 Found 1650 hits with Last Name = 'xiong' and Initial = 'j'
Found 1650 hits with Last Name = 'xiong' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM194780

(7-(4-(4-(1-benzothiophen-4-yl)piperazin-1-yl)butox...)Show SMILES O=c1ccc2ccc(OCCCCN3CCN(CC3)c3cccc4sccc34)cc2[nH]1 Show InChI InChI=1S/C25H27N3O2S/c29-25-9-7-19-6-8-20(18-22(19)26-25)30-16-2-1-11-27-12-14-28(15-13-27)23-4-3-5-24-21(23)10-17-31-24/h3-10,17-18H,1-2,11-16H2,(H,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H](+)8-OH-DPAT from human 5HT1A receptor expressed in human HeLa cells measured after 60 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112709
BindingDB Entry DOI: 10.7270/Q2XK8K6M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]ketanserin from rat cerebral cortex 5HT2A receptor measured after 15 mins by liquid scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112709
BindingDB Entry DOI: 10.7270/Q2XK8K6M |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50001019
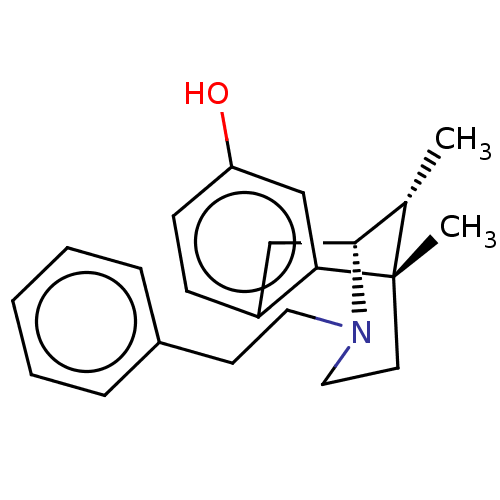
(6,11-Dimethyl-3-phenethyl-1,2,3,4,5,6-hexahydro-2,...)Show SMILES C[C@H]1[C@H]2Cc3ccc(O)cc3[C@@]1(C)CCN2CCc1ccccc1 |TLB:16:15:1:10.4.3| Show InChI InChI=1S/C22H27NO/c1-16-21-14-18-8-9-19(24)15-20(18)22(16,2)11-13-23(21)12-10-17-6-4-3-5-7-17/h3-9,15-16,21,24H,10-14H2,1-2H3/t16-,21+,22-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50001019

(6,11-Dimethyl-3-phenethyl-1,2,3,4,5,6-hexahydro-2,...)Show SMILES C[C@H]1[C@H]2Cc3ccc(O)cc3[C@@]1(C)CCN2CCc1ccccc1 |TLB:16:15:1:10.4.3| Show InChI InChI=1S/C22H27NO/c1-16-21-14-18-8-9-19(24)15-20(18)22(16,2)11-13-23(21)12-10-17-6-4-3-5-7-17/h3-9,15-16,21,24H,10-14H2,1-2H3/t16-,21+,22-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114649
BindingDB Entry DOI: 10.7270/Q2F47T3Z |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50001043
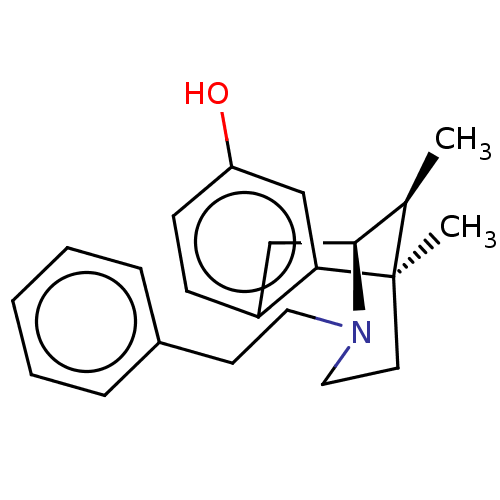
(6,11-Dimethyl-3-phenethyl-1,2,3,4,5,6-hexahydro-2,...)Show SMILES C[C@@H]1[C@@H]2Cc3ccc(O)cc3[C@]1(C)CCN2CCc1ccccc1 |TLB:16:15:1:10.4.3| Show InChI InChI=1S/C22H27NO/c1-16-21-14-18-8-9-19(24)15-20(18)22(16,2)11-13-23(21)12-10-17-6-4-3-5-7-17/h3-9,15-16,21,24H,10-14H2,1-2H3/t16-,21+,22-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in guinea pig brain membrane incubated for 2.5 hrs by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112144
BindingDB Entry DOI: 10.7270/Q2WM1J54 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50581486

(CHEMBL5082165) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from MOR in Sprague-Dawley rat brain membranes measured after 90 mins by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113879
BindingDB Entry DOI: 10.7270/Q26W9FZS |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM194780

(7-(4-(4-(1-benzothiophen-4-yl)piperazin-1-yl)butox...)Show SMILES O=c1ccc2ccc(OCCCCN3CCN(CC3)c3cccc4sccc34)cc2[nH]1 Show InChI InChI=1S/C25H27N3O2S/c29-25-9-7-19-6-8-20(18-22(19)26-25)30-16-2-1-11-27-12-14-28(15-13-27)23-4-3-5-24-21(23)10-17-31-24/h3-10,17-18H,1-2,11-16H2,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
| DrugBank
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]raclopride from human D2 long receptor expressed in CHO cells measured after 60 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112709
BindingDB Entry DOI: 10.7270/Q2XK8K6M |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50008984

(4-(4-Chloro-benzyl)-2-(1-methyl-azepan-4-yl)-2H-ph...)Show InChI InChI=1S/C22H28N2O/c1-2-22(25)24(20-11-7-4-8-12-20)21-14-17-23(18-15-21)16-13-19-9-5-3-6-10-19/h3-12,21H,2,13-18H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of sigma 1 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112144
BindingDB Entry DOI: 10.7270/Q2WM1J54 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM194780

(7-(4-(4-(1-benzothiophen-4-yl)piperazin-1-yl)butox...)Show SMILES O=c1ccc2ccc(OCCCCN3CCN(CC3)c3cccc4sccc34)cc2[nH]1 Show InChI InChI=1S/C25H27N3O2S/c29-25-9-7-19-6-8-20(18-22(19)26-25)30-16-2-1-11-27-12-14-28(15-13-27)23-4-3-5-24-21(23)10-17-31-24/h3-10,17-18H,1-2,11-16H2,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]ketanserin from human 5HT2A receptor expressed in CHO-K1 cells measured after 20 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112709
BindingDB Entry DOI: 10.7270/Q2XK8K6M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM423299
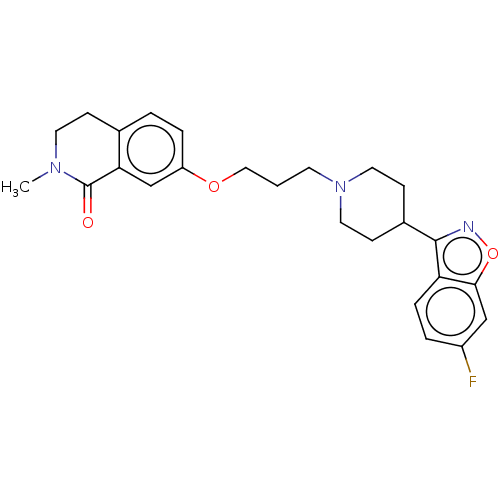
(US10501452, Compound 5)Show SMILES CN1CCc2ccc(OCCCN3CCC(CC3)c3noc4cc(F)ccc34)cc2C1=O Show InChI InChI=1S/C25H28FN3O3/c1-28-11-7-17-3-5-20(16-22(17)25(28)30)31-14-2-10-29-12-8-18(9-13-29)24-21-6-4-19(26)15-23(21)32-27-24/h3-6,15-16,18H,2,7-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]ketanserin from rat cerebral cortex 5HT2A receptor measured after 15 mins by liquid scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112709
BindingDB Entry DOI: 10.7270/Q2XK8K6M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50469504

(CHEMBL4293999)Show SMILES Fc1ccc2c(noc2c1)C1CCN(CCCCC(=O)c2cc3CCN4c3c(CCC4=O)c2)CC1 Show InChI InChI=1S/C28H30FN3O3/c29-22-5-6-23-25(17-22)35-30-27(23)18-8-12-31(13-9-18)11-2-1-3-24(33)21-15-19-4-7-26(34)32-14-10-20(16-21)28(19)32/h5-6,15-18H,1-4,7-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to 5HT2A receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112709
BindingDB Entry DOI: 10.7270/Q2XK8K6M |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50581505
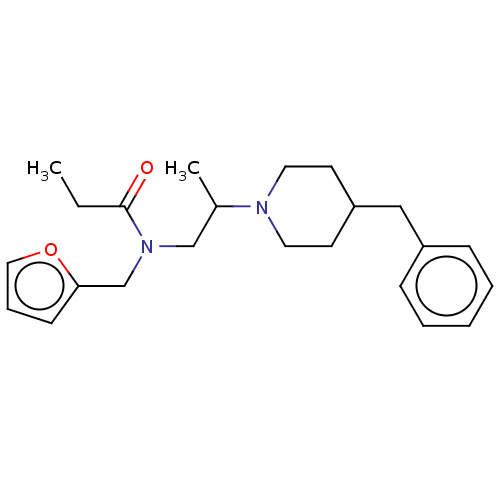
(CHEMBL5094445) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from MOR in Sprague-Dawley rat brain membranes measured after 90 mins by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113879
BindingDB Entry DOI: 10.7270/Q26W9FZS |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50564197

(CHEMBL4776178)Show SMILES CCC(=O)N(CCN1CCC(Cc2ccc(F)cc2)CC1)c1ccc(F)cc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-(+)-pnetazocin from sigma1 receptor in guinea pig brain membrane incubated for 150 mins by liquid scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112144
BindingDB Entry DOI: 10.7270/Q2WM1J54 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM111939

(US8618107, 105)Show SMILES Cn1cc(cc(Nc2ccncn2)c1=O)-c1cccc(N2CCc3c4CC(C)(C)Cc4sc3C2=O)c1CO Show InChI InChI=1S/C29H29N5O3S/c1-29(2)12-20-19-8-10-34(28(37)26(19)38-24(20)13-29)23-6-4-5-18(21(23)15-35)17-11-22(27(36)33(3)14-17)32-25-7-9-30-16-31-25/h4-7,9,11,14,16,35H,8,10,12-13,15H2,1-3H3,(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc., Research and Early Development, 1 DNA Way, South San Francisco, California 94080, United States.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length His-tagged BTK expressed in baculovirus expression system by Z-LYTE assay |
ACS Med Chem Lett 8: 608-613 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00103
BindingDB Entry DOI: 10.7270/Q24M96ZH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50564176
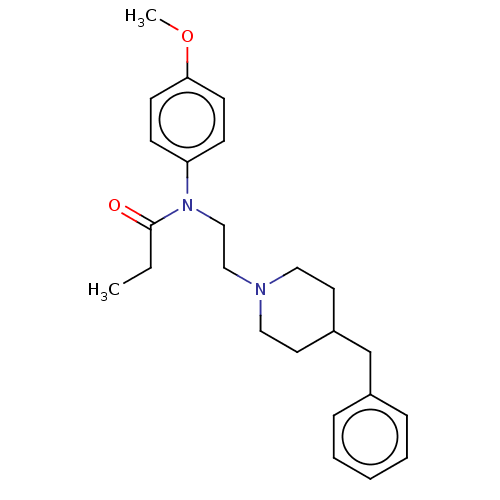
(CHEMBL4797982)Show SMILES CCC(=O)N(CCN1CCC(Cc2ccccc2)CC1)c1ccc(OC)cc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-(+)-pnetazocin from sigma1 receptor in guinea pig brain membrane incubated for 150 mins by liquid scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112144
BindingDB Entry DOI: 10.7270/Q2WM1J54 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50564177
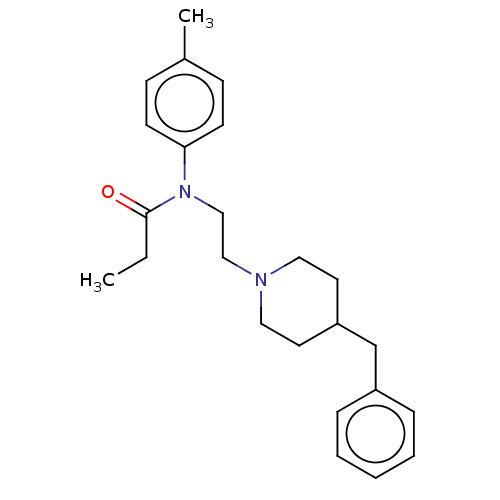
(CHEMBL4778918) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-(+)-pnetazocin from sigma1 receptor in guinea pig brain membrane incubated for 150 mins by liquid scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112144
BindingDB Entry DOI: 10.7270/Q2WM1J54 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50469504

(CHEMBL4293999)Show SMILES Fc1ccc2c(noc2c1)C1CCN(CCCCC(=O)c2cc3CCN4c3c(CCC4=O)c2)CC1 Show InChI InChI=1S/C28H30FN3O3/c29-22-5-6-23-25(17-22)35-30-27(23)18-8-12-31(13-9-18)11-2-1-3-24(33)21-15-19-4-7-26(34)32-14-10-20(16-21)28(19)32/h5-6,15-18H,1-4,7-14H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to D3 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112709
BindingDB Entry DOI: 10.7270/Q2XK8K6M |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50038419
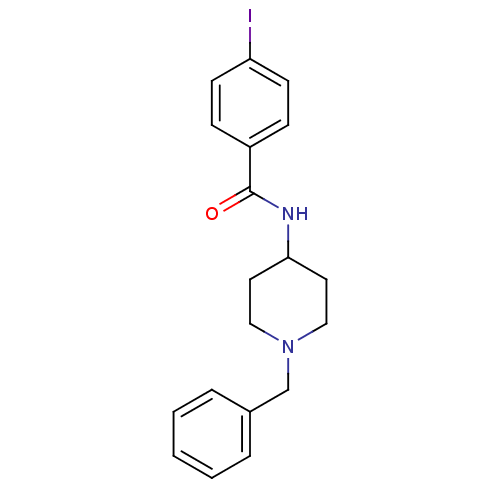
(CHEMBL50112 | N-(1-Benzyl-piperidin-4-yl)-4-iodo-b...)Show InChI InChI=1S/C19H21IN2O/c20-17-8-6-16(7-9-17)19(23)21-18-10-12-22(13-11-18)14-15-4-2-1-3-5-15/h1-9,18H,10-14H2,(H,21,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114649
BindingDB Entry DOI: 10.7270/Q2F47T3Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50564193
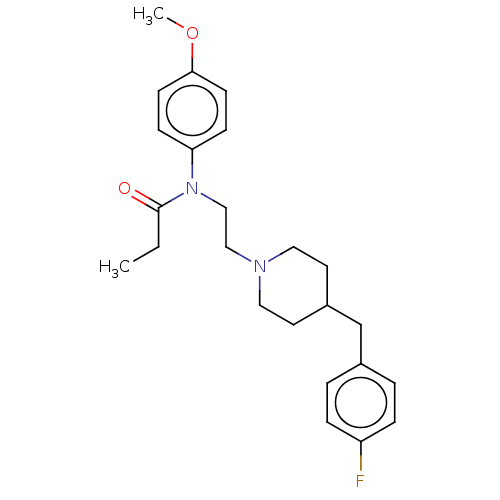
(CHEMBL4789321)Show SMILES CCC(=O)N(CCN1CCC(Cc2ccc(F)cc2)CC1)c1ccc(OC)cc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-(+)-pnetazocin from sigma1 receptor in guinea pig brain membrane incubated for 150 mins by liquid scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112144
BindingDB Entry DOI: 10.7270/Q2WM1J54 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50564193

(CHEMBL4789321)Show SMILES CCC(=O)N(CCN1CCC(Cc2ccc(F)cc2)CC1)c1ccc(OC)cc1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114649
BindingDB Entry DOI: 10.7270/Q2F47T3Z |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50564193

(CHEMBL4789321)Show SMILES CCC(=O)N(CCN1CCC(Cc2ccc(F)cc2)CC1)c1ccc(OC)cc1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-diprenorphin from human mu opioid receptor expressed in CHO cell membrane incubated for 150 mins by liquid scintillation countin... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112144
BindingDB Entry DOI: 10.7270/Q2WM1J54 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50564193

(CHEMBL4789321)Show SMILES CCC(=O)N(CCN1CCC(Cc2ccc(F)cc2)CC1)c1ccc(OC)cc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114649
BindingDB Entry DOI: 10.7270/Q2F47T3Z |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50564186
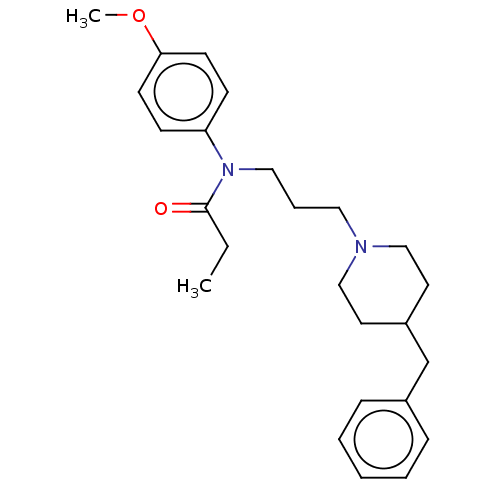
(CHEMBL4797005)Show SMILES CCC(=O)N(CCCN1CCC(Cc2ccccc2)CC1)c1ccc(OC)cc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-(+)-pnetazocin from sigma1 receptor in guinea pig brain membrane incubated for 150 mins by liquid scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112144
BindingDB Entry DOI: 10.7270/Q2WM1J54 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50564195
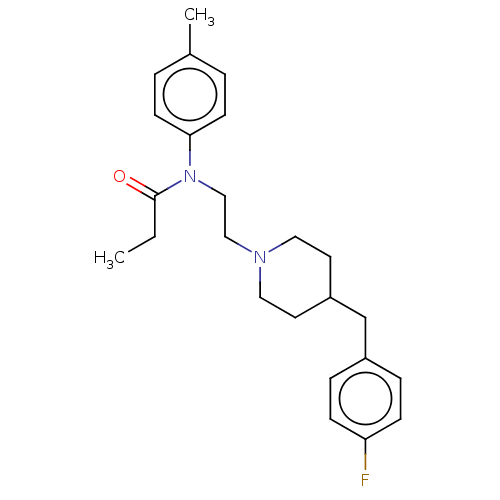
(CHEMBL4789528)Show SMILES CCC(=O)N(CCN1CCC(Cc2ccc(F)cc2)CC1)c1ccc(C)cc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-(+)-pnetazocin from sigma1 receptor in guinea pig brain membrane incubated for 150 mins by liquid scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112144
BindingDB Entry DOI: 10.7270/Q2WM1J54 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50590791
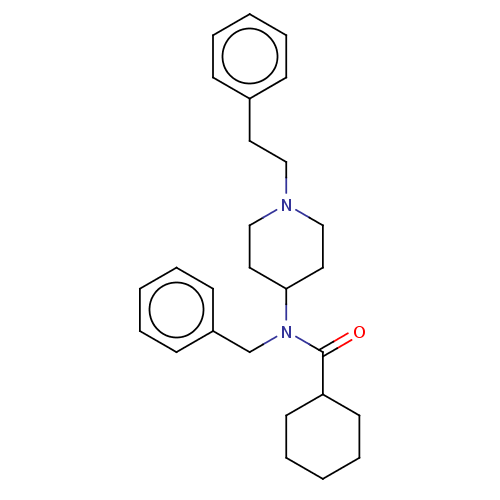
(CHEMBL5192526)Show SMILES O=C(C1CCCCC1)N(Cc1ccccc1)C1CCN(CCc2ccccc2)CC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114649
BindingDB Entry DOI: 10.7270/Q2F47T3Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50469504

(CHEMBL4293999)Show SMILES Fc1ccc2c(noc2c1)C1CCN(CCCCC(=O)c2cc3CCN4c3c(CCC4=O)c2)CC1 Show InChI InChI=1S/C28H30FN3O3/c29-22-5-6-23-25(17-22)35-30-27(23)18-8-12-31(13-9-18)11-2-1-3-24(33)21-15-19-4-7-26(34)32-14-10-20(16-21)28(19)32/h5-6,15-18H,1-4,7-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to D2 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112709
BindingDB Entry DOI: 10.7270/Q2XK8K6M |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM21015

((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N(C)[C@@H](Cc1ccccc1)C(=O)NCCO Show InChI InChI=1S/C26H35N5O6/c1-17(30-25(36)21(27)14-19-8-10-20(33)11-9-19)24(35)29-16-23(34)31(2)22(26(37)28-12-13-32)15-18-6-4-3-5-7-18/h3-11,17,21-22,32-33H,12-16,27H2,1-2H3,(H,28,37)(H,29,35)(H,30,36)/t17-,21+,22+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114649
BindingDB Entry DOI: 10.7270/Q2F47T3Z |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]prazosin from rat cerebral cortex alpha1 adrenergic receptor measured after 60 mins by liquid scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112709
BindingDB Entry DOI: 10.7270/Q2XK8K6M |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50008984

(4-(4-Chloro-benzyl)-2-(1-methyl-azepan-4-yl)-2H-ph...)Show InChI InChI=1S/C22H28N2O/c1-2-22(25)24(20-11-7-4-8-12-20)21-14-17-23(18-15-21)16-13-19-9-5-3-6-10-19/h3-12,21H,2,13-18H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114649
BindingDB Entry DOI: 10.7270/Q2F47T3Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50590778
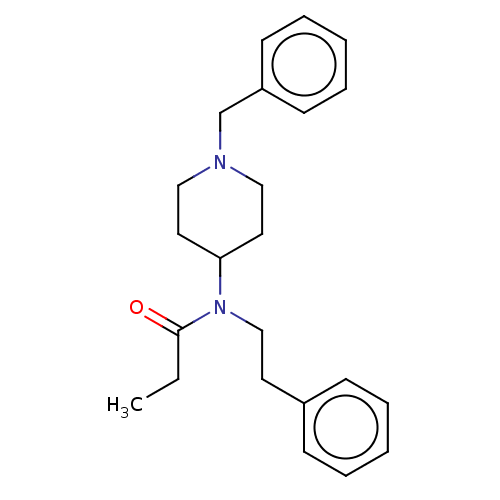
(CHEMBL5182393) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114649
BindingDB Entry DOI: 10.7270/Q2F47T3Z |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50581499
(CHEMBL5086596)Show SMILES CCC(=O)N(CC(C)N1CCC(Cc2ccccc2)CC1)c1ccc(Cl)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from MOR in Sprague-Dawley rat brain membranes measured after 90 mins by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113879
BindingDB Entry DOI: 10.7270/Q26W9FZS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50556105
(CHEMBL4743324)Show SMILES Cn1ccc2ccc(OCCCN3CCC(CC3)c3noc4cc(F)ccc34)cc2c1=O | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]ketanserin from rat cerebral cortex 5HT2A receptor measured after 15 mins by liquid scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112709
BindingDB Entry DOI: 10.7270/Q2XK8K6M |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50564175
(CHEMBL4788719) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-diprenorphin from human mu opioid receptor expressed in CHO cell membrane incubated for 150 mins by liquid scintillation countin... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112144
BindingDB Entry DOI: 10.7270/Q2WM1J54 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]spiperone from D2 receptor in rat striatum measured after 15 mins by liquid scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112709
BindingDB Entry DOI: 10.7270/Q2XK8K6M |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50564175

(CHEMBL4788719) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from MOR in Sprague-Dawley rat brain membranes measured after 90 mins by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113879
BindingDB Entry DOI: 10.7270/Q26W9FZS |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50581497
(CHEMBL5075339)Show SMILES CC(CN(C(=O)c1ccco1)c1ccccc1)N1CCC(Cc2ccccc2)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from MOR in Sprague-Dawley rat brain membranes measured after 90 mins by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113879
BindingDB Entry DOI: 10.7270/Q26W9FZS |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50001043

(6,11-Dimethyl-3-phenethyl-1,2,3,4,5,6-hexahydro-2,...)Show SMILES C[C@@H]1[C@@H]2Cc3ccc(O)cc3[C@]1(C)CCN2CCc1ccccc1 |TLB:16:15:1:10.4.3| Show InChI InChI=1S/C22H27NO/c1-16-21-14-18-8-9-19(24)15-20(18)22(16,2)11-13-23(21)12-10-17-6-4-3-5-7-17/h3-9,15-16,21,24H,10-14H2,1-2H3/t16-,21+,22-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50001043

(6,11-Dimethyl-3-phenethyl-1,2,3,4,5,6-hexahydro-2,...)Show SMILES C[C@@H]1[C@@H]2Cc3ccc(O)cc3[C@]1(C)CCN2CCc1ccccc1 |TLB:16:15:1:10.4.3| Show InChI InChI=1S/C22H27NO/c1-16-21-14-18-8-9-19(24)15-20(18)22(16,2)11-13-23(21)12-10-17-6-4-3-5-7-17/h3-9,15-16,21,24H,10-14H2,1-2H3/t16-,21+,22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114649
BindingDB Entry DOI: 10.7270/Q2F47T3Z |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50001019

(6,11-Dimethyl-3-phenethyl-1,2,3,4,5,6-hexahydro-2,...)Show SMILES C[C@H]1[C@H]2Cc3ccc(O)cc3[C@@]1(C)CCN2CCc1ccccc1 |TLB:16:15:1:10.4.3| Show InChI InChI=1S/C22H27NO/c1-16-21-14-18-8-9-19(24)15-20(18)22(16,2)11-13-23(21)12-10-17-6-4-3-5-7-17/h3-9,15-16,21,24H,10-14H2,1-2H3/t16-,21+,22-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-(+)-pnetazocin from sigma1 receptor in guinea pig brain membrane incubated for 120 mins by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112144
BindingDB Entry DOI: 10.7270/Q2WM1J54 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50564178
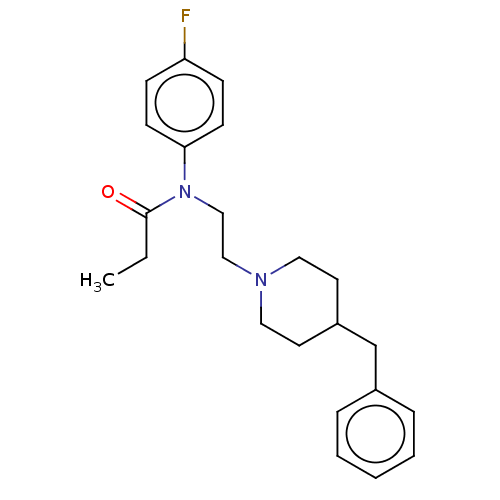
(CHEMBL4785048) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-(+)-pnetazocin from sigma1 receptor in guinea pig brain membrane incubated for 150 mins by liquid scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112144
BindingDB Entry DOI: 10.7270/Q2WM1J54 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50581508
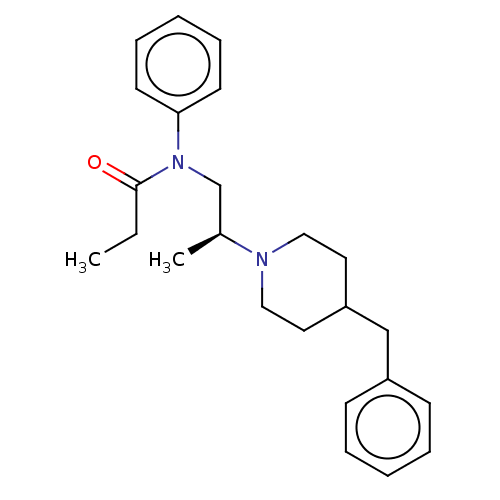
(CHEMBL5087973)Show SMILES CCC(=O)N(C[C@H](C)N1CCC(Cc2ccccc2)CC1)c1ccccc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from MOR in Sprague-Dawley rat brain membranes measured after 90 mins by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113879
BindingDB Entry DOI: 10.7270/Q26W9FZS |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50581508

(CHEMBL5087973)Show SMILES CCC(=O)N(C[C@H](C)N1CCC(Cc2ccccc2)CC1)c1ccccc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114649
BindingDB Entry DOI: 10.7270/Q2F47T3Z |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50581498
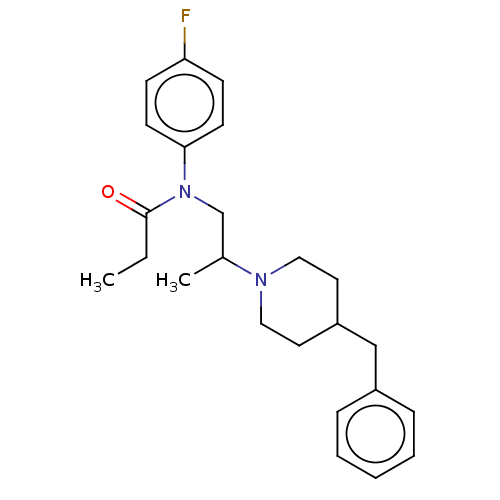
(CHEMBL5079619)Show SMILES CCC(=O)N(CC(C)N1CCC(Cc2ccccc2)CC1)c1ccc(F)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from MOR in Sprague-Dawley rat brain membranes measured after 90 mins by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113879
BindingDB Entry DOI: 10.7270/Q26W9FZS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM423299

(US10501452, Compound 5)Show SMILES CN1CCc2ccc(OCCCN3CCC(CC3)c3noc4cc(F)ccc34)cc2C1=O Show InChI InChI=1S/C25H28FN3O3/c1-28-11-7-17-3-5-20(16-22(17)25(28)30)31-14-2-10-29-12-8-18(9-13-29)24-21-6-4-19(26)15-23(21)32-27-24/h3-6,15-16,18H,2,7-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]5-CT from rat cerebral cortex 5HT7 receptor measured after 30 mins by liquid scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112709
BindingDB Entry DOI: 10.7270/Q2XK8K6M |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50581496
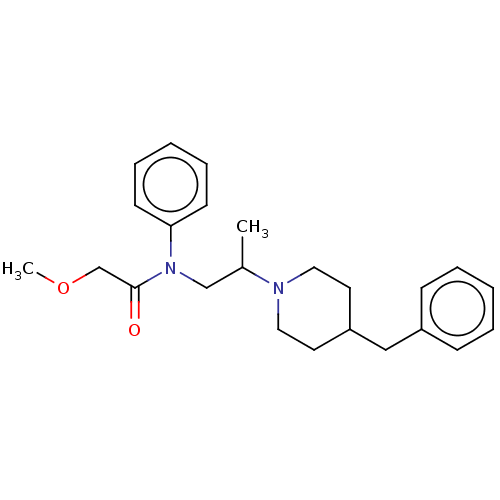
(CHEMBL5087044)Show SMILES COCC(=O)N(CC(C)N1CCC(Cc2ccccc2)CC1)c1ccccc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from MOR in Sprague-Dawley rat brain membranes measured after 90 mins by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113879
BindingDB Entry DOI: 10.7270/Q26W9FZS |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50581498

(CHEMBL5079619)Show SMILES CCC(=O)N(CC(C)N1CCC(Cc2ccccc2)CC1)c1ccc(F)cc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-(+)-pentazocine from sigma-1 receptor in guinea pig brain membranes incubated for 90 mins by liquid scintillation counting metho... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113879
BindingDB Entry DOI: 10.7270/Q26W9FZS |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50064196

((2S,6R,11S)-6,11-Dimethyl-3-[2-(methyl-phenyl-amin...)Show SMILES C[C@@H]1[C@@H]2Cc3ccc(O)cc3[C@]1(C)CCN2CCN(C)c1ccccc1 |TLB:16:15:1:10.4.3| Show InChI InChI=1S/C23H30N2O/c1-17-22-15-18-9-10-20(26)16-21(18)23(17,2)11-12-25(22)14-13-24(3)19-7-5-4-6-8-19/h4-10,16-17,22,26H,11-15H2,1-3H3/t17-,22+,23-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50564197

(CHEMBL4776178)Show SMILES CCC(=O)N(CCN1CCC(Cc2ccc(F)cc2)CC1)c1ccc(F)cc1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-diprenorphin from human mu opioid receptor expressed in CHO cell membrane incubated for 150 mins by liquid scintillation countin... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112144
BindingDB Entry DOI: 10.7270/Q2WM1J54 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50469504

(CHEMBL4293999)Show SMILES Fc1ccc2c(noc2c1)C1CCN(CCCCC(=O)c2cc3CCN4c3c(CCC4=O)c2)CC1 Show InChI InChI=1S/C28H30FN3O3/c29-22-5-6-23-25(17-22)35-30-27(23)18-8-12-31(13-9-18)11-2-1-3-24(33)21-15-19-4-7-26(34)32-14-10-20(16-21)28(19)32/h5-6,15-18H,1-4,7-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to 5HT6 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112709
BindingDB Entry DOI: 10.7270/Q2XK8K6M |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50064195

((2S,6R,11S)-6,11-Dimethyl-3-(2-phenylamino-ethyl)-...)Show SMILES C[C@@H]1[C@@H]2Cc3ccc(O)cc3[C@]1(C)CCN2CCNc1ccccc1 |TLB:16:15:1:10.4.3| Show InChI InChI=1S/C22H28N2O/c1-16-21-14-17-8-9-19(25)15-20(17)22(16,2)10-12-24(21)13-11-23-18-6-4-3-5-7-18/h3-9,15-16,21,23,25H,10-14H2,1-2H3/t16-,21+,22-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data