18)25)32-24-23-16(2)21(13-30(23)27-14-26-24)31-11-8-17-6-9-29(3)10-7-17/h4-5,12-14,17,28H,6-11H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against VEGFR2 |
J Med Chem 49: 2143-6 (2006)
Article DOI: 10.1021/jm051106d
BindingDB Entry DOI: 10.7270/Q2WW7H7N |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50184817
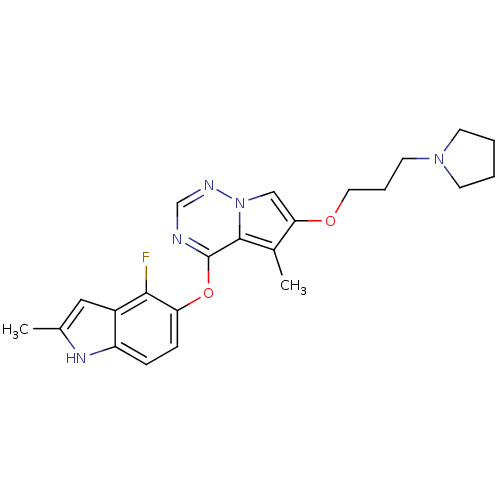
(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-methyl-6-...)Show SMILES Cc1cc2c(F)c(Oc3ncnn4cc(OCCCN5CCCC5)c(C)c34)ccc2[nH]1 Show InChI InChI=1S/C23H26FN5O2/c1-15-12-17-18(27-15)6-7-19(21(17)24)31-23-22-16(2)20(13-29(22)26-14-25-23)30-11-5-10-28-8-3-4-9-28/h6-7,12-14,27H,3-5,8-11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against VEGFR2 |
J Med Chem 49: 2143-6 (2006)
Article DOI: 10.1021/jm051106d
BindingDB Entry DOI: 10.7270/Q2WW7H7N |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50066561
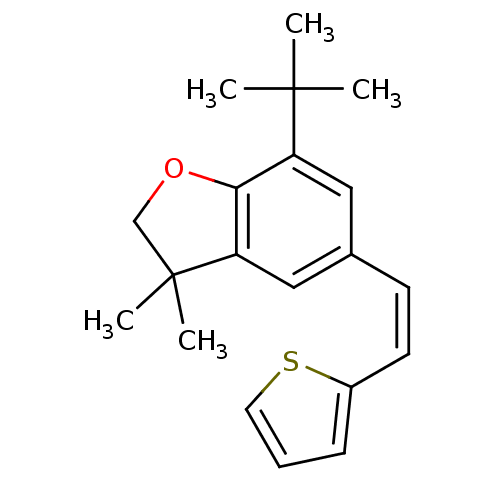
(7-tert-Butyl-3,3-dimethyl-5-((Z)-2-thiophen-2-yl-v...)Show InChI InChI=1S/C20H24OS/c1-19(2,3)16-11-14(8-9-15-7-6-10-22-15)12-17-18(16)21-13-20(17,4)5/h6-12H,13H2,1-5H3/b9-8- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 (COX-1) |
J Med Chem 41: 3515-29 (1998)
Article DOI: 10.1021/jm9802416
BindingDB Entry DOI: 10.7270/Q2V98763 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50184811
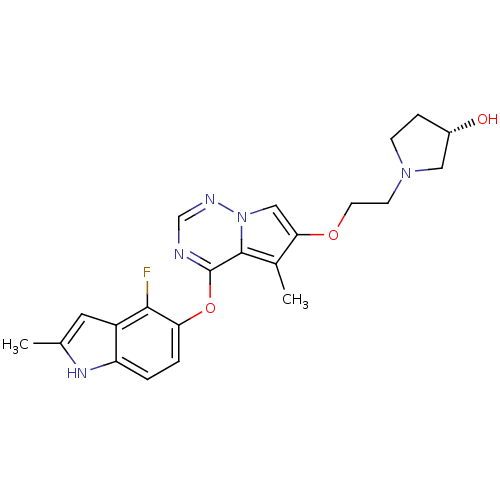
((R)-1-(2-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5...)Show SMILES Cc1cc2c(F)c(Oc3ncnn4cc(OCCN5CC[C@H](O)C5)c(C)c34)ccc2[nH]1 Show InChI InChI=1S/C22H24FN5O3/c1-13-9-16-17(26-13)3-4-18(20(16)23)31-22-21-14(2)19(11-28(21)25-12-24-22)30-8-7-27-6-5-15(29)10-27/h3-4,9,11-12,15,26,29H,5-8,10H2,1-2H3/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against VEGFR2 |
J Med Chem 49: 2143-6 (2006)
Article DOI: 10.1021/jm051106d
BindingDB Entry DOI: 10.7270/Q2WW7H7N |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50184816
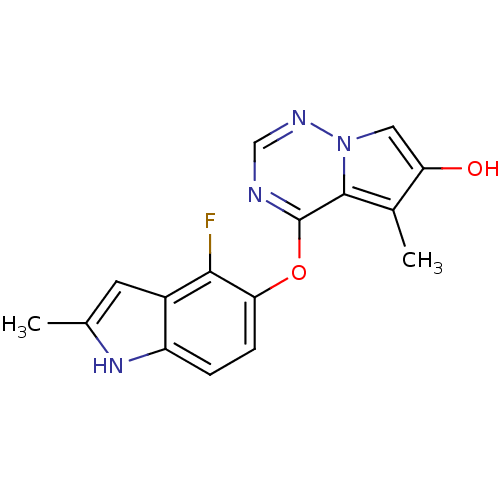
(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-methylpyr...)Show InChI InChI=1S/C16H13FN4O2/c1-8-5-10-11(20-8)3-4-13(14(10)17)23-16-15-9(2)12(22)6-21(15)19-7-18-16/h3-7,20,22H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against VEGFR2 |
J Med Chem 49: 2143-6 (2006)
Article DOI: 10.1021/jm051106d
BindingDB Entry DOI: 10.7270/Q2WW7H7N |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50066564
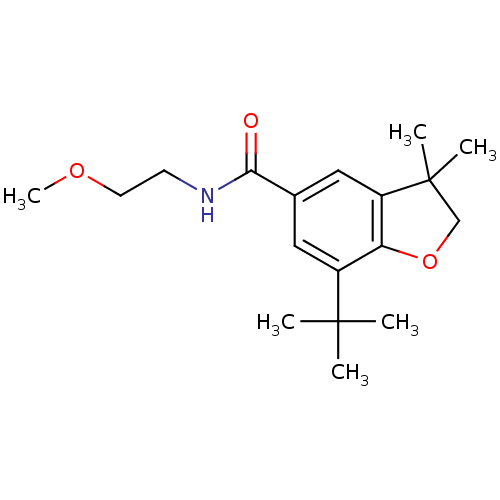
(7-tert-Butyl-3,3-dimethyl-2,3-dihydro-benzofuran-5...)Show InChI InChI=1S/C18H27NO3/c1-17(2,3)13-9-12(16(20)19-7-8-21-6)10-14-15(13)22-11-18(14,4)5/h9-10H,7-8,11H2,1-6H3,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 41: 3515-29 (1998)
Article DOI: 10.1021/jm9802416
BindingDB Entry DOI: 10.7270/Q2V98763 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Rattus norvegicus-Rattus norvegicus (rat)) | BDBM13319

(BMS-386914 | CHEMBL183536 | [[6-Cyano-1-(3-methyl-...)Show SMILES Cn1cnc(c1)S(=O)(=O)N(CC(=O)OC(C)(C)C)C1CN(Cc2cncn2C)c2ccc(cc2C1)C#N Show InChI InChI=1S/C25H31N7O4S/c1-25(2,3)36-24(33)15-32(37(34,35)23-14-29(4)17-28-23)20-9-19-8-18(10-26)6-7-22(19)31(12-20)13-21-11-27-16-30(21)5/h6-8,11,14,16-17,20H,9,12-13,15H2,1-5H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of cellular reversion in H-ras transformed Rat-1 cells |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50066561

(7-tert-Butyl-3,3-dimethyl-5-((Z)-2-thiophen-2-yl-v...)Show InChI InChI=1S/C20H24OS/c1-19(2,3)16-11-14(8-9-15-7-6-10-22-15)12-17-18(16)21-13-20(17,4)5/h6-12H,13H2,1-5H3/b9-8- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 41: 3515-29 (1998)
Article DOI: 10.1021/jm9802416
BindingDB Entry DOI: 10.7270/Q2V98763 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50066569
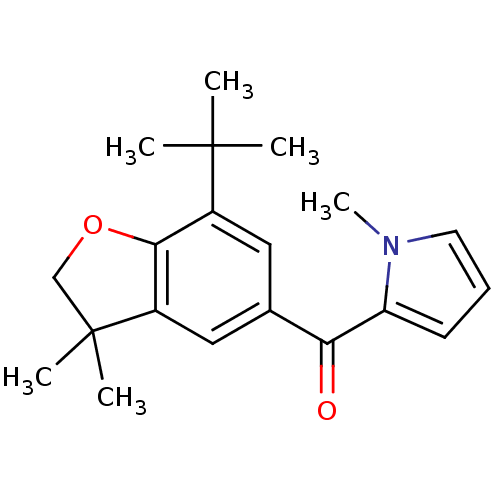
((7-tert-Butyl-3,3-dimethyl-2,3-dihydro-benzofuran-...)Show InChI InChI=1S/C20H25NO2/c1-19(2,3)14-10-13(17(22)16-8-7-9-21(16)6)11-15-18(14)23-12-20(15,4)5/h7-11H,12H2,1-6H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 (COX-1) |
J Med Chem 41: 3515-29 (1998)
Article DOI: 10.1021/jm9802416
BindingDB Entry DOI: 10.7270/Q2V98763 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data