 Found 747 hits with Last Name = 'xiang' and Initial = 'm'
Found 747 hits with Last Name = 'xiang' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM14361

((R,S)-Rolipram | 4-(3-cyclopentyloxy-4-methoxy-phe...)Show InChI InChI=1S/C16H21NO3/c1-19-14-7-6-11(12-9-16(18)17-10-12)8-15(14)20-13-4-2-3-5-13/h6-8,12-13H,2-5,9-10H2,1H3,(H,17,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.00230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD
Curated by ChEMBL
| Assay Description
Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate |
Bioorg Med Chem Lett 13: 2355-8 (2003)
BindingDB Entry DOI: 10.7270/Q2CZ39CG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50220995

(CHEMBL77788)Show InChI InChI=1S/C15H20N2O3/c1-11(18)17-16-10-12-7-8-14(19-2)15(9-12)20-13-5-3-4-6-13/h7-10,13H,3-6H2,1-2H3,(H,17,18)/b16-10+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD
Curated by ChEMBL
| Assay Description
Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate |
Bioorg Med Chem Lett 13: 2355-8 (2003)
BindingDB Entry DOI: 10.7270/Q2CZ39CG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50221005
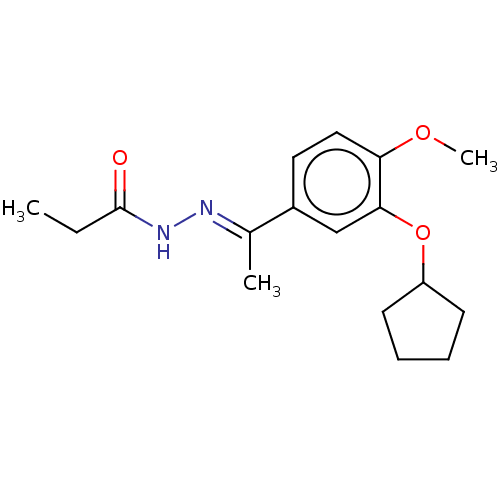
(CHEMBL75684)Show InChI InChI=1S/C17H24N2O3/c1-4-17(20)19-18-12(2)13-9-10-15(21-3)16(11-13)22-14-7-5-6-8-14/h9-11,14H,4-8H2,1-3H3,(H,19,20)/b18-12+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD
Curated by ChEMBL
| Assay Description
Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate |
Bioorg Med Chem Lett 13: 2355-8 (2003)
BindingDB Entry DOI: 10.7270/Q2CZ39CG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50220998
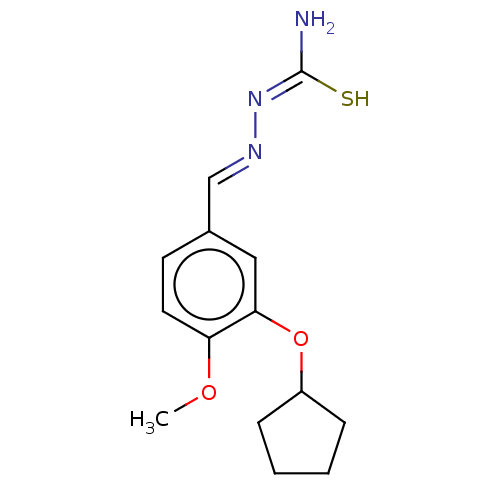
(CHEMBL76382)Show InChI InChI=1S/C14H19N3O2S/c1-18-12-7-6-10(9-16-17-14(15)20)8-13(12)19-11-4-2-3-5-11/h6-9,11H,2-5H2,1H3,(H3,15,17,20)/b16-9+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD
Curated by ChEMBL
| Assay Description
Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate |
Bioorg Med Chem Lett 13: 2355-8 (2003)
BindingDB Entry DOI: 10.7270/Q2CZ39CG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50221003
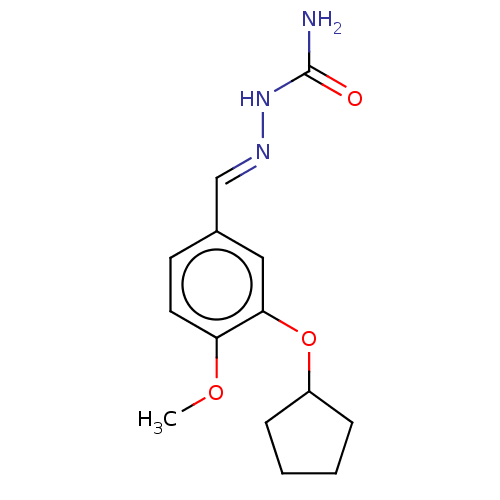
(CHEMBL432348)Show InChI InChI=1S/C14H19N3O3/c1-19-12-7-6-10(9-16-17-14(15)18)8-13(12)20-11-4-2-3-5-11/h6-9,11H,2-5H2,1H3,(H3,15,17,18)/b16-9+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD
Curated by ChEMBL
| Assay Description
Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate |
Bioorg Med Chem Lett 13: 2355-8 (2003)
BindingDB Entry DOI: 10.7270/Q2CZ39CG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50220997

(CHEMBL78237)Show InChI InChI=1S/C16H22N2O3/c1-11(17-18-12(2)19)13-8-9-15(20-3)16(10-13)21-14-6-4-5-7-14/h8-10,14H,4-7H2,1-3H3,(H,18,19)/b17-11+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD
Curated by ChEMBL
| Assay Description
Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate |
Bioorg Med Chem Lett 13: 2355-8 (2003)
BindingDB Entry DOI: 10.7270/Q2CZ39CG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50221006
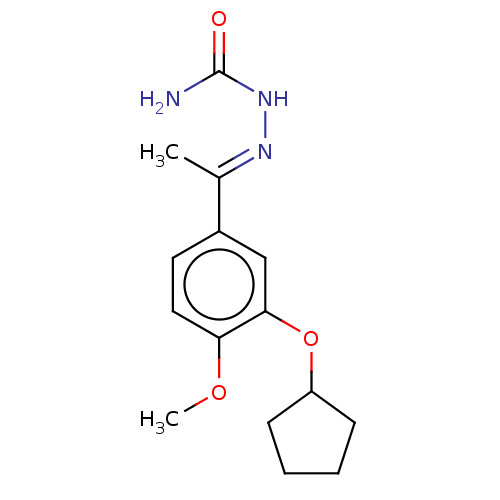
(CHEMBL77358)Show InChI InChI=1S/C15H21N3O3/c1-10(17-18-15(16)19)11-7-8-13(20-2)14(9-11)21-12-5-3-4-6-12/h7-9,12H,3-6H2,1-2H3,(H3,16,18,19)/b17-10+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.146 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD
Curated by ChEMBL
| Assay Description
Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate |
Bioorg Med Chem Lett 13: 2355-8 (2003)
BindingDB Entry DOI: 10.7270/Q2CZ39CG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50220999

(CHEMBL77745)Show InChI InChI=1S/C15H21N3O2S/c1-10(17-18-15(16)21)11-7-8-13(19-2)14(9-11)20-12-5-3-4-6-12/h7-9,12H,3-6H2,1-2H3,(H3,16,18,21)/b17-10+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.336 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD
Curated by ChEMBL
| Assay Description
Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate |
Bioorg Med Chem Lett 13: 2355-8 (2003)
BindingDB Entry DOI: 10.7270/Q2CZ39CG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50220996

(CHEMBL76635)Show InChI InChI=1S/C20H23N3O3/c1-25-17-12-11-15(13-18(17)26-16-9-5-6-10-16)19(22-23-20(21)24)14-7-3-2-4-8-14/h2-4,7-8,11-13,16H,5-6,9-10H2,1H3,(H3,21,23,24)/b22-19+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD
Curated by ChEMBL
| Assay Description
Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate |
Bioorg Med Chem Lett 13: 2355-8 (2003)
BindingDB Entry DOI: 10.7270/Q2CZ39CG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50221004
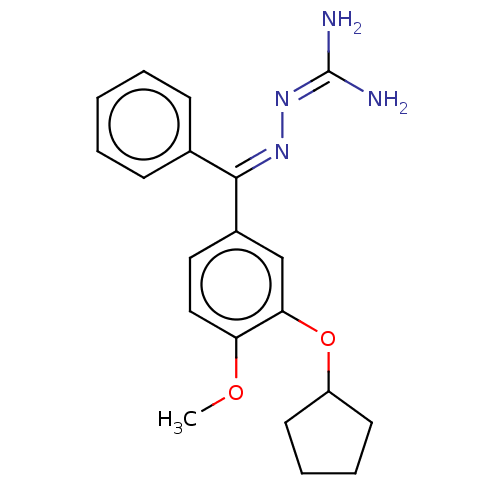
(CHEMBL77999)Show SMILES [#6]-[#8]-c1ccc(cc1-[#8]-[#6]-1-[#6]-[#6]-[#6]-[#6]-1)-[#6](=[#7]\[#7]=[#6](/[#7])-[#7])\c1ccccc1 Show InChI InChI=1S/C20H24N4O2/c1-25-17-12-11-15(13-18(17)26-16-9-5-6-10-16)19(23-24-20(21)22)14-7-3-2-4-8-14/h2-4,7-8,11-13,16H,5-6,9-10H2,1H3,(H4,21,22,24)/b23-19+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD
Curated by ChEMBL
| Assay Description
Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate |
Bioorg Med Chem Lett 13: 2355-8 (2003)
BindingDB Entry DOI: 10.7270/Q2CZ39CG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50221001
(CHEMBL76257)Show InChI InChI=1S/C21H24N2O3/c1-15(24)22-23-21(16-8-4-3-5-9-16)17-12-13-19(25-2)20(14-17)26-18-10-6-7-11-18/h3-5,8-9,12-14,18H,6-7,10-11H2,1-2H3,(H,22,24)/b23-21+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD
Curated by ChEMBL
| Assay Description
Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate |
Bioorg Med Chem Lett 13: 2355-8 (2003)
BindingDB Entry DOI: 10.7270/Q2CZ39CG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50220993

(CHEMBL78238)Show SMILES COc1ccc(cc1OC1CCCC1)C(=N\N=C(\N)S)\c1ccccc1 Show InChI InChI=1S/C20H23N3O2S/c1-24-17-12-11-15(13-18(17)25-16-9-5-6-10-16)19(22-23-20(21)26)14-7-3-2-4-8-14/h2-4,7-8,11-13,16H,5-6,9-10H2,1H3,(H3,21,23,26)/b22-19+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD
Curated by ChEMBL
| Assay Description
Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate |
Bioorg Med Chem Lett 13: 2355-8 (2003)
BindingDB Entry DOI: 10.7270/Q2CZ39CG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50221007
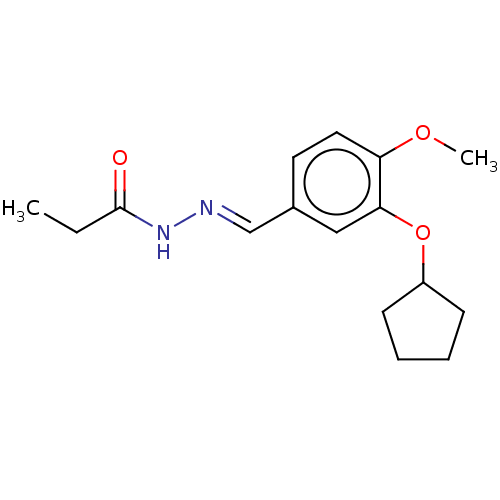
(CHEMBL80258)Show InChI InChI=1S/C16H22N2O3/c1-3-16(19)18-17-11-12-8-9-14(20-2)15(10-12)21-13-6-4-5-7-13/h8-11,13H,3-7H2,1-2H3,(H,18,19)/b17-11+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD
Curated by ChEMBL
| Assay Description
Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate |
Bioorg Med Chem Lett 13: 2355-8 (2003)
BindingDB Entry DOI: 10.7270/Q2CZ39CG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50221002

(CHEMBL306320)Show SMILES [#6]-[#8]-c1ccc(cc1-[#8]-[#6]-1-[#6]-[#6]-[#6]-[#6]-1)-[#6](\[#6])=[#7]\[#7]=[#6](/[#7])-[#7] Show InChI InChI=1S/C15H22N4O2/c1-10(18-19-15(16)17)11-7-8-13(20-2)14(9-11)21-12-5-3-4-6-12/h7-9,12H,3-6H2,1-2H3,(H4,16,17,19)/b18-10+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD
Curated by ChEMBL
| Assay Description
Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate |
Bioorg Med Chem Lett 13: 2355-8 (2003)
BindingDB Entry DOI: 10.7270/Q2CZ39CG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50221000
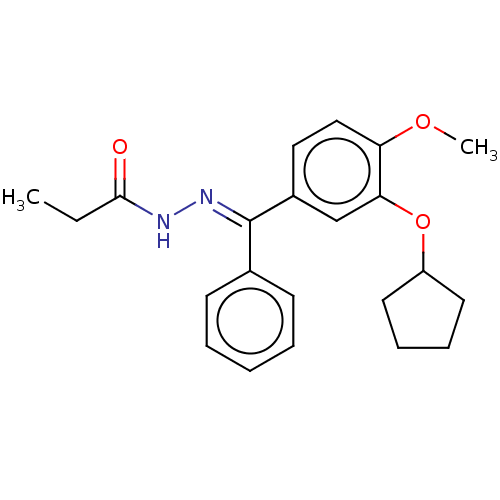
(CHEMBL431962)Show SMILES CCC(=O)N\N=C(/c1ccccc1)c1ccc(OC)c(OC2CCCC2)c1 Show InChI InChI=1S/C22H26N2O3/c1-3-21(25)23-24-22(16-9-5-4-6-10-16)17-13-14-19(26-2)20(15-17)27-18-11-7-8-12-18/h4-6,9-10,13-15,18H,3,7-8,11-12H2,1-2H3,(H,23,25)/b24-22+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD
Curated by ChEMBL
| Assay Description
Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate |
Bioorg Med Chem Lett 13: 2355-8 (2003)
BindingDB Entry DOI: 10.7270/Q2CZ39CG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50220994
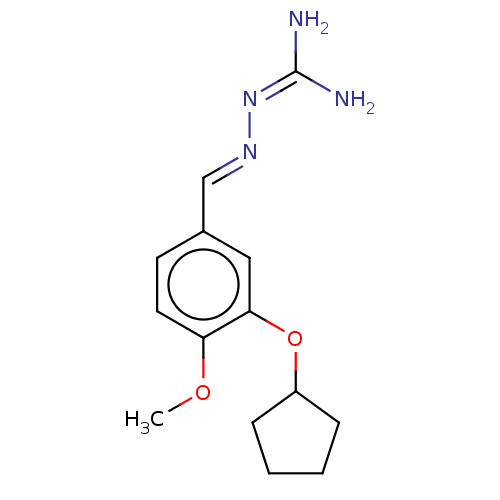
(CHEMBL77177)Show SMILES [#6]-[#8]-c1ccc(\[#6]=[#7]\[#7]=[#6](/[#7])-[#7])cc1-[#8]-[#6]-1-[#6]-[#6]-[#6]-[#6]-1 Show InChI InChI=1S/C14H20N4O2/c1-19-12-7-6-10(9-17-18-14(15)16)8-13(12)20-11-4-2-3-5-11/h6-9,11H,2-5H2,1H3,(H4,15,16,18)/b17-9+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD
Curated by ChEMBL
| Assay Description
Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate |
Bioorg Med Chem Lett 13: 2355-8 (2003)
BindingDB Entry DOI: 10.7270/Q2CZ39CG |
More data for this
Ligand-Target Pair | |
Fatty acid-binding protein, adipocyte
(Rattus norvegicus) | BDBM50212873
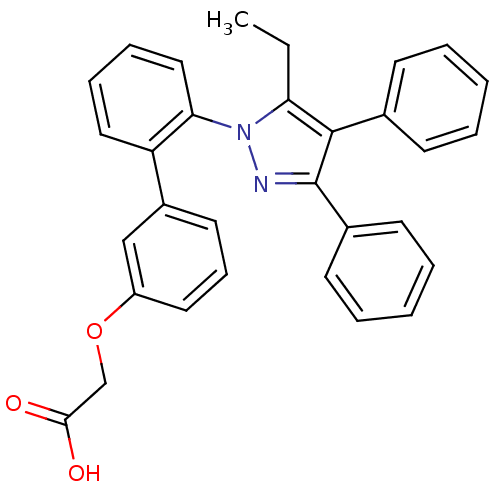
(((2'-(5-ETHYL-3,4-DIPHENYL-1H-PYRAZOL-1-YL)-3-BIPH...)Show SMILES CCc1c(c(nn1-c1ccccc1-c1cccc(OCC(O)=O)c1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C31H26N2O3/c1-2-27-30(22-12-5-3-6-13-22)31(23-14-7-4-8-15-23)32-33(27)28-19-10-9-18-26(28)24-16-11-17-25(20-24)36-21-29(34)35/h3-20H,2,21H2,1H3,(H,34,35) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of rat ap2 by fluorescent 1,8-anilino-8-naphthalene sulfonate assay |
Eur J Med Chem 52: 70-81 (2012)
Article DOI: 10.1016/j.ejmech.2012.03.006
BindingDB Entry DOI: 10.7270/Q26H4JD5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Fatty acid-binding protein, adipocyte
(Rattus norvegicus) | BDBM50381857
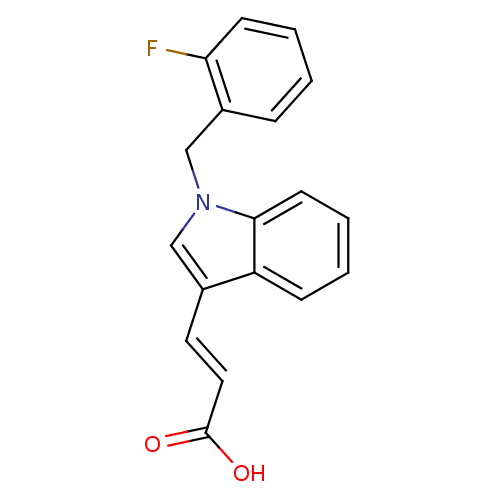
(CHEMBL2023268)Show InChI InChI=1S/C18H14FNO2/c19-16-7-3-1-5-14(16)12-20-11-13(9-10-18(21)22)15-6-2-4-8-17(15)20/h1-11H,12H2,(H,21,22)/b10-9+ | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of rat ap2 by fluorescent 1,8-anilino-8-naphthalene sulfonate assay |
Eur J Med Chem 52: 70-81 (2012)
Article DOI: 10.1016/j.ejmech.2012.03.006
BindingDB Entry DOI: 10.7270/Q26H4JD5 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50146577
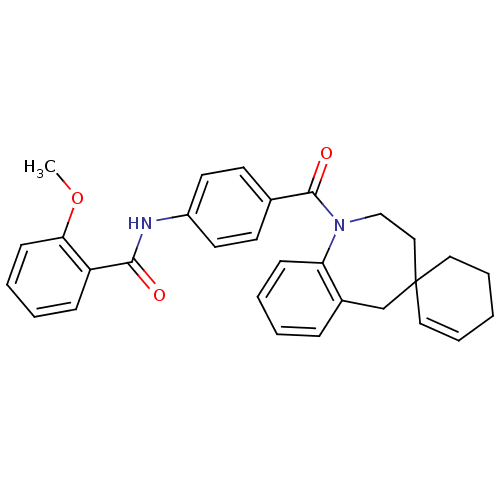
(CHEMBL102311 | spirobenzoxazines analogues)Show SMILES COc1ccccc1C(=O)Nc1ccc(cc1)C(=O)N1CCC2(CCCC=C2)Cc2ccccc12 |c:28| Show InChI InChI=1S/C30H30N2O3/c1-35-27-12-6-4-10-25(27)28(33)31-24-15-13-22(14-16-24)29(34)32-20-19-30(17-7-2-8-18-30)21-23-9-3-5-11-26(23)32/h3-7,9-17H,2,8,18-21H2,1H3,(H,31,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against [3H]-AVP binding to cloned human vasopressin receptor |
Bioorg Med Chem Lett 14: 2987-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.103
BindingDB Entry DOI: 10.7270/Q2BK1BSD |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM14361

((R,S)-Rolipram | 4-(3-cyclopentyloxy-4-methoxy-phe...)Show InChI InChI=1S/C16H21NO3/c1-19-14-7-6-11(12-9-16(18)17-10-12)8-15(14)20-13-4-2-3-5-13/h6-8,12-13H,2-5,9-10H2,1H3,(H,17,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified rat liver phosphodiesterase 4 |
Bioorg Med Chem Lett 13: 2355-8 (2003)
BindingDB Entry DOI: 10.7270/Q2CZ39CG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BLK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2017.12.079
BindingDB Entry DOI: 10.7270/Q2P272TR |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50220996

(CHEMBL76635)Show InChI InChI=1S/C20H23N3O3/c1-25-17-12-11-15(13-18(17)26-16-9-5-6-10-16)19(22-23-20(21)24)14-7-3-2-4-8-14/h2-4,7-8,11-13,16H,5-6,9-10H2,1H3,(H3,21,23,24)/b22-19+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified rat liver phosphodiesterase 4 |
Bioorg Med Chem Lett 13: 2355-8 (2003)
BindingDB Entry DOI: 10.7270/Q2CZ39CG |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-4
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center
Curated by ChEMBL
| Assay Description
Inhibition of HER4 (unknown origin) |
Eur J Med Chem 169: 121-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.077
BindingDB Entry DOI: 10.7270/Q2PR80BX |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50221003

(CHEMBL432348)Show InChI InChI=1S/C14H19N3O3/c1-19-12-7-6-10(9-16-17-14(15)18)8-13(12)20-11-4-2-3-5-11/h6-9,11H,2-5H2,1H3,(H3,15,17,18)/b16-9+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified rat liver phosphodiesterase 4 |
Bioorg Med Chem Lett 13: 2355-8 (2003)
BindingDB Entry DOI: 10.7270/Q2CZ39CG |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50572387
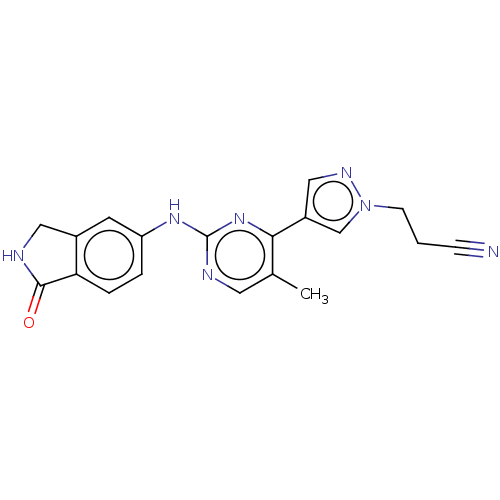
(CHEMBL4845662)Show SMILES Cc1cnc(Nc2ccc3C(=O)NCc3c2)nc1-c1cnn(CCC#N)c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human TYK2 (875 to end residues) using GGMEDIYFEFMGG as substrate incubated for 40 mins in presence of [gamma33P]ATP by sci... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01468
BindingDB Entry DOI: 10.7270/Q23N2750 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50505817

(CHEMBL4447631)Show SMILES CC(C)n1cc(cn1)-c1nc(Nc2ccc(N3CCC(CC3)N(C)CCO)c(F)c2)ncc1C Show InChI InChI=1S/C25H34FN7O/c1-17(2)33-16-19(15-28-33)24-18(3)14-27-25(30-24)29-20-5-6-23(22(26)13-20)32-9-7-21(8-10-32)31(4)11-12-34/h5-6,13-17,21,34H,7-12H2,1-4H3,(H,27,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human JAK2 (808 to end residues) KTFCGTPEYLAPE as substrate measured after 40 mins in presence of [gamm33P]ATP by scintilla... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01468
BindingDB Entry DOI: 10.7270/Q23N2750 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50505817

(CHEMBL4447631)Show SMILES CC(C)n1cc(cn1)-c1nc(Nc2ccc(N3CCC(CC3)N(C)CCO)c(F)c2)ncc1C Show InChI InChI=1S/C25H34FN7O/c1-17(2)33-16-19(15-28-33)24-18(3)14-27-25(30-24)29-20-5-6-23(22(26)13-20)32-9-7-21(8-10-32)31(4)11-12-34/h5-6,13-17,21,34H,7-12H2,1-4H3,(H,27,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
West China Hospital of Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human JAK2 (808 to end residues) using KTFCGTPEYLAP as substrate after 40 mins in presence of [gamma33P]-ATP by scintillation counting ... |
J Med Chem 62: 10305-10320 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01348
BindingDB Entry DOI: 10.7270/Q25X2D7B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of human FGFR1 |
Bioorg Med Chem 26: 5479-5493 (2018)
Article DOI: 10.1016/j.bmc.2018.09.027
BindingDB Entry DOI: 10.7270/Q2319ZMZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BMX (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2017.12.079
BindingDB Entry DOI: 10.7270/Q2P272TR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50559293
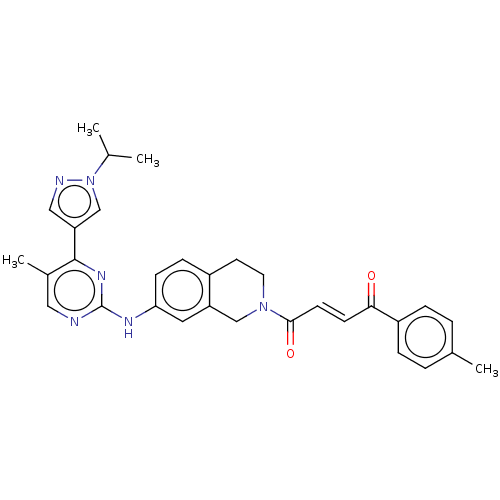
(CHEMBL4789273)Show SMILES CC(C)n1cc(cn1)-c1nc(Nc2ccc3CCN(Cc3c2)C(=O)\C=C\C(=O)c2ccc(C)cc2)ncc1C | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human JAK2 (808 to end residues) KTFCGTPEYLAPEVRREPRILSEEEQEMFRDFDYIADWC as substrate measured after 40 mins in presence of... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01488
BindingDB Entry DOI: 10.7270/Q28P6472 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50505819
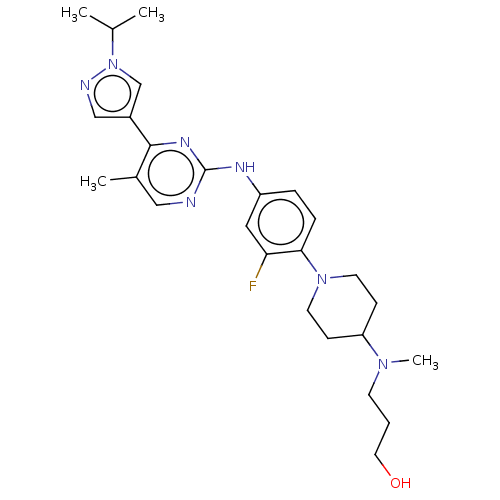
(CHEMBL4560698)Show SMILES CC(C)n1cc(cn1)-c1nc(Nc2ccc(N3CCC(CC3)N(C)CCCO)c(F)c2)ncc1C Show InChI InChI=1S/C26H36FN7O/c1-18(2)34-17-20(16-29-34)25-19(3)15-28-26(31-25)30-21-6-7-24(23(27)14-21)33-11-8-22(9-12-33)32(4)10-5-13-35/h6-7,14-18,22,35H,5,8-13H2,1-4H3,(H,28,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
West China Hospital of Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human JAK2 (808 to end residues) using KTFCGTPEYLAP as substrate after 40 mins in presence of [gamma33P]-ATP by scintillation counting ... |
J Med Chem 62: 10305-10320 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01348
BindingDB Entry DOI: 10.7270/Q25X2D7B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50572387

(CHEMBL4845662)Show SMILES Cc1cnc(Nc2ccc3C(=O)NCc3c2)nc1-c1cnn(CCC#N)c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human JAK2 (808 to end residues) KTFCGTPEYLAPE as substrate measured after 40 mins in presence of [gamm33P]ATP by scintilla... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01468
BindingDB Entry DOI: 10.7270/Q23N2750 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) in the presence of ATP by caliper mobility shift assay |
Eur J Med Chem 169: 121-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.077
BindingDB Entry DOI: 10.7270/Q2PR80BX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50220998

(CHEMBL76382)Show InChI InChI=1S/C14H19N3O2S/c1-18-12-7-6-10(9-16-17-14(15)20)8-13(12)19-11-4-2-3-5-11/h6-9,11H,2-5H2,1H3,(H3,15,17,20)/b16-9+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified rat liver phosphodiesterase 4 |
Bioorg Med Chem Lett 13: 2355-8 (2003)
BindingDB Entry DOI: 10.7270/Q2CZ39CG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
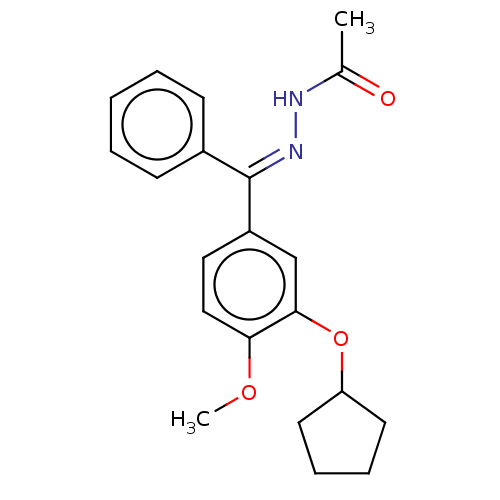
(Rattus norvegicus) | BDBM50221004

(CHEMBL77999)Show SMILES [#6]-[#8]-c1ccc(cc1-[#8]-[#6]-1-[#6]-[#6]-[#6]-[#6]-1)-[#6](=[#7]\[#7]=[#6](/[#7])-[#7])\c1ccccc1 Show InChI InChI=1S/C20H24N4O2/c1-25-17-12-11-15(13-18(17)26-16-9-5-6-10-16)19(23-24-20(21)22)14-7-3-2-4-8-14/h2-4,7-8,11-13,16H,5-6,9-10H2,1H3,(H4,21,22,24)/b23-19+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified rat liver phosphodiesterase 4 |
Bioorg Med Chem Lett 13: 2355-8 (2003)
BindingDB Entry DOI: 10.7270/Q2CZ39CG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50220993

(CHEMBL78238)Show SMILES COc1ccc(cc1OC1CCCC1)C(=N\N=C(\N)S)\c1ccccc1 Show InChI InChI=1S/C20H23N3O2S/c1-24-17-12-11-15(13-18(17)25-16-9-5-6-10-16)19(22-23-20(21)26)14-7-3-2-4-8-14/h2-4,7-8,11-13,16H,5-6,9-10H2,1H3,(H3,21,23,26)/b22-19+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified rat liver phosphodiesterase 4 |
Bioorg Med Chem Lett 13: 2355-8 (2003)
BindingDB Entry DOI: 10.7270/Q2CZ39CG |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50572360

(CHEMBL4867814)Show SMILES CCS(=O)(=O)N1CCc2cc(Nc3ncc(C)c(n3)-c3cnn(c3)C(C)C)ccc12 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human TYK2 (875 to end residues) using GGMEDIYFEFMGG as substrate incubated for 40 mins in presence of [gamma33P]ATP by sci... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01468
BindingDB Entry DOI: 10.7270/Q23N2750 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of human KDR |
Bioorg Med Chem 26: 5479-5493 (2018)
Article DOI: 10.1016/j.bmc.2018.09.027
BindingDB Entry DOI: 10.7270/Q2319ZMZ |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50572367
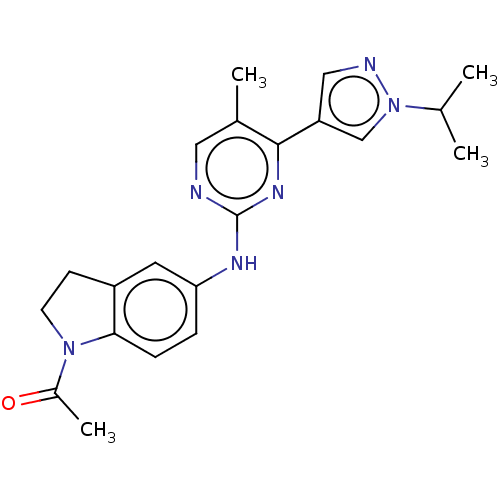
(CHEMBL4869302)Show SMILES CC(C)n1cc(cn1)-c1nc(Nc2ccc3N(CCc3c2)C(C)=O)ncc1C | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human TYK2 (875 to end residues) using GGMEDIYFEFMGG as substrate incubated for 40 mins in presence of [gamma33P]ATP by sci... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01468
BindingDB Entry DOI: 10.7270/Q23N2750 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50220995

(CHEMBL77788)Show InChI InChI=1S/C15H20N2O3/c1-11(18)17-16-10-12-7-8-14(19-2)15(9-12)20-13-5-3-4-6-13/h7-10,13H,3-6H2,1-2H3,(H,17,18)/b16-10+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified rat liver phosphodiesterase 4 |
Bioorg Med Chem Lett 13: 2355-8 (2003)
BindingDB Entry DOI: 10.7270/Q2CZ39CG |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50572385

(CHEMBL4851075) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human TYK2 (875 to end residues) using GGMEDIYFEFMGG as substrate incubated for 40 mins in presence of [gamma33P]ATP by sci... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01468
BindingDB Entry DOI: 10.7270/Q23N2750 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50572383
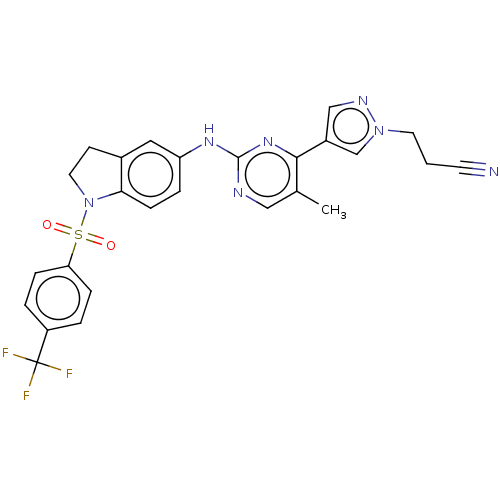
(CHEMBL4873919)Show SMILES Cc1cnc(Nc2ccc3N(CCc3c2)S(=O)(=O)c2ccc(cc2)C(F)(F)F)nc1-c1cnn(CCC#N)c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human TYK2 (875 to end residues) using GGMEDIYFEFMGG as substrate incubated for 40 mins in presence of [gamma33P]ATP by sci... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01468
BindingDB Entry DOI: 10.7270/Q23N2750 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50572374
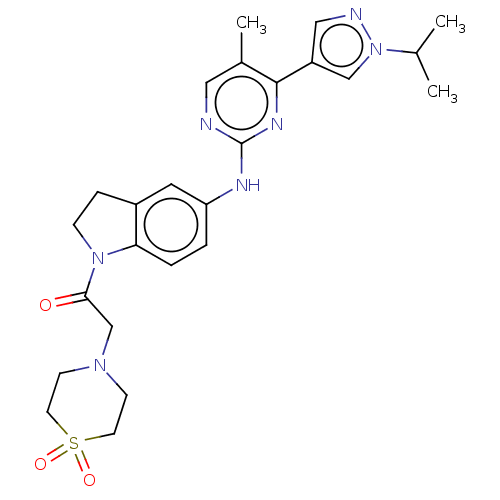
(CHEMBL4862108)Show SMILES CC(C)n1cc(cn1)-c1nc(Nc2ccc3N(CCc3c2)C(=O)CN2CCS(=O)(=O)CC2)ncc1C | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human TYK2 (875 to end residues) using GGMEDIYFEFMGG as substrate incubated for 40 mins in presence of [gamma33P]ATP by sci... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01468
BindingDB Entry DOI: 10.7270/Q23N2750 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50559279
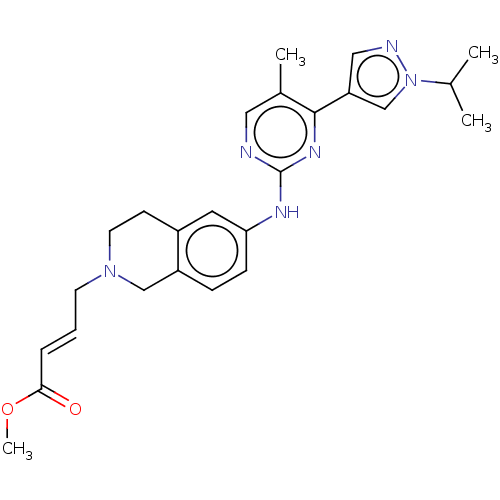
(CHEMBL4754856)Show SMILES COC(=O)\C=C\CN1CCc2cc(Nc3ncc(C)c(n3)-c3cnn(c3)C(C)C)ccc2C1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human JAK2 (808 to end residues) KTFCGTPEYLAPEVRREPRILSEEEQEMFRDFDYIADWC as substrate measured after 40 mins in presence of... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01488
BindingDB Entry DOI: 10.7270/Q28P6472 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50225162
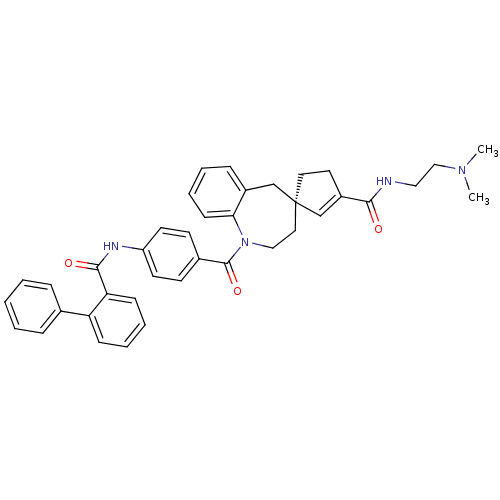
((4R)-N-[2-(dimethylamino)ethyl]-1-({4-[(2-phenylbe...)Show SMILES CN(C)CCNC(=O)C1=C[C@@]2(CC1)CCN(C(=O)c1ccc(NC(=O)c3ccccc3-c3ccccc3)cc1)c1ccccc1C2 |t:8| Show InChI InChI=1S/C39H40N4O3/c1-42(2)25-23-40-36(44)31-20-21-39(27-31)22-24-43(35-15-9-6-12-30(35)26-39)38(46)29-16-18-32(19-17-29)41-37(45)34-14-8-7-13-33(34)28-10-4-3-5-11-28/h3-19,27H,20-26H2,1-2H3,(H,40,44)(H,41,45)/t39-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Arg-vasopressin from human recombinant vasopressin V1a receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 6623-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.059
BindingDB Entry DOI: 10.7270/Q2T72H5P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50505820
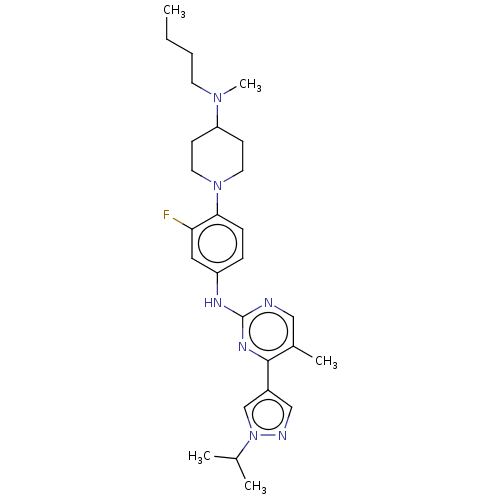
(CHEMBL4436572)Show SMILES CCCCN(C)C1CCN(CC1)c1ccc(Nc2ncc(C)c(n2)-c2cnn(c2)C(C)C)cc1F Show InChI InChI=1S/C27H38FN7/c1-6-7-12-33(5)23-10-13-34(14-11-23)25-9-8-22(15-24(25)28)31-27-29-16-20(4)26(32-27)21-17-30-35(18-21)19(2)3/h8-9,15-19,23H,6-7,10-14H2,1-5H3,(H,29,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
West China Hospital of Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human JAK2 (808 to end residues) using KTFCGTPEYLAP as substrate after 40 mins in presence of [gamma33P]-ATP by scintillation counting ... |
J Med Chem 62: 10305-10320 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01348
BindingDB Entry DOI: 10.7270/Q25X2D7B |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50366915
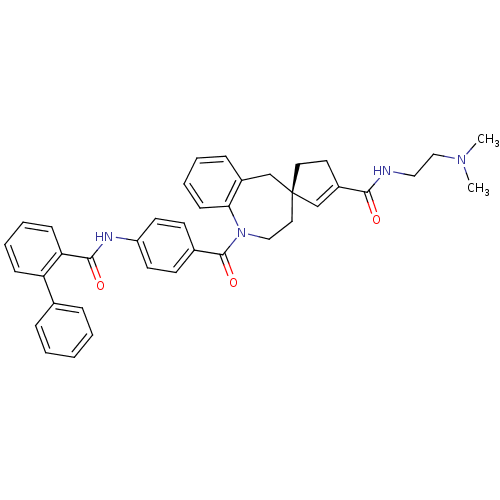
(CHEMBL1788220)Show SMILES CN(C)CCNC(=O)C1=C[C@]2(CC1)CCN(C(=O)c1ccc(NC(=O)c3ccccc3-c3ccccc3)cc1)c1ccccc1C2 |r,t:8| Show InChI InChI=1S/C39H40N4O3/c1-42(2)25-23-40-36(44)31-20-21-39(27-31)22-24-43(35-15-9-6-12-30(35)26-39)38(46)29-16-18-32(19-17-29)41-37(45)34-14-8-7-13-33(34)28-10-4-3-5-11-28/h3-19,27H,20-26H2,1-2H3,(H,40,44)(H,41,45)/t39-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity measured by inhibition of 3[H] AVP binding to cloned human vasopressin V1a receptor |
Bioorg Med Chem Lett 14: 3143-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.016
BindingDB Entry DOI: 10.7270/Q2Z60PMQ |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50505817

(CHEMBL4447631)Show SMILES CC(C)n1cc(cn1)-c1nc(Nc2ccc(N3CCC(CC3)N(C)CCO)c(F)c2)ncc1C Show InChI InChI=1S/C25H34FN7O/c1-17(2)33-16-19(15-28-33)24-18(3)14-27-25(30-24)29-20-5-6-23(22(26)13-20)32-9-7-21(8-10-32)31(4)11-12-34/h5-6,13-17,21,34H,7-12H2,1-4H3,(H,27,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human TYK2 (875 to end residues) using GGMEDIYFEFMGG as substrate incubated for 40 mins in presence of [gamma33P]ATP by sci... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01468
BindingDB Entry DOI: 10.7270/Q23N2750 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50572392
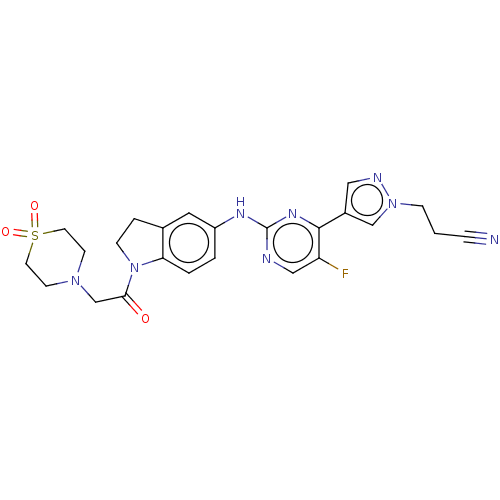
(CHEMBL4865102)Show SMILES Fc1cnc(Nc2ccc3N(CCc3c2)C(=O)CN2CCS(=O)(=O)CC2)nc1-c1cnn(CCC#N)c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human TYK2 (875 to end residues) using GGMEDIYFEFMGG as substrate incubated for 40 mins in presence of [gamma33P]ATP by sci... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01468
BindingDB Entry DOI: 10.7270/Q23N2750 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50559295
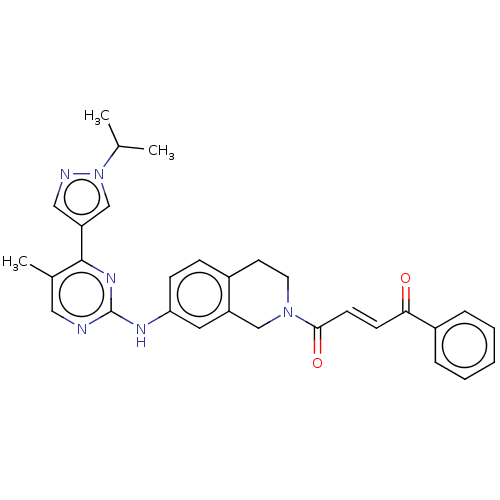
(CHEMBL4741075)Show SMILES CC(C)n1cc(cn1)-c1nc(Nc2ccc3CCN(Cc3c2)C(=O)\C=C\C(=O)c2ccccc2)ncc1C | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human JAK2 (808 to end residues) KTFCGTPEYLAPEVRREPRILSEEEQEMFRDFDYIADWC as substrate measured after 40 mins in presence of... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01488
BindingDB Entry DOI: 10.7270/Q28P6472 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data