

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50314073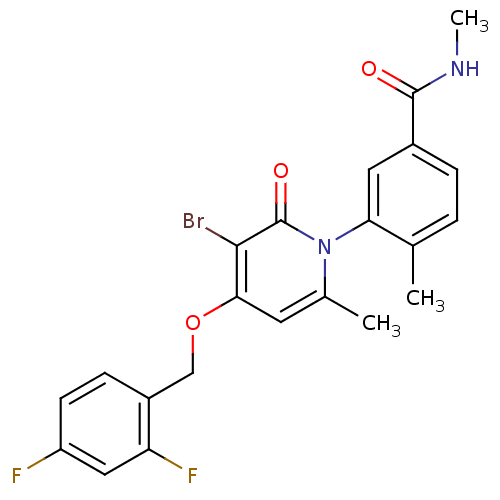 (3-(3-bromo-4-(2,4-difluorobenzyloxy)-6-methyl-2-ox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of p38alpha kinase | Bioorg Med Chem Lett 21: 3856-60 (2011) Article DOI: 10.1016/j.bmcl.2011.05.006 BindingDB Entry DOI: 10.7270/Q2G73F2S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 11 (Homo sapiens (Human)) | BDBM50346920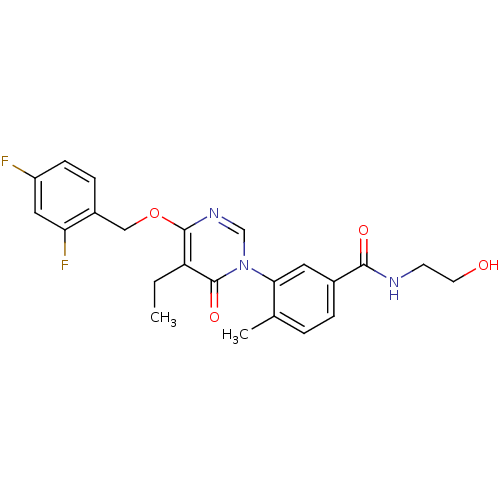 (CHEMBL1795686) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of p38beta kinase | Bioorg Med Chem Lett 21: 3856-60 (2011) Article DOI: 10.1016/j.bmcl.2011.05.006 BindingDB Entry DOI: 10.7270/Q2G73F2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 11 (Homo sapiens (Human)) | BDBM50346919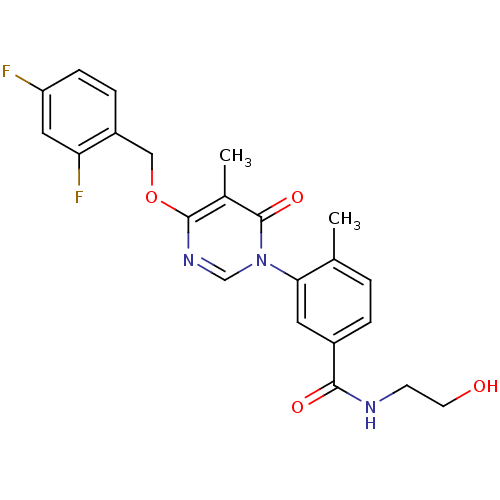 (CHEMBL1795685) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of p38beta kinase | Bioorg Med Chem Lett 21: 3856-60 (2011) Article DOI: 10.1016/j.bmcl.2011.05.006 BindingDB Entry DOI: 10.7270/Q2G73F2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466897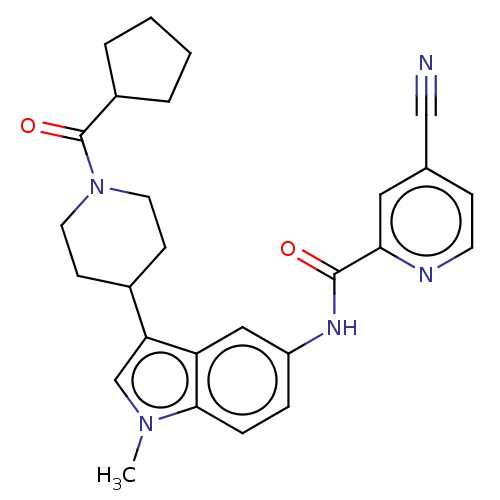 (CHEMBL4287715) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466891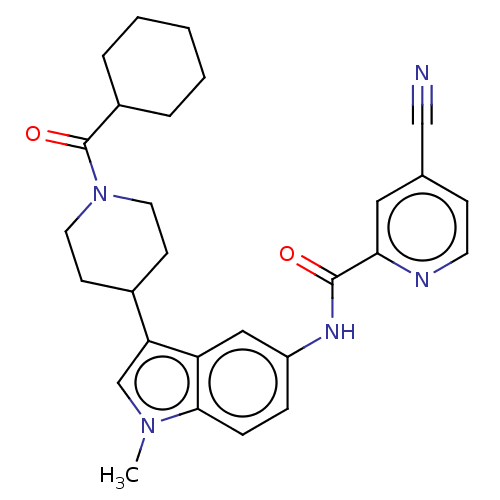 (CHEMBL4281109) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 11 (Homo sapiens (Human)) | BDBM50314073 (3-(3-bromo-4-(2,4-difluorobenzyloxy)-6-methyl-2-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of p38beta kinase | Bioorg Med Chem Lett 21: 3856-60 (2011) Article DOI: 10.1016/j.bmcl.2011.05.006 BindingDB Entry DOI: 10.7270/Q2G73F2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466893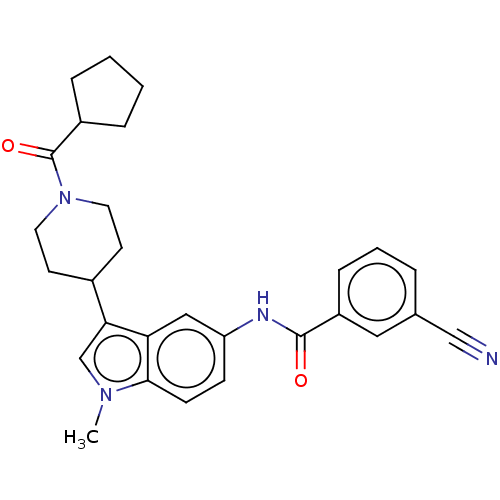 (CHEMBL4291727) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50346918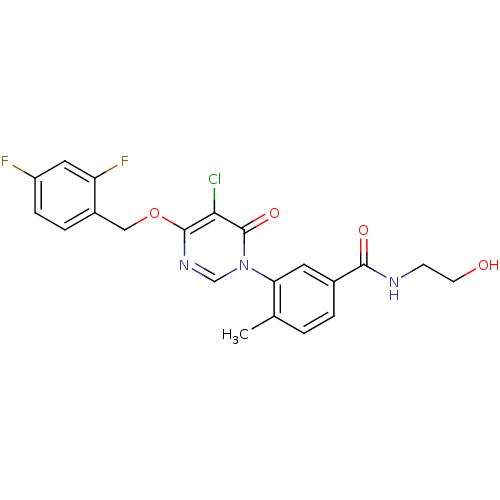 (CHEMBL1738710) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 193 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of p38alpha kinase | Bioorg Med Chem Lett 21: 3856-60 (2011) Article DOI: 10.1016/j.bmcl.2011.05.006 BindingDB Entry DOI: 10.7270/Q2G73F2S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466892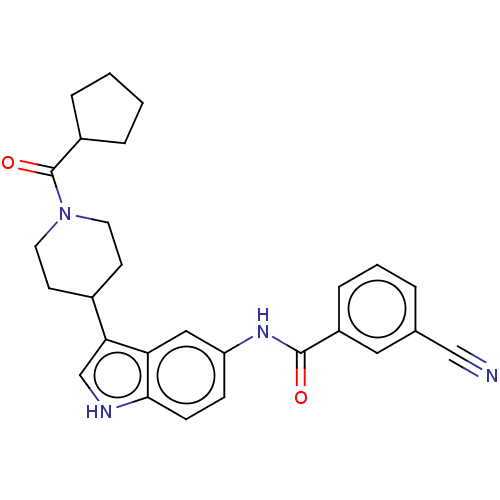 (CHEMBL4283871) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466913 (CHEMBL4289304) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 9 (Homo sapiens (Human)) | BDBM50314073 (3-(3-bromo-4-(2,4-difluorobenzyloxy)-6-methyl-2-ox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of JNK2 | Bioorg Med Chem Lett 21: 3856-60 (2011) Article DOI: 10.1016/j.bmcl.2011.05.006 BindingDB Entry DOI: 10.7270/Q2G73F2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of full-length human His-tagged BTK expressed in baculovirus expression system using FAM-Srctide peptide as substrate preincubated for 1 h... | ACS Med Chem Lett 10: 80-85 (2019) Article DOI: 10.1021/acsmedchemlett.8b00461 BindingDB Entry DOI: 10.7270/Q28G8Q30 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50356214 (CHEMBL1910471) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of MMP13 | Bioorg Med Chem Lett 21: 6800-3 (2011) Article DOI: 10.1016/j.bmcl.2011.09.036 BindingDB Entry DOI: 10.7270/Q2BV7H23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50356215 (CHEMBL1910473) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of MMP13 | Bioorg Med Chem Lett 21: 6800-3 (2011) Article DOI: 10.1016/j.bmcl.2011.09.036 BindingDB Entry DOI: 10.7270/Q2BV7H23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377836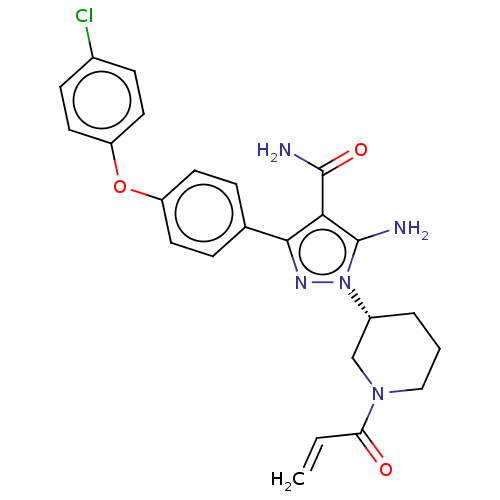 (1-[(3R)-1-acryloyl piperidin-3-yl]-5-amino-3-[4-(4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Claudius Regaud | Assay Description TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide peptid... | J Med Chem 48: 287-91 (2005) BindingDB Entry DOI: 10.7270/Q2P271FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM470468 (1-[(3R)-1-acryloylpiperidin-3-yl]-5-amino-3-[4-(4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description BTK: TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide p... | US Patent US10815213 (2020) BindingDB Entry DOI: 10.7270/Q2W95D87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377836 (1-[(3R)-1-acryloyl piperidin-3-yl]-5-amino-3-[4-(4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of full-length human His-tagged BTK expressed in baculovirus expression system using FAM-Srctide peptide as substrate preincubated for 1 h... | ACS Med Chem Lett 10: 80-85 (2019) Article DOI: 10.1021/acsmedchemlett.8b00461 BindingDB Entry DOI: 10.7270/Q28G8Q30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM303543 (US10138229, Example 54 | US10266513, Example 127 |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Claudius Regaud | Assay Description TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide peptid... | J Med Chem 48: 287-91 (2005) BindingDB Entry DOI: 10.7270/Q2P271FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM303543 (US10138229, Example 54 | US10266513, Example 127 |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description BTK: TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide p... | US Patent US10815213 (2020) BindingDB Entry DOI: 10.7270/Q2W95D87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377842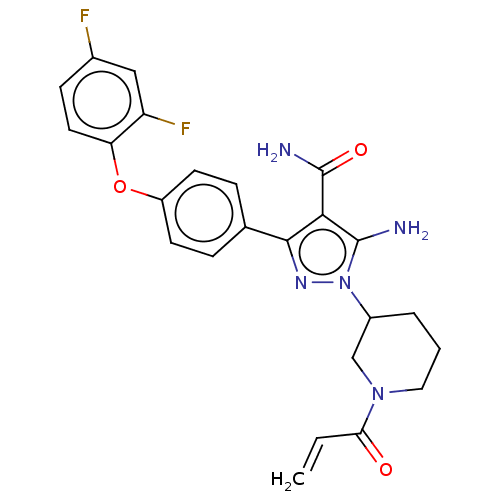 (1-[(3S)-1-acryloyl piperidin-3-yl]-5-amino-3-[4-(2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Claudius Regaud | Assay Description TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide peptid... | J Med Chem 48: 287-91 (2005) BindingDB Entry DOI: 10.7270/Q2P271FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50521321 (CHEMBL4442732) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of full-length human His-tagged BTK expressed in baculovirus expression system using FAM-Srctide peptide as substrate preincubated for 1 h... | ACS Med Chem Lett 10: 80-85 (2019) Article DOI: 10.1021/acsmedchemlett.8b00461 BindingDB Entry DOI: 10.7270/Q28G8Q30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377842 (1-[(3S)-1-acryloyl piperidin-3-yl]-5-amino-3-[4-(2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description BTK: TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide p... | US Patent US10815213 (2020) BindingDB Entry DOI: 10.7270/Q2W95D87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription factor ETV6/Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50531864 (CHEMBL4524568) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai University Curated by ChEMBL | Assay Description Inhibition of TEL/AXL (unknown origin) expressed in mouse BA/F3 cells assessed as growth inhibition after 72 hrs by SRB assay | Bioorg Med Chem Lett 29: 836-838 (2019) Article DOI: 10.1016/j.bmcl.2019.01.018 BindingDB Entry DOI: 10.7270/Q25B05Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50521322 (CHEMBL4436118) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of full-length human His-tagged BTK expressed in baculovirus expression system using FAM-Srctide peptide as substrate preincubated for 1 h... | ACS Med Chem Lett 10: 80-85 (2019) Article DOI: 10.1021/acsmedchemlett.8b00461 BindingDB Entry DOI: 10.7270/Q28G8Q30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377707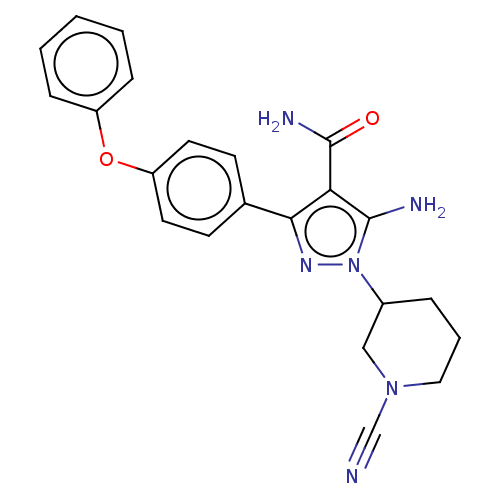 (5-amino-1-(1-cyanopiperidin-3-yl)-3-(4-phenoxyphen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Claudius Regaud | Assay Description TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide peptid... | J Med Chem 48: 287-91 (2005) BindingDB Entry DOI: 10.7270/Q2P271FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377707 (5-amino-1-(1-cyanopiperidin-3-yl)-3-(4-phenoxyphen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description BTK: TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide p... | US Patent US10815213 (2020) BindingDB Entry DOI: 10.7270/Q2W95D87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377839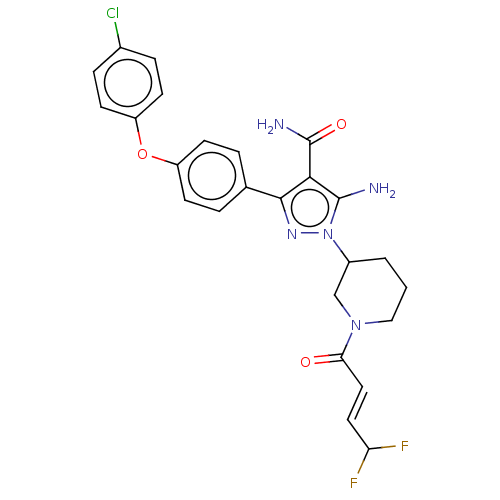 (5-amino-3-[4-(4-chlorophenoxy)phenyl]-1-{(3R)-1-[(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Claudius Regaud | Assay Description TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide peptid... | J Med Chem 48: 287-91 (2005) BindingDB Entry DOI: 10.7270/Q2P271FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377839 (5-amino-3-[4-(4-chlorophenoxy)phenyl]-1-{(3R)-1-[(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description BTK: TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide p... | US Patent US10815213 (2020) BindingDB Entry DOI: 10.7270/Q2W95D87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377831 (5-amino-1-(1-cyanopiperidin-3-yl)-3-[4-(phenylthio...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Claudius Regaud | Assay Description TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide peptid... | J Med Chem 48: 287-91 (2005) BindingDB Entry DOI: 10.7270/Q2P271FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377831 (5-amino-1-(1-cyanopiperidin-3-yl)-3-[4-(phenylthio...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description BTK: TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide p... | US Patent US10815213 (2020) BindingDB Entry DOI: 10.7270/Q2W95D87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377722 (5-amino-1-(1-cyanopiperidin-3-yl)-3-[4-(3,4-dimeth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Claudius Regaud | Assay Description TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide peptid... | J Med Chem 48: 287-91 (2005) BindingDB Entry DOI: 10.7270/Q2P271FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM470355 ((R)-5-amino-1-(1-cyanopiperidin-3-yl)-3-[4-(3,4-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description BTK: TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide p... | US Patent US10815213 (2020) BindingDB Entry DOI: 10.7270/Q2W95D87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377850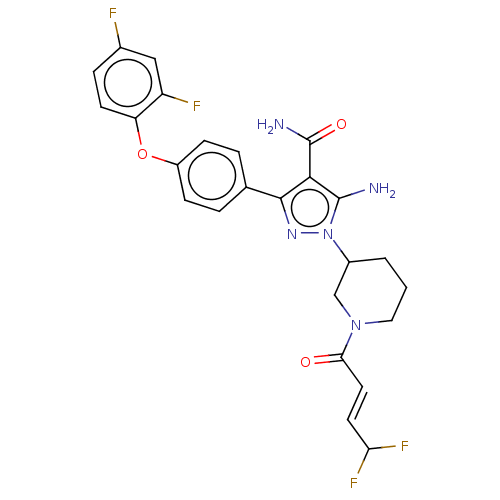 (5-amino-1-{(3R)-1-[(2E)-4,4-difluorobut-2-enoyl]pi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description BTK: TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide p... | US Patent US10815213 (2020) BindingDB Entry DOI: 10.7270/Q2W95D87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377850 (5-amino-1-{(3R)-1-[(2E)-4,4-difluorobut-2-enoyl]pi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Claudius Regaud | Assay Description TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide peptid... | J Med Chem 48: 287-91 (2005) BindingDB Entry DOI: 10.7270/Q2P271FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50521317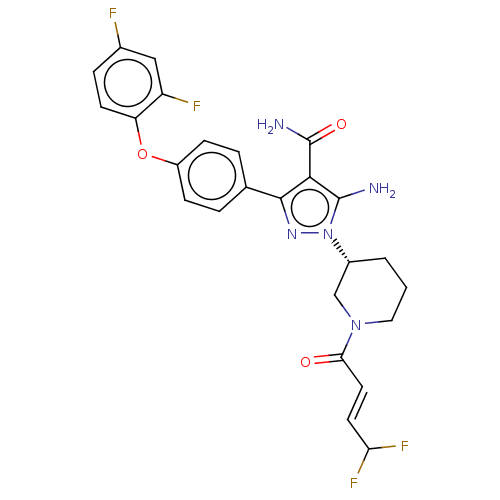 (CHEMBL4559065) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of full-length human His-tagged BTK expressed in baculovirus expression system using FAM-Srctide peptide as substrate preincubated for 1 h... | ACS Med Chem Lett 10: 80-85 (2019) Article DOI: 10.1021/acsmedchemlett.8b00461 BindingDB Entry DOI: 10.7270/Q28G8Q30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase ROS (Homo sapiens (Human)) | BDBM50512149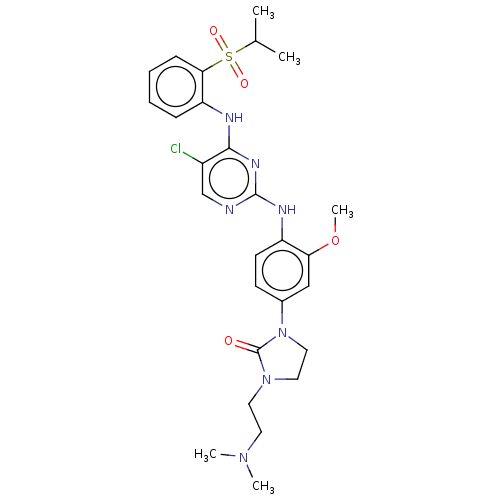 (CHEMBL4470529) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged human ROS1 cytoplasmic domain (1883 to 2347 residues) expressed in Baculovirus expression system using IRS1 as su... | Eur J Med Chem 171: 297-309 (2019) Article DOI: 10.1016/j.ejmech.2019.03.038 BindingDB Entry DOI: 10.7270/Q28G8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377734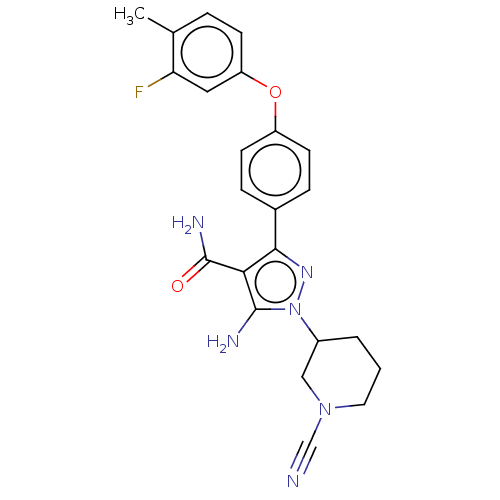 (5-amino-1-(1-cyanopiperidin-3-yl)-3-[4-(3-fluoro-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description BTK: TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide p... | US Patent US10815213 (2020) BindingDB Entry DOI: 10.7270/Q2W95D87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377734 (5-amino-1-(1-cyanopiperidin-3-yl)-3-[4-(3-fluoro-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Claudius Regaud | Assay Description TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide peptid... | J Med Chem 48: 287-91 (2005) BindingDB Entry DOI: 10.7270/Q2P271FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377754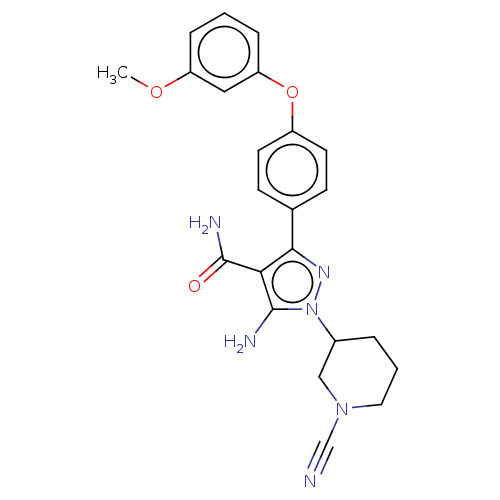 (5-amino-1-(1-cyanopiperidin-3-yl)- 3-[4-(3-methoxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description BTK: TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide p... | US Patent US10815213 (2020) BindingDB Entry DOI: 10.7270/Q2W95D87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377754 (5-amino-1-(1-cyanopiperidin-3-yl)- 3-[4-(3-methoxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Claudius Regaud | Assay Description TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide peptid... | J Med Chem 48: 287-91 (2005) BindingDB Entry DOI: 10.7270/Q2P271FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377827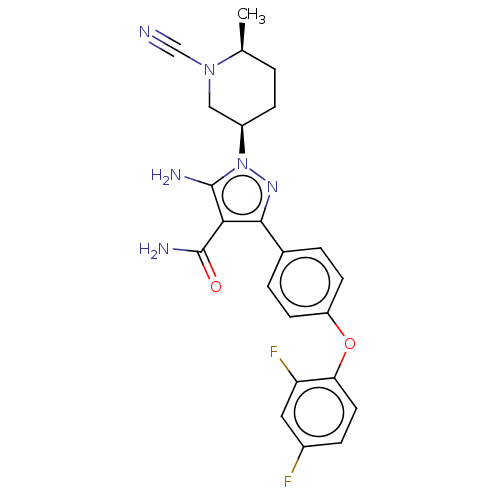 (5-amino-1-[(3R*,6S*)-1-cyano-6-methylpiperidin-3-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Claudius Regaud | Assay Description TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide peptid... | J Med Chem 48: 287-91 (2005) BindingDB Entry DOI: 10.7270/Q2P271FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377827 (5-amino-1-[(3R*,6S*)-1-cyano-6-methylpiperidin-3-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description BTK: TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide p... | US Patent US10815213 (2020) BindingDB Entry DOI: 10.7270/Q2W95D87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM470491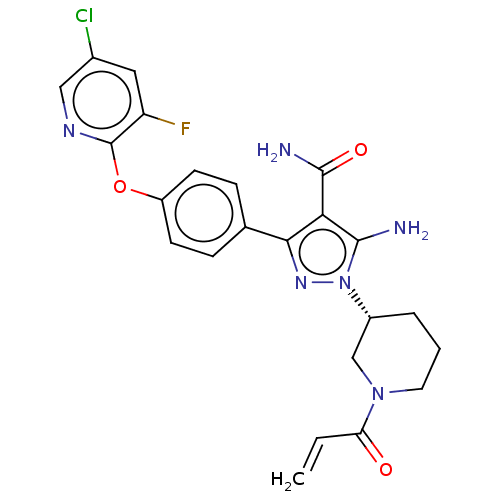 (1-[(3R)-1-acryloylpiperidin-3-yl]-5-amino-3-{4-[(5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description BTK: TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide p... | US Patent US10815213 (2020) BindingDB Entry DOI: 10.7270/Q2W95D87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377859 (1-[(3R)-1-acryloyl piperidin-3-yl]-5-amino-3-{4-[(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Claudius Regaud | Assay Description TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide peptid... | J Med Chem 48: 287-91 (2005) BindingDB Entry DOI: 10.7270/Q2P271FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM50468395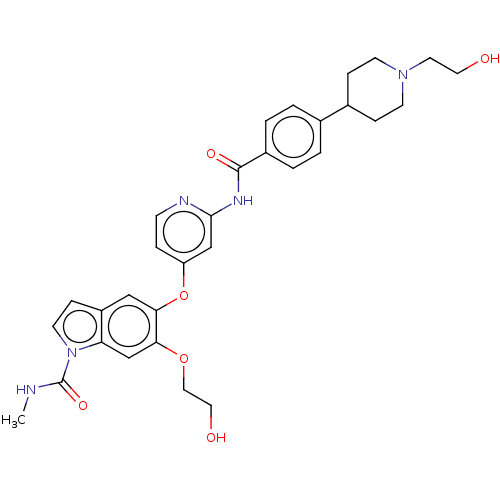 (CHEMBL4294625) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged FGFR2 (399 to 821 residues) cytoplasmic domain expressed in baculovirus expression system after 60 mins by ... | Eur J Med Chem 154: 9-28 (2018) Article DOI: 10.1016/j.ejmech.2018.05.005 BindingDB Entry DOI: 10.7270/Q2736TM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM50468395 (CHEMBL4294625) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged FGFR1 (398 to 822 residues) cytoplasmic domain expressed in baculovirus expression system after 60 mins by ... | Eur J Med Chem 154: 9-28 (2018) Article DOI: 10.1016/j.ejmech.2018.05.005 BindingDB Entry DOI: 10.7270/Q2736TM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM50468395 (CHEMBL4294625) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged FGFR3 (436 to 806 residues) cytoplasmic domain expressed in baculovirus expression system after 60 mins by ... | Eur J Med Chem 154: 9-28 (2018) Article DOI: 10.1016/j.ejmech.2018.05.005 BindingDB Entry DOI: 10.7270/Q2736TM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantetheinase (Homo sapiens (Human)) | BDBM394655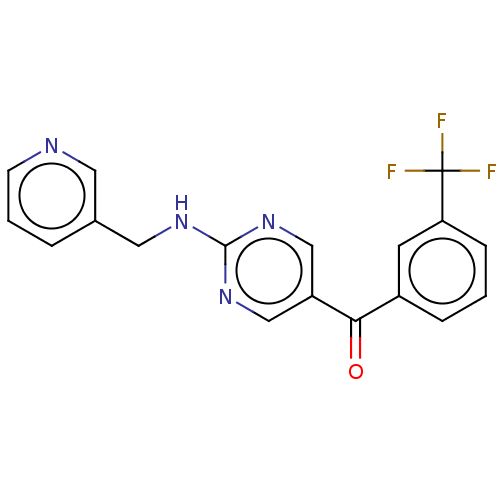 (US10308615, Example 12 | preparation of {2-[(pyrid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The vanin-1 protein was prepared in-house from a construct expressing the extracellular domain of human vanin-1 (GenBank ID NM_004666) preceded N-ter... | Bioorg Med Chem Lett 19: 3664-8 (2009) BindingDB Entry DOI: 10.7270/Q22809Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377778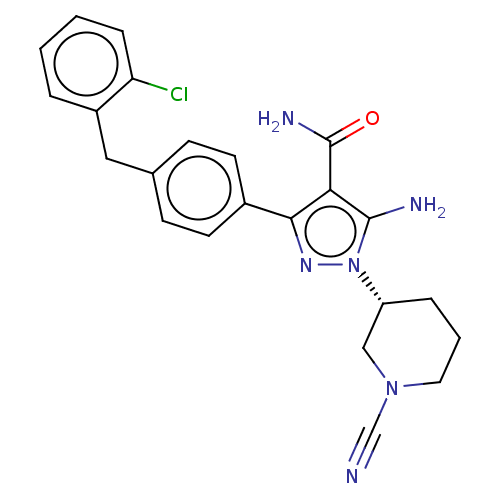 ((R)-5-amino-3-(4-(2-chlorobenzyl)phenyl)-1-(1-cyan...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Claudius Regaud | Assay Description TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide peptid... | J Med Chem 48: 287-91 (2005) BindingDB Entry DOI: 10.7270/Q2P271FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377778 ((R)-5-amino-3-(4-(2-chlorobenzyl)phenyl)-1-(1-cyan...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description BTK: TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide p... | US Patent US10815213 (2020) BindingDB Entry DOI: 10.7270/Q2W95D87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 6994 total ) | Next | Last >> |