 Found 294 hits with Last Name = 'yang' and Initial = 'jj'
Found 294 hits with Last Name = 'yang' and Initial = 'jj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein cereblon
(Homo sapiens (Human)) | BDBM50586185
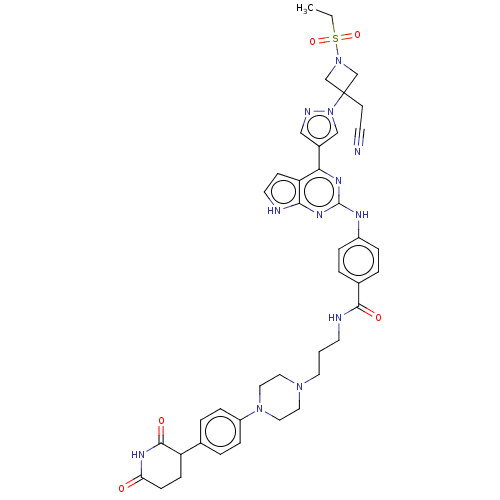
(CHEMBL5090416)Show SMILES CCS(=O)(=O)N1CC(CC#N)(C1)n1cc(cn1)-c1nc(Nc2ccc(cc2)C(=O)NCCCN2CCN(CC2)c2ccc(cc2)C2CCC(=O)NC2=O)nc2[nH]ccc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to CRBN (unknown origin) measured by Fluorescent Polarization assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00650
BindingDB Entry DOI: 10.7270/Q2D79GBF |
More data for this
Ligand-Target Pair | |
Protein cereblon
(Homo sapiens (Human)) | BDBM50586179
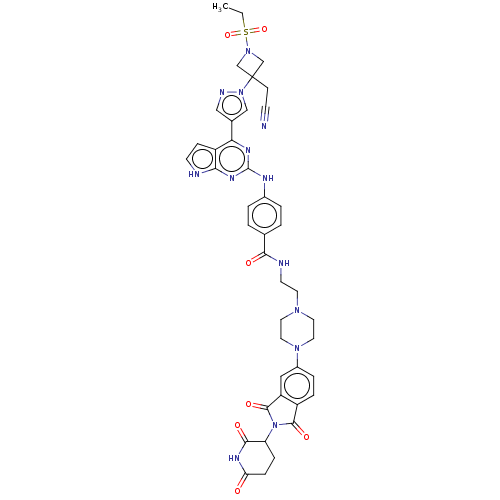
(CHEMBL4744617)Show SMILES CCS(=O)(=O)N1CC(CC#N)(C1)n1cc(cn1)-c1nc(Nc2ccc(cc2)C(=O)NCCN2CCN(CC2)c2ccc3C(=O)N(C4CCC(=O)NC4=O)C(=O)c3c2)nc2[nH]ccc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to CRBN (unknown origin) measured by Fluorescent Polarization assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00650
BindingDB Entry DOI: 10.7270/Q2D79GBF |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50520104
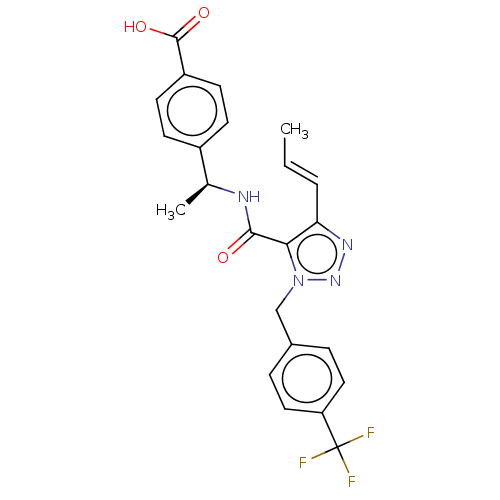
(CHEMBL4535971)Show SMILES C\C=C\c1nnn(Cc2ccc(cc2)C(F)(F)F)c1C(=O)N[C@@H](C)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C23H21F3N4O3/c1-3-4-19-20(21(31)27-14(2)16-7-9-17(10-8-16)22(32)33)30(29-28-19)13-15-5-11-18(12-6-15)23(24,25)26/h3-12,14H,13H2,1-2H3,(H,27,31)(H,32,33)/b4-3+/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human EP4 transfected in human HEK293 cells co transfected with SmBit-beta-arrestin. assessed as reduction in PGE2 induced-beta-arresti... |
J Med Chem 63: 569-590 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01269
BindingDB Entry DOI: 10.7270/Q2N58QRT |
More data for this
Ligand-Target Pair | |
Protein cereblon
(Homo sapiens (Human)) | BDBM50586184

(CHEMBL5078429)Show SMILES CCS(=O)(=O)N1CC(CC#N)(C1)n1cc(cn1)-c1nc(Nc2ccc(cc2)C(=O)NCCN2CCN(CC2)c2ccc(cc2)C2CCC(=O)NC2=O)nc2[nH]ccc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to CRBN (unknown origin) measured by Fluorescent Polarization assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00650
BindingDB Entry DOI: 10.7270/Q2D79GBF |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50339608

(3-(1-Amino-1-methylethyl)-N-{(2R)-7-[(7-oxo-5,6,7,...)Show SMILES CC(C)(N)c1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C29H29F3N4O3/c1-28(2,33)19-11-18(12-20(15-19)29(30,31)32)27(38)35-21-5-3-16-4-6-22(14-17(16)13-21)39-24-9-10-34-26-23(24)7-8-25(37)36-26/h4,6,9-12,14-15,21H,3,5,7-8,13,33H2,1-2H3,(H,35,38)(H,34,36,37)/t21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of RET |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
Protein cereblon
(Homo sapiens (Human)) | BDBM50586180

(CHEMBL5082689)Show SMILES CCS(=O)(=O)N1CC(CC#N)(C1)n1cc(cn1)-c1nc(Nc2ccc(cc2)C(=O)NCCCCNc2cccc(c2)C2CCC(=O)NC2=O)nc2[nH]ccc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to CRBN (unknown origin) measured by Fluorescent Polarization assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00650
BindingDB Entry DOI: 10.7270/Q2D79GBF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50339609

(3-[(Dimethylamino)methyl]-N-{(2R)-7-[(7-oxo-5,6,7,...)Show SMILES CN(C)Cc1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C29H29F3N4O3/c1-36(2)16-17-11-20(13-21(12-17)29(30,31)32)28(38)34-22-5-3-18-4-6-23(15-19(18)14-22)39-25-9-10-33-27-24(25)7-8-26(37)35-27/h4,6,9-13,15,22H,3,5,7-8,14,16H2,1-2H3,(H,34,38)(H,33,35,37)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Abl1 |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50520069

(CHEMBL4457944)Show SMILES C[C@H](NC(=O)c1c(nnn1CCOc1cccc(c1)C(F)(F)F)-c1ccccc1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C27H23F3N4O4/c1-17(18-10-12-20(13-11-18)26(36)37)31-25(35)24-23(19-6-3-2-4-7-19)32-33-34(24)14-15-38-22-9-5-8-21(16-22)27(28,29)30/h2-13,16-17H,14-15H2,1H3,(H,31,35)(H,36,37)/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP4 expressed in CHO cells coexpressing G16-alpha assessed as intracellular calcium flux preincubated for 15 mins follow... |
J Med Chem 63: 569-590 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01269
BindingDB Entry DOI: 10.7270/Q2N58QRT |
More data for this
Ligand-Target Pair | |
Protein cereblon
(Homo sapiens (Human)) | BDBM50586176
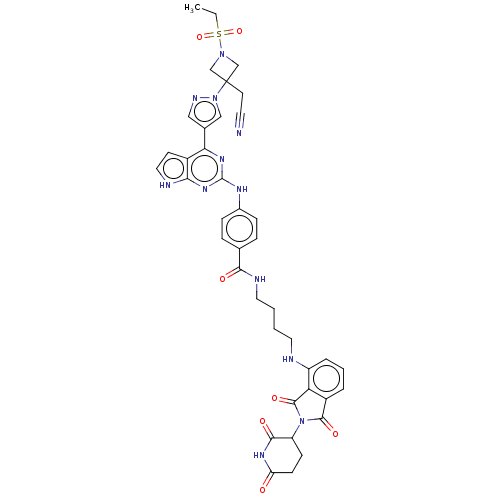
(CHEMBL4742130)Show SMILES CCS(=O)(=O)N1CC(CC#N)(C1)n1cc(cn1)-c1nc(Nc2ccc(cc2)C(=O)NCCCCNc2cccc3C(=O)N(C4CCC(=O)NC4=O)C(=O)c23)nc2[nH]ccc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to CRBN (unknown origin) measured by Fluorescent Polarization assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00650
BindingDB Entry DOI: 10.7270/Q2D79GBF |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50339609

(3-[(Dimethylamino)methyl]-N-{(2R)-7-[(7-oxo-5,6,7,...)Show SMILES CN(C)Cc1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C29H29F3N4O3/c1-36(2)16-17-11-20(13-21(12-17)29(30,31)32)28(38)34-22-5-3-18-4-6-23(15-19(18)14-22)39-25-9-10-33-27-24(25)7-8-26(37)35-27/h4,6,9-13,15,22H,3,5,7-8,14,16H2,1-2H3,(H,34,38)(H,33,35,37)/t22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of RET |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50339609

(3-[(Dimethylamino)methyl]-N-{(2R)-7-[(7-oxo-5,6,7,...)Show SMILES CN(C)Cc1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C29H29F3N4O3/c1-36(2)16-17-11-20(13-21(12-17)29(30,31)32)28(38)34-22-5-3-18-4-6-23(15-19(18)14-22)39-25-9-10-33-27-24(25)7-8-26(37)35-27/h4,6,9-13,15,22H,3,5,7-8,14,16H2,1-2H3,(H,34,38)(H,33,35,37)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of C-Raf |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50339608

(3-(1-Amino-1-methylethyl)-N-{(2R)-7-[(7-oxo-5,6,7,...)Show SMILES CC(C)(N)c1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C29H29F3N4O3/c1-28(2,33)19-11-18(12-20(15-19)29(30,31)32)27(38)35-21-5-3-16-4-6-22(14-17(16)13-21)39-24-9-10-34-26-23(24)7-8-25(37)36-26/h4,6,9-12,14-15,21H,3,5,7-8,13,33H2,1-2H3,(H,35,38)(H,34,36,37)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of C-Raf |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
Discoidin domain-containing receptor 2
(Homo sapiens (Human)) | BDBM50339609

(3-[(Dimethylamino)methyl]-N-{(2R)-7-[(7-oxo-5,6,7,...)Show SMILES CN(C)Cc1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C29H29F3N4O3/c1-36(2)16-17-11-20(13-21(12-17)29(30,31)32)28(38)34-22-5-3-18-4-6-23(15-19(18)14-22)39-25-9-10-33-27-24(25)7-8-26(37)35-27/h4,6,9-13,15,22H,3,5,7-8,14,16H2,1-2H3,(H,34,38)(H,33,35,37)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of DDR2 |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50520101
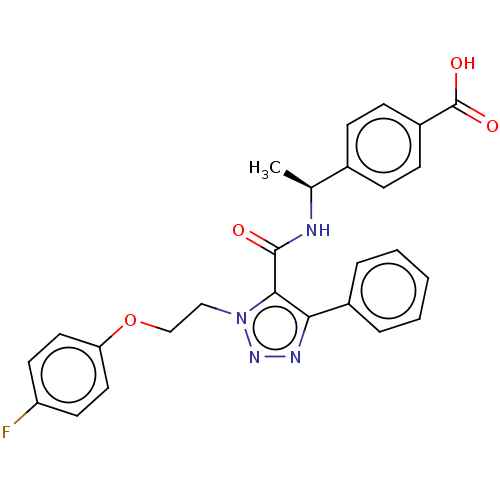
(CHEMBL4439228)Show SMILES C[C@H](NC(=O)c1c(nnn1CCOc1ccc(F)cc1)-c1ccccc1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C26H23FN4O4/c1-17(18-7-9-20(10-8-18)26(33)34)28-25(32)24-23(19-5-3-2-4-6-19)29-30-31(24)15-16-35-22-13-11-21(27)12-14-22/h2-14,17H,15-16H2,1H3,(H,28,32)(H,33,34)/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP4 expressed in CHO cells coexpressing G16-alpha assessed as intracellular calcium flux preincubated for 15 mins follow... |
J Med Chem 63: 569-590 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01269
BindingDB Entry DOI: 10.7270/Q2N58QRT |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50339615

((2S)-7-({2-[(Cyclopropylcarbonyl)amino]pyridin-4-y...)Show SMILES CN(C)Cc1cc(NC(=O)[C@H]2CCc3ccc(Oc4ccnc(NC(=O)C5CC5)c4)cc3C2)cc(c1)C(F)(F)F |r| Show InChI InChI=1S/C30H31F3N4O3/c1-37(2)17-18-11-23(30(31,32)33)15-24(12-18)35-29(39)21-6-3-19-7-8-25(14-22(19)13-21)40-26-9-10-34-27(16-26)36-28(38)20-4-5-20/h7-12,14-16,20-21H,3-6,13,17H2,1-2H3,(H,35,39)(H,34,36,38)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of C-Raf assessed as reduction in [33P]ATP incorporation into biotinylated substrate peptide after 3 hrs by flash plate assay |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
Protein cereblon
(Homo sapiens (Human)) | BDBM50586177

(CHEMBL5090604)Show SMILES CCS(=O)(=O)N1CC(CC#N)(C1)n1cc(cn1)-c1nc(Nc2ccc(cc2)C(=O)NCCCCOc2cccc3C(=O)N(C4CCC(=O)NC4=O)C(=O)c23)nc2[nH]ccc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to CRBN (unknown origin) measured by Fluorescent Polarization assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00650
BindingDB Entry DOI: 10.7270/Q2D79GBF |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50339608

(3-(1-Amino-1-methylethyl)-N-{(2R)-7-[(7-oxo-5,6,7,...)Show SMILES CC(C)(N)c1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C29H29F3N4O3/c1-28(2,33)19-11-18(12-20(15-19)29(30,31)32)27(38)35-21-5-3-16-4-6-22(14-17(16)13-21)39-24-9-10-34-26-23(24)7-8-25(37)36-26/h4,6,9-12,14-15,21H,3,5,7-8,13,33H2,1-2H3,(H,35,38)(H,34,36,37)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild-type B-Raf |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50339613

((2S)-N-[3-(aminomethyl)-5-(trifluoromethyl)phenyl]...)Show SMILES NCc1cc(NC(=O)[C@H]2CCc3ccc(Oc4ccnc(NC(=O)C5CC5)c4)cc3C2)cc(c1)C(F)(F)F |r| Show InChI InChI=1S/C28H27F3N4O3/c29-28(30,31)21-9-16(15-32)10-22(13-21)34-27(37)19-4-1-17-5-6-23(12-20(17)11-19)38-24-7-8-33-25(14-24)35-26(36)18-2-3-18/h5-10,12-14,18-19H,1-4,11,15,32H2,(H,34,37)(H,33,35,36)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of C-Raf assessed as reduction in [33P]ATP incorporation into biotinylated substrate peptide after 3 hrs by flash plate assay |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50339616

(7-({2-[(Cyclopropylcarbonyl)amino]pyridin-4-yl}oxy...)Show SMILES CC(C)NCc1cc(NC(=O)C2CCc3ccc(Oc4ccnc(NC(=O)C5CC5)c4)cc3C2)cc(c1)C(F)(F)F Show InChI InChI=1S/C31H33F3N4O3/c1-18(2)36-17-19-11-24(31(32,33)34)15-25(12-19)37-30(40)22-6-3-20-7-8-26(14-23(20)13-22)41-27-9-10-35-28(16-27)38-29(39)21-4-5-21/h7-12,14-16,18,21-22,36H,3-6,13,17H2,1-2H3,(H,37,40)(H,35,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of C-Raf assessed as reduction in [33P]ATP incorporation into biotinylated substrate peptide after 3 hrs by flash plate assay |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50520098
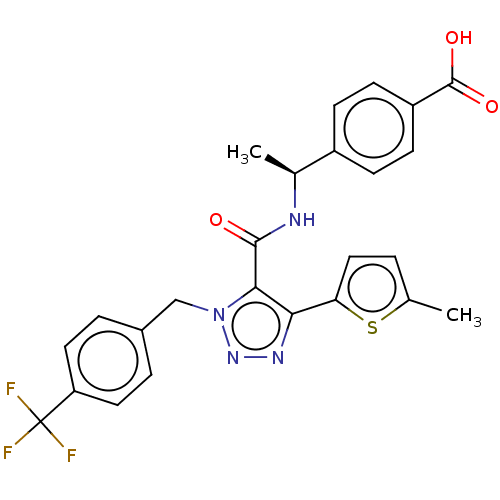
(CHEMBL4548291)Show SMILES C[C@H](NC(=O)c1c(nnn1Cc1ccc(cc1)C(F)(F)F)-c1ccc(C)s1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C25H21F3N4O3S/c1-14-3-12-20(36-14)21-22(23(33)29-15(2)17-6-8-18(9-7-17)24(34)35)32(31-30-21)13-16-4-10-19(11-5-16)25(26,27)28/h3-12,15H,13H2,1-2H3,(H,29,33)(H,34,35)/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP4 expressed in CHO cells coexpressing G16-alpha assessed as intracellular calcium flux preincubated for 15 mins follow... |
J Med Chem 63: 569-590 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01269
BindingDB Entry DOI: 10.7270/Q2N58QRT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50339609

(3-[(Dimethylamino)methyl]-N-{(2R)-7-[(7-oxo-5,6,7,...)Show SMILES CN(C)Cc1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C29H29F3N4O3/c1-36(2)16-17-11-20(13-21(12-17)29(30,31)32)28(38)34-22-5-3-18-4-6-23(15-19(18)14-22)39-25-9-10-33-27-24(25)7-8-26(37)35-27/h4,6,9-13,15,22H,3,5,7-8,14,16H2,1-2H3,(H,34,38)(H,33,35,37)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild-type B-Raf |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50520104

(CHEMBL4535971)Show SMILES C\C=C\c1nnn(Cc2ccc(cc2)C(F)(F)F)c1C(=O)N[C@@H](C)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C23H21F3N4O3/c1-3-4-19-20(21(31)27-14(2)16-7-9-17(10-8-16)22(32)33)30(29-28-19)13-15-5-11-18(12-6-15)23(24,25)26/h3-12,14H,13H2,1-2H3,(H,27,31)(H,32,33)/b4-3+/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human EP4 transfected in human HEK293 cells co transfected with CRE-luciferase assessed as reduction in PGE2-induced luciferase express... |
J Med Chem 63: 569-590 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01269
BindingDB Entry DOI: 10.7270/Q2N58QRT |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of C-Raf |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50339611
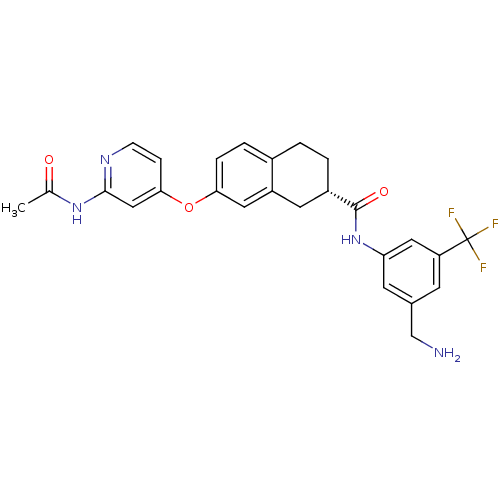
((2S)-7-{[2-(acetylamino)pyridin-4-yl]oxy}-N-[3-(am...)Show SMILES CC(=O)Nc1cc(Oc2ccc3CC[C@@H](Cc3c2)C(=O)Nc2cc(CN)cc(c2)C(F)(F)F)ccn1 |r| Show InChI InChI=1S/C26H25F3N4O3/c1-15(34)32-24-13-23(6-7-31-24)36-22-5-4-17-2-3-18(10-19(17)11-22)25(35)33-21-9-16(14-30)8-20(12-21)26(27,28)29/h4-9,11-13,18H,2-3,10,14,30H2,1H3,(H,33,35)(H,31,32,34)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of C-Raf assessed as reduction in [33P]ATP incorporation into biotinylated substrate peptide after 3 hrs by flash plate assay |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50339614

((2R)-N-[3-(aminomethyl)-5-(trifluoromethyl)phenyl]...)Show SMILES NCc1cc(NC(=O)[C@@H]2CCc3ccc(Oc4ccnc(NC(=O)C5CC5)c4)cc3C2)cc(c1)C(F)(F)F |r| Show InChI InChI=1S/C28H27F3N4O3/c29-28(30,31)21-9-16(15-32)10-22(13-21)34-27(37)19-4-1-17-5-6-23(12-20(17)11-19)38-24-7-8-33-25(14-24)35-26(36)18-2-3-18/h5-10,12-14,18-19H,1-4,11,15,32H2,(H,34,37)(H,33,35,36)/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of C-Raf assessed as reduction in [33P]ATP incorporation into biotinylated substrate peptide after 3 hrs by flash plate assay |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50520104

(CHEMBL4535971)Show SMILES C\C=C\c1nnn(Cc2ccc(cc2)C(F)(F)F)c1C(=O)N[C@@H](C)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C23H21F3N4O3/c1-3-4-19-20(21(31)27-14(2)16-7-9-17(10-8-16)22(32)33)30(29-28-19)13-15-5-11-18(12-6-15)23(24,25)26/h3-12,14H,13H2,1-2H3,(H,27,31)(H,32,33)/b4-3+/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP4 expressed in CHO cells coexpressing G16-alpha assessed as intracellular calcium flux preincubated for 15 mins follow... |
J Med Chem 63: 569-590 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01269
BindingDB Entry DOI: 10.7270/Q2N58QRT |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50339612

(CHEMBL1688868 | N-[3-(Aminomethyl)-5-(trifluoromet...)Show SMILES NCc1cc(NC(=O)C2CCc3ccc(Oc4ccnc(NC(=O)C5CC5)c4)cc3C2)cc(c1)C(F)(F)F Show InChI InChI=1S/C28H27F3N4O3/c29-28(30,31)21-9-16(15-32)10-22(13-21)34-27(37)19-4-1-17-5-6-23(12-20(17)11-19)38-24-7-8-33-25(14-24)35-26(36)18-2-3-18/h5-10,12-14,18-19H,1-4,11,15,32H2,(H,34,37)(H,33,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of C-Raf assessed as reduction in [33P]ATP incorporation into biotinylated substrate peptide after 3 hrs by flash plate assay |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50339608

(3-(1-Amino-1-methylethyl)-N-{(2R)-7-[(7-oxo-5,6,7,...)Show SMILES CC(C)(N)c1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C29H29F3N4O3/c1-28(2,33)19-11-18(12-20(15-19)29(30,31)32)27(38)35-21-5-3-16-4-6-22(14-17(16)13-21)39-24-9-10-34-26-23(24)7-8-25(37)36-26/h4,6,9-12,14-15,21H,3,5,7-8,13,33H2,1-2H3,(H,35,38)(H,34,36,37)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
Discoidin domain-containing receptor 2
(Homo sapiens (Human)) | BDBM50339608

(3-(1-Amino-1-methylethyl)-N-{(2R)-7-[(7-oxo-5,6,7,...)Show SMILES CC(C)(N)c1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C29H29F3N4O3/c1-28(2,33)19-11-18(12-20(15-19)29(30,31)32)27(38)35-21-5-3-16-4-6-22(14-17(16)13-21)39-24-9-10-34-26-23(24)7-8-25(37)36-26/h4,6,9-12,14-15,21H,3,5,7-8,13,33H2,1-2H3,(H,35,38)(H,34,36,37)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of DDR2 |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM119448
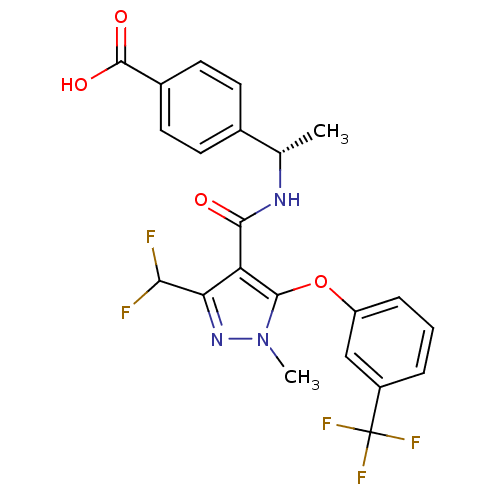
(US8686018, 107)Show SMILES C[C@H](NC(=O)c1c(nn(C)c1Oc1cccc(c1)C(F)(F)F)C(F)F)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C22H18F5N3O4/c1-11(12-6-8-13(9-7-12)21(32)33)28-19(31)16-17(18(23)24)29-30(2)20(16)34-15-5-3-4-14(10-15)22(25,26)27/h3-11,18H,1-2H3,(H,28,31)(H,32,33)/t11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP4 expressed in CHO cells coexpressing G16-alpha assessed as intracellular calcium flux preincubated for 15 mins follow... |
J Med Chem 63: 569-590 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01269
BindingDB Entry DOI: 10.7270/Q2N58QRT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50520086

(CHEMBL4463343)Show SMILES C[C@H](NC(=O)c1c(nnn1Cc1ccc(cc1)C(F)(F)F)-c1ccccc1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C26H21F3N4O3/c1-16(18-9-11-20(12-10-18)25(35)36)30-24(34)23-22(19-5-3-2-4-6-19)31-32-33(23)15-17-7-13-21(14-8-17)26(27,28)29/h2-14,16H,15H2,1H3,(H,30,34)(H,35,36)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP4 expressed in CHO cells coexpressing G16-alpha assessed as intracellular calcium flux preincubated for 15 mins follow... |
J Med Chem 63: 569-590 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01269
BindingDB Entry DOI: 10.7270/Q2N58QRT |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50339623

(4-[3-(3-{[4-Chloro-3-(trifluoromethyl)phenyl]amino...)Show SMILES CNC(=O)c1cc(Oc2cccc(CCC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)c2)ccn1 Show InChI InChI=1S/C23H19ClF3N3O3/c1-28-22(32)20-13-17(9-10-29-20)33-16-4-2-3-14(11-16)5-8-21(31)30-15-6-7-19(24)18(12-15)23(25,26)27/h2-4,6-7,9-13H,5,8H2,1H3,(H,28,32)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of C-Raf assessed as reduction in [33P]ATP incorporation into biotinylated substrate peptide after 3 hrs by flash plate assay |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50520114
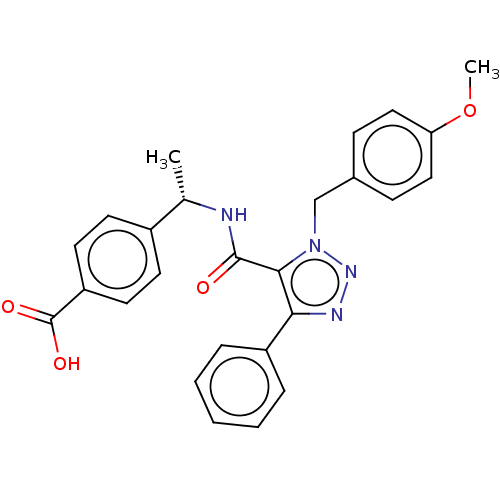
(CHEMBL4462095)Show SMILES COc1ccc(Cn2nnc(c2C(=O)N[C@@H](C)c2ccc(cc2)C(O)=O)-c2ccccc2)cc1 |r| Show InChI InChI=1S/C26H24N4O4/c1-17(19-10-12-21(13-11-19)26(32)33)27-25(31)24-23(20-6-4-3-5-7-20)28-29-30(24)16-18-8-14-22(34-2)15-9-18/h3-15,17H,16H2,1-2H3,(H,27,31)(H,32,33)/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP4 expressed in CHO cells coexpressing G16-alpha assessed as intracellular calcium flux preincubated for 15 mins follow... |
J Med Chem 63: 569-590 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01269
BindingDB Entry DOI: 10.7270/Q2N58QRT |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50339622
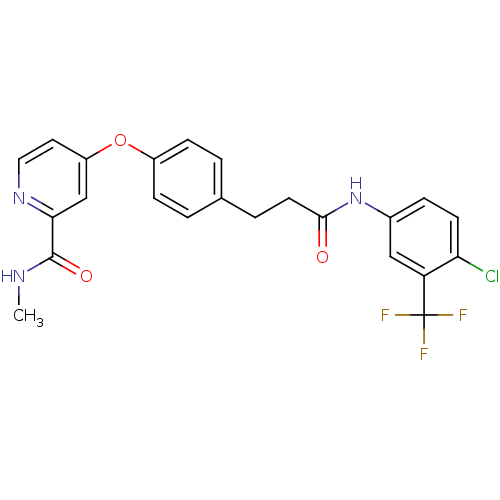
(4-[4-(3-{[4-Chloro-3-(trifluoromethyl)phenyl]amino...)Show SMILES CNC(=O)c1cc(Oc2ccc(CCC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C23H19ClF3N3O3/c1-28-22(32)20-13-17(10-11-29-20)33-16-6-2-14(3-7-16)4-9-21(31)30-15-5-8-19(24)18(12-15)23(25,26)27/h2-3,5-8,10-13H,4,9H2,1H3,(H,28,32)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of C-Raf assessed as reduction in [33P]ATP incorporation into biotinylated substrate peptide after 3 hrs by flash plate assay |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50520103

(CHEMBL4455965)Show SMILES C\C=C\c1nnn(Cc2ccc(cc2)C(F)(F)F)c1C(=O)NCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C22H19F3N4O3/c1-2-3-18-19(20(30)26-12-14-4-8-16(9-5-14)21(31)32)29(28-27-18)13-15-6-10-17(11-7-15)22(23,24)25/h2-11H,12-13H2,1H3,(H,26,30)(H,31,32)/b3-2+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP4 expressed in CHO cells coexpressing G16-alpha assessed as intracellular calcium flux preincubated for 15 mins follow... |
J Med Chem 63: 569-590 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01269
BindingDB Entry DOI: 10.7270/Q2N58QRT |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50339608

(3-(1-Amino-1-methylethyl)-N-{(2R)-7-[(7-oxo-5,6,7,...)Show SMILES CC(C)(N)c1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C29H29F3N4O3/c1-28(2,33)19-11-18(12-20(15-19)29(30,31)32)27(38)35-21-5-3-16-4-6-22(14-17(16)13-21)39-24-9-10-34-26-23(24)7-8-25(37)36-26/h4,6,9-12,14-15,21H,3,5,7-8,13,33H2,1-2H3,(H,35,38)(H,34,36,37)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EPHA2 |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50339610
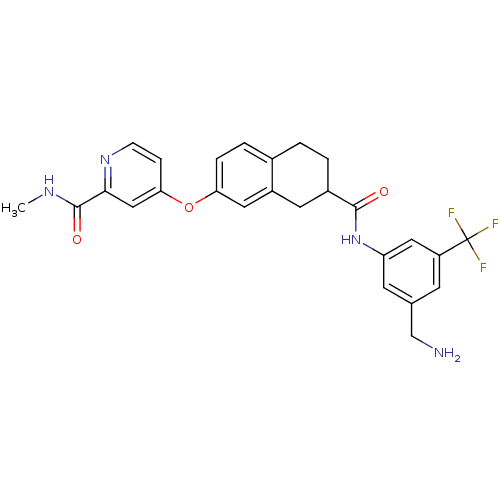
(4-{[7-({[3-(Aminomethyl)-5-(trifluoromethyl)phenyl...)Show SMILES CNC(=O)c1cc(Oc2ccc3CCC(Cc3c2)C(=O)Nc2cc(CN)cc(c2)C(F)(F)F)ccn1 Show InChI InChI=1S/C26H25F3N4O3/c1-31-25(35)23-13-22(6-7-32-23)36-21-5-4-16-2-3-17(10-18(16)11-21)24(34)33-20-9-15(14-30)8-19(12-20)26(27,28)29/h4-9,11-13,17H,2-3,10,14,30H2,1H3,(H,31,35)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of C-Raf assessed as reduction in [33P]ATP incorporation into biotinylated substrate peptide after 3 hrs by flash plate assay |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50520097
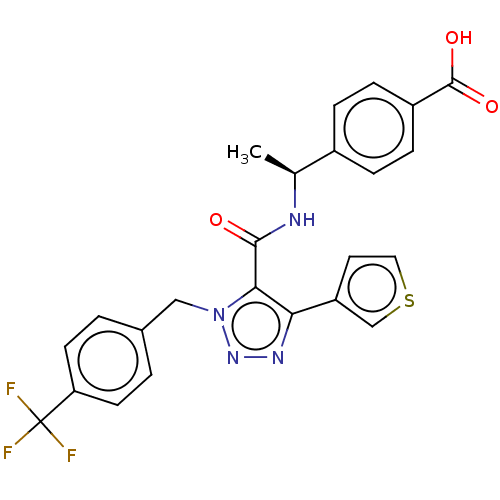
(CHEMBL4564092)Show SMILES C[C@H](NC(=O)c1c(nnn1Cc1ccc(cc1)C(F)(F)F)-c1ccsc1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C24H19F3N4O3S/c1-14(16-4-6-17(7-5-16)23(33)34)28-22(32)21-20(18-10-11-35-13-18)29-30-31(21)12-15-2-8-19(9-3-15)24(25,26)27/h2-11,13-14H,12H2,1H3,(H,28,32)(H,33,34)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP4 expressed in CHO cells coexpressing G16-alpha assessed as intracellular calcium flux preincubated for 15 mins follow... |
J Med Chem 63: 569-590 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01269
BindingDB Entry DOI: 10.7270/Q2N58QRT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50520072

(CHEMBL4550862)Show SMILES C\C=C\c1nnn(Cc2ccc(cc2)C(F)(F)F)c1C(=O)NCC1CCC(CC1)C(O)=O |(8.5,-22.36,;8.83,-23.87,;10.29,-24.35,;10.61,-25.86,;9.58,-27,;10.36,-28.33,;11.86,-28.01,;13,-29.04,;12.69,-30.55,;11.22,-31.02,;10.91,-32.52,;12.06,-33.55,;13.53,-33.06,;13.84,-31.56,;11.74,-35.06,;10.28,-35.54,;12.89,-36.08,;11.33,-36.54,;12.02,-26.48,;13.35,-25.7,;13.35,-24.16,;14.69,-26.48,;16.01,-25.7,;17.35,-26.47,;17.35,-28.02,;18.68,-28.79,;20.02,-28.02,;20.01,-26.47,;18.68,-25.7,;21.36,-28.78,;22.69,-28.01,;21.36,-30.33,)| Show InChI InChI=1S/C22H25F3N4O3/c1-2-3-18-19(20(30)26-12-14-4-8-16(9-5-14)21(31)32)29(28-27-18)13-15-6-10-17(11-7-15)22(23,24)25/h2-3,6-7,10-11,14,16H,4-5,8-9,12-13H2,1H3,(H,26,30)(H,31,32)/b3-2+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP4 expressed in CHO cells coexpressing G16-alpha assessed as intracellular calcium flux preincubated for 15 mins follow... |
J Med Chem 63: 569-590 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01269
BindingDB Entry DOI: 10.7270/Q2N58QRT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50520100

(CHEMBL4576681)Show SMILES C\C=C\c1nnn(Cc2ccc(cc2)C(F)(F)F)c1C(=O)NC1(CC1)c1ccc(cc1)C(O)=O Show InChI InChI=1S/C24H21F3N4O3/c1-2-3-19-20(31(30-29-19)14-15-4-8-18(9-5-15)24(25,26)27)21(32)28-23(12-13-23)17-10-6-16(7-11-17)22(33)34/h2-11H,12-14H2,1H3,(H,28,32)(H,33,34)/b3-2+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP4 expressed in CHO cells coexpressing G16-alpha assessed as intracellular calcium flux preincubated for 15 mins follow... |
J Med Chem 63: 569-590 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01269
BindingDB Entry DOI: 10.7270/Q2N58QRT |
More data for this
Ligand-Target Pair | |
Protein cereblon
(Homo sapiens (Human)) | BDBM50586187
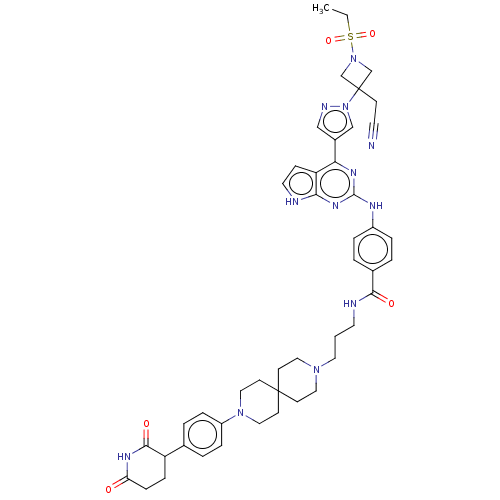
(CHEMBL5074539)Show SMILES CCS(=O)(=O)N1CC(CC#N)(C1)n1cc(cn1)-c1nc(Nc2ccc(cc2)C(=O)NCCCN2CCC3(CC2)CCN(CC3)c2ccc(cc2)C2CCC(=O)NC2=O)nc2[nH]ccc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to CRBN (unknown origin) measured by Fluorescent Polarization assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00650
BindingDB Entry DOI: 10.7270/Q2D79GBF |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Mus musculus (Mouse)) | BDBM50520104

(CHEMBL4535971)Show SMILES C\C=C\c1nnn(Cc2ccc(cc2)C(F)(F)F)c1C(=O)N[C@@H](C)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C23H21F3N4O3/c1-3-4-19-20(21(31)27-14(2)16-7-9-17(10-8-16)22(32)33)30(29-28-19)13-15-5-11-18(12-6-15)23(24,25)26/h3-12,14H,13H2,1-2H3,(H,27,31)(H,32,33)/b4-3+/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse EP4 expressed in CHO cells coexpressing G16-alpha assessed as intracellular calcium flux preincubated for 15 mins follow... |
J Med Chem 63: 569-590 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01269
BindingDB Entry DOI: 10.7270/Q2N58QRT |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50339625

(4-{[7-({[4-(Aminomethyl)-3-(trifluoromethyl)phenyl...)Show SMILES CNC(=O)c1cc(Oc2ccc3CCC(Cc3c2)C(=O)Nc2ccc(CN)c(c2)C(F)(F)F)ccn1 Show InChI InChI=1S/C26H25F3N4O3/c1-31-25(35)23-13-21(8-9-32-23)36-20-7-5-15-2-3-16(10-18(15)11-20)24(34)33-19-6-4-17(14-30)22(12-19)26(27,28)29/h4-9,11-13,16H,2-3,10,14,30H2,1H3,(H,31,35)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of C-Raf assessed as reduction in [33P]ATP incorporation into biotinylated substrate peptide after 3 hrs by flash plate assay |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50343948
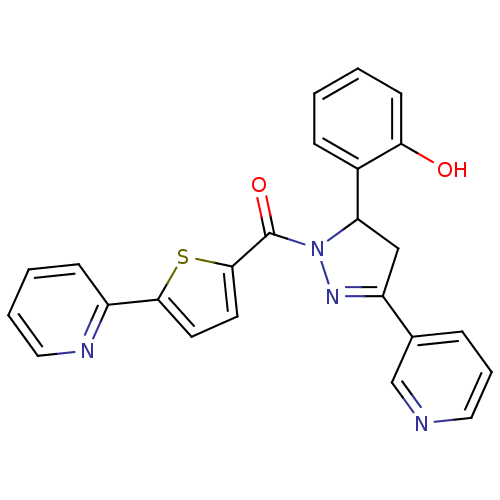
((5-(2-hydroxyphenyl)-3-(pyridin-3-yl)-4,5-dihydro-...)Show SMILES Oc1ccccc1C1CC(=NN1C(=O)c1ccc(s1)-c1ccccn1)c1cccnc1 |c:10| Show InChI InChI=1S/C24H18N4O2S/c29-21-9-2-1-7-17(21)20-14-19(16-6-5-12-25-15-16)27-28(20)24(30)23-11-10-22(31-23)18-8-3-4-13-26-18/h1-13,15,20,29H,14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf |
Bioorg Med Chem Lett 20: 4800-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.113
BindingDB Entry DOI: 10.7270/Q2MP53M9 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50339609

(3-[(Dimethylamino)methyl]-N-{(2R)-7-[(7-oxo-5,6,7,...)Show SMILES CN(C)Cc1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C29H29F3N4O3/c1-36(2)16-17-11-20(13-21(12-17)29(30,31)32)28(38)34-22-5-3-18-4-6-23(15-19(18)14-22)39-25-9-10-33-27-24(25)7-8-26(37)35-27/h4,6,9-13,15,22H,3,5,7-8,14,16H2,1-2H3,(H,34,38)(H,33,35,37)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EPHA2 |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
Protein cereblon
(Homo sapiens (Human)) | BDBM50586182

(CHEMBL5073737)Show SMILES CCS(=O)(=O)N1CC(CC#N)(C1)n1cc(cn1)-c1nc(Nc2ccc(cc2)C(=O)NCCCCOc2cccc(c2)C2CCC(=O)NC2=O)nc2[nH]ccc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to CRBN (unknown origin) measured by Fluorescent Polarization assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00650
BindingDB Entry DOI: 10.7270/Q2D79GBF |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50520104

(CHEMBL4535971)Show SMILES C\C=C\c1nnn(Cc2ccc(cc2)C(F)(F)F)c1C(=O)N[C@@H](C)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C23H21F3N4O3/c1-3-4-19-20(21(31)27-14(2)16-7-9-17(10-8-16)22(32)33)30(29-28-19)13-15-5-11-18(12-6-15)23(24,25)26/h3-12,14H,13H2,1-2H3,(H,27,31)(H,32,33)/b4-3+/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human EP4 transfected in human HEK293 cells assessed as reduction in PGE2-induced cAMP level incubated for 15 mins followed by PGE2 sti... |
J Med Chem 63: 569-590 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01269
BindingDB Entry DOI: 10.7270/Q2N58QRT |
More data for this
Ligand-Target Pair | |
Protein cereblon
(Homo sapiens (Human)) | BDBM50586178

(CHEMBL5092577)Show SMILES CCS(=O)(=O)N1CC(CC#N)(C1)n1cc(cn1)-c1nc(Nc2ccc(cc2)C(=O)NC2CCN(CC2)c2ccc3C(=O)N(C4CCC(=O)NC4=O)C(=O)c3c2)nc2[nH]ccc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to CRBN (unknown origin) measured by Fluorescent Polarization assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00650
BindingDB Entry DOI: 10.7270/Q2D79GBF |
More data for this
Ligand-Target Pair | |
Protein cereblon
(Homo sapiens (Human)) | BDBM50586181
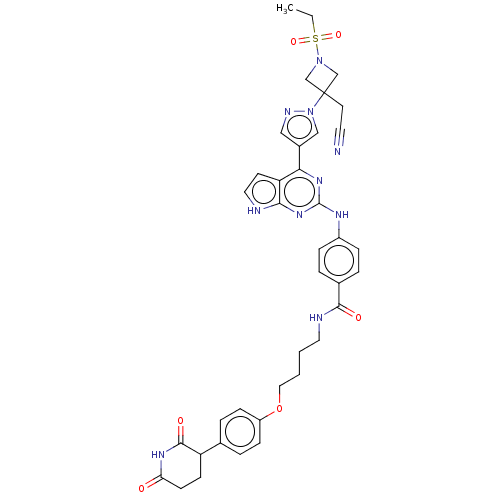
(CHEMBL5082136)Show SMILES CCS(=O)(=O)N1CC(CC#N)(C1)n1cc(cn1)-c1nc(Nc2ccc(cc2)C(=O)NCCCCOc2ccc(cc2)C2CCC(=O)NC2=O)nc2[nH]ccc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to CRBN (unknown origin) measured by Fluorescent Polarization assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00650
BindingDB Entry DOI: 10.7270/Q2D79GBF |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50520110
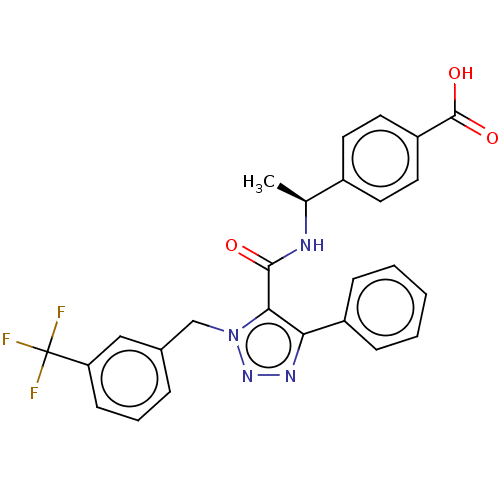
(CHEMBL4569593)Show SMILES C[C@H](NC(=O)c1c(nnn1Cc1cccc(c1)C(F)(F)F)-c1ccccc1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C26H21F3N4O3/c1-16(18-10-12-20(13-11-18)25(35)36)30-24(34)23-22(19-7-3-2-4-8-19)31-32-33(23)15-17-6-5-9-21(14-17)26(27,28)29/h2-14,16H,15H2,1H3,(H,30,34)(H,35,36)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP4 expressed in CHO cells coexpressing G16-alpha assessed as intracellular calcium flux preincubated for 15 mins follow... |
J Med Chem 63: 569-590 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01269
BindingDB Entry DOI: 10.7270/Q2N58QRT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data