

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16047 ((4S)-4-[(2S)-2-[(2R,4S,5S)-5-[(2S)-2-[(2S)-2-[(4S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human beta-secretase | Bioorg Med Chem Lett 13: 4335-9 (2003) BindingDB Entry DOI: 10.7270/Q2513ZR3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16250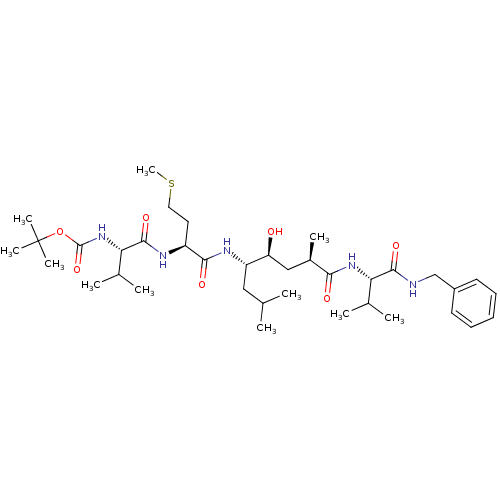 (CHEMBL290001 | N-(tert-butoxycarbonyl)-L-valyl-N-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Beta-secretase-1 in HEK293 (Human Embryonic Kidney) cell line. | Bioorg Med Chem Lett 14: 239-43 (2003) BindingDB Entry DOI: 10.7270/Q2MW2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16047 ((4S)-4-[(2S)-2-[(2R,4S,5S)-5-[(2S)-2-[(2S)-2-[(4S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Beta-secretase-1 in HEK293 (Human Embryonic Kidney) cell line. | Bioorg Med Chem Lett 14: 239-43 (2003) BindingDB Entry DOI: 10.7270/Q2MW2HP3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50250527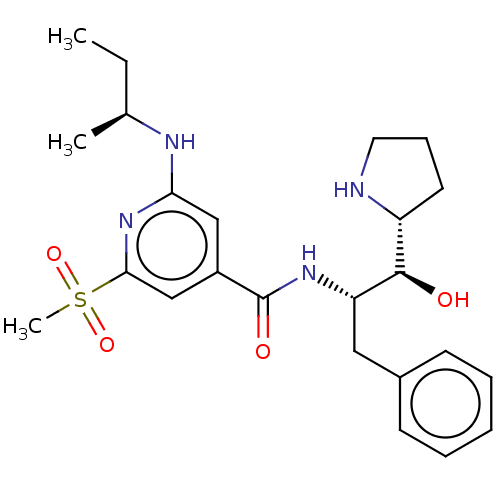 (CHEMBL4066837) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly SA Curated by ChEMBL | Assay Description Inhibition of Fc-fused BACE1 (1 to 460 residues) (unknown origin) expressed in HEK293 cells using mcaFRET peptide as substrate after 20 hrs by fluore... | J Med Chem 60: 9807-9820 (2017) Article DOI: 10.1021/acs.jmedchem.7b01304 BindingDB Entry DOI: 10.7270/Q29S1TGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50250537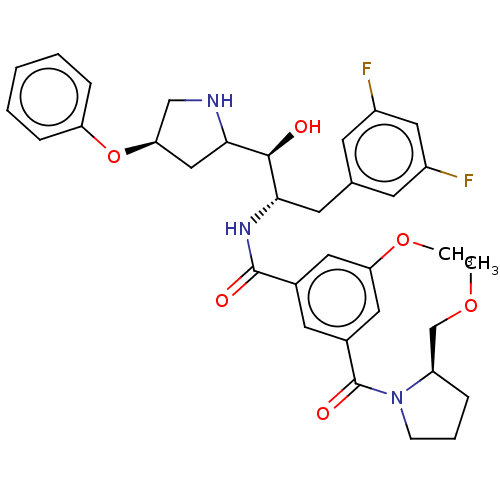 (CHEMBL4071843) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly SA Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) expressed in HEK293-APP751swe cells assessed as reduction in amyloid beta (1 to 40) level by sandwich-ELISA meth... | J Med Chem 60: 9807-9820 (2017) Article DOI: 10.1021/acs.jmedchem.7b01304 BindingDB Entry DOI: 10.7270/Q29S1TGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50397678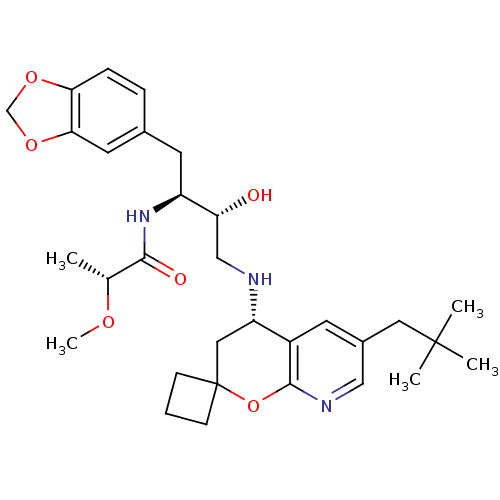 (CHEMBL2181880) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly SA Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) expressed in HEK293-APP751swe cells assessed as reduction in amyloid beta (1 to 40) level by sandwich-ELISA meth... | J Med Chem 60: 9807-9820 (2017) Article DOI: 10.1021/acs.jmedchem.7b01304 BindingDB Entry DOI: 10.7270/Q29S1TGX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50137678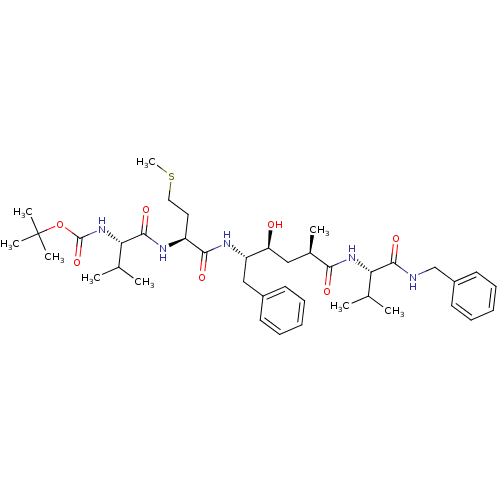 (CHEMBL312277 | [(S)-1-((S)-1-{(3S,4S)-1-Benzyl-4-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Beta-secretase-1 in HEK293 (Human Embryonic Kidney) cell line. | Bioorg Med Chem Lett 14: 239-43 (2003) BindingDB Entry DOI: 10.7270/Q2MW2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50366891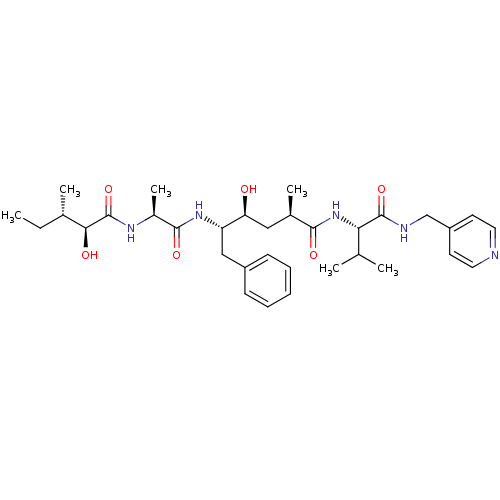 (CHEMBL1790644) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Beta-secretase 1 in human HEK293 cells | Bioorg Med Chem Lett 14: 245-50 (2003) BindingDB Entry DOI: 10.7270/Q2H41S0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50137699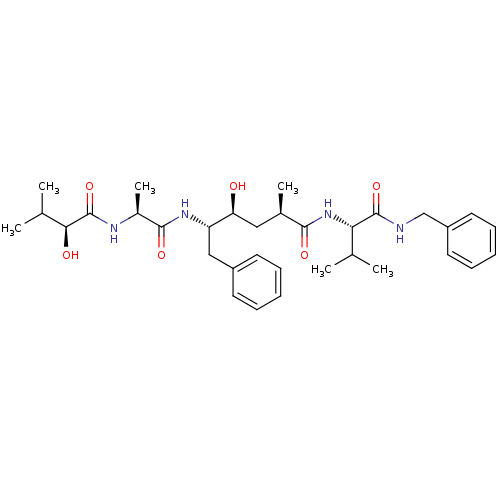 ((S)-4-(S)-Hydroxy-5-[(S)-2-((S)-2-hydroxy-3-methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Beta-secretase 1 in human HEK293 cells | Bioorg Med Chem Lett 14: 245-50 (2003) BindingDB Entry DOI: 10.7270/Q2H41S0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50137686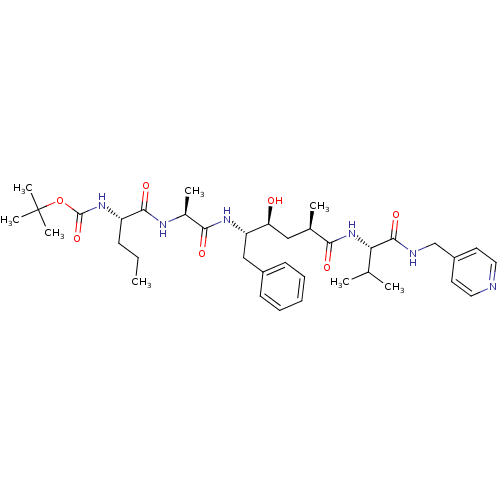 (CHEMBL78781 | {(S)-1-[(S)-1-((2S,4S)-1-Benzyl-2-hy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Beta-secretase-1 in HEK293 (Human Embryonic Kidney) cell line. | Bioorg Med Chem Lett 14: 239-43 (2003) BindingDB Entry DOI: 10.7270/Q2MW2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50366890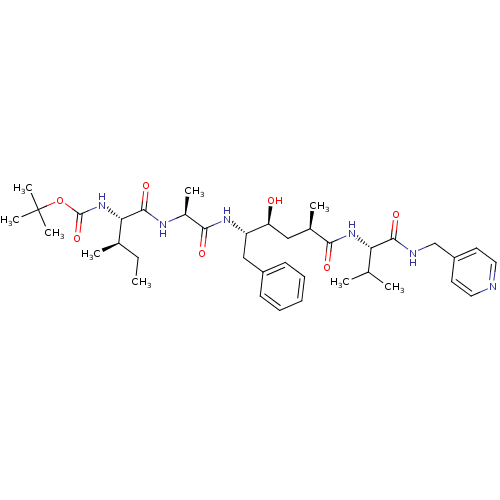 (CHEMBL1790594) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Beta-secretase-1 in HEK293 (Human Embryonic Kidney) cell line. | Bioorg Med Chem Lett 14: 239-43 (2003) BindingDB Entry DOI: 10.7270/Q2MW2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50137708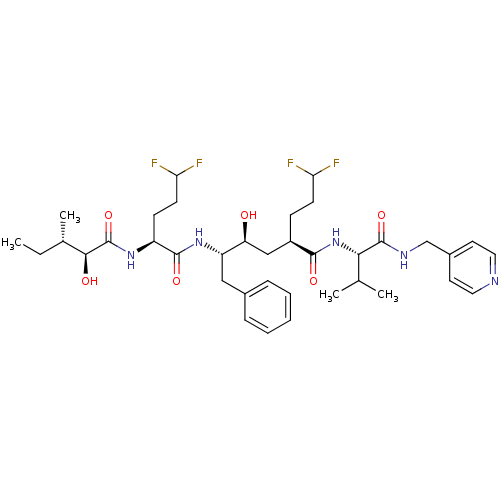 ((S)-5-[(S)-5,5-Difluoro-2-((2S,3S)-2-hydroxy-3-met...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Beta-secretase 1 in human HEK293 cells | Bioorg Med Chem Lett 14: 245-50 (2003) BindingDB Entry DOI: 10.7270/Q2H41S0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50137681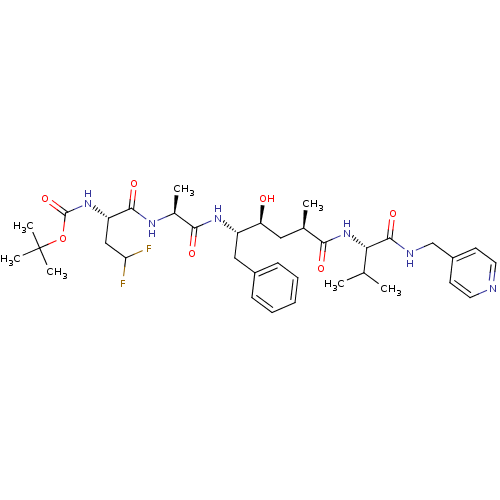 (CHEMBL81490 | {(S)-1-[(S)-1-((2S,4S)-1-Benzyl-2-hy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Beta-secretase-1 in HEK293 (Human Embryonic Kidney) cell line. | Bioorg Med Chem Lett 14: 239-43 (2003) BindingDB Entry DOI: 10.7270/Q2MW2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50136343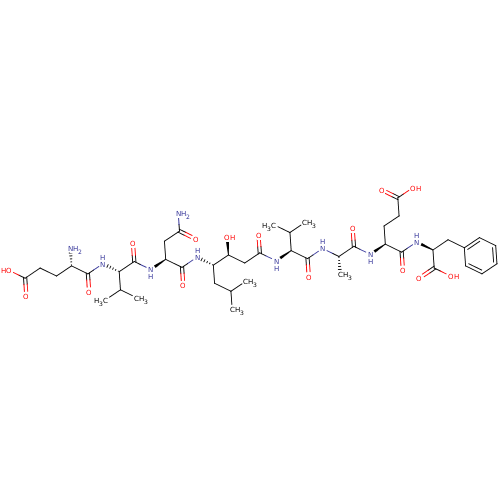 (CHEMBL137755 | Glu-Val-Asn-statine-Val-Ala-Glu-Phe) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human beta-secretase | Bioorg Med Chem Lett 13: 4335-9 (2003) BindingDB Entry DOI: 10.7270/Q2513ZR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50137691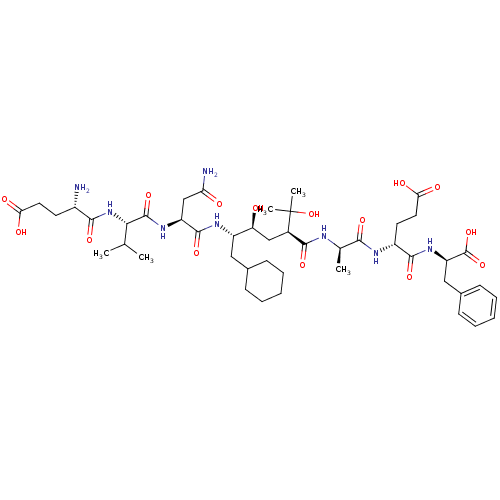 ((S)-5-{(S)-2-[(S)-2-((S)-2-Amino-4-carboxy-butyryl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Beta-secretase-1 in HEK293 (Human Embryonic Kidney) cell line. | Bioorg Med Chem Lett 14: 239-43 (2003) BindingDB Entry DOI: 10.7270/Q2MW2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50136347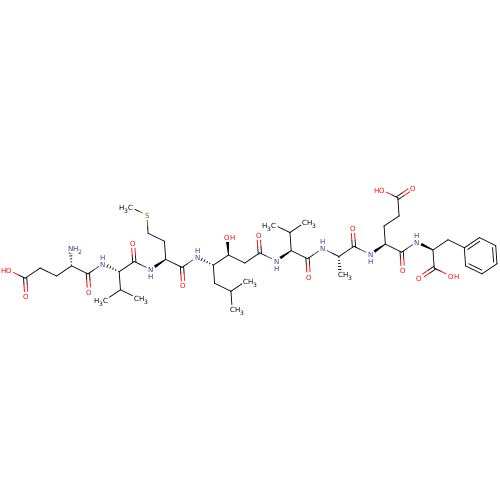 (CHEMBL335837 | Glu-Val-Met-statine-Val-Ala-Glu-Phe) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human beta-secretase | Bioorg Med Chem Lett 13: 4335-9 (2003) BindingDB Entry DOI: 10.7270/Q2513ZR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50137693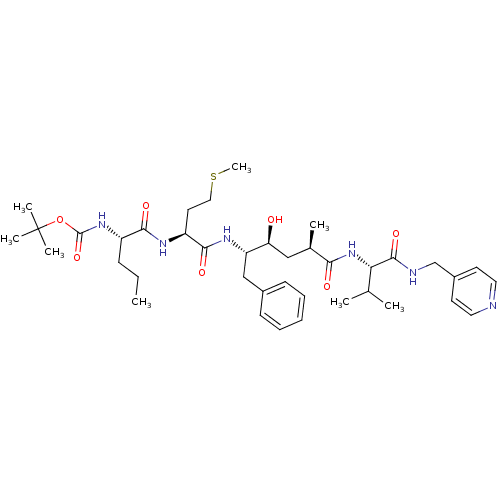 (CHEMBL81037 | {(S)-1-[(S)-1-((2S,4S)-1-Benzyl-2-hy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Beta-secretase-1 in HEK293 (Human Embryonic Kidney) cell line. | Bioorg Med Chem Lett 14: 239-43 (2003) BindingDB Entry DOI: 10.7270/Q2MW2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50137697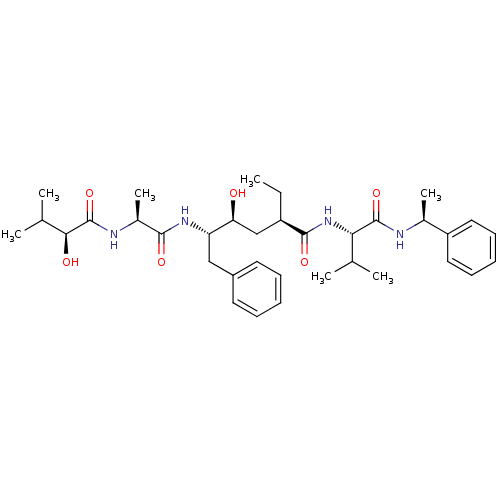 ((S)-2-Ethyl-4-(S)-hydroxy-5-[(S)-2-((S)-2-hydroxy-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Beta-secretase 1 in human HEK293 cells | Bioorg Med Chem Lett 14: 245-50 (2003) BindingDB Entry DOI: 10.7270/Q2H41S0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50137683 (CHEMBL309884 | {(S)-1-[(S)-1-((2S,4S)-1-Benzyl-2-h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Beta-secretase-1 in HEK293 (Human Embryonic Kidney) cell line. | Bioorg Med Chem Lett 14: 239-43 (2003) BindingDB Entry DOI: 10.7270/Q2MW2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM15805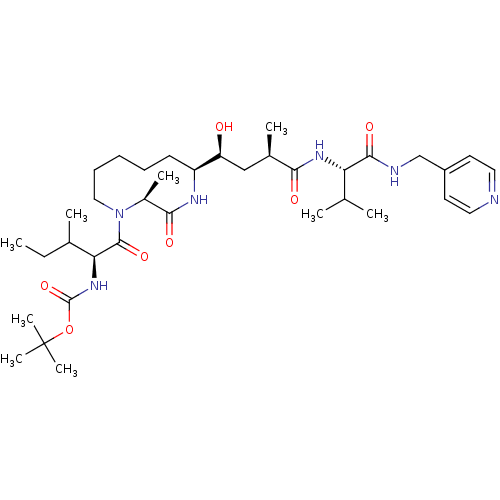 (Macrocyclic BACE inhibitor 7 | tert-butyl N-[(2S)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | 4.6 | 22 |
Lilly S.A. | Assay Description The substrate is linked to a fluorescent dye on one end and to a quenching group on its other end. The fluorescence of the substrate is significantly... | Bioorg Med Chem Lett 16: 191-5 (2006) Article DOI: 10.1016/j.bmcl.2005.09.003 BindingDB Entry DOI: 10.7270/Q2FJ2F26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50137706 ((S)-5-[(S)-5-Fluoro-2-((2S,3S)-2-hydroxy-3-methyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Beta-secretase 1 in human HEK293 cells | Bioorg Med Chem Lett 14: 245-50 (2003) BindingDB Entry DOI: 10.7270/Q2H41S0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50366850 (CHEMBL1791017) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human beta-secretase | Bioorg Med Chem Lett 13: 4335-9 (2003) BindingDB Entry DOI: 10.7270/Q2513ZR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50137690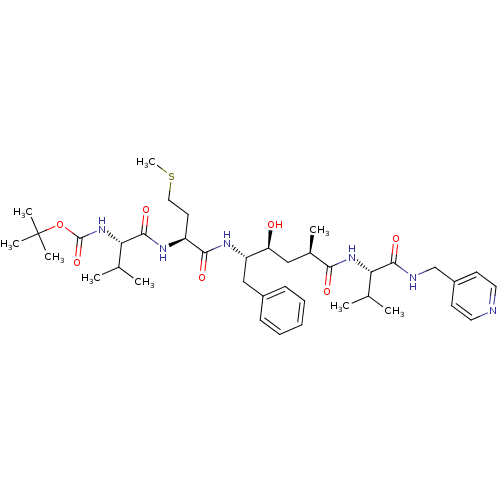 (CHEMBL310452 | {(S)-1-[(S)-1-((2S,4S)-1-Benzyl-2-h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Beta-secretase-1 in HEK293 (Human Embryonic Kidney) cell line. | Bioorg Med Chem Lett 14: 239-43 (2003) BindingDB Entry DOI: 10.7270/Q2MW2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50137684 (CHEMBL78459 | {(S)-1-[(S)-1-((2S,4S)-1-Benzyl-2-hy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Beta-secretase-1 in HEK293 (Human Embryonic Kidney) cell line. | Bioorg Med Chem Lett 14: 239-43 (2003) BindingDB Entry DOI: 10.7270/Q2MW2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50250536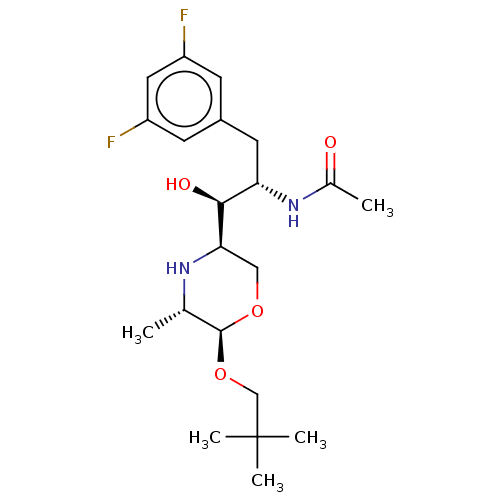 (CHEMBL4077530) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly SA Curated by ChEMBL | Assay Description Inhibition of Fc-fused BACE1 (1 to 460 residues) (unknown origin) expressed in HEK293 cells using mcaFRET peptide as substrate after 20 hrs by fluore... | J Med Chem 60: 9807-9820 (2017) Article DOI: 10.1021/acs.jmedchem.7b01304 BindingDB Entry DOI: 10.7270/Q29S1TGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM15799 (CHEMBL81671 | Macrocyclic BACE inhibitor 1 | tert-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Beta-secretase-1 in HEK293 (Human Embryonic Kidney) cell line. | Bioorg Med Chem Lett 14: 239-43 (2003) BindingDB Entry DOI: 10.7270/Q2MW2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM15799 (CHEMBL81671 | Macrocyclic BACE inhibitor 1 | tert-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Beta-secretase 1 in human HEK293 cells | Bioorg Med Chem Lett 14: 245-50 (2003) BindingDB Entry DOI: 10.7270/Q2H41S0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM15799 (CHEMBL81671 | Macrocyclic BACE inhibitor 1 | tert-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | 4.6 | 22 |
Lilly S.A. | Assay Description The substrate is linked to a fluorescent dye on one end and to a quenching group on its other end. The fluorescence of the substrate is significantly... | Bioorg Med Chem Lett 16: 191-5 (2006) Article DOI: 10.1016/j.bmcl.2005.09.003 BindingDB Entry DOI: 10.7270/Q2FJ2F26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50366842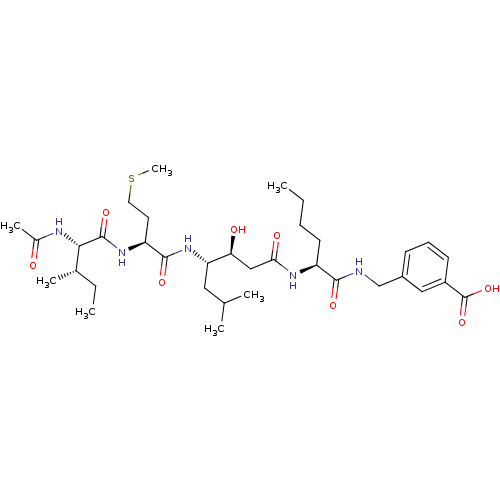 (CHEMBL1791018) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human beta-secretase | Bioorg Med Chem Lett 13: 4335-9 (2003) BindingDB Entry DOI: 10.7270/Q2513ZR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50250536 (CHEMBL4077530) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly SA Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) expressed in HEK293-APP751swe cells assessed as reduction in amyloid beta (1 to 40) level by sandwich-ELISA meth... | J Med Chem 60: 9807-9820 (2017) Article DOI: 10.1021/acs.jmedchem.7b01304 BindingDB Entry DOI: 10.7270/Q29S1TGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50366843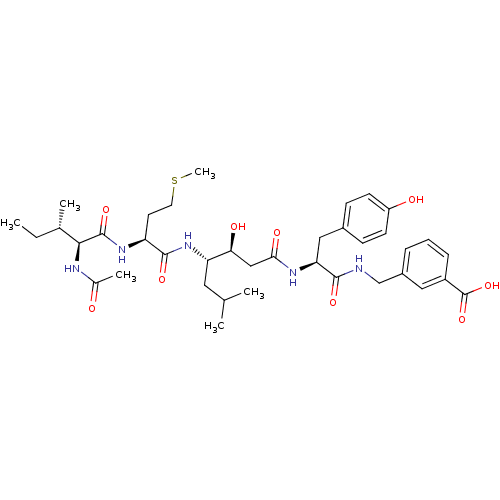 (CHEMBL1791010) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human beta-secretase | Bioorg Med Chem Lett 13: 4335-9 (2003) BindingDB Entry DOI: 10.7270/Q2513ZR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50250530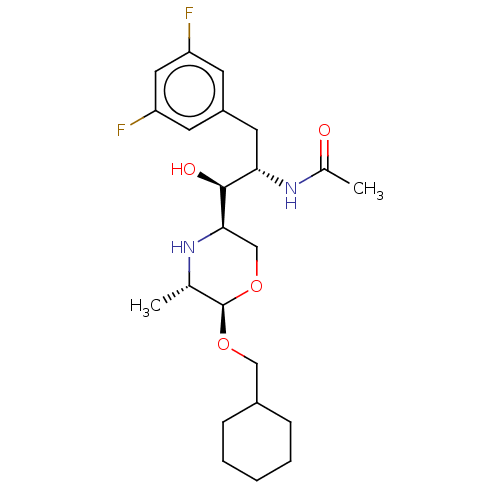 (CHEMBL4081363) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly SA Curated by ChEMBL | Assay Description Inhibition of Fc-fused BACE1 (1 to 460 residues) (unknown origin) expressed in HEK293 cells using mcaFRET peptide as substrate after 20 hrs by fluore... | J Med Chem 60: 9807-9820 (2017) Article DOI: 10.1021/acs.jmedchem.7b01304 BindingDB Entry DOI: 10.7270/Q29S1TGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50137694 ((S)-2-Ethyl-4-(S)-hydroxy-5-[(S)-2-((S)-2-hydroxy-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Beta-secretase 1 in human HEK293 cells | Bioorg Med Chem Lett 14: 245-50 (2003) BindingDB Entry DOI: 10.7270/Q2H41S0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50250527 (CHEMBL4066837) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly SA Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) expressed in HEK293-APP751swe cells assessed as reduction in amyloid beta (1 to 40) level by sandwich-ELISA meth... | J Med Chem 60: 9807-9820 (2017) Article DOI: 10.1021/acs.jmedchem.7b01304 BindingDB Entry DOI: 10.7270/Q29S1TGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50136339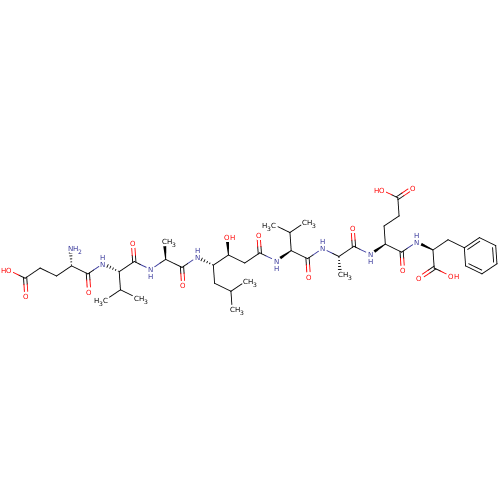 ((S)-4-{(S)-2-[(S)-2-((3S,4S)-4-{(S)-2-[(S)-2-((S)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human beta-secretase | Bioorg Med Chem Lett 13: 4335-9 (2003) BindingDB Entry DOI: 10.7270/Q2513ZR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50136333 ((S)-4-{(S)-2-[(S)-2-((3S,4S)-4-{(S)-2-[(S)-2-((S)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibitory activity against human brain beta-APP (amyloid precursor protein) cleaving enzyme Beta-secretase | Bioorg Med Chem Lett 13: 4335-9 (2003) BindingDB Entry DOI: 10.7270/Q2513ZR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50366847 (CHEMBL1791012) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human beta-secretase | Bioorg Med Chem Lett 13: 4335-9 (2003) BindingDB Entry DOI: 10.7270/Q2513ZR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50137682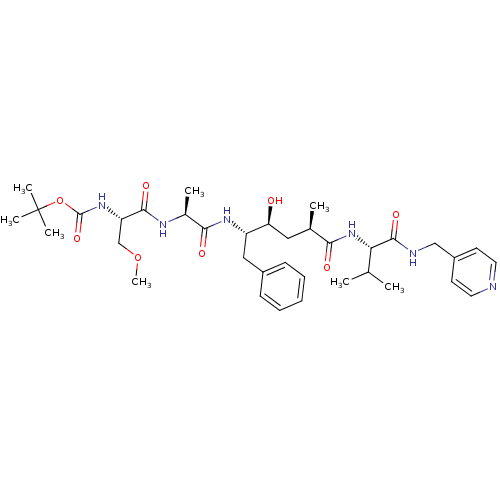 (CHEMBL82594 | {(S)-1-[(S)-1-((2S,4S)-1-Benzyl-2-hy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 118 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Beta-secretase-1 in HEK293 (Human Embryonic Kidney) cell line. | Bioorg Med Chem Lett 14: 239-43 (2003) BindingDB Entry DOI: 10.7270/Q2MW2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50137688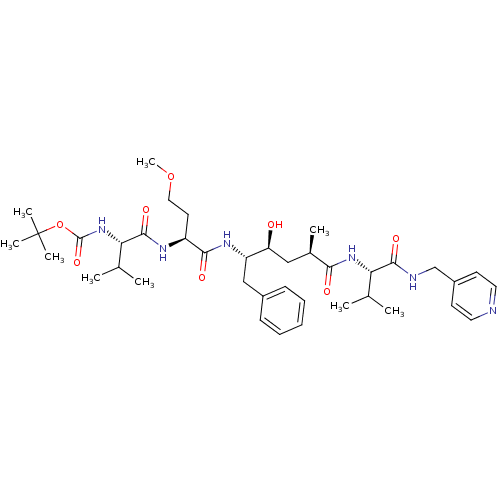 (CHEMBL314014 | {(S)-1-[(S)-1-((2S,4S)-1-Benzyl-2-h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Beta-secretase-1 in HEK293 (Human Embryonic Kidney) cell line. | Bioorg Med Chem Lett 14: 239-43 (2003) BindingDB Entry DOI: 10.7270/Q2MW2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50366851 (CHEMBL1791016) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human beta-secretase | Bioorg Med Chem Lett 13: 4335-9 (2003) BindingDB Entry DOI: 10.7270/Q2513ZR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50137710 ((2S,3S)-4-Hydroxy-5-[(S)-2-((S)-2-hydroxy-3-methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Beta-secretase 1 in human HEK293 cells | Bioorg Med Chem Lett 14: 245-50 (2003) BindingDB Entry DOI: 10.7270/Q2H41S0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50366849 (CHEMBL1791011) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human beta-secretase | Bioorg Med Chem Lett 13: 4335-9 (2003) BindingDB Entry DOI: 10.7270/Q2513ZR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50136342 (CHEMBL134730 | Glu-Val-Met-statine-Leu-Ala-Glu-Phe) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human beta-secretase | Bioorg Med Chem Lett 13: 4335-9 (2003) BindingDB Entry DOI: 10.7270/Q2513ZR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50137695 ((S)-4-(S)-Hydroxy-5-[(S)-2-((2S,3S)-2-hydroxy-3-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 176 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Beta-secretase 1 in human HEK293 cells | Bioorg Med Chem Lett 14: 245-50 (2003) BindingDB Entry DOI: 10.7270/Q2H41S0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50366846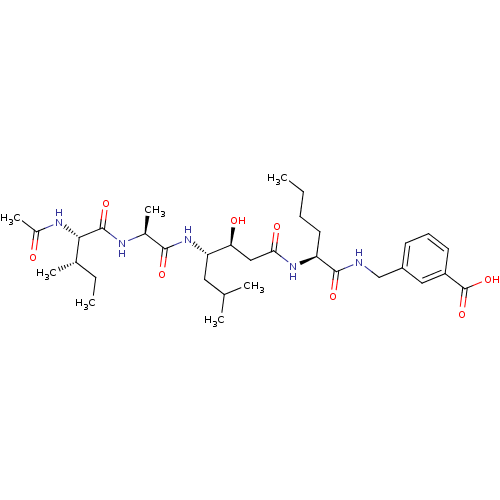 (CHEMBL1791009) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human beta-secretase | Bioorg Med Chem Lett 13: 4335-9 (2003) BindingDB Entry DOI: 10.7270/Q2513ZR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50137705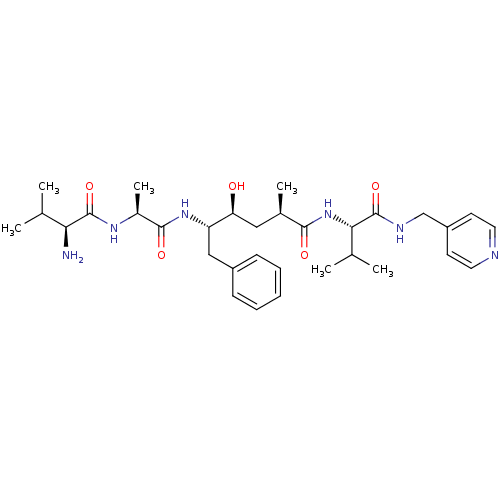 ((S)-5-[(S)-2-((S)-2-Amino-3-methyl-butyrylamino)-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Beta-secretase 1 in human HEK293 cells | Bioorg Med Chem Lett 14: 245-50 (2003) BindingDB Entry DOI: 10.7270/Q2H41S0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50250530 (CHEMBL4081363) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 205 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly SA Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) expressed in HEK293-APP751swe cells assessed as reduction in amyloid beta (1 to 40) level by sandwich-ELISA meth... | J Med Chem 60: 9807-9820 (2017) Article DOI: 10.1021/acs.jmedchem.7b01304 BindingDB Entry DOI: 10.7270/Q29S1TGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50250529 (CHEMBL4089395) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly SA Curated by ChEMBL | Assay Description Inhibition of Fc-fused BACE1 (1 to 460 residues) (unknown origin) expressed in HEK293 cells using mcaFRET peptide as substrate after 20 hrs by fluore... | J Med Chem 60: 9807-9820 (2017) Article DOI: 10.1021/acs.jmedchem.7b01304 BindingDB Entry DOI: 10.7270/Q29S1TGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50366848 (CHEMBL1791021) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human beta-secretase | Bioorg Med Chem Lett 13: 4335-9 (2003) BindingDB Entry DOI: 10.7270/Q2513ZR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50137704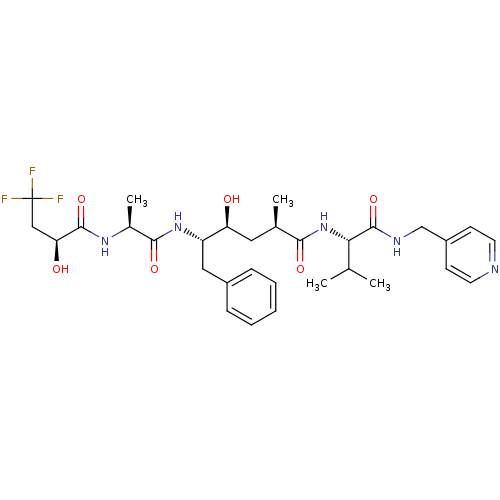 ((S)-4-(S)-Hydroxy-2-methyl-6-phenyl-5-[(S)-2-((S)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Beta-secretase 1 in human HEK293 cells | Bioorg Med Chem Lett 14: 245-50 (2003) BindingDB Entry DOI: 10.7270/Q2H41S0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 99 total ) | Next | Last >> |