

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Broad substrate specificity ATP-binding cassette transporter ABCG2 (Homo sapiens (Human)) | BDBM106221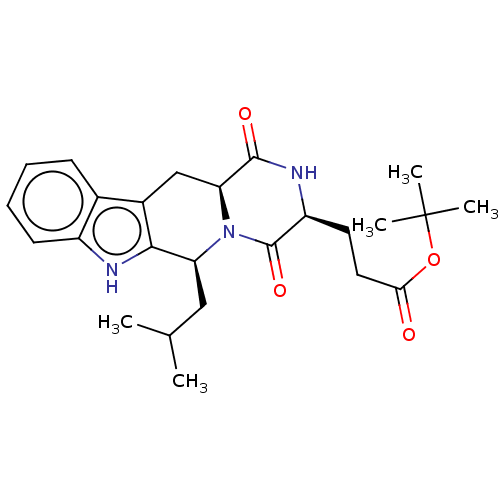 (US9695174, Ko134) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. Curated by ChEMBL | Assay Description Inhibition of BCRP in human Caco2 cells using 3H-Estron-3-sulfate as substrate assessed as Ko143 IC50 measured at 30 to 120 mins by microplate scinti... | Bioorg Med Chem Lett 26: 551-555 (2016) Article DOI: 10.1016/j.bmcl.2015.11.077 BindingDB Entry DOI: 10.7270/Q2HX1GPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Broad substrate specificity ATP-binding cassette transporter ABCG2 (Homo sapiens (Human)) | BDBM50500541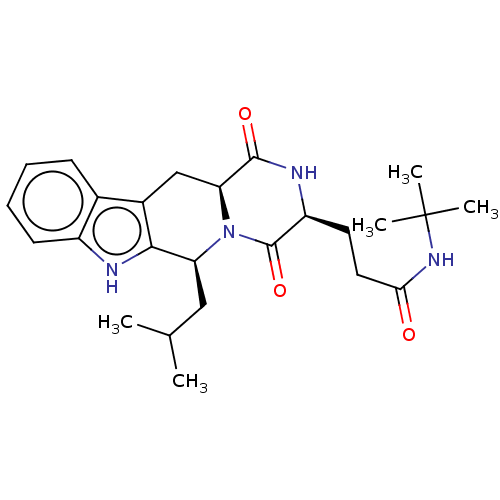 (CHEMBL3747380) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. Curated by ChEMBL | Assay Description Inhibition of BCRP in human Caco2 cells using 3H-Estron-3-sulfate as substrate assessed as Ko143 IC50 measured at 30 to 120 mins by microplate scinti... | Bioorg Med Chem Lett 26: 551-555 (2016) Article DOI: 10.1016/j.bmcl.2015.11.077 BindingDB Entry DOI: 10.7270/Q2HX1GPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Broad substrate specificity ATP-binding cassette transporter ABCG2 (Homo sapiens (Human)) | BDBM50500540 (CHEMBL3747767) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. Curated by ChEMBL | Assay Description Inhibition of BCRP in human Caco2 cells using 3H-Estron-3-sulfate as substrate assessed as Ko143 IC50 measured at 30 to 120 mins by microplate scinti... | Bioorg Med Chem Lett 26: 551-555 (2016) Article DOI: 10.1016/j.bmcl.2015.11.077 BindingDB Entry DOI: 10.7270/Q2HX1GPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Broad substrate specificity ATP-binding cassette transporter ABCG2 (Homo sapiens (Human)) | BDBM106203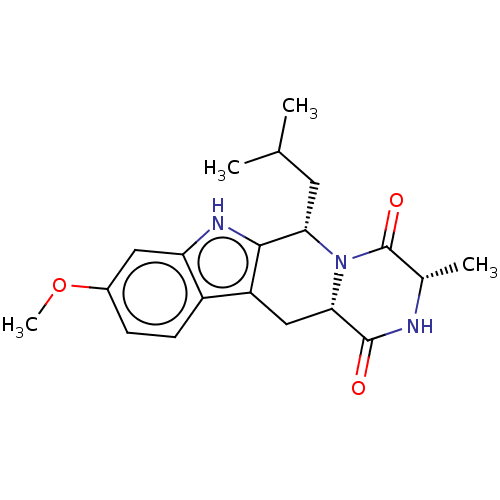 (US9695174, I-1) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 600 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description To determine potency of compounds on inhibition of transporters, bi-directional transport studies are performed in Caco-2 cells (American Type Cultur... | US Patent US9695174 (2017) BindingDB Entry DOI: 10.7270/Q2DN436B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Broad substrate specificity ATP-binding cassette transporter ABCG2 (Homo sapiens (Human)) | BDBM106203 (US9695174, I-1) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. Curated by ChEMBL | Assay Description Inhibition of BCRP in human Caco2 cells using 3H-Estron-3-sulfate as substrate measured at 30 to 120 mins by microplate scintillation and luminescenc... | Bioorg Med Chem Lett 26: 551-555 (2016) Article DOI: 10.1016/j.bmcl.2015.11.077 BindingDB Entry DOI: 10.7270/Q2HX1GPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Broad substrate specificity ATP-binding cassette transporter ABCG2 (Homo sapiens (Human)) | BDBM106203 (US9695174, I-1) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. Curated by ChEMBL | Assay Description Inhibition of BCRP in human Caco2 cells using 3H-Estron-3-sulfate as substrate assessed as Ko143 IC50 measured at 30 to 120 mins by microplate scinti... | Bioorg Med Chem Lett 26: 551-555 (2016) Article DOI: 10.1016/j.bmcl.2015.11.077 BindingDB Entry DOI: 10.7270/Q2HX1GPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Broad substrate specificity ATP-binding cassette transporter ABCG2 (Homo sapiens (Human)) | BDBM106209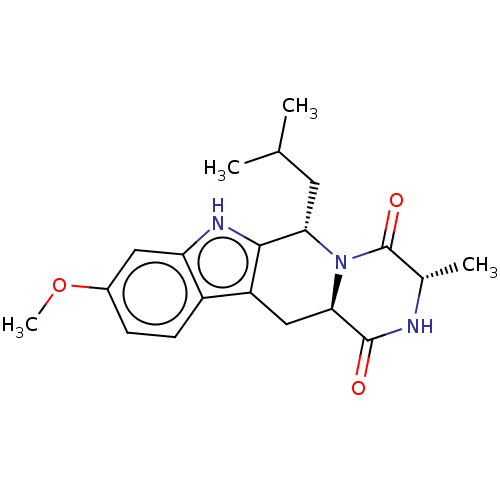 (US9695174, 4) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. Curated by ChEMBL | Assay Description Inhibition of BCRP in human Caco2 cells using 3H-Estron-3-sulfate as substrate assessed as Ko143 IC50 measured at 30 to 120 mins by microplate scinti... | Bioorg Med Chem Lett 26: 551-555 (2016) Article DOI: 10.1016/j.bmcl.2015.11.077 BindingDB Entry DOI: 10.7270/Q2HX1GPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Broad substrate specificity ATP-binding cassette transporter ABCG2 (Homo sapiens (Human)) | BDBM50500539 (CHEMBL3746825) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. Curated by ChEMBL | Assay Description Inhibition of BCRP in human Caco2 cells using 3H-Estron-3-sulfate as substrate assessed as Ko143 IC50 measured at 30 to 120 mins by microplate scinti... | Bioorg Med Chem Lett 26: 551-555 (2016) Article DOI: 10.1016/j.bmcl.2015.11.077 BindingDB Entry DOI: 10.7270/Q2HX1GPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Broad substrate specificity ATP-binding cassette transporter ABCG2 (Homo sapiens (Human)) | BDBM106157 (US9695174, 8) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. Curated by ChEMBL | Assay Description Inhibition of BCRP in human Caco2 cells using 3H-Estron-3-sulfate as substrate assessed as Ko143 IC50 measured at 30 to 120 mins by microplate scinti... | Bioorg Med Chem Lett 26: 551-555 (2016) Article DOI: 10.1016/j.bmcl.2015.11.077 BindingDB Entry DOI: 10.7270/Q2HX1GPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Broad substrate specificity ATP-binding cassette transporter ABCG2 (Homo sapiens (Human)) | BDBM106207 (US9695174, 12) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. Curated by ChEMBL | Assay Description Inhibition of BCRP in human Caco2 cells using 3H-Estron-3-sulfate as substrate assessed as Ko143 IC50 measured at 30 to 120 mins by microplate scinti... | Bioorg Med Chem Lett 26: 551-555 (2016) Article DOI: 10.1016/j.bmcl.2015.11.077 BindingDB Entry DOI: 10.7270/Q2HX1GPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Broad substrate specificity ATP-binding cassette transporter ABCG2 (Homo sapiens (Human)) | BDBM106216 (US9695174, 12A) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. Curated by ChEMBL | Assay Description Inhibition of BCRP in human Caco2 cells using 3H-Estron-3-sulfate as substrate assessed as Ko143 IC50 measured at 30 to 120 mins by microplate scinti... | Bioorg Med Chem Lett 26: 551-555 (2016) Article DOI: 10.1016/j.bmcl.2015.11.077 BindingDB Entry DOI: 10.7270/Q2HX1GPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Broad substrate specificity ATP-binding cassette transporter ABCG2 (Homo sapiens (Human)) | BDBM106207 (US9695174, 12) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.98E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description To determine potency of compounds on inhibition of transporters, bi-directional transport studies are performed in Caco-2 cells (American Type Cultur... | US Patent US9695174 (2017) BindingDB Entry DOI: 10.7270/Q2DN436B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Broad substrate specificity ATP-binding cassette transporter ABCG2 (Homo sapiens (Human)) | BDBM106207 (US9695174, 12) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. Curated by ChEMBL | Assay Description Inhibition of BCRP in human Caco2 cells using 3H-Estron-3-sulfate as substrate measured at 30 to 120 mins by microplate scintillation and luminescenc... | Bioorg Med Chem Lett 26: 551-555 (2016) Article DOI: 10.1016/j.bmcl.2015.11.077 BindingDB Entry DOI: 10.7270/Q2HX1GPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Broad substrate specificity ATP-binding cassette transporter ABCG2 (Homo sapiens (Human)) | BDBM106221 (US9695174, Ko134) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.25E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description To determine potency of compounds on inhibition of transporters, bi-directional transport studies are performed in Caco-2 cells (American Type Cultur... | US Patent US9695174 (2017) BindingDB Entry DOI: 10.7270/Q2DN436B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Broad substrate specificity ATP-binding cassette transporter ABCG2 (Homo sapiens (Human)) | BDBM106221 (US9695174, Ko134) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. Curated by ChEMBL | Assay Description Inhibition of BCRP in human Caco2 cells using 3H-Estron-3-sulfate as substrate measured at 30 to 120 mins by microplate scintillation and luminescenc... | Bioorg Med Chem Lett 26: 551-555 (2016) Article DOI: 10.1016/j.bmcl.2015.11.077 BindingDB Entry DOI: 10.7270/Q2HX1GPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Broad substrate specificity ATP-binding cassette transporter ABCG2 (Homo sapiens (Human)) | BDBM50500541 (CHEMBL3747380) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. Curated by ChEMBL | Assay Description Inhibition of BCRP in human Caco2 cells using 3H-Estron-3-sulfate as substrate measured at 30 to 120 mins by microplate scintillation and luminescenc... | Bioorg Med Chem Lett 26: 551-555 (2016) Article DOI: 10.1016/j.bmcl.2015.11.077 BindingDB Entry DOI: 10.7270/Q2HX1GPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50305083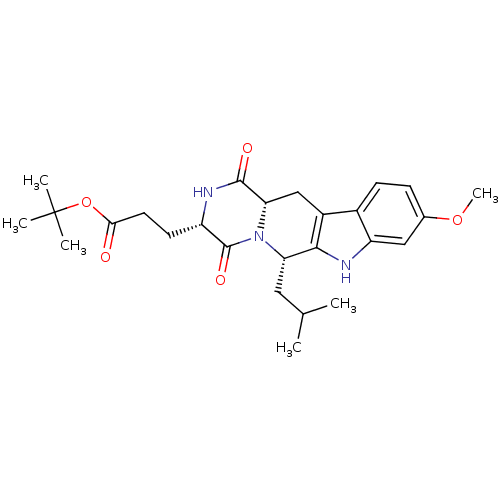 (3-((3S,6S)-6-Isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description Human liver microsomes (HLMs) (0.3 mg/mL in 0.1-M potassium phosphate buffer, pH 7.4) are incubated with CYP (cytochromes P450) isozyme-selective sub... | US Patent US9695174 (2017) BindingDB Entry DOI: 10.7270/Q2DN436B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Broad substrate specificity ATP-binding cassette transporter ABCG2 (Homo sapiens (Human)) | BDBM106157 (US9695174, 8) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. Curated by ChEMBL | Assay Description Inhibition of BCRP in human Caco2 cells using 3H-Estron-3-sulfate as substrate measured at 30 to 120 mins by microplate scintillation and luminescenc... | Bioorg Med Chem Lett 26: 551-555 (2016) Article DOI: 10.1016/j.bmcl.2015.11.077 BindingDB Entry DOI: 10.7270/Q2HX1GPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Broad substrate specificity ATP-binding cassette transporter ABCG2 (Homo sapiens (Human)) | BDBM106209 (US9695174, 4) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. Curated by ChEMBL | Assay Description Inhibition of BCRP in human Caco2 cells using 3H-Estron-3-sulfate as substrate measured at 30 to 120 mins by microplate scintillation and luminescenc... | Bioorg Med Chem Lett 26: 551-555 (2016) Article DOI: 10.1016/j.bmcl.2015.11.077 BindingDB Entry DOI: 10.7270/Q2HX1GPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Broad substrate specificity ATP-binding cassette transporter ABCG2 (Homo sapiens (Human)) | BDBM50500540 (CHEMBL3747767) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. Curated by ChEMBL | Assay Description Inhibition of BCRP in human Caco2 cells using 3H-Estron-3-sulfate as substrate measured at 30 to 120 mins by microplate scintillation and luminescenc... | Bioorg Med Chem Lett 26: 551-555 (2016) Article DOI: 10.1016/j.bmcl.2015.11.077 BindingDB Entry DOI: 10.7270/Q2HX1GPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Broad substrate specificity ATP-binding cassette transporter ABCG2 (Homo sapiens (Human)) | BDBM106216 (US9695174, 12A) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description To determine potency of compounds on inhibition of transporters, bi-directional transport studies are performed in Caco-2 cells (American Type Cultur... | US Patent US9695174 (2017) BindingDB Entry DOI: 10.7270/Q2DN436B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Broad substrate specificity ATP-binding cassette transporter ABCG2 (Homo sapiens (Human)) | BDBM106158 (US9695174, 8A) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description To determine potency of compounds on inhibition of transporters, bi-directional transport studies are performed in Caco-2 cells (American Type Cultur... | US Patent US9695174 (2017) BindingDB Entry DOI: 10.7270/Q2DN436B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Broad substrate specificity ATP-binding cassette transporter ABCG2 (Homo sapiens (Human)) | BDBM106157 (US9695174, 8) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description To determine potency of compounds on inhibition of transporters, bi-directional transport studies are performed in Caco-2 cells (American Type Cultur... | US Patent US9695174 (2017) BindingDB Entry DOI: 10.7270/Q2DN436B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Broad substrate specificity ATP-binding cassette transporter ABCG2 (Homo sapiens (Human)) | BDBM106209 (US9695174, 4) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description To determine potency of compounds on inhibition of transporters, bi-directional transport studies are performed in Caco-2 cells (American Type Cultur... | US Patent US9695174 (2017) BindingDB Entry DOI: 10.7270/Q2DN436B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Broad substrate specificity ATP-binding cassette transporter ABCG2 (Homo sapiens (Human)) | BDBM106216 (US9695174, 12A) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. Curated by ChEMBL | Assay Description Inhibition of BCRP in human Caco2 cells using 3H-Estron-3-sulfate as substrate measured at 30 to 120 mins by microplate scintillation and luminescenc... | Bioorg Med Chem Lett 26: 551-555 (2016) Article DOI: 10.1016/j.bmcl.2015.11.077 BindingDB Entry DOI: 10.7270/Q2HX1GPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Broad substrate specificity ATP-binding cassette transporter ABCG2 (Homo sapiens (Human)) | BDBM50500539 (CHEMBL3746825) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. Curated by ChEMBL | Assay Description Inhibition of BCRP in human Caco2 cells using 3H-Estron-3-sulfate as substrate measured at 30 to 120 mins by microplate scintillation and luminescenc... | Bioorg Med Chem Lett 26: 551-555 (2016) Article DOI: 10.1016/j.bmcl.2015.11.077 BindingDB Entry DOI: 10.7270/Q2HX1GPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50305083 (3-((3S,6S)-6-Isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.47E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description Human liver microsomes (HLMs) (0.3 mg/mL in 0.1-M potassium phosphate buffer, pH 7.4) are incubated with CYP (cytochromes P450) isozyme-selective sub... | US Patent US9695174 (2017) BindingDB Entry DOI: 10.7270/Q2DN436B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM106203 (US9695174, I-1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.54E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description Human liver microsomes (HLMs) (0.3 mg/mL in 0.1-M potassium phosphate buffer, pH 7.4) are incubated with CYP (cytochromes P450) isozyme-selective sub... | US Patent US9695174 (2017) BindingDB Entry DOI: 10.7270/Q2DN436B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50305083 (3-((3S,6S)-6-Isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description Human liver microsomes (HLMs) (0.3 mg/mL in 0.1-M potassium phosphate buffer, pH 7.4) are incubated with CYP (cytochromes P450) isozyme-selective sub... | US Patent US9695174 (2017) BindingDB Entry DOI: 10.7270/Q2DN436B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM106203 (US9695174, I-1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.85E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description Human liver microsomes (HLMs) (0.3 mg/mL in 0.1-M potassium phosphate buffer, pH 7.4) are incubated with CYP (cytochromes P450) isozyme-selective sub... | US Patent US9695174 (2017) BindingDB Entry DOI: 10.7270/Q2DN436B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50305083 (3-((3S,6S)-6-Isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description To determine potency of compounds on inhibition of transporters, bi-directional transport studies are performed in Caco-2 cells (American Type Cultur... | US Patent US9695174 (2017) BindingDB Entry DOI: 10.7270/Q2DN436B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM106203 (US9695174, I-1) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description Human liver microsomes (HLMs) (0.3 mg/mL in 0.1-M potassium phosphate buffer, pH 7.4) are incubated with CYP (cytochromes P450) isozyme-selective sub... | US Patent US9695174 (2017) BindingDB Entry DOI: 10.7270/Q2DN436B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4/3A5 (Homo sapiens (Human)) | BDBM50305083 (3-((3S,6S)-6-Isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description Human liver microsomes (HLMs) (0.3 mg/mL in 0.1-M potassium phosphate buffer, pH 7.4) are incubated with CYP (cytochromes P450) isozyme-selective sub... | US Patent US9695174 (2017) BindingDB Entry DOI: 10.7270/Q2DN436B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4/3A5 (Homo sapiens (Human)) | BDBM106203 (US9695174, I-1) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description Human liver microsomes (HLMs) (0.3 mg/mL in 0.1-M potassium phosphate buffer, pH 7.4) are incubated with CYP (cytochromes P450) isozyme-selective sub... | US Patent US9695174 (2017) BindingDB Entry DOI: 10.7270/Q2DN436B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50305083 (3-((3S,6S)-6-Isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description Human liver microsomes (HLMs) (0.3 mg/mL in 0.1-M potassium phosphate buffer, pH 7.4) are incubated with CYP (cytochromes P450) isozyme-selective sub... | US Patent US9695174 (2017) BindingDB Entry DOI: 10.7270/Q2DN436B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM106203 (US9695174, I-1) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description To determine potency of compounds on inhibition of transporters, bi-directional transport studies are performed in Caco-2 cells (American Type Cultur... | US Patent US9695174 (2017) BindingDB Entry DOI: 10.7270/Q2DN436B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM106203 (US9695174, I-1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description Human liver microsomes (HLMs) (0.3 mg/mL in 0.1-M potassium phosphate buffer, pH 7.4) are incubated with CYP (cytochromes P450) isozyme-selective sub... | US Patent US9695174 (2017) BindingDB Entry DOI: 10.7270/Q2DN436B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50305083 (3-((3S,6S)-6-Isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description Human liver microsomes (HLMs) (0.3 mg/mL in 0.1-M potassium phosphate buffer, pH 7.4) are incubated with CYP (cytochromes P450) isozyme-selective sub... | US Patent US9695174 (2017) BindingDB Entry DOI: 10.7270/Q2DN436B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM106203 (US9695174, I-1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description Human liver microsomes (HLMs) (0.3 mg/mL in 0.1-M potassium phosphate buffer, pH 7.4) are incubated with CYP (cytochromes P450) isozyme-selective sub... | US Patent US9695174 (2017) BindingDB Entry DOI: 10.7270/Q2DN436B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||