 Found 1962 hits with Last Name = 'yoon' and Initial = 's'
Found 1962 hits with Last Name = 'yoon' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prothrombin
(Homo sapiens (Human)) | BDBM50111110
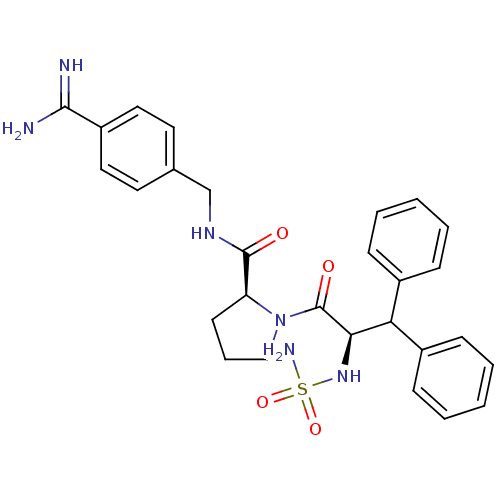
(2N-(4-Benzamidinemethyl)-1-[2-aminosulfonamido-3,3...)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2CCCN2C(=O)[C@H](NS(N)(=O)=O)C(c2ccccc2)c2ccccc2)cc1 Show InChI InChI=1S/C28H32N6O4S/c29-26(30)22-15-13-19(14-16-22)18-32-27(35)23-12-7-17-34(23)28(36)25(33-39(31,37)38)24(20-8-3-1-4-9-20)21-10-5-2-6-11-21/h1-6,8-11,13-16,23-25,33H,7,12,17-18H2,(H3,29,30)(H,32,35)(H2,31,37,38)/t23-,25+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition constant (Ki) against human thrombin |
J Med Chem 46: 3612-22 (2003)
Article DOI: 10.1021/jm030025j
BindingDB Entry DOI: 10.7270/Q28K79T3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50111101
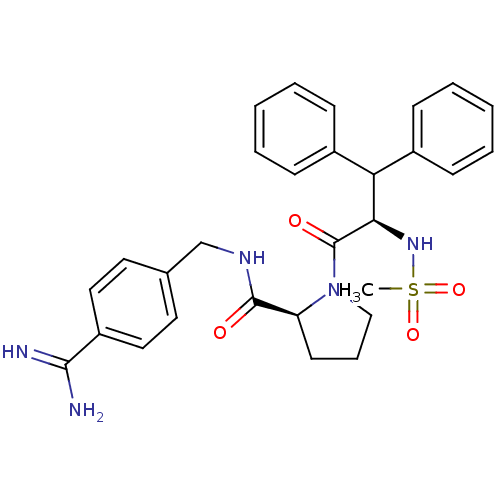
((S)-1-((R)-2-Methanesulfonylamino-3,3-diphenyl-pro...)Show SMILES CS(=O)(=O)N[C@H](C(c1ccccc1)c1ccccc1)C(=O)N1CCC[C@H]1C(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C29H33N5O4S/c1-39(37,38)33-26(25(21-9-4-2-5-10-21)22-11-6-3-7-12-22)29(36)34-18-8-13-24(34)28(35)32-19-20-14-16-23(17-15-20)27(30)31/h2-7,9-12,14-17,24-26,33H,8,13,18-19H2,1H3,(H3,30,31)(H,32,35)/t24-,26+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition constant (Ki) against human thrombin |
J Med Chem 46: 3612-22 (2003)
Article DOI: 10.1021/jm030025j
BindingDB Entry DOI: 10.7270/Q28K79T3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131789
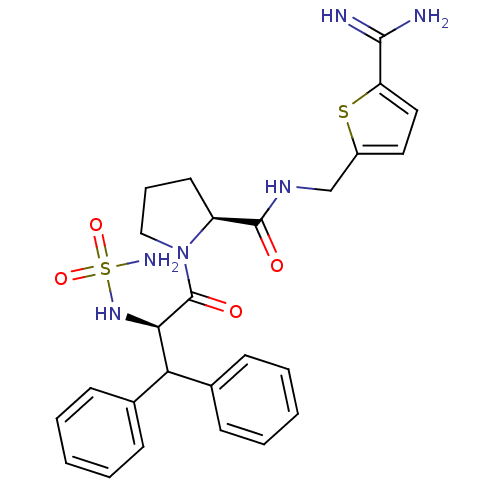
(1-(2-sulfonamide-amino-3,3-diphenyl-propionyl)-pyr...)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2CCCN2C(=O)[C@H](NS(N)(=O)=O)C(c2ccccc2)c2ccccc2)s1 Show InChI InChI=1S/C26H30N6O4S2/c27-24(28)21-14-13-19(37-21)16-30-25(33)20-12-7-15-32(20)26(34)23(31-38(29,35)36)22(17-8-3-1-4-9-17)18-10-5-2-6-11-18/h1-6,8-11,13-14,20,22-23,31H,7,12,15-16H2,(H3,27,28)(H,30,33)(H2,29,35,36)/t20-,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition constant (Ki) against human thrombin |
J Med Chem 46: 3612-22 (2003)
Article DOI: 10.1021/jm030025j
BindingDB Entry DOI: 10.7270/Q28K79T3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50111105

((S)-1-((R)-2-Methanesulfonylamino-3,3-diphenyl-pro...)Show SMILES CS(=O)(=O)N[C@H](C(c1ccccc1)c1ccccc1)C(=O)N1CCC[C@H]1C(=O)NCc1ccc(s1)C(N)=N Show InChI InChI=1S/C27H31N5O4S2/c1-38(35,36)31-24(23(18-9-4-2-5-10-18)19-11-6-3-7-12-19)27(34)32-16-8-13-21(32)26(33)30-17-20-14-15-22(37-20)25(28)29/h2-7,9-12,14-15,21,23-24,31H,8,13,16-17H2,1H3,(H3,28,29)(H,30,33)/t21-,24+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition constant (Ki) against human thrombin |
J Med Chem 46: 3612-22 (2003)
Article DOI: 10.1021/jm030025j
BindingDB Entry DOI: 10.7270/Q28K79T3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131790
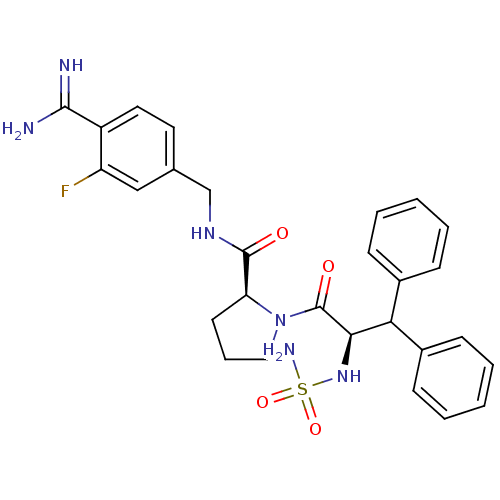
(1-(2-sulfonamideamino-3,3-diphenyl-propionyl)-pyrr...)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2CCCN2C(=O)[C@H](NS(N)(=O)=O)C(c2ccccc2)c2ccccc2)cc1F Show InChI InChI=1S/C28H31FN6O4S/c29-22-16-18(13-14-21(22)26(30)31)17-33-27(36)23-12-7-15-35(23)28(37)25(34-40(32,38)39)24(19-8-3-1-4-9-19)20-10-5-2-6-11-20/h1-6,8-11,13-14,16,23-25,34H,7,12,15,17H2,(H3,30,31)(H,33,36)(H2,32,38,39)/t23-,25+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition constant (Ki) against human thrombin |
J Med Chem 46: 3612-22 (2003)
Article DOI: 10.1021/jm030025j
BindingDB Entry DOI: 10.7270/Q28K79T3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131795
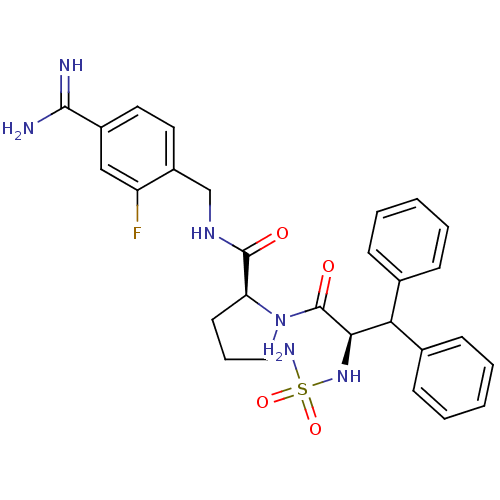
(1-(2-sulfonamideamino-3,3-diphenyl-propionyl)-pyrr...)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2CCCN2C(=O)[C@H](NS(N)(=O)=O)C(c2ccccc2)c2ccccc2)c(F)c1 Show InChI InChI=1S/C28H31FN6O4S/c29-22-16-20(26(30)31)13-14-21(22)17-33-27(36)23-12-7-15-35(23)28(37)25(34-40(32,38)39)24(18-8-3-1-4-9-18)19-10-5-2-6-11-19/h1-6,8-11,13-14,16,23-25,34H,7,12,15,17H2,(H3,30,31)(H,33,36)(H2,32,38,39)/t23-,25+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition constant (Ki) against human thrombin |
J Med Chem 46: 3612-22 (2003)
Article DOI: 10.1021/jm030025j
BindingDB Entry DOI: 10.7270/Q28K79T3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131778
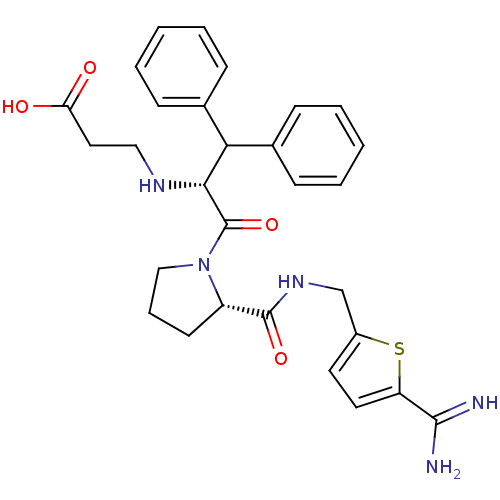
(3-(1-Benzhydryl-2-{2-[(5-carbamimidoyl-thiophen-2-...)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2CCCN2C(=O)[C@H](NCCC(O)=O)C(c2ccccc2)c2ccccc2)s1 Show InChI InChI=1S/C29H33N5O4S/c30-27(31)23-14-13-21(39-23)18-33-28(37)22-12-7-17-34(22)29(38)26(32-16-15-24(35)36)25(19-8-3-1-4-9-19)20-10-5-2-6-11-20/h1-6,8-11,13-14,22,25-26,32H,7,12,15-18H2,(H3,30,31)(H,33,37)(H,35,36)/t22-,26+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition constant (Ki) against human thrombin |
J Med Chem 46: 3612-22 (2003)
Article DOI: 10.1021/jm030025j
BindingDB Entry DOI: 10.7270/Q28K79T3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50111120
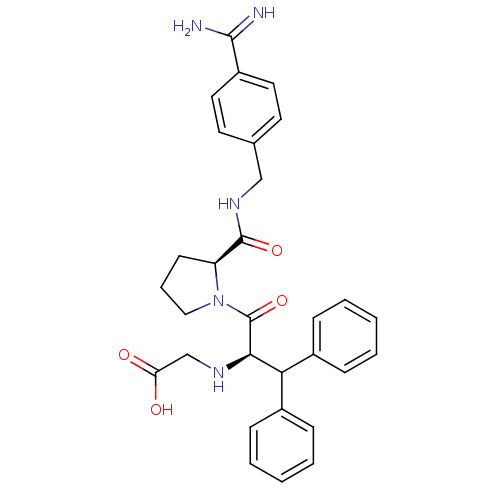
(2N-(4-Benzamidinemethyl)-1-[2-Aminoaceticacid-3,3-...)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2CCCN2C(=O)[C@H](NCC(O)=O)C(c2ccccc2)c2ccccc2)cc1 Show InChI InChI=1S/C30H33N5O4/c31-28(32)23-15-13-20(14-16-23)18-34-29(38)24-12-7-17-35(24)30(39)27(33-19-25(36)37)26(21-8-3-1-4-9-21)22-10-5-2-6-11-22/h1-6,8-11,13-16,24,26-27,33H,7,12,17-19H2,(H3,31,32)(H,34,38)(H,36,37)/t24-,27+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition constant (Ki) against human thrombin |
J Med Chem 46: 3612-22 (2003)
Article DOI: 10.1021/jm030025j
BindingDB Entry DOI: 10.7270/Q28K79T3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131796
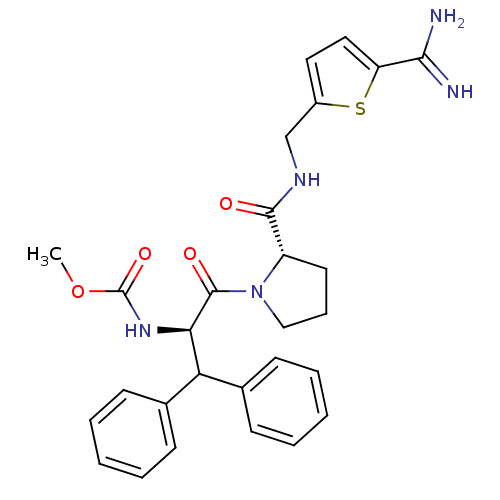
((1-Benzhydryl-2-{2-[(5-carbamimidoyl-thiophen-2-yl...)Show SMILES COC(=O)N[C@H](C(c1ccccc1)c1ccccc1)C(=O)N1CCC[C@H]1C(=O)NCc1ccc(s1)C(N)=N Show InChI InChI=1S/C28H31N5O4S/c1-37-28(36)32-24(23(18-9-4-2-5-10-18)19-11-6-3-7-12-19)27(35)33-16-8-13-21(33)26(34)31-17-20-14-15-22(38-20)25(29)30/h2-7,9-12,14-15,21,23-24H,8,13,16-17H2,1H3,(H3,29,30)(H,31,34)(H,32,36)/t21-,24+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition constant (Ki) against human thrombin |
J Med Chem 46: 3612-22 (2003)
Article DOI: 10.1021/jm030025j
BindingDB Entry DOI: 10.7270/Q28K79T3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131791
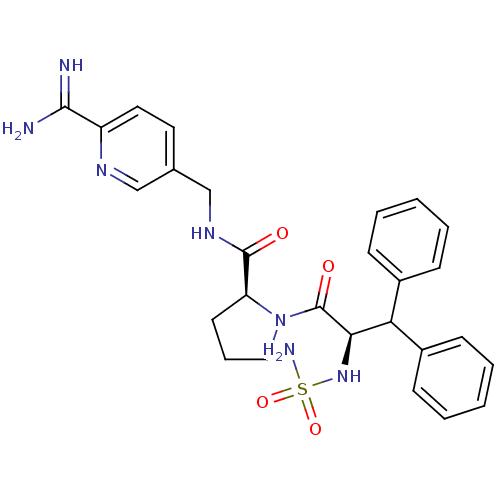
(1-(2-sulfonamideamino-3,3-diphenyl-propionyl)-pyrr...)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2CCCN2C(=O)[C@H](NS(N)(=O)=O)C(c2ccccc2)c2ccccc2)cn1 Show InChI InChI=1S/C27H31N7O4S/c28-25(29)21-14-13-18(16-31-21)17-32-26(35)22-12-7-15-34(22)27(36)24(33-39(30,37)38)23(19-8-3-1-4-9-19)20-10-5-2-6-11-20/h1-6,8-11,13-14,16,22-24,33H,7,12,15,17H2,(H3,28,29)(H,32,35)(H2,30,37,38)/t22-,24+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition constant (Ki) against human thrombin |
J Med Chem 46: 3612-22 (2003)
Article DOI: 10.1021/jm030025j
BindingDB Entry DOI: 10.7270/Q28K79T3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131780
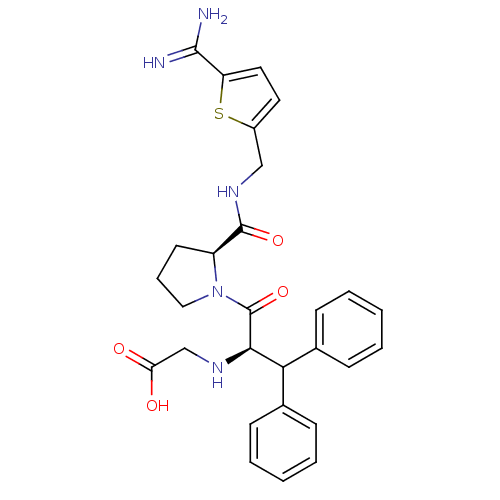
((1-Benzhydryl-2-{2-[(5-carbamimidoyl-thiophen-2-yl...)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2CCCN2C(=O)[C@H](NCC(O)=O)C(c2ccccc2)c2ccccc2)s1 Show InChI InChI=1S/C28H31N5O4S/c29-26(30)22-14-13-20(38-22)16-32-27(36)21-12-7-15-33(21)28(37)25(31-17-23(34)35)24(18-8-3-1-4-9-18)19-10-5-2-6-11-19/h1-6,8-11,13-14,21,24-25,31H,7,12,15-17H2,(H3,29,30)(H,32,36)(H,34,35)/t21-,25+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition constant (Ki) against human thrombin |
J Med Chem 46: 3612-22 (2003)
Article DOI: 10.1021/jm030025j
BindingDB Entry DOI: 10.7270/Q28K79T3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131792

((1-Benzhydryl-2-{2-[(6-carbamimidoyl-pyridin-3-ylm...)Show SMILES COC(=O)N[C@H](C(c1ccccc1)c1ccccc1)C(=O)N1CCC[C@H]1C(=O)NCc1ccc(nc1)C(N)=N Show InChI InChI=1S/C29H32N6O4/c1-39-29(38)34-25(24(20-9-4-2-5-10-20)21-11-6-3-7-12-21)28(37)35-16-8-13-23(35)27(36)33-18-19-14-15-22(26(30)31)32-17-19/h2-7,9-12,14-15,17,23-25H,8,13,16,18H2,1H3,(H3,30,31)(H,33,36)(H,34,38)/t23-,25+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition constant (Ki) against human thrombin |
J Med Chem 46: 3612-22 (2003)
Article DOI: 10.1021/jm030025j
BindingDB Entry DOI: 10.7270/Q28K79T3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131798

(1-(2-Methylamino-3,3-diphenyl-propionyl)-pyrrolidi...)Show SMILES NC(=N)c1cc(CNC(=O)[C@@H]2CCCN2C(=O)[C@H](NS(N)(=O)=O)C(c2ccccc2)c2ccccc2)co1 Show InChI InChI=1S/C26H30N6O5S/c27-24(28)21-14-17(16-37-21)15-30-25(33)20-12-7-13-32(20)26(34)23(31-38(29,35)36)22(18-8-3-1-4-9-18)19-10-5-2-6-11-19/h1-6,8-11,14,16,20,22-23,31H,7,12-13,15H2,(H3,27,28)(H,30,33)(H2,29,35,36)/t20-,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition constant (Ki) against human thrombin |
J Med Chem 46: 3612-22 (2003)
Article DOI: 10.1021/jm030025j
BindingDB Entry DOI: 10.7270/Q28K79T3 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50073160
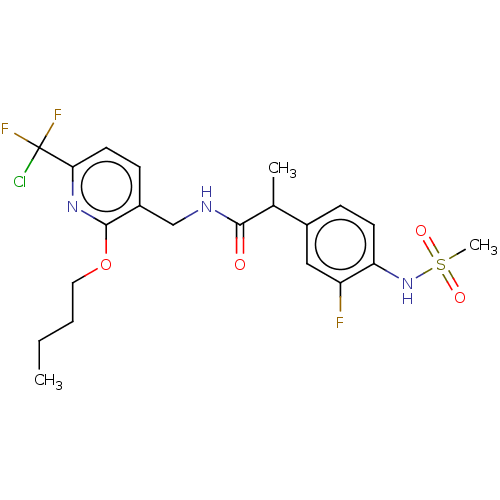
(CHEMBL3407762)Show SMILES CCCCOc1nc(ccc1CNC(=O)C(C)c1ccc(NS(C)(=O)=O)c(F)c1)C(F)(F)Cl Show InChI InChI=1S/C21H25ClF3N3O4S/c1-4-5-10-32-20-15(7-9-18(27-20)21(22,24)25)12-26-19(29)13(2)14-6-8-17(16(23)11-14)28-33(3,30)31/h6-9,11,13,28H,4-5,10,12H2,1-3H3,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of N-arachidonoyl dopamine-induced activity by FLIPR assay |
Eur J Med Chem 93: 101-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.001
BindingDB Entry DOI: 10.7270/Q2N0188S |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131782
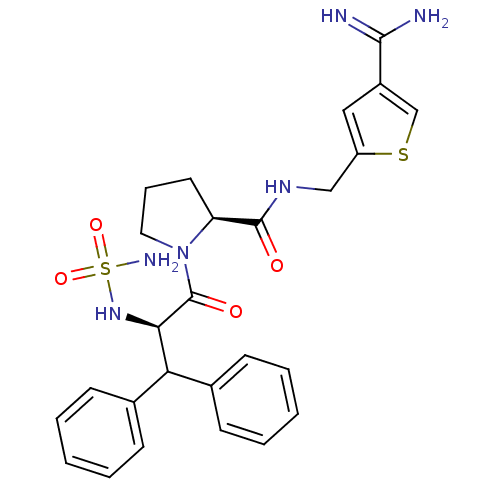
(1-(2-sulfonamide-amino-3,3-diphenyl-propionyl)-pyr...)Show SMILES NC(=N)c1csc(CNC(=O)[C@@H]2CCCN2C(=O)[C@H](NS(N)(=O)=O)C(c2ccccc2)c2ccccc2)c1 Show InChI InChI=1S/C26H30N6O4S2/c27-24(28)19-14-20(37-16-19)15-30-25(33)21-12-7-13-32(21)26(34)23(31-38(29,35)36)22(17-8-3-1-4-9-17)18-10-5-2-6-11-18/h1-6,8-11,14,16,21-23,31H,7,12-13,15H2,(H3,27,28)(H,30,33)(H2,29,35,36)/t21-,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition constant (Ki) against human thrombin |
J Med Chem 46: 3612-22 (2003)
Article DOI: 10.1021/jm030025j
BindingDB Entry DOI: 10.7270/Q28K79T3 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50061298

(CHEMBL3393837)Show SMILES CC1CCN(CC1)c1nc(ccc1CNC(=O)Nc1cccc2cnccc12)C(F)(F)F Show InChI InChI=1S/C23H24F3N5O/c1-15-8-11-31(12-9-15)21-17(5-6-20(30-21)23(24,25)26)14-28-22(32)29-19-4-2-3-16-13-27-10-7-18(16)19/h2-7,10,13,15H,8-9,11-12,14H2,1H3,(H2,28,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 assessed as inhibition of NADA-induced effect at 1 uM by FLIPR assay |
Bioorg Med Chem Lett 25: 803-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.086
BindingDB Entry DOI: 10.7270/Q2JD4ZG3 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50049553
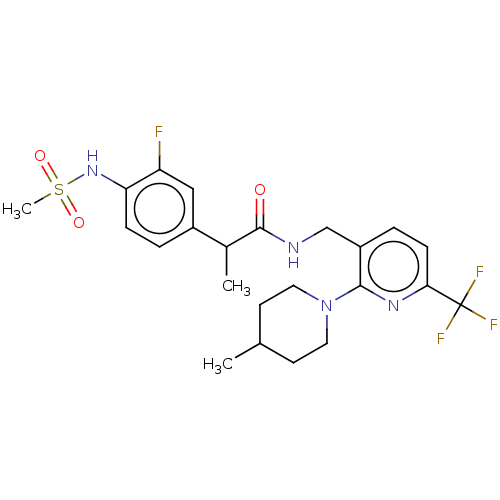
(CHEMBL2177428)Show SMILES CC(C(=O)NCc1ccc(nc1N1CCC(C)CC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C23H28F4N4O3S/c1-14-8-10-31(11-9-14)21-17(5-7-20(29-21)23(25,26)27)13-28-22(32)15(2)16-4-6-19(18(24)12-16)30-35(3,33)34/h4-7,12,14-15,30H,8-11,13H2,1-3H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of N-arachidonoyl dopamine-induced activity by FLIPR assay |
Eur J Med Chem 93: 101-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.001
BindingDB Entry DOI: 10.7270/Q2N0188S |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50111122
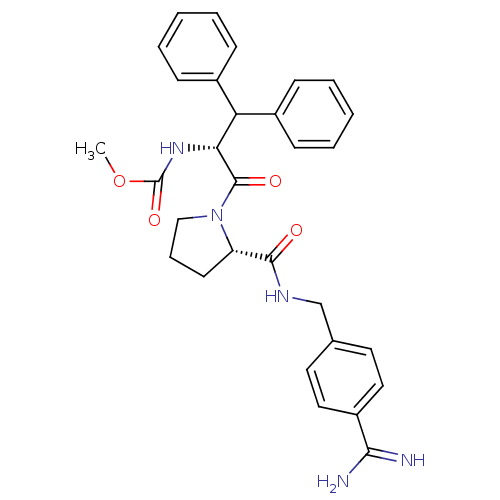
(2N-(4-Benzamidinemethyl)-1-[2-Carbamicacidmethyles...)Show SMILES COC(=O)N[C@H](C(c1ccccc1)c1ccccc1)C(=O)N1CCC[C@H]1C(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C30H33N5O4/c1-39-30(38)34-26(25(21-9-4-2-5-10-21)22-11-6-3-7-12-22)29(37)35-18-8-13-24(35)28(36)33-19-20-14-16-23(17-15-20)27(31)32/h2-7,9-12,14-17,24-26H,8,13,18-19H2,1H3,(H3,31,32)(H,33,36)(H,34,38)/t24-,26+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition constant (Ki) against human thrombin |
J Med Chem 46: 3612-22 (2003)
Article DOI: 10.1021/jm030025j
BindingDB Entry DOI: 10.7270/Q28K79T3 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50073159
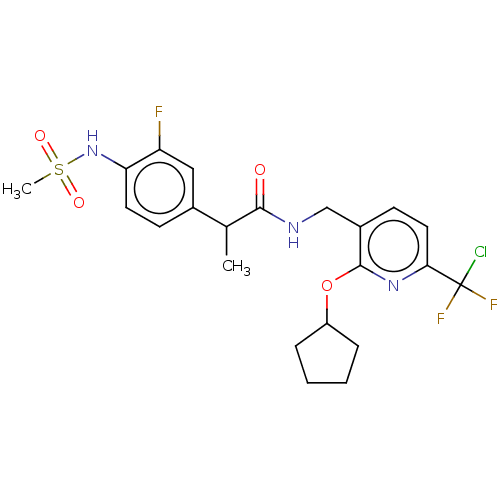
(CHEMBL3407765)Show SMILES CC(C(=O)NCc1ccc(nc1OC1CCCC1)C(F)(F)Cl)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C22H25ClF3N3O4S/c1-13(14-7-9-18(17(24)11-14)29-34(2,31)32)20(30)27-12-15-8-10-19(22(23,25)26)28-21(15)33-16-5-3-4-6-16/h7-11,13,16,29H,3-6,12H2,1-2H3,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of N-arachidonoyl dopamine-induced activity by FLIPR assay |
Eur J Med Chem 93: 101-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.001
BindingDB Entry DOI: 10.7270/Q2N0188S |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131787
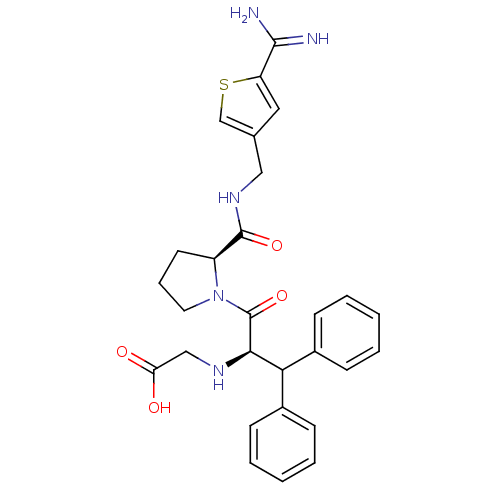
((1-Benzhydryl-2-{2-[(5-carbamimidoyl-thiophen-3-yl...)Show SMILES NC(=N)c1cc(CNC(=O)[C@@H]2CCCN2C(=O)[C@H](NCC(O)=O)C(c2ccccc2)c2ccccc2)cs1 Show InChI InChI=1S/C28H31N5O4S/c29-26(30)22-14-18(17-38-22)15-32-27(36)21-12-7-13-33(21)28(37)25(31-16-23(34)35)24(19-8-3-1-4-9-19)20-10-5-2-6-11-20/h1-6,8-11,14,17,21,24-25,31H,7,12-13,15-16H2,(H3,29,30)(H,32,36)(H,34,35)/t21-,25+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition constant (Ki) against human thrombin |
J Med Chem 46: 3612-22 (2003)
Article DOI: 10.1021/jm030025j
BindingDB Entry DOI: 10.7270/Q28K79T3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131781
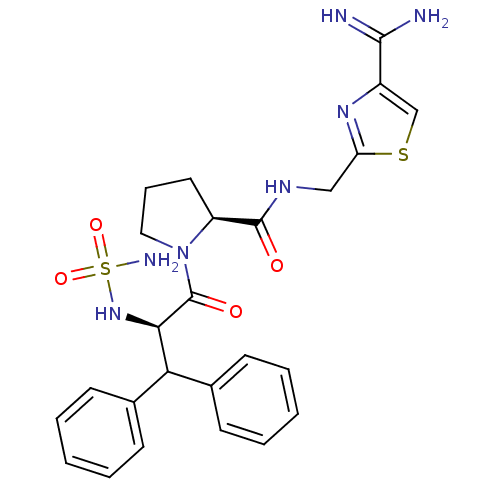
(1-(2-sulfonamide-amino-3,3-diphenyl-propionyl)-pyr...)Show SMILES NC(=N)c1csc(CNC(=O)[C@@H]2CCCN2C(=O)[C@H](NS(N)(=O)=O)C(c2ccccc2)c2ccccc2)n1 Show InChI InChI=1S/C25H29N7O4S2/c26-23(27)18-15-37-20(30-18)14-29-24(33)19-12-7-13-32(19)25(34)22(31-38(28,35)36)21(16-8-3-1-4-9-16)17-10-5-2-6-11-17/h1-6,8-11,15,19,21-22,31H,7,12-14H2,(H3,26,27)(H,29,33)(H2,28,35,36)/t19-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition constant (Ki) against human thrombin |
J Med Chem 46: 3612-22 (2003)
Article DOI: 10.1021/jm030025j
BindingDB Entry DOI: 10.7270/Q28K79T3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131797

(1-(2-sulfonamide-amino-3,3-diphenyl-propionyl)-pyr...)Show SMILES NC(=N)c1cc(CNC(=O)[C@@H]2CCCN2C(=O)[C@H](NS(N)(=O)=O)C(c2ccccc2)c2ccccc2)cs1 Show InChI InChI=1S/C26H30N6O4S2/c27-24(28)21-14-17(16-37-21)15-30-25(33)20-12-7-13-32(20)26(34)23(31-38(29,35)36)22(18-8-3-1-4-9-18)19-10-5-2-6-11-19/h1-6,8-11,14,16,20,22-23,31H,7,12-13,15H2,(H3,27,28)(H,30,33)(H2,29,35,36)/t20-,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition constant (Ki) against human thrombin |
J Med Chem 46: 3612-22 (2003)
Article DOI: 10.1021/jm030025j
BindingDB Entry DOI: 10.7270/Q28K79T3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131779
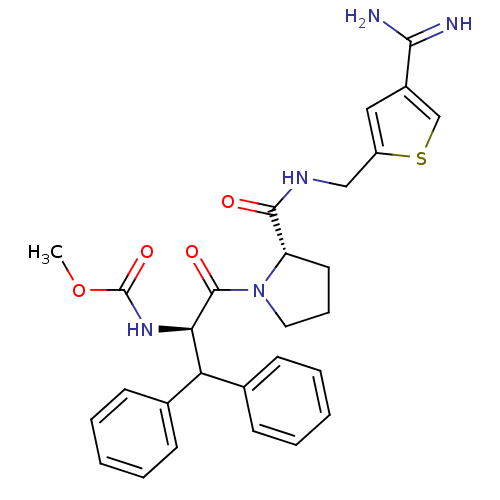
((1-Benzhydryl-2-{2-[(4-carbamimidoyl-thiophen-2-yl...)Show SMILES COC(=O)N[C@H](C(c1ccccc1)c1ccccc1)C(=O)N1CCC[C@H]1C(=O)NCc1cc(cs1)C(N)=N Show InChI InChI=1S/C28H31N5O4S/c1-37-28(36)32-24(23(18-9-4-2-5-10-18)19-11-6-3-7-12-19)27(35)33-14-8-13-22(33)26(34)31-16-21-15-20(17-38-21)25(29)30/h2-7,9-12,15,17,22-24H,8,13-14,16H2,1H3,(H3,29,30)(H,31,34)(H,32,36)/t22-,24+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition constant (Ki) against human thrombin |
J Med Chem 46: 3612-22 (2003)
Article DOI: 10.1021/jm030025j
BindingDB Entry DOI: 10.7270/Q28K79T3 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50061298

(CHEMBL3393837)Show SMILES CC1CCN(CC1)c1nc(ccc1CNC(=O)Nc1cccc2cnccc12)C(F)(F)F Show InChI InChI=1S/C23H24F3N5O/c1-15-8-11-31(12-9-15)21-17(5-6-20(30-21)23(24,25)26)14-28-22(32)29-19-4-2-3-16-13-27-10-7-18(16)19/h2-7,10,13,15H,8-9,11-12,14H2,1H3,(H2,28,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 assessed as inhibition of capsaicin-induced effect by FLIPR assay |
Bioorg Med Chem Lett 25: 803-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.086
BindingDB Entry DOI: 10.7270/Q2JD4ZG3 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50073160

(CHEMBL3407762)Show SMILES CCCCOc1nc(ccc1CNC(=O)C(C)c1ccc(NS(C)(=O)=O)c(F)c1)C(F)(F)Cl Show InChI InChI=1S/C21H25ClF3N3O4S/c1-4-5-10-32-20-15(7-9-18(27-20)21(22,24)25)12-26-19(29)13(2)14-6-8-17(16(23)11-14)28-33(3,30)31/h6-9,11,13,28H,4-5,10,12H2,1-3H3,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced activity by FLIPR assay |
Eur J Med Chem 93: 101-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.001
BindingDB Entry DOI: 10.7270/Q2N0188S |
More data for this
Ligand-Target Pair | |
Serine protease 1/Trypsin-2
(Homo sapiens (Human)) | BDBM50131780

((1-Benzhydryl-2-{2-[(5-carbamimidoyl-thiophen-2-yl...)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2CCCN2C(=O)[C@H](NCC(O)=O)C(c2ccccc2)c2ccccc2)s1 Show InChI InChI=1S/C28H31N5O4S/c29-26(30)22-14-13-20(38-22)16-32-27(36)21-12-7-15-33(21)28(37)25(31-17-23(34)35)24(18-8-3-1-4-9-18)19-10-5-2-6-11-19/h1-6,8-11,13-14,21,24-25,31H,7,12,15-17H2,(H3,29,30)(H,32,36)(H,34,35)/t21-,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition constant (Ki) against human human trypsin was determined |
J Med Chem 46: 3612-22 (2003)
Article DOI: 10.1021/jm030025j
BindingDB Entry DOI: 10.7270/Q28K79T3 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50398494

(CHEMBL2177429)Show SMILES C[C@H](C(=O)NCc1ccc(nc1N1CCC(C)CC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 |r| Show InChI InChI=1S/C23H28F4N4O3S/c1-14-8-10-31(11-9-14)21-17(5-7-20(29-21)23(25,26)27)13-28-22(32)15(2)16-4-6-19(18(24)12-16)30-35(3,33)34/h4-7,12,14-15,30H,8-11,13H2,1-3H3,(H,28,32)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced TRPV1 activation by FLIPR method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128266
BindingDB Entry DOI: 10.7270/Q2CV4NG9 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM176555
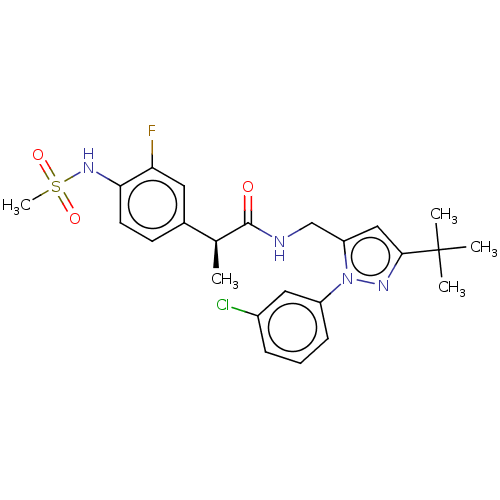
(US9120756, 17)Show SMILES C[C@H](C(=O)NCc1cc(nn1-c1cccc(Cl)c1)C(C)(C)C)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C24H28ClFN4O3S/c1-15(16-9-10-21(20(26)11-16)29-34(5,32)33)23(31)27-14-19-13-22(24(2,3)4)28-30(19)18-8-6-7-17(25)12-18/h6-13,15,29H,14H2,1-5H3,(H,27,31)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced TRPV1 activation by FLIPR method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128266
BindingDB Entry DOI: 10.7270/Q2CV4NG9 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
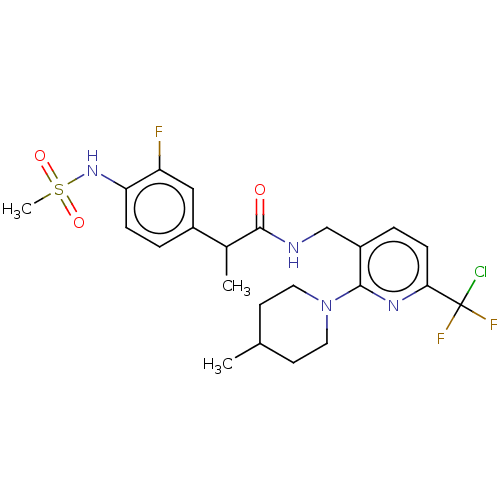
(Homo sapiens (Human)) | BDBM50073156
(CHEMBL3407753)Show SMILES CC(C(=O)NCc1ccc(nc1N1CCC(C)CC1)C(F)(F)Cl)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C23H28ClF3N4O3S/c1-14-8-10-31(11-9-14)21-17(5-7-20(29-21)23(24,26)27)13-28-22(32)15(2)16-4-6-19(18(25)12-16)30-35(3,33)34/h4-7,12,14-15,30H,8-11,13H2,1-3H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced activity by FLIPR assay |
Eur J Med Chem 93: 101-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.001
BindingDB Entry DOI: 10.7270/Q2N0188S |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50049553

(CHEMBL2177428)Show SMILES CC(C(=O)NCc1ccc(nc1N1CCC(C)CC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C23H28F4N4O3S/c1-14-8-10-31(11-9-14)21-17(5-7-20(29-21)23(25,26)27)13-28-22(32)15(2)16-4-6-19(18(24)12-16)30-35(3,33)34/h4-7,12,14-15,30H,8-11,13H2,1-3H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced activity by FLIPR assay |
Eur J Med Chem 93: 101-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.001
BindingDB Entry DOI: 10.7270/Q2N0188S |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM176564
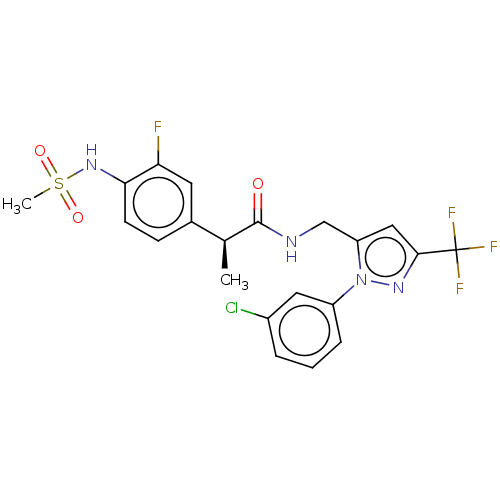
(US9120756, 26)Show SMILES C[C@H](C(=O)NCc1cc(nn1-c1cccc(Cl)c1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C21H19ClF4N4O3S/c1-12(13-6-7-18(17(23)8-13)29-34(2,32)33)20(31)27-11-16-10-19(21(24,25)26)28-30(16)15-5-3-4-14(22)9-15/h3-10,12,29H,11H2,1-2H3,(H,27,31)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced TRPV1 activation by FLIPR method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128266
BindingDB Entry DOI: 10.7270/Q2CV4NG9 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50061352
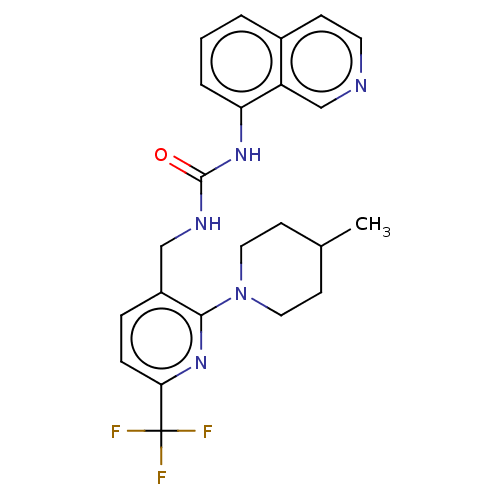
(CHEMBL3393836)Show SMILES CC1CCN(CC1)c1nc(ccc1CNC(=O)Nc1cccc2ccncc12)C(F)(F)F Show InChI InChI=1S/C23H24F3N5O/c1-15-8-11-31(12-9-15)21-17(5-6-20(30-21)23(24,25)26)13-28-22(32)29-19-4-2-3-16-7-10-27-14-18(16)19/h2-7,10,14-15H,8-9,11-13H2,1H3,(H2,28,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 assessed as inhibition of capsaicin-induced effect by FLIPR assay |
Bioorg Med Chem Lett 25: 803-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.086
BindingDB Entry DOI: 10.7270/Q2JD4ZG3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131784
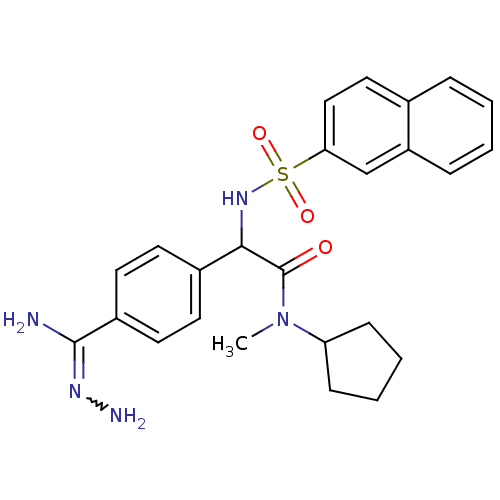
(2-{4-[(Z)-amino(hydrazono)methyl]phenyl}-N-cyclope...)Show SMILES CN(C1CCCC1)C(=O)C(NS(=O)(=O)c1ccc2ccccc2c1)c1ccc(cc1)C(N)=NN |w:32.36| Show InChI InChI=1S/C25H29N5O3S/c1-30(21-8-4-5-9-21)25(31)23(18-10-12-19(13-11-18)24(26)28-27)29-34(32,33)22-15-14-17-6-2-3-7-20(17)16-22/h2-3,6-7,10-16,21,23,29H,4-5,8-9,27H2,1H3,(H2,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition constant (Ki) against human thrombin |
J Med Chem 46: 3612-22 (2003)
Article DOI: 10.1021/jm030025j
BindingDB Entry DOI: 10.7270/Q28K79T3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131783
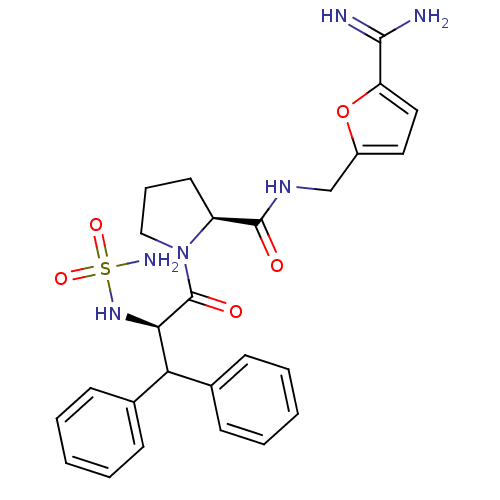
(1-(2-sulfonamide-amino-3,3-diphenyl-propionyl)-pyr...)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2CCCN2C(=O)[C@H](NS(N)(=O)=O)C(c2ccccc2)c2ccccc2)o1 Show InChI InChI=1S/C26H30N6O5S/c27-24(28)21-14-13-19(37-21)16-30-25(33)20-12-7-15-32(20)26(34)23(31-38(29,35)36)22(17-8-3-1-4-9-17)18-10-5-2-6-11-18/h1-6,8-11,13-14,20,22-23,31H,7,12,15-16H2,(H3,27,28)(H,30,33)(H2,29,35,36)/t20-,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition constant (Ki) against human thrombin |
J Med Chem 46: 3612-22 (2003)
Article DOI: 10.1021/jm030025j
BindingDB Entry DOI: 10.7270/Q28K79T3 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50263407
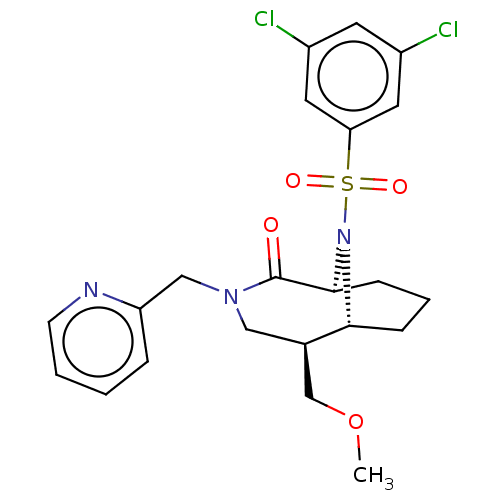
(CHEMBL4090599)Show SMILES [H][C@]12CCC[C@]([H])(N1S(=O)(=O)c1cc(Cl)cc(Cl)c1)C(=O)N(Cc1ccccn1)C[C@@H]2COC |r,TLB:31:30:7:2.3.4,20:19:7:2.3.4,THB:22:21:7:2.3.4| Show InChI InChI=1S/C22H25Cl2N3O4S/c1-31-14-15-12-26(13-18-5-2-3-8-25-18)22(28)21-7-4-6-20(15)27(21)32(29,30)19-10-16(23)9-17(24)11-19/h2-3,5,8-11,15,20-21H,4,6-7,12-14H2,1H3/t15-,20-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany.
Curated by ChEMBL
| Assay Description
Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... |
J Med Chem 61: 3660-3673 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00137
BindingDB Entry DOI: 10.7270/Q2833VGW |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50073183
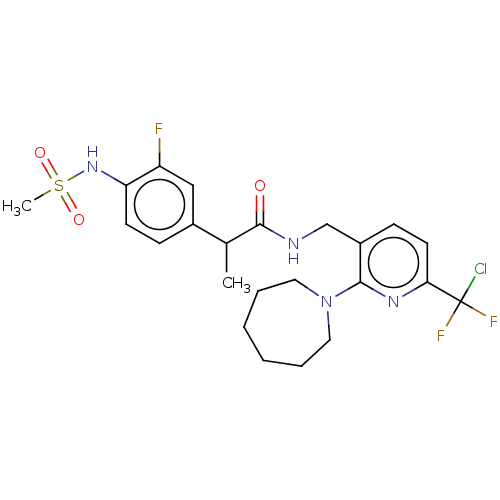
(CHEMBL3407760)Show SMILES CC(C(=O)NCc1ccc(nc1N1CCCCCC1)C(F)(F)Cl)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C23H28ClF3N4O3S/c1-15(16-7-9-19(18(25)13-16)30-35(2,33)34)22(32)28-14-17-8-10-20(23(24,26)27)29-21(17)31-11-5-3-4-6-12-31/h7-10,13,15,30H,3-6,11-12,14H2,1-2H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced activity by FLIPR assay |
Eur J Med Chem 93: 101-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.001
BindingDB Entry DOI: 10.7270/Q2N0188S |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50073159

(CHEMBL3407765)Show SMILES CC(C(=O)NCc1ccc(nc1OC1CCCC1)C(F)(F)Cl)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C22H25ClF3N3O4S/c1-13(14-7-9-18(17(24)11-14)29-34(2,31)32)20(30)27-12-15-8-10-19(22(23,25)26)28-21(15)33-16-5-3-4-6-16/h7-11,13,16,29H,3-6,12H2,1-2H3,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced activity by FLIPR assay |
Eur J Med Chem 93: 101-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.001
BindingDB Entry DOI: 10.7270/Q2N0188S |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50151114

(CHEMBL3770682)Show SMILES C[C@H](C(=O)NCc1ccc(nc1N(Cc1ccccc1)S(=O)(=O)c1ccccc1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 |r| Show InChI InChI=1S/C30H28F4N4O5S2/c1-20(22-13-15-26(25(31)17-22)37-44(2,40)41)29(39)35-18-23-14-16-27(30(32,33)34)36-28(23)38(19-21-9-5-3-6-10-21)45(42,43)24-11-7-4-8-12-24/h3-17,20,37H,18-19H2,1-2H3,(H,35,39)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX from human TRPV1 expressed in CHO cells after 60 mins by scintillation counting analysis |
Bioorg Med Chem 24: 1231-40 (2016)
Article DOI: 10.1016/j.bmc.2016.01.051
BindingDB Entry DOI: 10.7270/Q25D8TP7 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50073150
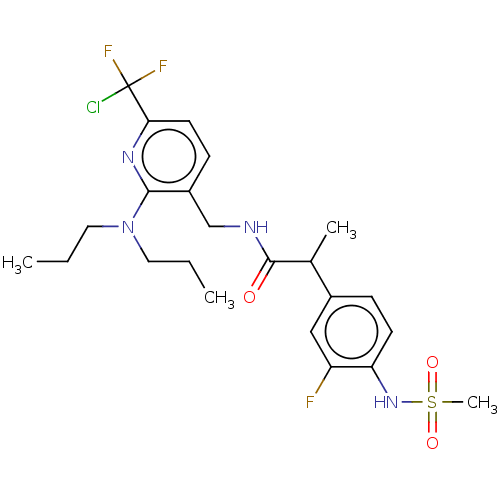
(CHEMBL3407758)Show SMILES CCCN(CCC)c1nc(ccc1CNC(=O)C(C)c1ccc(NS(C)(=O)=O)c(F)c1)C(F)(F)Cl Show InChI InChI=1S/C23H30ClF3N4O3S/c1-5-11-31(12-6-2)21-17(8-10-20(29-21)23(24,26)27)14-28-22(32)15(3)16-7-9-19(18(25)13-16)30-35(4,33)34/h7-10,13,15,30H,5-6,11-12,14H2,1-4H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced activity by FLIPR assay |
Eur J Med Chem 93: 101-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.001
BindingDB Entry DOI: 10.7270/Q2N0188S |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131788
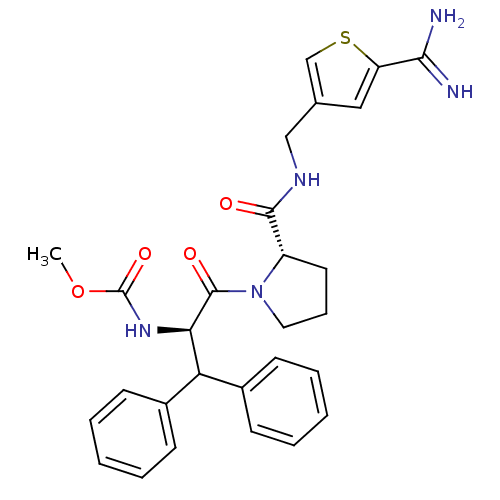
((1-Benzhydryl-2-{2-[(5-carbamimidoyl-thiophen-3-yl...)Show SMILES COC(=O)N[C@H](C(c1ccccc1)c1ccccc1)C(=O)N1CCC[C@H]1C(=O)NCc1csc(c1)C(N)=N Show InChI InChI=1S/C28H31N5O4S/c1-37-28(36)32-24(23(19-9-4-2-5-10-19)20-11-6-3-7-12-20)27(35)33-14-8-13-21(33)26(34)31-16-18-15-22(25(29)30)38-17-18/h2-7,9-12,15,17,21,23-24H,8,13-14,16H2,1H3,(H3,29,30)(H,31,34)(H,32,36)/t21-,24+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition constant (Ki) against human thrombin |
J Med Chem 46: 3612-22 (2003)
Article DOI: 10.1021/jm030025j
BindingDB Entry DOI: 10.7270/Q28K79T3 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50073174
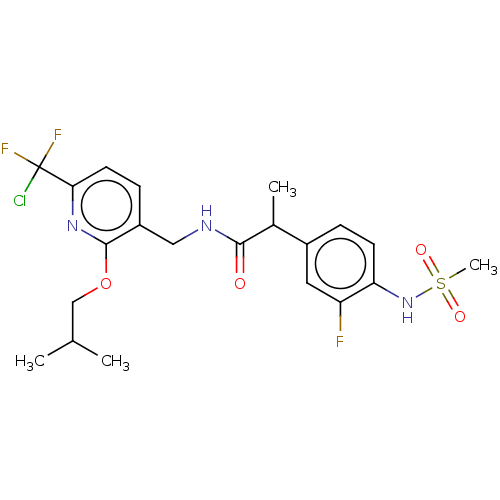
(CHEMBL3407764)Show SMILES CC(C)COc1nc(ccc1CNC(=O)C(C)c1ccc(NS(C)(=O)=O)c(F)c1)C(F)(F)Cl Show InChI InChI=1S/C21H25ClF3N3O4S/c1-12(2)11-32-20-15(6-8-18(27-20)21(22,24)25)10-26-19(29)13(3)14-5-7-17(16(23)9-14)28-33(4,30)31/h5-9,12-13,28H,10-11H2,1-4H3,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced activity by FLIPR assay |
Eur J Med Chem 93: 101-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.001
BindingDB Entry DOI: 10.7270/Q2N0188S |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131793
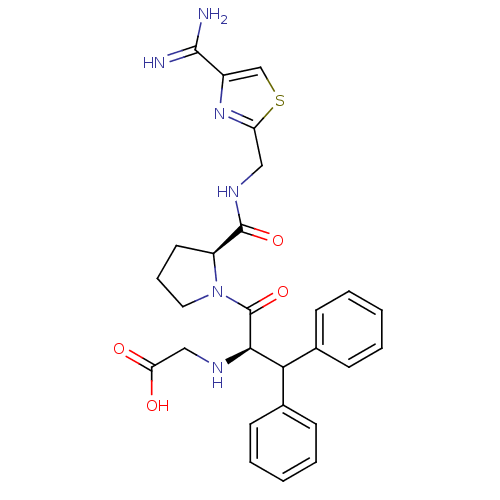
((1-Benzhydryl-2-{2-[(4-carbamimidoyl-thiazol-2-ylm...)Show SMILES NC(=N)c1csc(CNC(=O)[C@@H]2CCCN2C(=O)[C@H](NCC(O)=O)C(c2ccccc2)c2ccccc2)n1 Show InChI InChI=1S/C27H30N6O4S/c28-25(29)19-16-38-21(32-19)14-31-26(36)20-12-7-13-33(20)27(37)24(30-15-22(34)35)23(17-8-3-1-4-9-17)18-10-5-2-6-11-18/h1-6,8-11,16,20,23-24,30H,7,12-15H2,(H3,28,29)(H,31,36)(H,34,35)/t20-,24+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition constant (Ki) against human thrombin |
J Med Chem 46: 3612-22 (2003)
Article DOI: 10.1021/jm030025j
BindingDB Entry DOI: 10.7270/Q28K79T3 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50061319
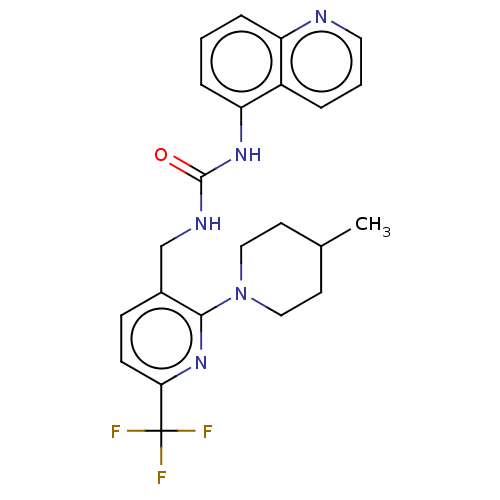
(CHEMBL3393838)Show SMILES CC1CCN(CC1)c1nc(ccc1CNC(=O)Nc1cccc2ncccc12)C(F)(F)F Show InChI InChI=1S/C23H24F3N5O/c1-15-9-12-31(13-10-15)21-16(7-8-20(30-21)23(24,25)26)14-28-22(32)29-19-6-2-5-18-17(19)4-3-11-27-18/h2-8,11,15H,9-10,12-14H2,1H3,(H2,28,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 assessed as inhibition of capsaicin-induced effect by FLIPR assay |
Bioorg Med Chem Lett 25: 803-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.086
BindingDB Entry DOI: 10.7270/Q2JD4ZG3 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50150646

(CHEMBL3770091)Show SMILES CC(C(=O)NCc1ccc(nc1N(Cc1ccc(F)cc1)S(=O)(=O)c1ccc(F)cc1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C30H26F6N4O5S2/c1-18(20-5-13-26(25(33)15-20)39-46(2,42)43)29(41)37-16-21-6-14-27(30(34,35)36)38-28(21)40(17-19-3-7-22(31)8-4-19)47(44,45)24-11-9-23(32)10-12-24/h3-15,18,39H,16-17H2,1-2H3,(H,37,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX from human TRPV1 expressed in CHO cells after 60 mins by scintillation counting analysis |
Bioorg Med Chem 24: 1231-40 (2016)
Article DOI: 10.1016/j.bmc.2016.01.051
BindingDB Entry DOI: 10.7270/Q25D8TP7 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50151119
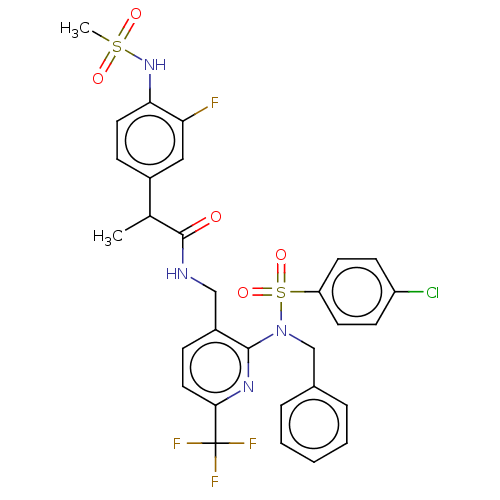
(CHEMBL3769821)Show SMILES CC(C(=O)NCc1ccc(nc1N(Cc1ccccc1)S(=O)(=O)c1ccc(Cl)cc1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C30H27ClF4N4O5S2/c1-19(21-8-14-26(25(32)16-21)38-45(2,41)42)29(40)36-17-22-9-15-27(30(33,34)35)37-28(22)39(18-20-6-4-3-5-7-20)46(43,44)24-12-10-23(31)11-13-24/h3-16,19,38H,17-18H2,1-2H3,(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX from human TRPV1 expressed in CHO cells after 60 mins by scintillation counting analysis |
Bioorg Med Chem 24: 1231-40 (2016)
Article DOI: 10.1016/j.bmc.2016.01.051
BindingDB Entry DOI: 10.7270/Q25D8TP7 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50073178
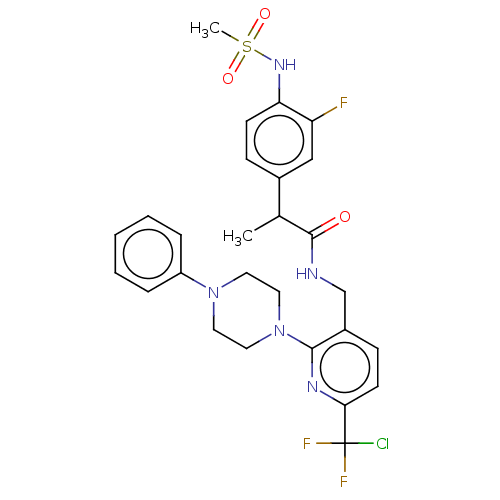
(CHEMBL3407761)Show SMILES CC(C(=O)NCc1ccc(nc1N1CCN(CC1)c1ccccc1)C(F)(F)Cl)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C27H29ClF3N5O3S/c1-18(19-8-10-23(22(29)16-19)34-40(2,38)39)26(37)32-17-20-9-11-24(27(28,30)31)33-25(20)36-14-12-35(13-15-36)21-6-4-3-5-7-21/h3-11,16,18,34H,12-15,17H2,1-2H3,(H,32,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced activity by FLIPR assay |
Eur J Med Chem 93: 101-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.001
BindingDB Entry DOI: 10.7270/Q2N0188S |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50073177
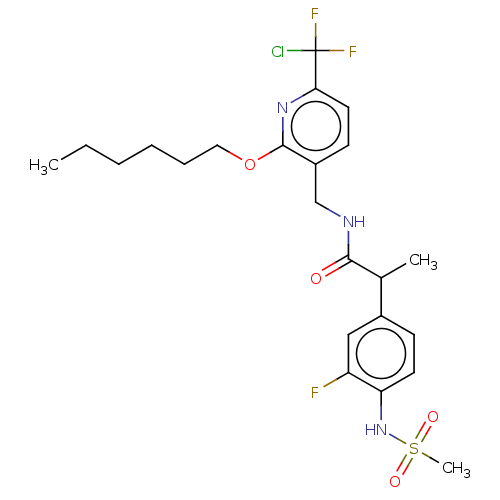
(CHEMBL3407763)Show SMILES CCCCCCOc1nc(ccc1CNC(=O)C(C)c1ccc(NS(C)(=O)=O)c(F)c1)C(F)(F)Cl Show InChI InChI=1S/C23H29ClF3N3O4S/c1-4-5-6-7-12-34-22-17(9-11-20(29-22)23(24,26)27)14-28-21(31)15(2)16-8-10-19(18(25)13-16)30-35(3,32)33/h8-11,13,15,30H,4-7,12,14H2,1-3H3,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced activity by FLIPR assay |
Eur J Med Chem 93: 101-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.001
BindingDB Entry DOI: 10.7270/Q2N0188S |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50073155

(CHEMBL3407754)Show SMILES CC(C(=O)NCc1ccc(nc1N1CCC(C)CC1)C1CC1)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C25H33FN4O3S/c1-16-10-12-30(13-11-16)24-20(7-8-22(28-24)18-4-5-18)15-27-25(31)17(2)19-6-9-23(21(26)14-19)29-34(3,32)33/h6-9,14,16-18,29H,4-5,10-13,15H2,1-3H3,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced activity by FLIPR assay |
Eur J Med Chem 93: 101-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.001
BindingDB Entry DOI: 10.7270/Q2N0188S |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50507371
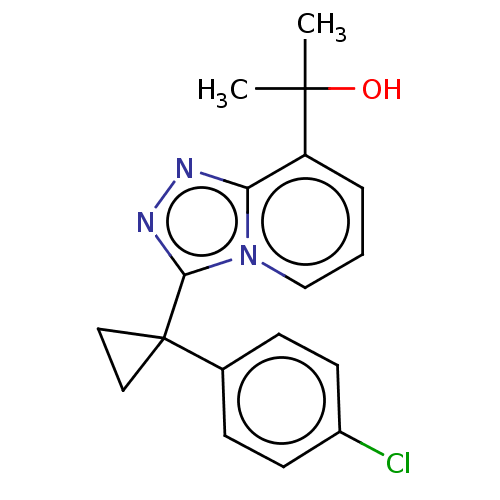
(BMS-823778)Show InChI InChI=1S/C18H18ClN3O/c1-17(2,23)14-4-3-11-22-15(14)20-21-16(22)18(9-10-18)12-5-7-13(19)8-6-12/h3-8,11,23H,9-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 11beta-HSD1 expressed in HEK293 cell microsomes using [3H]cortisone as substrate after 4 hrs by homogeneous immuno-ra... |
ACS Med Chem Lett 9: 1170-1174 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00307
BindingDB Entry DOI: 10.7270/Q20R9SP3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50073170
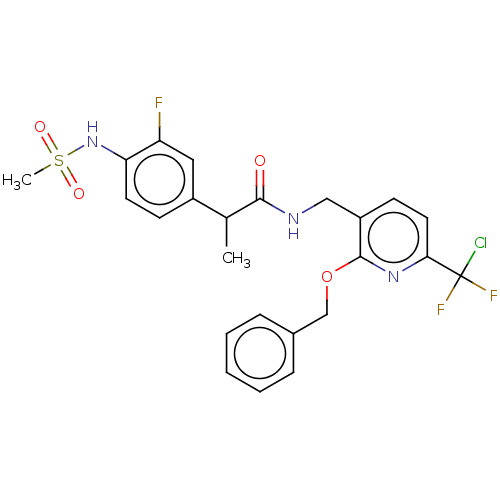
(CHEMBL3407950)Show SMILES CC(C(=O)NCc1ccc(nc1OCc1ccccc1)C(F)(F)Cl)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C24H23ClF3N3O4S/c1-15(17-8-10-20(19(26)12-17)31-36(2,33)34)22(32)29-13-18-9-11-21(24(25,27)28)30-23(18)35-14-16-6-4-3-5-7-16/h3-12,15,31H,13-14H2,1-2H3,(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced activity by FLIPR assay |
Eur J Med Chem 93: 101-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.001
BindingDB Entry DOI: 10.7270/Q2N0188S |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data