

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM729 ((2S,4R)-N-tert-butyl-1-[(2R,3S)-2-hydroxy-3-[(2S)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0310 | -62.4 | 4 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... | J Med Chem 43: 1094-108 (2000) Article DOI: 10.1021/jm990336n BindingDB Entry DOI: 10.7270/Q2BZ647F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [514-612] (Human immunodeficiency virus type 2) | BDBM729 ((2S,4R)-N-tert-butyl-1-[(2R,3S)-2-hydroxy-3-[(2S)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.130 | -58.7 | 10 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... | J Med Chem 43: 1094-108 (2000) Article DOI: 10.1021/jm990336n BindingDB Entry DOI: 10.7270/Q2BZ647F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50053967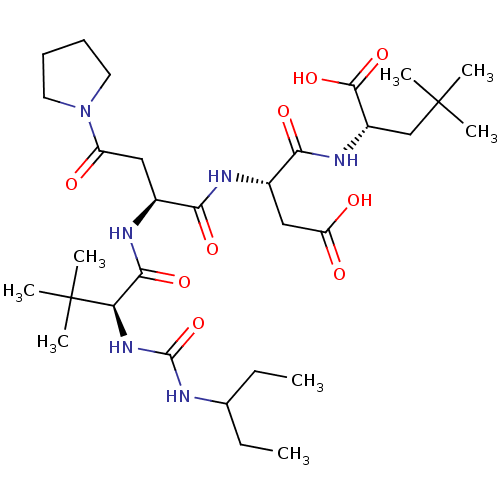 ((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-2-[3-(1-ethyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. | J Med Chem 39: 4173-80 (1996) Article DOI: 10.1021/jm960324r BindingDB Entry DOI: 10.7270/Q28W3DZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM748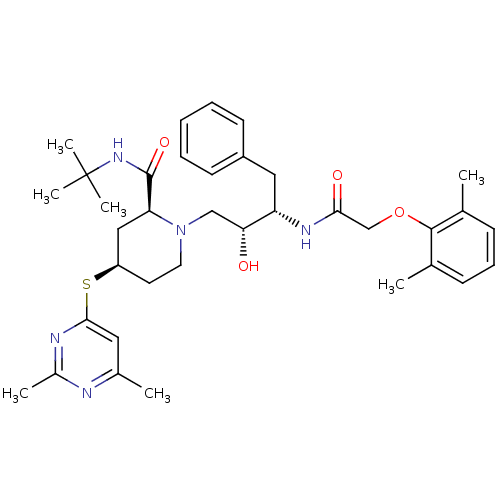 ((2S,4R)-N-tert-butyl-1-[(2R,3S)-3-[2-(2,6-dimethyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... | J Med Chem 43: 1094-108 (2000) Article DOI: 10.1021/jm990336n BindingDB Entry DOI: 10.7270/Q2BZ647F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM747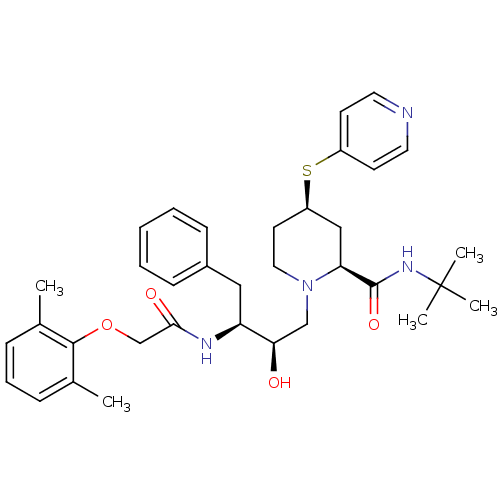 ((2S,4R)-N-tert-butyl-1-[(2R,3S)-3-[2-(2,6-dimethyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... | J Med Chem 43: 1094-108 (2000) Article DOI: 10.1021/jm990336n BindingDB Entry DOI: 10.7270/Q2BZ647F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM746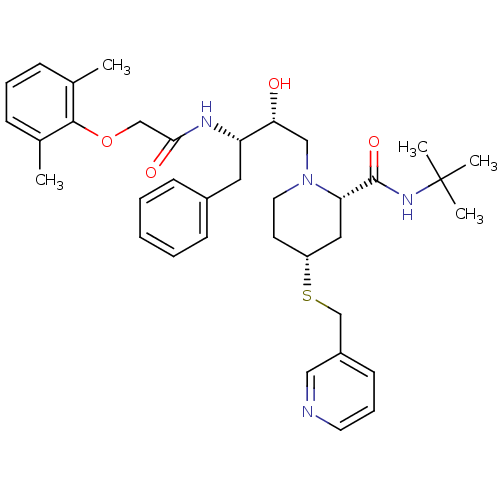 ((2S,4R)-N-tert-butyl-1-[(2R,3S)-3-[2-(2,6-dimethyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... | J Med Chem 43: 1094-108 (2000) Article DOI: 10.1021/jm990336n BindingDB Entry DOI: 10.7270/Q2BZ647F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM745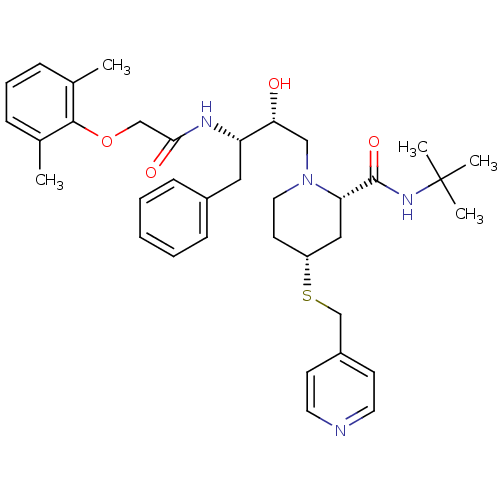 ((2S,4R)-N-tert-butyl-1-[(2R,3S)-3-[2-(2,6-dimethyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... | J Med Chem 43: 1094-108 (2000) Article DOI: 10.1021/jm990336n BindingDB Entry DOI: 10.7270/Q2BZ647F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27595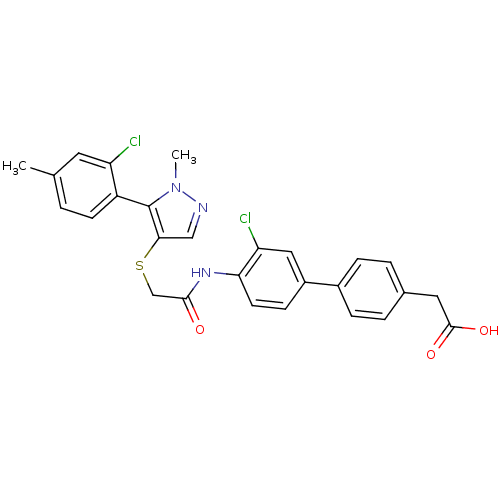 (2-{4-[3-chloro-4-(2-{[5-(2-chloro-4-methylphenyl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description IC50 values for wild-type and mutant RTs were obtained from a scintillation proximity assay using poly rC/biotin-dG15 and 3H-dGTP. Each value represe... | Bioorg Med Chem Lett 19: 1199-205 (2009) Article DOI: 10.1016/j.bmcl.2008.12.074 BindingDB Entry DOI: 10.7270/Q2ZG6QKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27583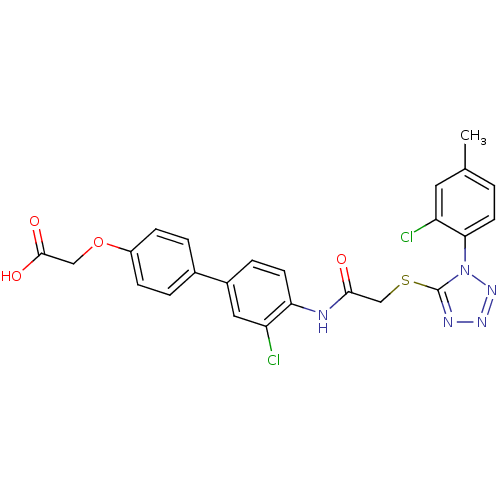 (2-{4-[3-chloro-4-(2-{[1-(2-chloro-4-methylphenyl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description IC50 values for wild-type and mutant RTs were obtained from a scintillation proximity assay using poly rC/biotin-dG15 and 3H-dGTP. Each value represe... | Bioorg Med Chem Lett 19: 1199-205 (2009) Article DOI: 10.1016/j.bmcl.2008.12.074 BindingDB Entry DOI: 10.7270/Q2ZG6QKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27599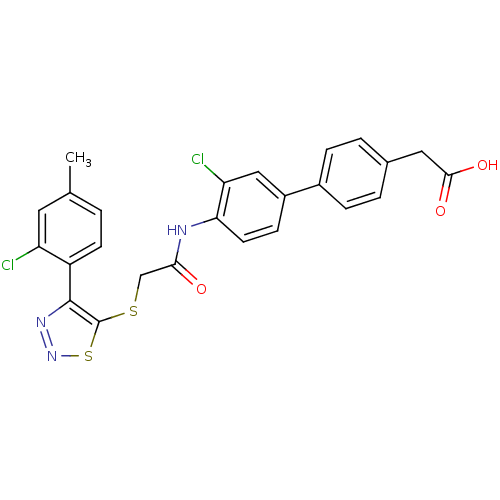 (2-{4-[3-chloro-4-(2-{[4-(2-chloro-4-methylphenyl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description IC50 values for wild-type and mutant RTs were obtained from a scintillation proximity assay using poly rC/biotin-dG15 and 3H-dGTP. Each value represe... | Bioorg Med Chem Lett 19: 1199-205 (2009) Article DOI: 10.1016/j.bmcl.2008.12.074 BindingDB Entry DOI: 10.7270/Q2ZG6QKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50476896 (CHEMBL232576) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by scintillation proximity assay | Bioorg Med Chem Lett 17: 4437-41 (2007) Article DOI: 10.1016/j.bmcl.2007.06.012 BindingDB Entry DOI: 10.7270/Q2BR8VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50369164 (CHEMBL1169533) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. | J Med Chem 39: 4173-80 (1996) Article DOI: 10.1021/jm960324r BindingDB Entry DOI: 10.7270/Q28W3DZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50369164 (CHEMBL1169533) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. | J Med Chem 39: 4173-80 (1996) Article DOI: 10.1021/jm960324r BindingDB Entry DOI: 10.7270/Q28W3DZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM10227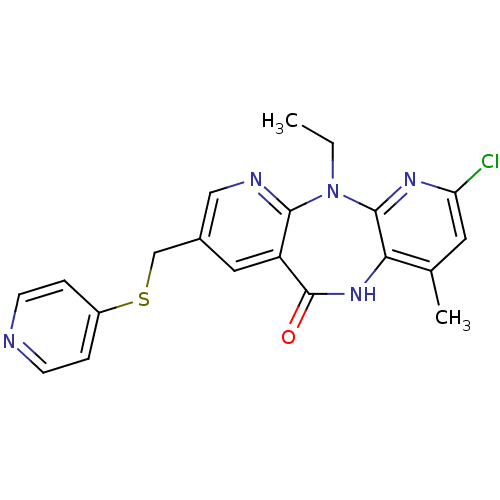 (5-chloro-2-ethyl-7-methyl-13-[(pyridin-4-ylsulfany...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description IC50 values for wild-type and mutant RTs were obtained from a scintillation proximity assay using poly rC/biotin-dG15 and 3H-dGTP. Each value represe... | Bioorg Med Chem Lett 14: 739-42 (2004) Article DOI: 10.1016/j.bmcl.2003.11.049 BindingDB Entry DOI: 10.7270/Q2C53J2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27591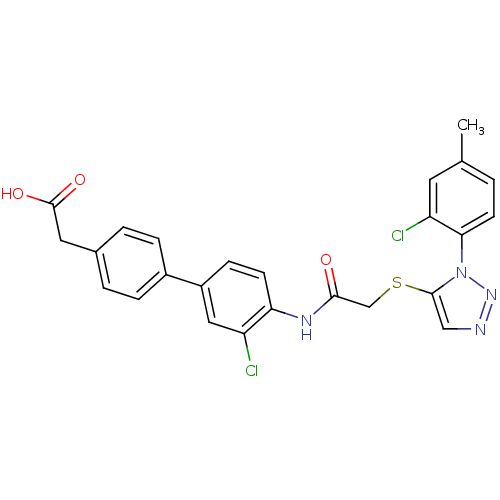 (2-{4-[3-chloro-4-(2-{[1-(2-chloro-4-methylphenyl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description IC50 values for wild-type and mutant RTs were obtained from a scintillation proximity assay using poly rC/biotin-dG15 and 3H-dGTP. Each value represe... | Bioorg Med Chem Lett 19: 1199-205 (2009) Article DOI: 10.1016/j.bmcl.2008.12.074 BindingDB Entry DOI: 10.7270/Q2ZG6QKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27584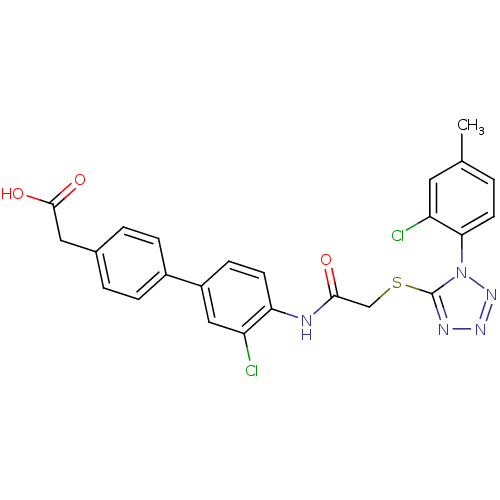 (2-{4-[3-chloro-4-(2-{[1-(2-chloro-4-methylphenyl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description IC50 values for wild-type and mutant RTs were obtained from a scintillation proximity assay using poly rC/biotin-dG15 and 3H-dGTP. Each value represe... | Bioorg Med Chem Lett 19: 1199-205 (2009) Article DOI: 10.1016/j.bmcl.2008.12.074 BindingDB Entry DOI: 10.7270/Q2ZG6QKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM577 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... | J Med Chem 43: 1094-108 (2000) Article DOI: 10.1021/jm990336n BindingDB Entry DOI: 10.7270/Q2BZ647F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50476895 (CHEMBL232379) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by scintillation proximity assay | Bioorg Med Chem Lett 17: 4437-41 (2007) Article DOI: 10.1016/j.bmcl.2007.06.012 BindingDB Entry DOI: 10.7270/Q2BR8VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50053968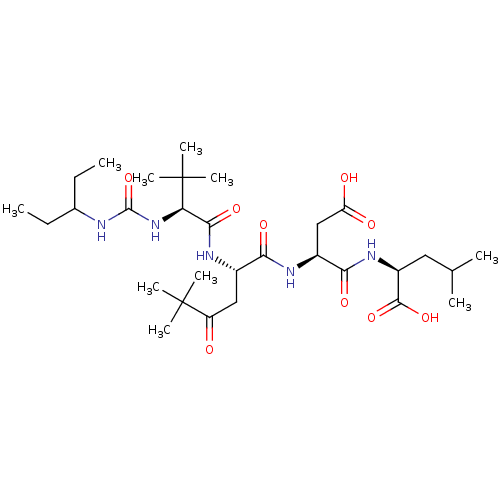 ((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-2-[3-(1-ethyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. | J Med Chem 39: 4173-80 (1996) Article DOI: 10.1021/jm960324r BindingDB Entry DOI: 10.7270/Q28W3DZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50050831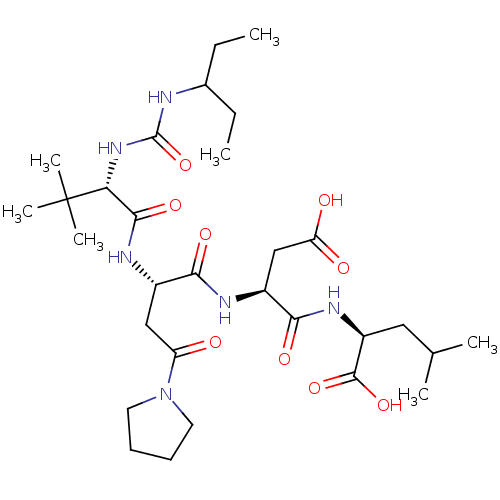 ((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-2-[3-(1-ethyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. | J Med Chem 39: 4173-80 (1996) Article DOI: 10.1021/jm960324r BindingDB Entry DOI: 10.7270/Q28W3DZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM2058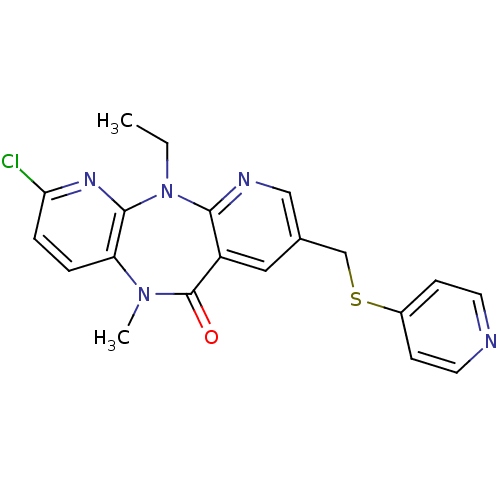 (2,8-disubstituted dipyridodiazepinone 41 | 2-Chlor...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description IC50 values for wild-type and mutant RTs were obtained from a scintillation proximity assay using poly rC/biotin-dG15 and 3H-dGTP. Each value represe... | Bioorg Med Chem Lett 14: 739-42 (2004) Article DOI: 10.1016/j.bmcl.2003.11.049 BindingDB Entry DOI: 10.7270/Q2C53J2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50476903 (CHEMBL230188) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by scintillation proximity assay | Bioorg Med Chem Lett 17: 4437-41 (2007) Article DOI: 10.1016/j.bmcl.2007.06.012 BindingDB Entry DOI: 10.7270/Q2BR8VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27607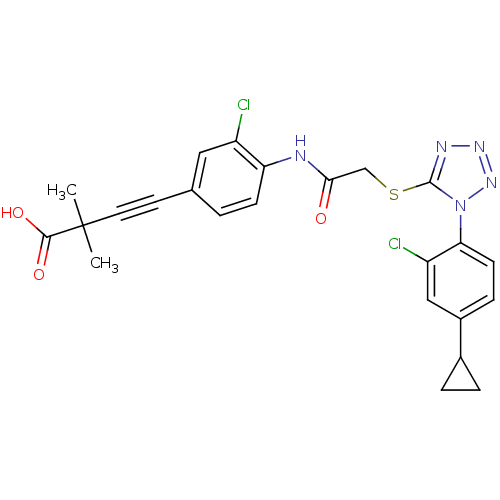 (4-[3-chloro-4-(2-{[1-(2-chloro-4-cyclopropylphenyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description IC50 values for wild-type and mutant RTs were obtained from a scintillation proximity assay using poly rC/biotin-dG15 and 3H-dGTP. Each value represe... | Bioorg Med Chem Lett 19: 1199-205 (2009) Article DOI: 10.1016/j.bmcl.2008.12.074 BindingDB Entry DOI: 10.7270/Q2ZG6QKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM10235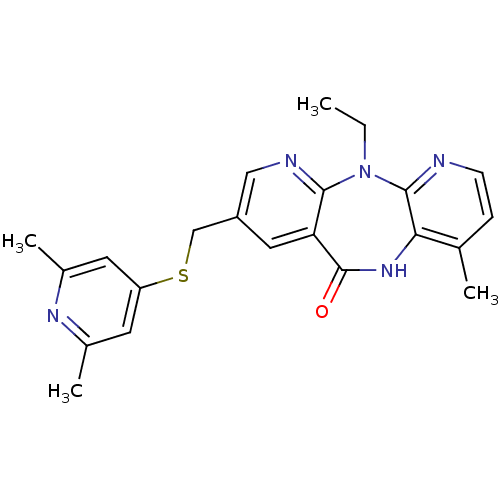 (13-{[(2,6-dimethylpyridin-4-yl)sulfanyl]methyl}-2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description IC50 values for wild-type and mutant RTs were obtained from a scintillation proximity assay using poly rC/biotin-dG15 and 3H-dGTP. Each value represe... | Bioorg Med Chem Lett 14: 739-42 (2004) Article DOI: 10.1016/j.bmcl.2003.11.049 BindingDB Entry DOI: 10.7270/Q2C53J2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50053981 ((S)-2-((S)-3-Carboxy-2-{(2R,5S)-2-(3,3-dimethyl-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. | J Med Chem 39: 4173-80 (1996) Article DOI: 10.1021/jm960324r BindingDB Entry DOI: 10.7270/Q28W3DZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50476897 (CHEMBL232178) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by scintillation proximity assay | Bioorg Med Chem Lett 17: 4437-41 (2007) Article DOI: 10.1016/j.bmcl.2007.06.012 BindingDB Entry DOI: 10.7270/Q2BR8VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50476898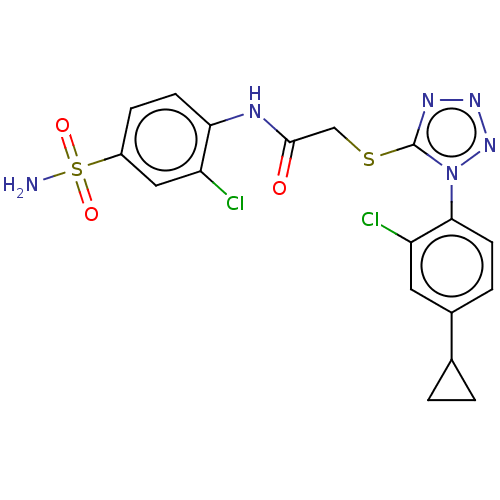 (CHEMBL232377) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by scintillation proximity assay | Bioorg Med Chem Lett 17: 4437-41 (2007) Article DOI: 10.1016/j.bmcl.2007.06.012 BindingDB Entry DOI: 10.7270/Q2BR8VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50476904 (CHEMBL232575) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by scintillation proximity assay | Bioorg Med Chem Lett 17: 4437-41 (2007) Article DOI: 10.1016/j.bmcl.2007.06.012 BindingDB Entry DOI: 10.7270/Q2BR8VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50476885 (CHEMBL231030) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by scintillation proximity assay | Bioorg Med Chem Lett 17: 4437-41 (2007) Article DOI: 10.1016/j.bmcl.2007.06.012 BindingDB Entry DOI: 10.7270/Q2BR8VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM10232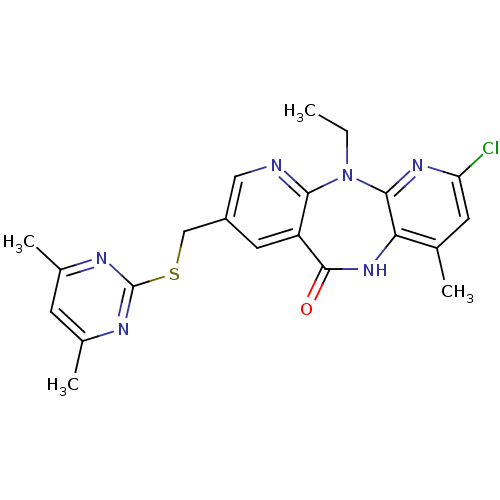 (5-chloro-13-{[(4,6-dimethylpyrimidin-2-yl)sulfanyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description IC50 values for wild-type and mutant RTs were obtained from a scintillation proximity assay using poly rC/biotin-dG15 and 3H-dGTP. Each value represe... | Bioorg Med Chem Lett 14: 739-42 (2004) Article DOI: 10.1016/j.bmcl.2003.11.049 BindingDB Entry DOI: 10.7270/Q2C53J2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50053973 ((S)-3-((S)-2-{(S)-2-[3-(1-Ethyl-propyl)-ureido]-3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. | J Med Chem 39: 4173-80 (1996) Article DOI: 10.1021/jm960324r BindingDB Entry DOI: 10.7270/Q28W3DZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM10234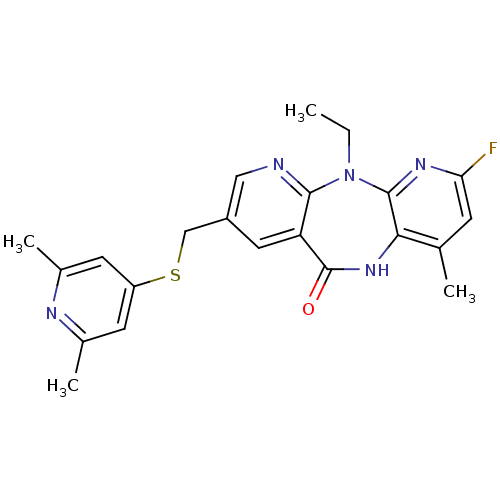 (13-{[(2,6-dimethylpyridin-4-yl)sulfanyl]methyl}-2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description IC50 values for wild-type and mutant RTs were obtained from a scintillation proximity assay using poly rC/biotin-dG15 and 3H-dGTP. Each value represe... | Bioorg Med Chem Lett 14: 739-42 (2004) Article DOI: 10.1016/j.bmcl.2003.11.049 BindingDB Entry DOI: 10.7270/Q2C53J2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM741 ((2S,4R)-N-tert-butyl-1-[(2R,3S)-3-[2-(2,6-dimethyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... | J Med Chem 43: 1094-108 (2000) Article DOI: 10.1021/jm990336n BindingDB Entry DOI: 10.7270/Q2BZ647F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50053984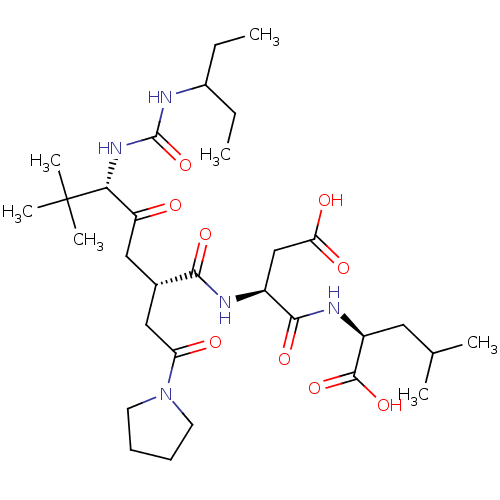 ((S)-2-{(S)-3-Carboxy-2-[(2S,5S)-5-[3-(1-ethyl-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. | J Med Chem 39: 4173-80 (1996) Article DOI: 10.1021/jm960324r BindingDB Entry DOI: 10.7270/Q28W3DZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50050828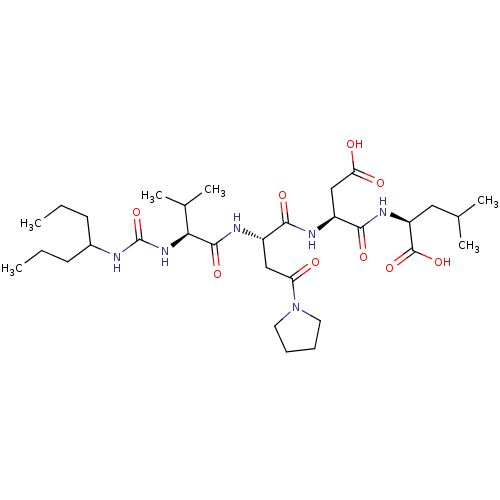 ((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-3-methyl-2-[3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. | J Med Chem 39: 4173-80 (1996) Article DOI: 10.1021/jm960324r BindingDB Entry DOI: 10.7270/Q28W3DZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM10230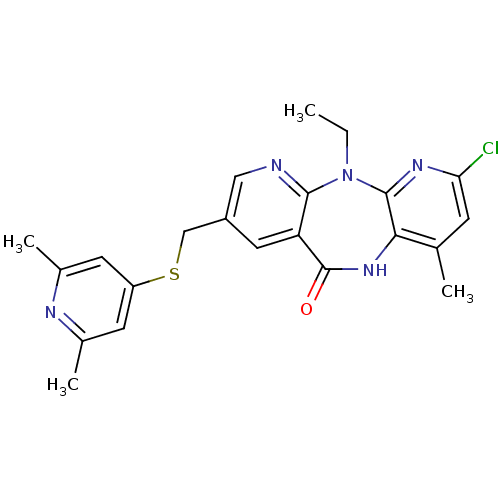 (5-chloro-13-{[(2,6-dimethylpyridin-4-yl)sulfanyl]m...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description IC50 values for wild-type and mutant RTs were obtained from a scintillation proximity assay using poly rC/biotin-dG15 and 3H-dGTP. Each value represe... | Bioorg Med Chem Lett 14: 739-42 (2004) Article DOI: 10.1016/j.bmcl.2003.11.049 BindingDB Entry DOI: 10.7270/Q2C53J2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27590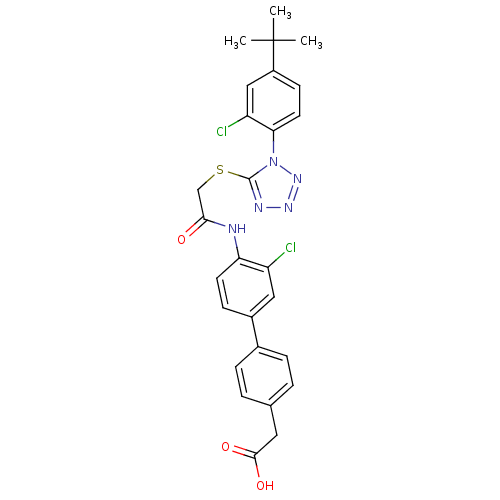 (2-{4-[4-(2-{[1-(4-tert-butyl-2-chlorophenyl)-1H-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description IC50 values for wild-type and mutant RTs were obtained from a scintillation proximity assay using poly rC/biotin-dG15 and 3H-dGTP. Each value represe... | Bioorg Med Chem Lett 19: 1199-205 (2009) Article DOI: 10.1016/j.bmcl.2008.12.074 BindingDB Entry DOI: 10.7270/Q2ZG6QKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50476894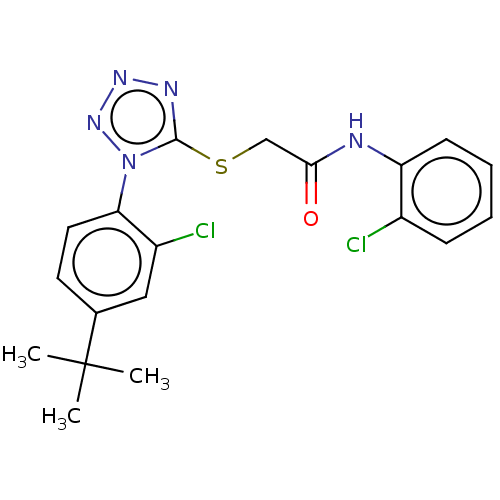 (CHEMBL230187) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by scintillation proximity assay | Bioorg Med Chem Lett 17: 4437-41 (2007) Article DOI: 10.1016/j.bmcl.2007.06.012 BindingDB Entry DOI: 10.7270/Q2BR8VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50476899 (CHEMBL232963) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N/Y181C mutant by scintillation proximity assay | Bioorg Med Chem Lett 17: 4437-41 (2007) Article DOI: 10.1016/j.bmcl.2007.06.012 BindingDB Entry DOI: 10.7270/Q2BR8VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27592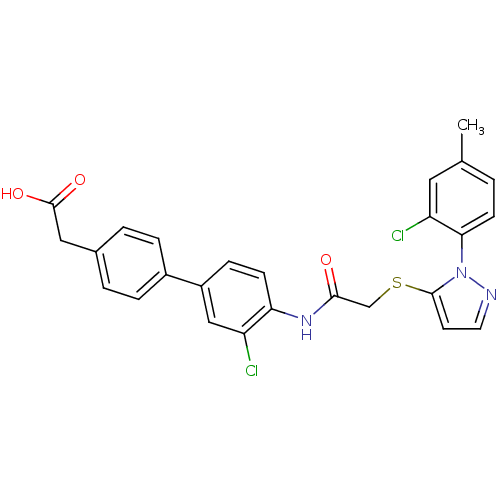 (2-{4-[3-chloro-4-(2-{[1-(2-chloro-4-methylphenyl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description IC50 values for wild-type and mutant RTs were obtained from a scintillation proximity assay using poly rC/biotin-dG15 and 3H-dGTP. Each value represe... | Bioorg Med Chem Lett 19: 1199-205 (2009) Article DOI: 10.1016/j.bmcl.2008.12.074 BindingDB Entry DOI: 10.7270/Q2ZG6QKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM10233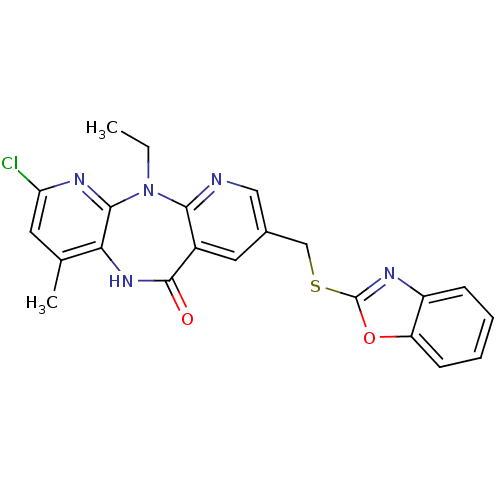 (13-[(1,3-benzoxazol-2-ylsulfanyl)methyl]-5-chloro-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description IC50 values for wild-type and mutant RTs were obtained from a scintillation proximity assay using poly rC/biotin-dG15 and 3H-dGTP. Each value represe... | Bioorg Med Chem Lett 14: 739-42 (2004) Article DOI: 10.1016/j.bmcl.2003.11.049 BindingDB Entry DOI: 10.7270/Q2C53J2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... | J Med Chem 43: 1094-108 (2000) Article DOI: 10.1021/jm990336n BindingDB Entry DOI: 10.7270/Q2BZ647F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27589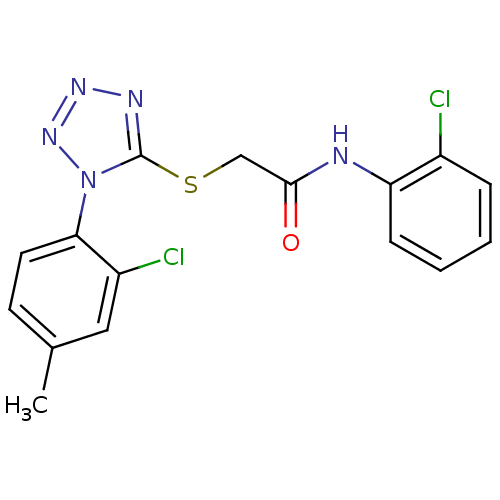 (2-{[1-(2-chloro-4-methylphenyl)-1H-1,2,3,4-tetrazo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description IC50 values for wild-type and mutant RTs were obtained from a scintillation proximity assay using poly rC/biotin-dG15 and 3H-dGTP. Each value represe... | Bioorg Med Chem Lett 19: 1199-205 (2009) Article DOI: 10.1016/j.bmcl.2008.12.074 BindingDB Entry DOI: 10.7270/Q2ZG6QKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50476896 (CHEMBL232576) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N/Y181C mutant by scintillation proximity assay | Bioorg Med Chem Lett 17: 4437-41 (2007) Article DOI: 10.1016/j.bmcl.2007.06.012 BindingDB Entry DOI: 10.7270/Q2BR8VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM27606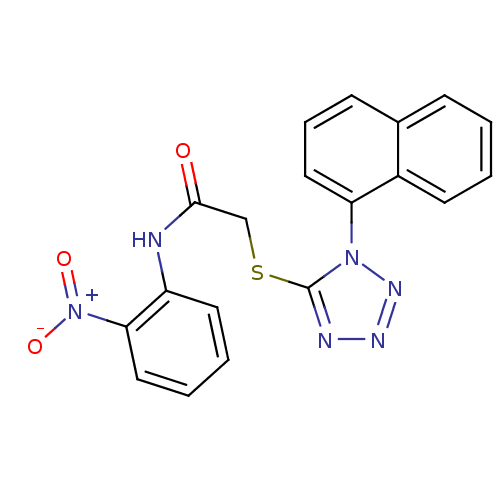 (2-{[1-(naphthalen-1-yl)-1H-1,2,3,4-tetrazol-5-yl]s...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by scintillation proximity assay | Bioorg Med Chem Lett 17: 4437-41 (2007) Article DOI: 10.1016/j.bmcl.2007.06.012 BindingDB Entry DOI: 10.7270/Q2BR8VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27606 (2-{[1-(naphthalen-1-yl)-1H-1,2,3,4-tetrazol-5-yl]s...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description IC50 values for wild-type and mutant RTs were obtained from a scintillation proximity assay using poly rC/biotin-dG15 and 3H-dGTP. Each value represe... | Bioorg Med Chem Lett 19: 1199-205 (2009) Article DOI: 10.1016/j.bmcl.2008.12.074 BindingDB Entry DOI: 10.7270/Q2ZG6QKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27597 (2-{4-[3-chloro-4-(2-{[1-(2-chloro-4-methylphenyl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description IC50 values for wild-type and mutant RTs were obtained from a scintillation proximity assay using poly rC/biotin-dG15 and 3H-dGTP. Each value represe... | Bioorg Med Chem Lett 19: 1199-205 (2009) Article DOI: 10.1016/j.bmcl.2008.12.074 BindingDB Entry DOI: 10.7270/Q2ZG6QKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50476909 (CHEMBL232378) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by scintillation proximity assay | Bioorg Med Chem Lett 17: 4437-41 (2007) Article DOI: 10.1016/j.bmcl.2007.06.012 BindingDB Entry DOI: 10.7270/Q2BR8VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50171982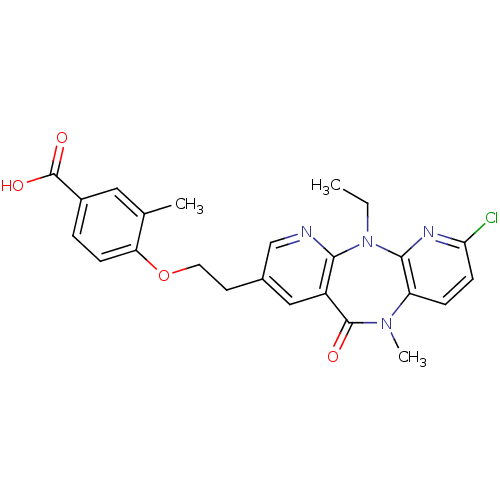 (4-[2-(7-Chloro-5-ethyl-10-methyl-11-oxo-10,11-dihy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibitory activity of compound dissolved in DMSO was determined against HIV-1 wild type reverse transcriptase (1-2 nM) by using [3H]-dGTP (71 nM) a... | J Med Chem 48: 5580-8 (2005) Article DOI: 10.1021/jm050255t BindingDB Entry DOI: 10.7270/Q29K49RC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50476893 (CHEMBL393190) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by scintillation proximity assay | Bioorg Med Chem Lett 17: 4437-41 (2007) Article DOI: 10.1016/j.bmcl.2007.06.012 BindingDB Entry DOI: 10.7270/Q2BR8VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 483 total ) | Next | Last >> |