

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cruzipain (Trypanosoma cruzi) | BDBM50229129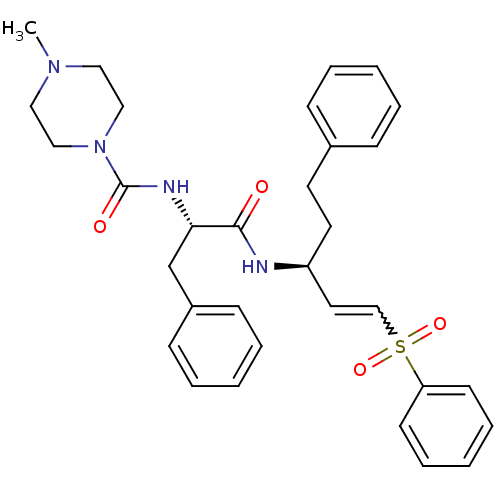 (4-Methyl-piperazine-1-carboxylic acid [(S)-1-((E)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Time dependent inhibition of Trypanosoma cruzi cruzain using Cbz-Phe-Arg-AMC as substrate assessed as inhibition constant for EI complex measured for... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00628 BindingDB Entry DOI: 10.7270/Q2445RC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50286450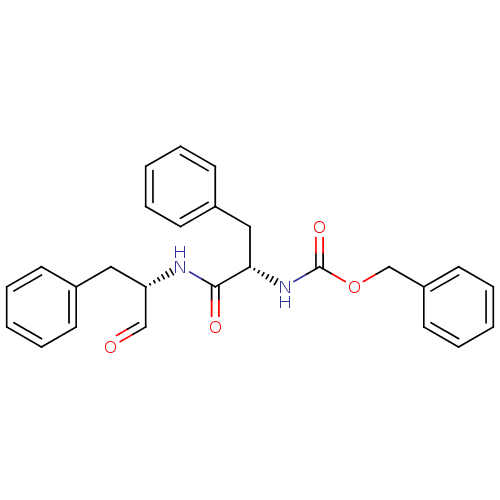 (CHEMBL128135 | [(S)-1-((S)-1-Benzyl-2-oxo-ethylcar...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Time dependent inhibition of Trypanosoma cruzi cruzain using Cbz-Phe-Arg-AMC as substrate assessed as inhibition constant for EI complex measured for... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00628 BindingDB Entry DOI: 10.7270/Q2445RC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50581372 (CHEMBL5079418) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Time dependent inhibition of Trypanosoma cruzi cruzain using Cbz-Phe-Arg-AMC as substrate assessed as inhibition constant for EI complex measured for... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00628 BindingDB Entry DOI: 10.7270/Q2445RC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496902 (CVD-0018409 | PF-07321332 | US11351149, Example 13...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00404 BindingDB Entry DOI: 10.7270/Q2222ZT1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM423466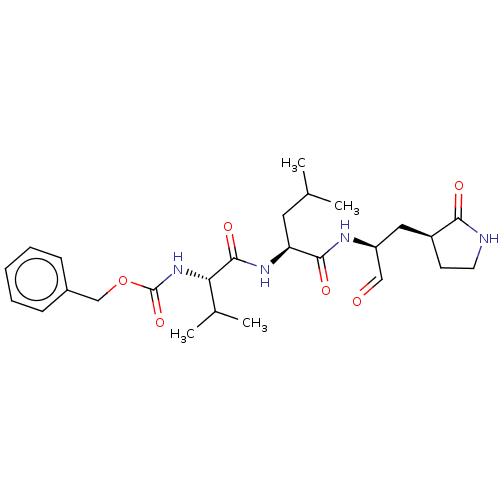 (WO2006061714, P39.1 | WO2006061714-ID-11 | cmdc.20...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.30 | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University | Assay Description The assays were carried out with 20 nM enzyme (except for MPI3, for which 10 nM enzyme was used) and 10 μM substrate at 37oC with continuous sha... | ChemMedChem (2020) Article DOI: 10.1002/cmdc.202000924 BindingDB Entry DOI: 10.7270/Q2G163V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM442754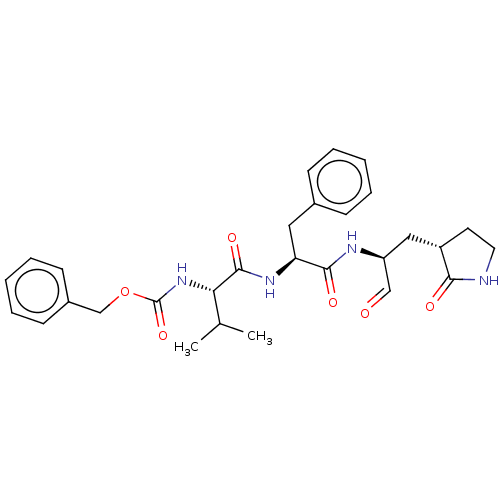 (MPI4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 15 | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University | Assay Description The assays were carried out with 20 nM enzyme (except for MPI3, for which 10 nM enzyme was used) and 10 μM substrate at 37oC with continuous sha... | ChemMedChem (2020) Article DOI: 10.1002/cmdc.202000924 BindingDB Entry DOI: 10.7270/Q2G163V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50581371 (CHEMBL5081709) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Time dependent inhibition of Trypanosoma cruzi cruzain using Cbz-Phe-Arg-AMC as substrate assessed as inhibition constant for EI complex measured for... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00628 BindingDB Entry DOI: 10.7270/Q2445RC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50581366 (CHEMBL5079571) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Time dependent inhibition of Trypanosoma cruzi cruzain using Cbz-Phe-Arg-AMC as substrate assessed as inhibition constant for EI complex measured for... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00628 BindingDB Entry DOI: 10.7270/Q2445RC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM420296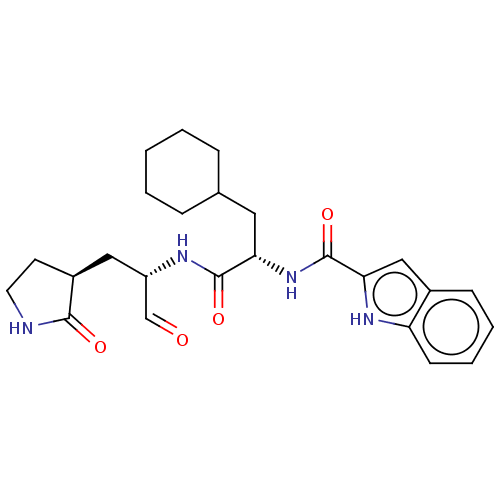 (Advanced SARS-CoV-2 Inhibitor 11a | MPI10 | acs.jm...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 30 | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University | Assay Description The assays were carried out with 20 nM enzyme (except for MPI3, for which 10 nM enzyme was used) and 10 μM substrate at 37oC with continuous sha... | ChemMedChem (2020) Article DOI: 10.1002/cmdc.202000924 BindingDB Entry DOI: 10.7270/Q2G163V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM419133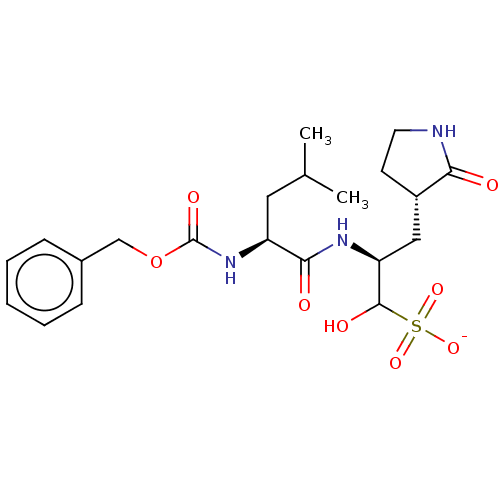 (BDBM429386 | GC376) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 30 | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University | Assay Description The assays were carried out with 20 nM enzyme (except for MPI3, for which 10 nM enzyme was used) and 10 μM substrate at 37oC with continuous sha... | ChemMedChem (2020) Article DOI: 10.1002/cmdc.202000924 BindingDB Entry DOI: 10.7270/Q2G163V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM442755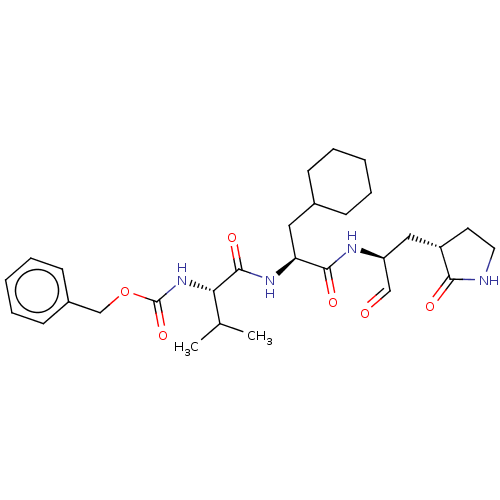 (MPI5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 32 | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University | Assay Description The assays were carried out with 20 nM enzyme (except for MPI3, for which 10 nM enzyme was used) and 10 μM substrate at 37oC with continuous sha... | ChemMedChem (2020) Article DOI: 10.1002/cmdc.202000924 BindingDB Entry DOI: 10.7270/Q2G163V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM442792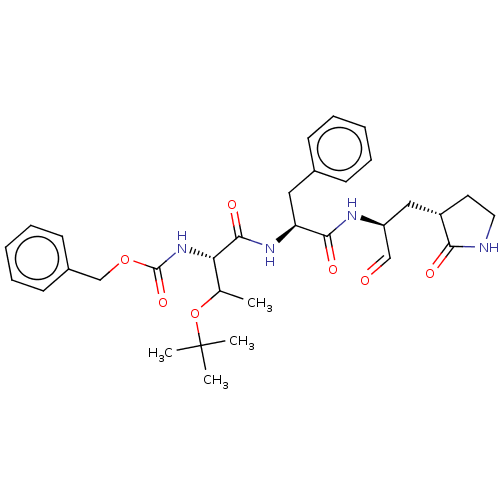 (MPI7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 46 | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University | Assay Description The assays were carried out with 20 nM enzyme (except for MPI3, for which 10 nM enzyme was used) and 10 μM substrate at 37oC with continuous sha... | ChemMedChem (2020) Article DOI: 10.1002/cmdc.202000924 BindingDB Entry DOI: 10.7270/Q2G163V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50581373 (CHEMBL5084365) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Time dependent inhibition of Trypanosoma cruzi cruzain using Cbz-Phe-Arg-AMC as substrate assessed as inhibition constant for EI complex measured for... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00628 BindingDB Entry DOI: 10.7270/Q2445RC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50581370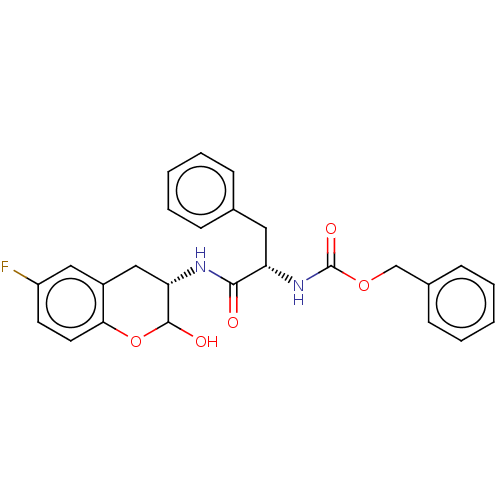 (CHEMBL5080091) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Time dependent inhibition of Trypanosoma cruzi cruzain using Cbz-Phe-Arg-AMC as substrate assessed as inhibition constant for EI complex measured for... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00628 BindingDB Entry DOI: 10.7270/Q2445RC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50581363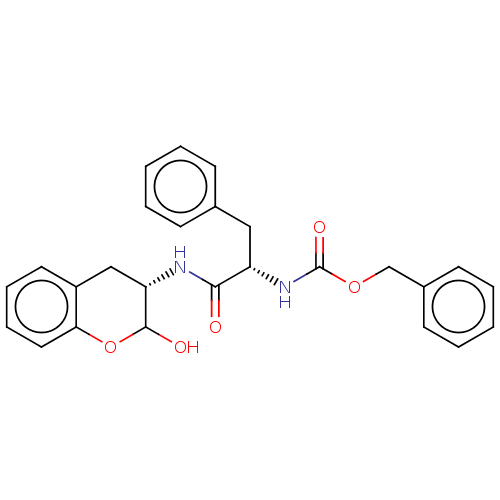 (CHEMBL5076256) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Time dependent inhibition of Trypanosoma cruzi cruzain using Cbz-Phe-Arg-AMC as substrate assessed as inhibition constant for EI complex measured for... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00628 BindingDB Entry DOI: 10.7270/Q2445RC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50581379 (CHEMBL5079773) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human Cathepsin L | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00628 BindingDB Entry DOI: 10.7270/Q2445RC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM442794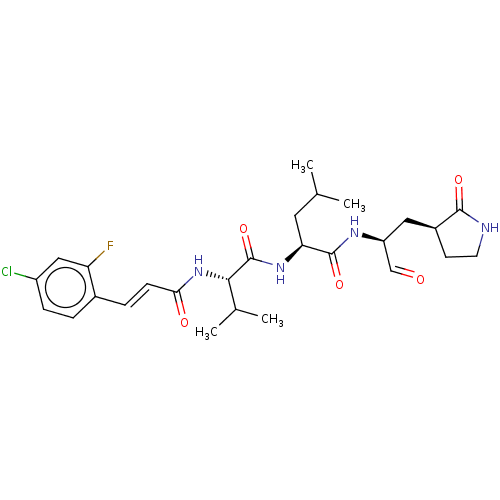 (MPI9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 55 | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University | Assay Description The assays were carried out with 20 nM enzyme (except for MPI3, for which 10 nM enzyme was used) and 10 μM substrate at 37oC with continuous sha... | ChemMedChem (2020) Article DOI: 10.1002/cmdc.202000924 BindingDB Entry DOI: 10.7270/Q2G163V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM442762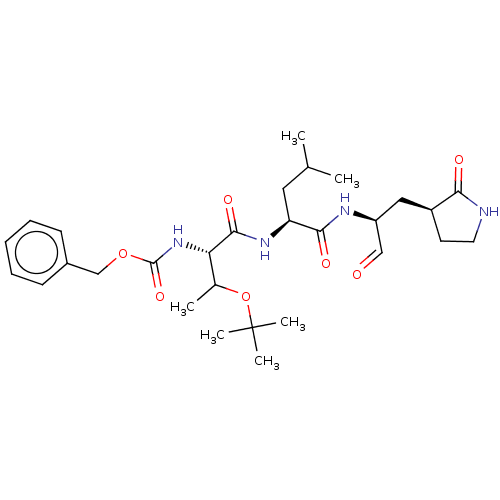 (MPI6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 59 | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University | Assay Description The assays were carried out with 20 nM enzyme (except for MPI3, for which 10 nM enzyme was used) and 10 μM substrate at 37oC with continuous sha... | ChemMedChem (2020) Article DOI: 10.1002/cmdc.202000924 BindingDB Entry DOI: 10.7270/Q2G163V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50581369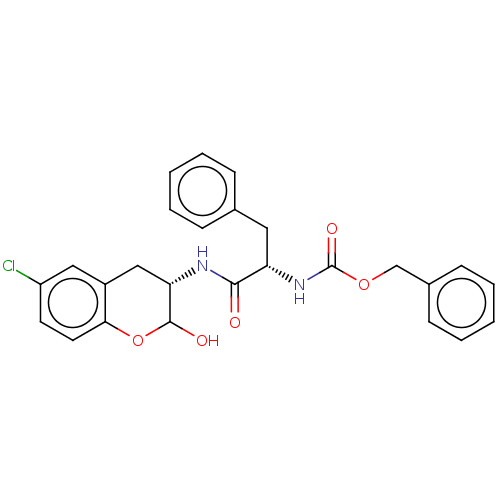 (CHEMBL5082439) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Time dependent inhibition of Trypanosoma cruzi cruzain using Cbz-Phe-Arg-AMC as substrate assessed as inhibition constant for EI complex measured for... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00628 BindingDB Entry DOI: 10.7270/Q2445RC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM442746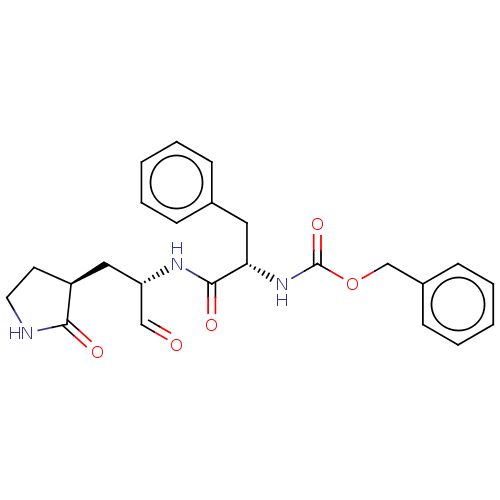 (MPI1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 98 | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University | Assay Description The assays were carried out with 20 nM enzyme (except for MPI3, for which 10 nM enzyme was used) and 10 μM substrate at 37oC with continuous sha... | ChemMedChem (2020) Article DOI: 10.1002/cmdc.202000924 BindingDB Entry DOI: 10.7270/Q2G163V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM429372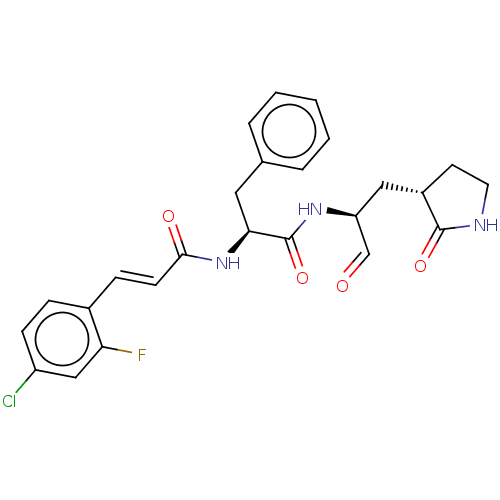 (MPI2 | med.21724, Compound 43) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 101 | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University | Assay Description The assays were carried out with 20 nM enzyme (except for MPI3, for which 10 nM enzyme was used) and 10 μM substrate at 37oC with continuous sha... | ChemMedChem (2020) Article DOI: 10.1002/cmdc.202000924 BindingDB Entry DOI: 10.7270/Q2G163V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM429100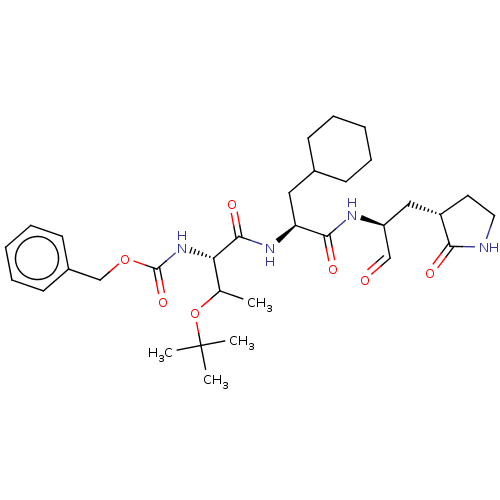 (MPI8 | jm5b01461, Compound 45) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 103 | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University | Assay Description The assays were carried out with 20 nM enzyme (except for MPI3, for which 10 nM enzyme was used) and 10 μM substrate at 37oC with continuous sha... | ChemMedChem (2020) Article DOI: 10.1002/cmdc.202000924 BindingDB Entry DOI: 10.7270/Q2G163V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50581368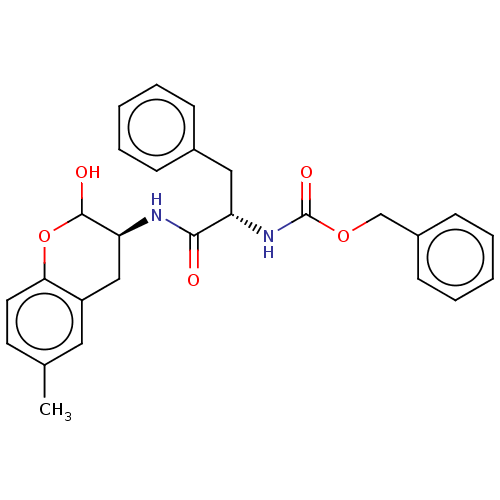 (CHEMBL5089591) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Time dependent inhibition of Trypanosoma cruzi cruzain using Cbz-Phe-Arg-AMC as substrate assessed as inhibition constant for EI complex measured for... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00628 BindingDB Entry DOI: 10.7270/Q2445RC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50581367 (CHEMBL5079029) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Time dependent inhibition of Trypanosoma cruzi cruzain using Cbz-Phe-Arg-AMC as substrate assessed as inhibition constant for EI complex measured for... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00628 BindingDB Entry DOI: 10.7270/Q2445RC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50581374 (CHEMBL5091908) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Time dependent inhibition of Trypanosoma cruzi cruzain using Cbz-Phe-Arg-AMC as substrate assessed as inhibition constant for EI complex measured for... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00628 BindingDB Entry DOI: 10.7270/Q2445RC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50581375 (CHEMBL5092503) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Time dependent inhibition of Trypanosoma cruzi cruzain using Cbz-Phe-Arg-AMC as substrate assessed as inhibition constant for EI complex measured for... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00628 BindingDB Entry DOI: 10.7270/Q2445RC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50581376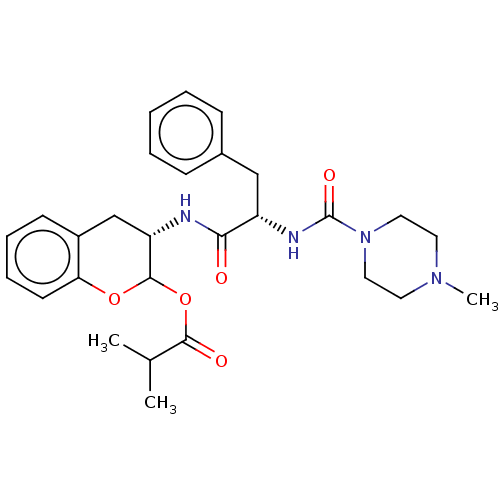 (CHEMBL5079476) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Time dependent inhibition of Trypanosoma cruzi cruzain using Cbz-Phe-Arg-AMC as substrate assessed as inhibition constant for EI complex measured for... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00628 BindingDB Entry DOI: 10.7270/Q2445RC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50581377 (CHEMBL5082504) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Time dependent inhibition of Trypanosoma cruzi cruzain using Cbz-Phe-Arg-AMC as substrate assessed as inhibition constant for EI complex measured for... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00628 BindingDB Entry DOI: 10.7270/Q2445RC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50581378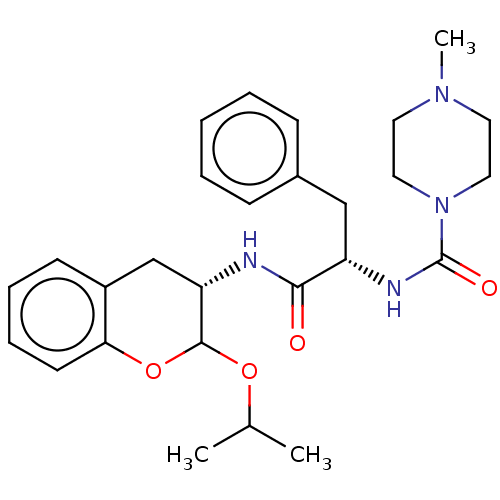 (CHEMBL5077312) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Time dependent inhibition of Trypanosoma cruzi cruzain using Cbz-Phe-Arg-AMC as substrate assessed as inhibition constant for EI complex measured for... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00628 BindingDB Entry DOI: 10.7270/Q2445RC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50581365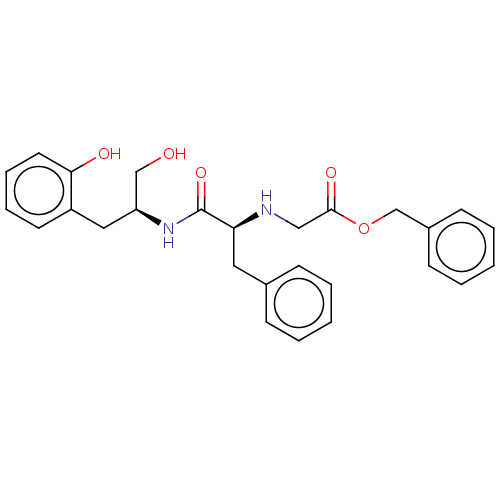 (CHEMBL5072701) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Time dependent inhibition of Trypanosoma cruzi cruzain using Cbz-Phe-Arg-AMC as substrate assessed as inhibition constant for EI complex measured for... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00628 BindingDB Entry DOI: 10.7270/Q2445RC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50581364 (CHEMBL5079046) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Time dependent inhibition of Trypanosoma cruzi cruzain using Cbz-Phe-Arg-AMC as substrate assessed as inhibition constant for EI complex measured for... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00628 BindingDB Entry DOI: 10.7270/Q2445RC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM452113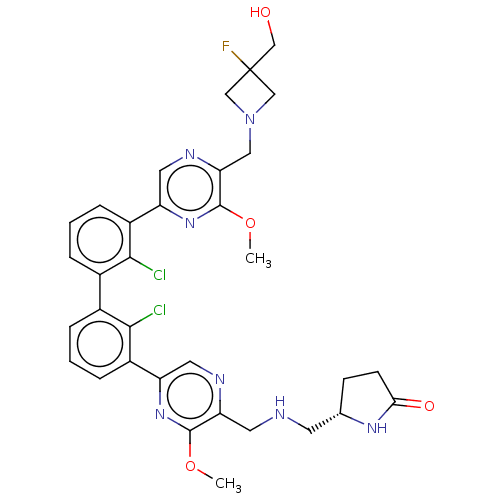 (US10710986, Example 252 | US11555029, No. 252) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were tested in biochemical protein-protein interaction assays to determine if they can specifically block the interaction between the extra... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P27329 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM452147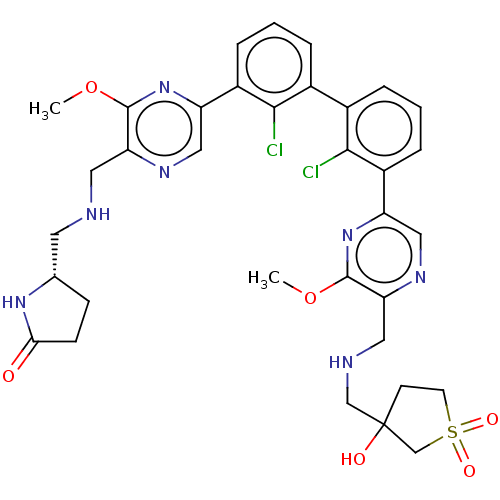 (US10710986, Example 286 | US11555029, No. 286) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were tested in biochemical protein-protein interaction assays to determine if they can specifically block the interaction between the extra... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P27329 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM452150 (US10710986, Example 289 | US11555029, No. 289) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were tested in biochemical protein-protein interaction assays to determine if they can specifically block the interaction between the extra... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P27329 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM452007 (US10710986, Example 147 | US11555029, No. 147) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were tested in biochemical protein-protein interaction assays to determine if they can specifically block the interaction between the extra... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P27329 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM452008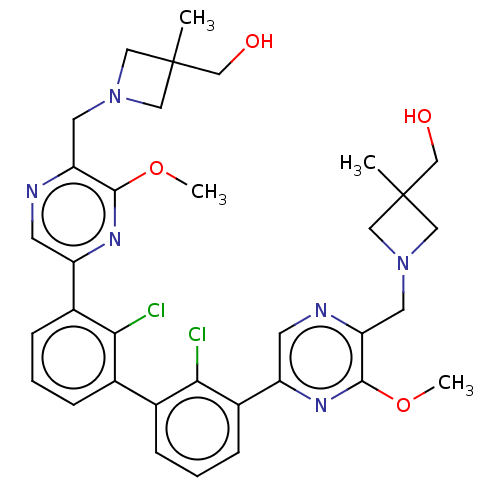 (US10710986, Example 148 | US11555029, No. 148) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were tested in biochemical protein-protein interaction assays to determine if they can specifically block the interaction between the extra... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P27329 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM451887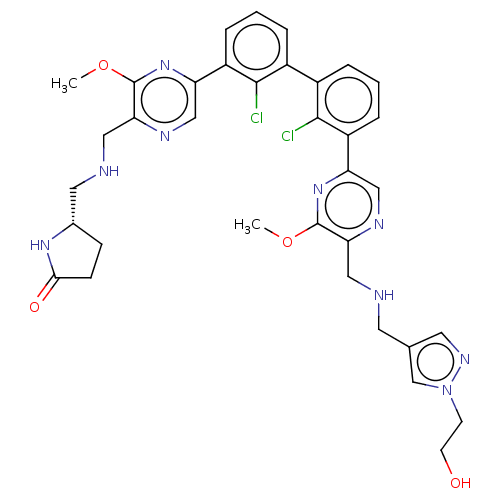 (US10710986, Example 26 | US11555029, No. 26) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were tested in biochemical protein-protein interaction assays to determine if they can specifically block the interaction between the extra... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P27329 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM452113 (US10710986, Example 252 | US11555029, No. 252) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in biochemical protein-protein interaction assays to determine if they can specifically block the interaction between the extra... | US Patent US10710986 (2020) BindingDB Entry DOI: 10.7270/Q28S4SZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM452147 (US10710986, Example 286 | US11555029, No. 286) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in biochemical protein-protein interaction assays to determine if they can specifically block the interaction between the extra... | US Patent US10710986 (2020) BindingDB Entry DOI: 10.7270/Q28S4SZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM452150 (US10710986, Example 289 | US11555029, No. 289) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in biochemical protein-protein interaction assays to determine if they can specifically block the interaction between the extra... | US Patent US10710986 (2020) BindingDB Entry DOI: 10.7270/Q28S4SZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM452007 (US10710986, Example 147 | US11555029, No. 147) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in biochemical protein-protein interaction assays to determine if they can specifically block the interaction between the extra... | US Patent US10710986 (2020) BindingDB Entry DOI: 10.7270/Q28S4SZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM452008 (US10710986, Example 148 | US11555029, No. 148) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in biochemical protein-protein interaction assays to determine if they can specifically block the interaction between the extra... | US Patent US10710986 (2020) BindingDB Entry DOI: 10.7270/Q28S4SZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM451887 (US10710986, Example 26 | US11555029, No. 26) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in biochemical protein-protein interaction assays to determine if they can specifically block the interaction between the extra... | US Patent US10710986 (2020) BindingDB Entry DOI: 10.7270/Q28S4SZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM452309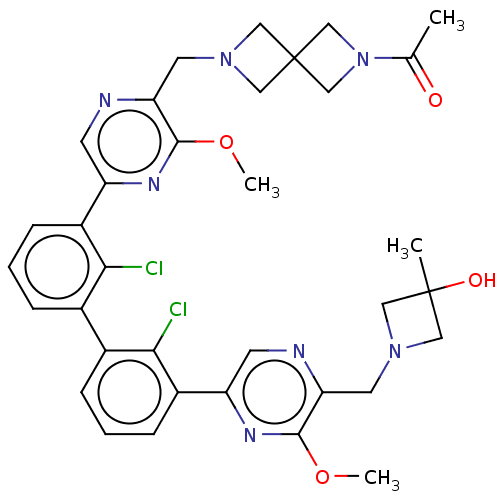 (US10710986, Example 447 | US11555029, No. 447) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in biochemical protein-protein interaction assays to determine if they can specifically block the interaction between the extra... | US Patent US10710986 (2020) BindingDB Entry DOI: 10.7270/Q28S4SZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM452312 (US10710986, Example 450 | US11555029, No. 450) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in biochemical protein-protein interaction assays to determine if they can specifically block the interaction between the extra... | US Patent US10710986 (2020) BindingDB Entry DOI: 10.7270/Q28S4SZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM452316 (US10710986, Example 454 | US11555029, No. 454) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in biochemical protein-protein interaction assays to determine if they can specifically block the interaction between the extra... | US Patent US10710986 (2020) BindingDB Entry DOI: 10.7270/Q28S4SZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM452340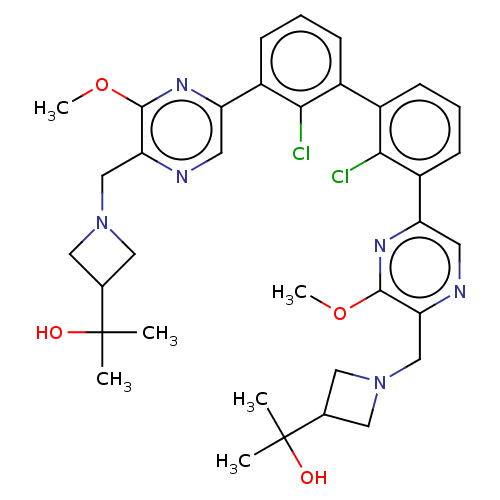 (US10710986, Example 478 | US11555029, No. 478) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in biochemical protein-protein interaction assays to determine if they can specifically block the interaction between the extra... | US Patent US10710986 (2020) BindingDB Entry DOI: 10.7270/Q28S4SZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM452342 (US10710986, Example 480 | US11555029, No. 480) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in biochemical protein-protein interaction assays to determine if they can specifically block the interaction between the extra... | US Patent US10710986 (2020) BindingDB Entry DOI: 10.7270/Q28S4SZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM452350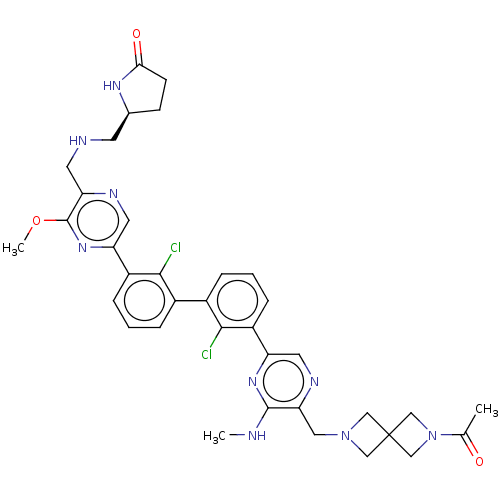 (US10710986, Example 488 | US11555029, No. 488) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in biochemical protein-protein interaction assays to determine if they can specifically block the interaction between the extra... | US Patent US10710986 (2020) BindingDB Entry DOI: 10.7270/Q28S4SZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM452353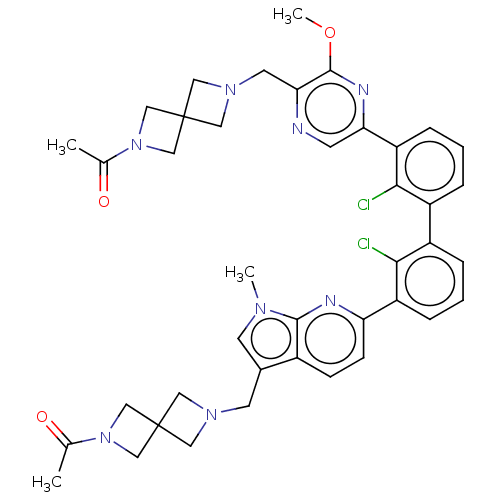 (US10710986, Example 491 | US11555029, No. 491) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in biochemical protein-protein interaction assays to determine if they can specifically block the interaction between the extra... | US Patent US10710986 (2020) BindingDB Entry DOI: 10.7270/Q28S4SZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2678 total ) | Next | Last >> |