

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sialidase (Clostridium perfringens) | BDBM50442400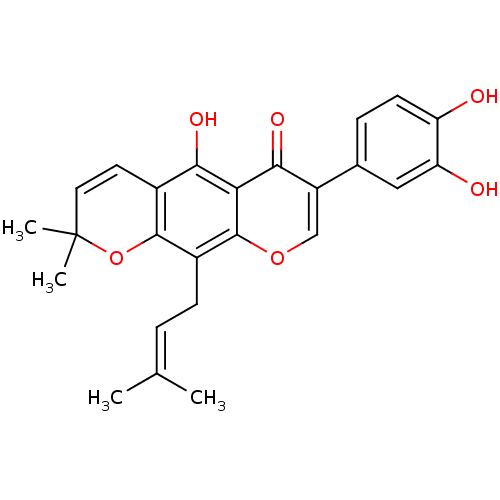 (AURICULASIN) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Non-competitive inhibition of Clostridium perfringens neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid sodium salt hydrate as... | Bioorg Med Chem 21: 6398-404 (2013) Article DOI: 10.1016/j.bmc.2013.08.049 BindingDB Entry DOI: 10.7270/Q2Z89DVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50495308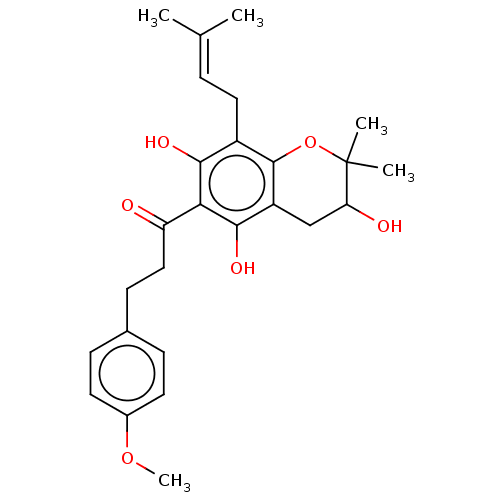 (CHEMBL3103546) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase using L-tyrosine as substrate preincubated for 10 mins with substrate followed by enzyme addition measu... | Bioorg Med Chem 22: 1115-20 (2014) Article DOI: 10.1016/j.bmc.2013.12.047 BindingDB Entry DOI: 10.7270/Q2222XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50495305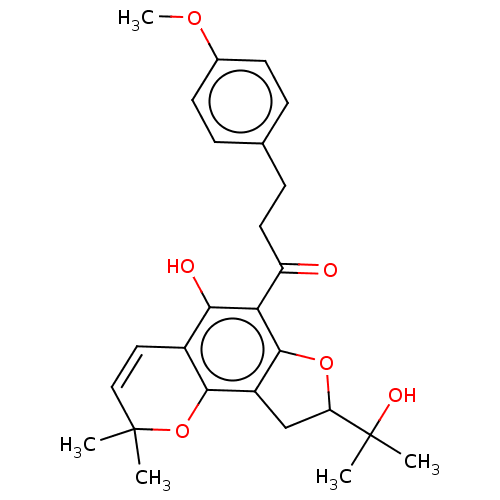 (CHEMBL3103544) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase using L-tyrosine as substrate preincubated for 10 mins with substrate followed by enzyme addition measu... | Bioorg Med Chem 22: 1115-20 (2014) Article DOI: 10.1016/j.bmc.2013.12.047 BindingDB Entry DOI: 10.7270/Q2222XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50495309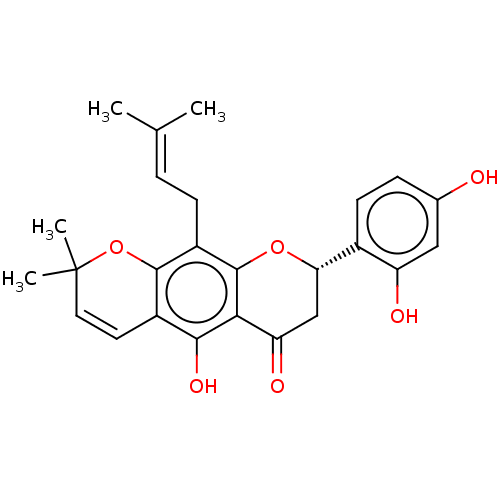 (Flemichin D) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase using L-tyrosine as substrate preincubated for 10 mins with substrate followed by enzyme addition measu... | Bioorg Med Chem 22: 1115-20 (2014) Article DOI: 10.1016/j.bmc.2013.12.047 BindingDB Entry DOI: 10.7270/Q2222XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50442401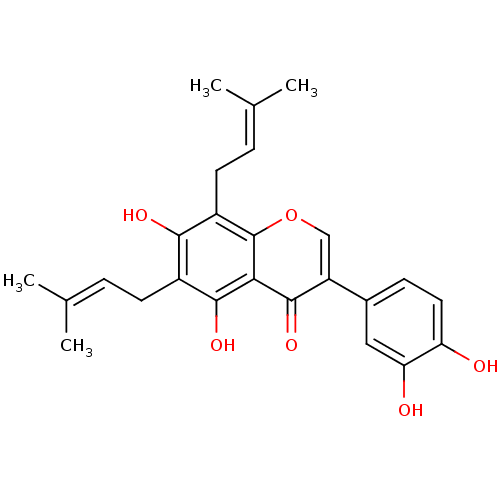 (CHEMBL2442947) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Non-competitive inhibition of Clostridium perfringens neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid sodium salt hydrate as... | Bioorg Med Chem 21: 6398-404 (2013) Article DOI: 10.1016/j.bmc.2013.08.049 BindingDB Entry DOI: 10.7270/Q2Z89DVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50380205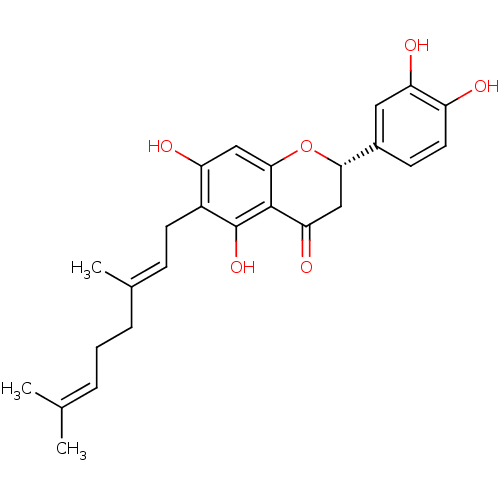 (NYMPHAEOL A) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Mixed type inhibition of equine BChE using butyrylthiocholine iodide as substrate by Lineweaver-Burk double-reciprocal-plot and dixon plot analysis | Bioorg Med Chem 20: 2595-602 (2012) Article DOI: 10.1016/j.bmc.2012.02.044 BindingDB Entry DOI: 10.7270/Q22808MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50442399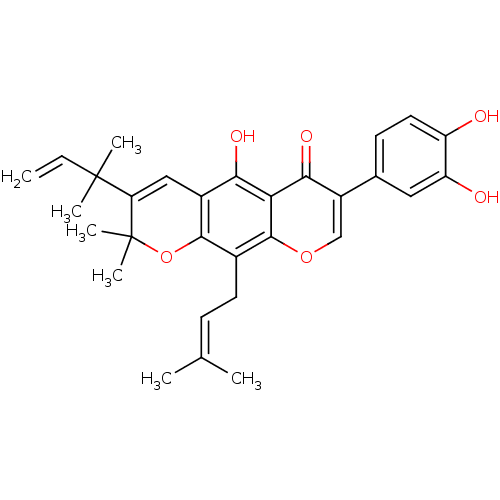 (CHEMBL2442948) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Non-competitive inhibition of Clostridium perfringens neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid sodium salt hydrate as... | Bioorg Med Chem 21: 6398-404 (2013) Article DOI: 10.1016/j.bmc.2013.08.049 BindingDB Entry DOI: 10.7270/Q2Z89DVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50495306 (khonklonginol H) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase using L-tyrosine as substrate preincubated for 10 mins with substrate followed by enzyme addition measu... | Bioorg Med Chem 22: 1115-20 (2014) Article DOI: 10.1016/j.bmc.2013.12.047 BindingDB Entry DOI: 10.7270/Q2222XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50442401 (CHEMBL2442947) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qiqihar University Curated by ChEMBL | Assay Description Non-competitive inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometry based Lineweaver-Burk plot | Bioorg Med Chem Lett 26: 318-21 (2016) Article DOI: 10.1016/j.bmcl.2015.12.021 BindingDB Entry DOI: 10.7270/Q2C82C4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50495305 (CHEMBL3103544) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase using L-DOPA as substrate preincubated for 10 mins with substrate followed by enzyme addition measured ... | Bioorg Med Chem 22: 1115-20 (2014) Article DOI: 10.1016/j.bmc.2013.12.047 BindingDB Entry DOI: 10.7270/Q2222XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50278899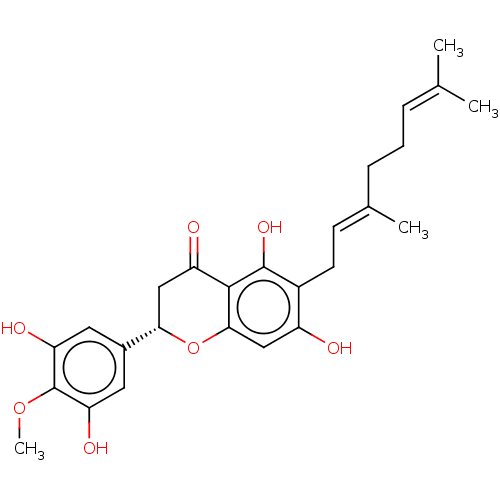 (CHEMBL4174694) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of human neutrophil elastase using varying levels of MeOSuc-AAPV-pNA as substrate measured after 30 mins by double-reciproc... | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50442397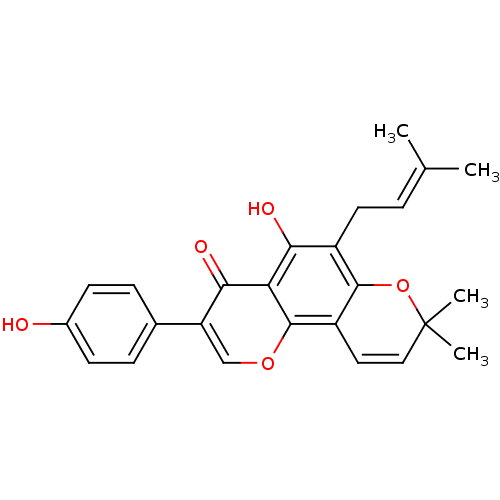 (OSAJIN) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Non-competitive inhibition of Clostridium perfringens neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid sodium salt hydrate as... | Bioorg Med Chem 21: 6398-404 (2013) Article DOI: 10.1016/j.bmc.2013.08.049 BindingDB Entry DOI: 10.7270/Q2Z89DVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50136569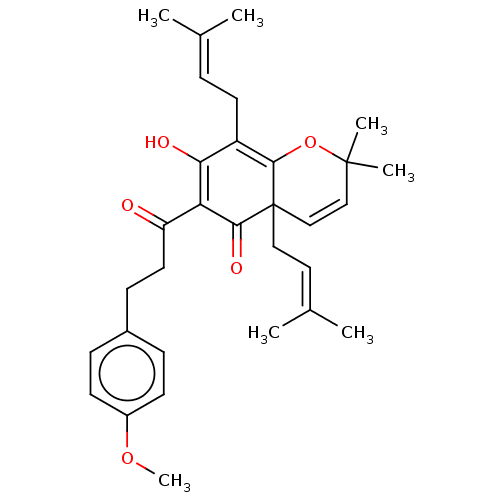 (CHEMBL3754629) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qiqihar University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometry based Lineweaver-Burk plot | Bioorg Med Chem Lett 26: 318-21 (2016) Article DOI: 10.1016/j.bmcl.2015.12.021 BindingDB Entry DOI: 10.7270/Q2C82C4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50442402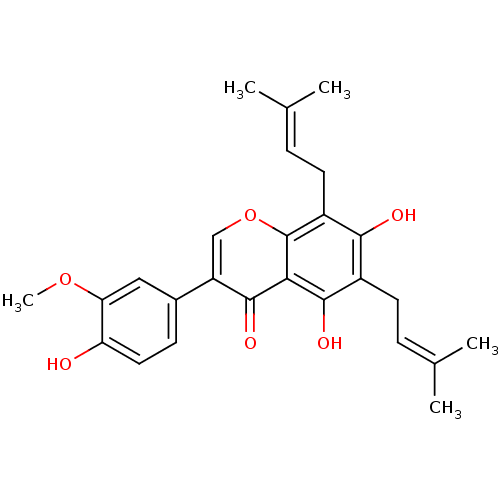 (FLEMINGSIN) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Non-competitive inhibition of Clostridium perfringens neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid sodium salt hydrate as... | Bioorg Med Chem 21: 6398-404 (2013) Article DOI: 10.1016/j.bmc.2013.08.049 BindingDB Entry DOI: 10.7270/Q2Z89DVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50495309 (Flemichin D) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase using L-DOPA as substrate preincubated for 10 mins with substrate followed by enzyme addition measured ... | Bioorg Med Chem 22: 1115-20 (2014) Article DOI: 10.1016/j.bmc.2013.12.047 BindingDB Entry DOI: 10.7270/Q2222XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50442403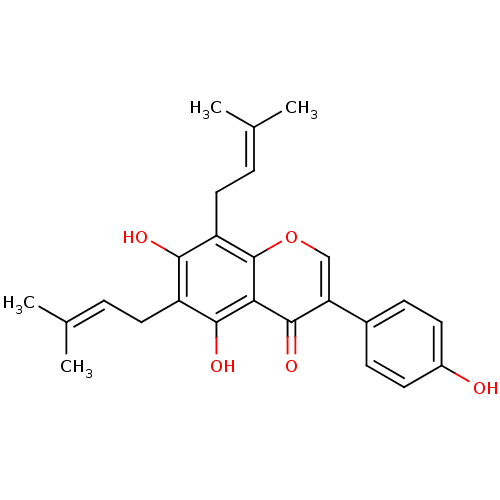 (CHEMBL494252) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 4.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Non-competitive inhibition of Clostridium perfringens neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid sodium salt hydrate as... | Bioorg Med Chem 21: 6398-404 (2013) Article DOI: 10.1016/j.bmc.2013.08.049 BindingDB Entry DOI: 10.7270/Q2Z89DVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50380203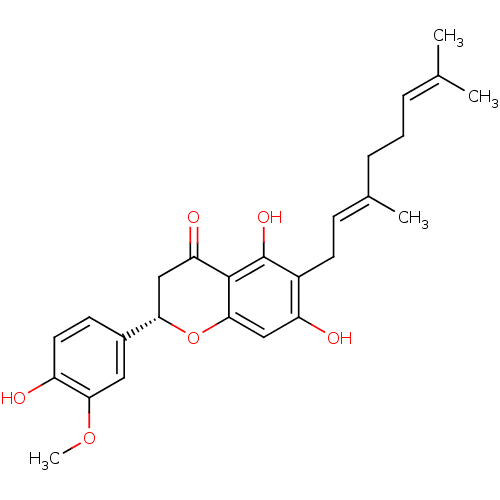 (CHEMBL253152) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of human neutrophil elastase using varying levels of MeOSuc-AAPV-pNA as substrate measured after 30 mins by double-reciproc... | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50442400 (AURICULASIN) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qiqihar University Curated by ChEMBL | Assay Description Non-competitive inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometry based Lineweaver-Burk plot | Bioorg Med Chem Lett 26: 318-21 (2016) Article DOI: 10.1016/j.bmcl.2015.12.021 BindingDB Entry DOI: 10.7270/Q2C82C4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50380201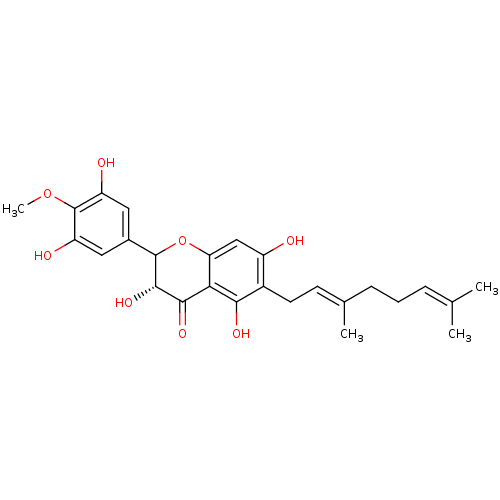 (CHEMBL2011403) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Mixed type inhibition of equine BChE using butyrylthiocholine iodide as substrate by Lineweaver-Burk double-reciprocal-plot and dixon plot analysis | Bioorg Med Chem 20: 2595-602 (2012) Article DOI: 10.1016/j.bmc.2012.02.044 BindingDB Entry DOI: 10.7270/Q22808MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50278908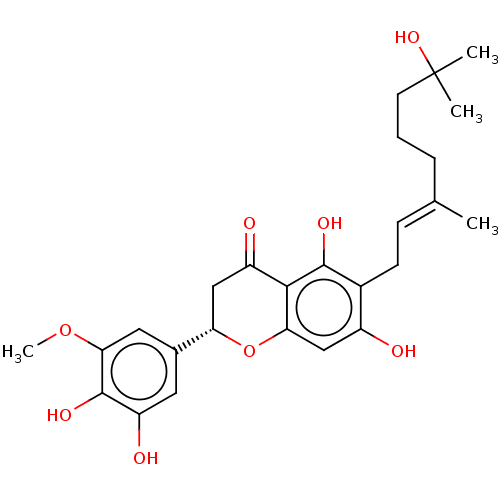 (CHEMBL4160635) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of human neutrophil elastase using varying levels of MeOSuc-AAPV-pNA as substrate measured after 30 mins by double-reciproc... | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50495310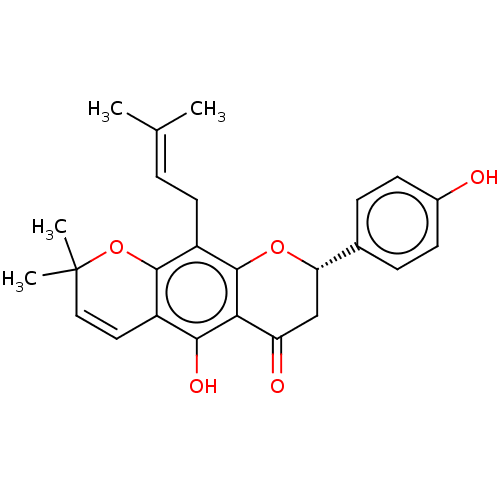 (Lupinifolin) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase using L-tyrosine as substrate preincubated for 10 mins with substrate followed by enzyme addition measu... | Bioorg Med Chem 22: 1115-20 (2014) Article DOI: 10.1016/j.bmc.2013.12.047 BindingDB Entry DOI: 10.7270/Q2222XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50278898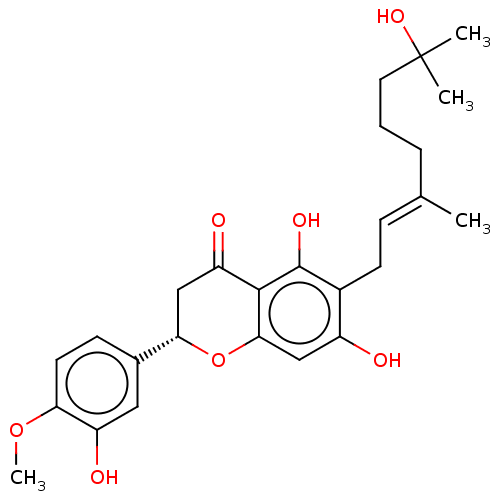 (CHEMBL4167885) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of human neutrophil elastase using varying levels of MeOSuc-AAPV-pNA as substrate measured after 30 mins by double-reciproc... | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50442398 (CHEMBL2442949) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Non-competitive inhibition of Clostridium perfringens neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid sodium salt hydrate as... | Bioorg Med Chem 21: 6398-404 (2013) Article DOI: 10.1016/j.bmc.2013.08.049 BindingDB Entry DOI: 10.7270/Q2Z89DVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50380204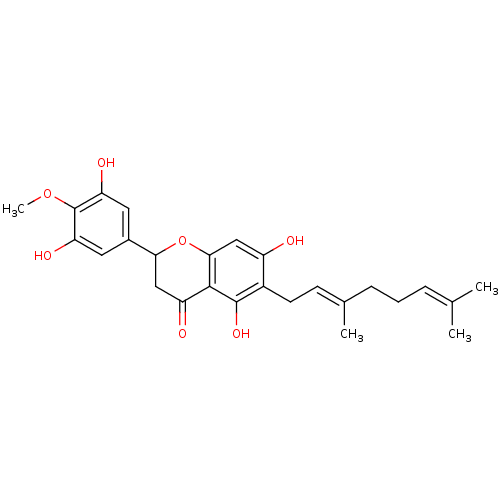 (CHEMBL2011405 | US10406136, Compound 6) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Mixed type inhibition of equine BChE using butyrylthiocholine iodide as substrate by Lineweaver-Burk double-reciprocal-plot and dixon plot analysis | Bioorg Med Chem 20: 2595-602 (2012) Article DOI: 10.1016/j.bmc.2012.02.044 BindingDB Entry DOI: 10.7270/Q22808MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50442396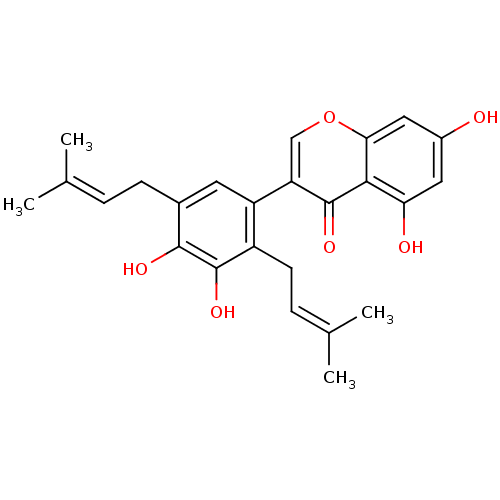 (CHEMBL2442950) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Non-competitive inhibition of Clostridium perfringens neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid sodium salt hydrate as... | Bioorg Med Chem 21: 6398-404 (2013) Article DOI: 10.1016/j.bmc.2013.08.049 BindingDB Entry DOI: 10.7270/Q2Z89DVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50495306 (khonklonginol H) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase using L-DOPA as substrate preincubated for 10 mins with substrate followed by enzyme addition measured ... | Bioorg Med Chem 22: 1115-20 (2014) Article DOI: 10.1016/j.bmc.2013.12.047 BindingDB Entry DOI: 10.7270/Q2222XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50495308 (CHEMBL3103546) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase using L-DOPA as substrate preincubated for 10 mins with substrate followed by enzyme addition measured ... | Bioorg Med Chem 22: 1115-20 (2014) Article DOI: 10.1016/j.bmc.2013.12.047 BindingDB Entry DOI: 10.7270/Q2222XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50278911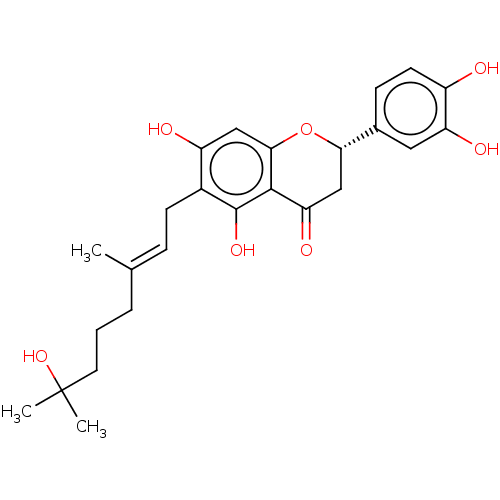 (CHEMBL4171240) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of human neutrophil elastase using varying levels of MeOSuc-AAPV-pNA as substrate measured after 30 mins by double-reciproc... | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50380205 (NYMPHAEOL A) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Mixed type inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate by Lineweaver-Burk double-reciprocal-plot and dixon plot ... | Bioorg Med Chem 20: 2595-602 (2012) Article DOI: 10.1016/j.bmc.2012.02.044 BindingDB Entry DOI: 10.7270/Q22808MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50495307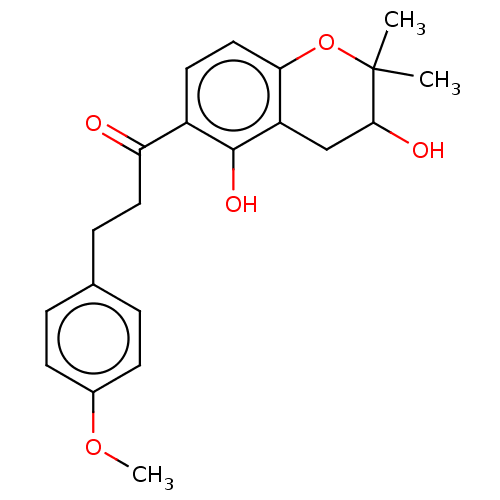 (CHEMBL3103545) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase using L-tyrosine as substrate preincubated for 10 mins with substrate followed by enzyme addition measu... | Bioorg Med Chem 22: 1115-20 (2014) Article DOI: 10.1016/j.bmc.2013.12.047 BindingDB Entry DOI: 10.7270/Q2222XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50442403 (CHEMBL494252) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qiqihar University Curated by ChEMBL | Assay Description Non-competitive inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometry based Lineweaver-Burk plot | Bioorg Med Chem Lett 26: 318-21 (2016) Article DOI: 10.1016/j.bmcl.2015.12.021 BindingDB Entry DOI: 10.7270/Q2C82C4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM7459 (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of human neutrophil elastase using varying levels of MeOSuc-AAPV-pNA as substrate measured after 30 mins by double-reciproc... | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50278902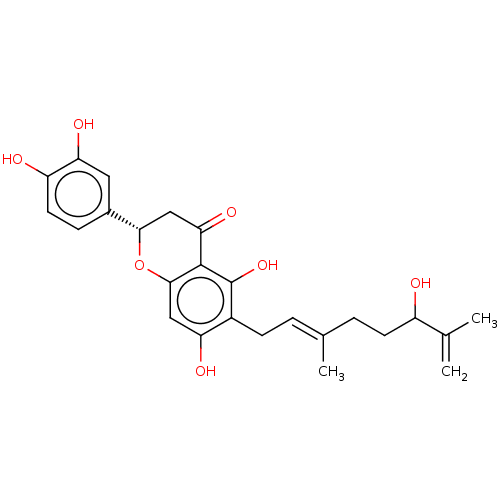 (CHEBI:66191 | tanariflavanone D) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of human neutrophil elastase using varying levels of MeOSuc-AAPV-pNA as substrate measured after 30 mins by double-reciproc... | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of human neutrophil elastase using varying levels of MeOSuc-AAPV-pNA as substrate measured after 30 mins by double-reciproc... | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50136568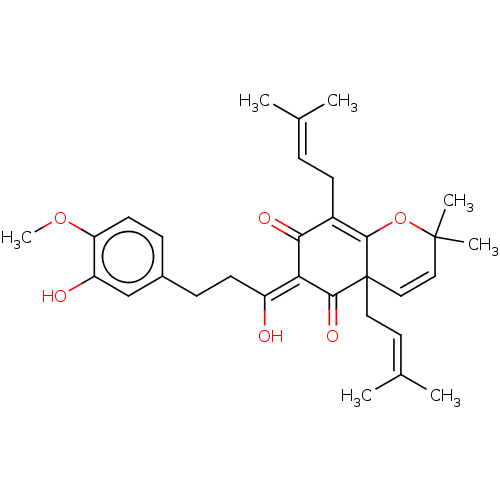 (CHEMBL3754570) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qiqihar University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometry based Lineweaver-Burk plot | Bioorg Med Chem Lett 26: 318-21 (2016) Article DOI: 10.1016/j.bmcl.2015.12.021 BindingDB Entry DOI: 10.7270/Q2C82C4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50278883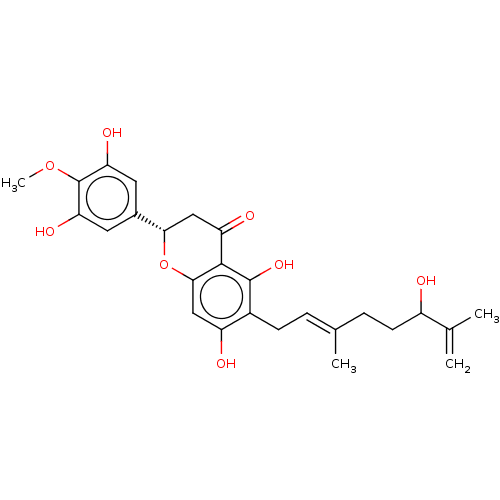 (CHEMBL4167043) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of human neutrophil elastase using varying levels of MeOSuc-AAPV-pNA as substrate measured after 30 mins by double-reciproc... | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50278905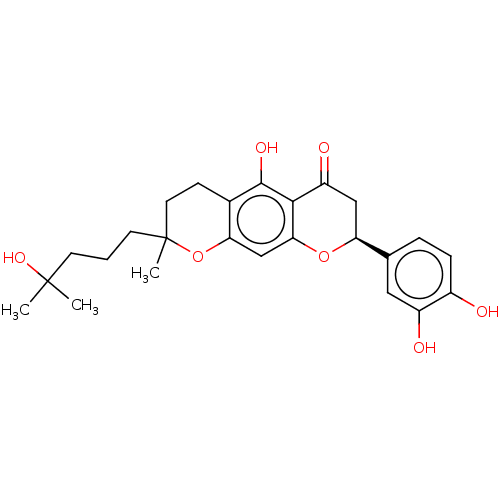 (CHEMBL2387711) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of human neutrophil elastase using varying levels of MeOSuc-AAPV-pNA as substrate measured after 30 mins by double-reciproc... | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50278904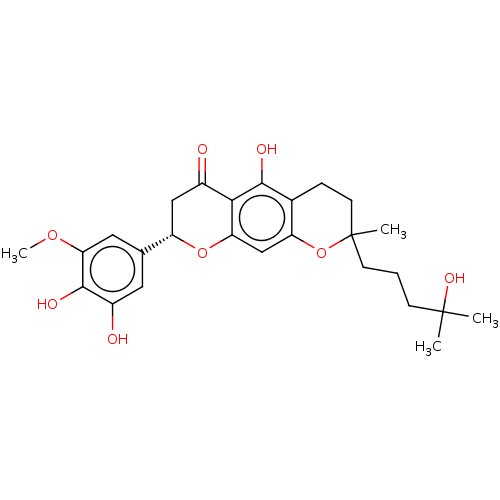 (CHEMBL4164709) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of high affinity radioligand binding to human alphaV-beta3 integrin | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50495307 (CHEMBL3103545) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase using L-DOPA as substrate preincubated for 10 mins with substrate followed by enzyme addition measured ... | Bioorg Med Chem 22: 1115-20 (2014) Article DOI: 10.1016/j.bmc.2013.12.047 BindingDB Entry DOI: 10.7270/Q2222XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50380205 (NYMPHAEOL A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of human neutrophil elastase using varying levels of MeOSuc-AAPV-pNA as substrate measured after 30 mins by double-reciproc... | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50380201 (CHEMBL2011403) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Mixed type inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate by Lineweaver-Burk double-reciprocal-plot and dixon plot ... | Bioorg Med Chem 20: 2595-602 (2012) Article DOI: 10.1016/j.bmc.2012.02.044 BindingDB Entry DOI: 10.7270/Q22808MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50136567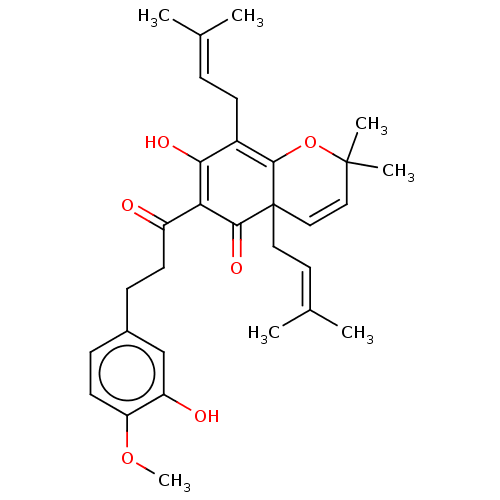 (CHEMBL3753821) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qiqihar University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometry based Lineweaver-Burk plot | Bioorg Med Chem Lett 26: 318-21 (2016) Article DOI: 10.1016/j.bmcl.2015.12.021 BindingDB Entry DOI: 10.7270/Q2C82C4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50380200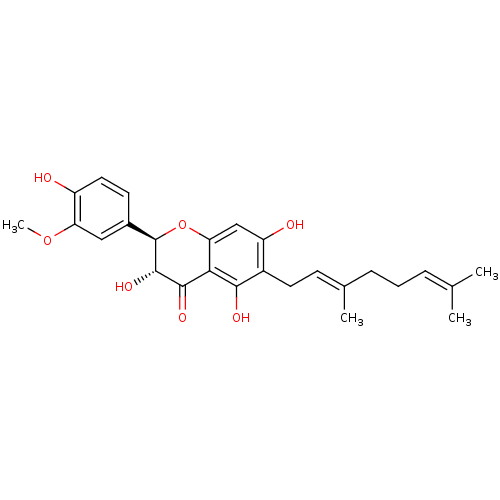 (CHEMBL459258) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Mixed type inhibition of equine BChE using butyrylthiocholine iodide as substrate by Lineweaver-Burk double-reciprocal-plot and dixon plot analysis | Bioorg Med Chem 20: 2595-602 (2012) Article DOI: 10.1016/j.bmc.2012.02.044 BindingDB Entry DOI: 10.7270/Q22808MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50380199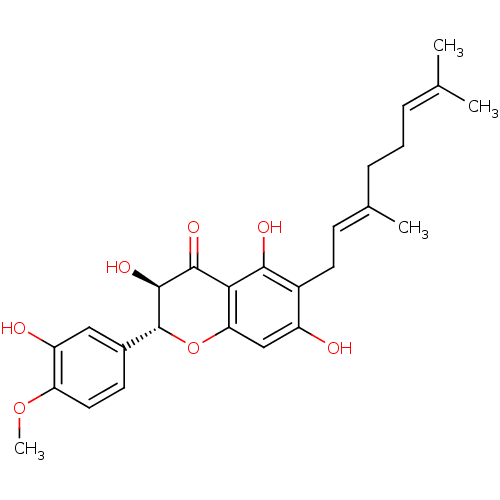 (CHEMBL2011402) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Mixed type inhibition of equine BChE using butyrylthiocholine iodide as substrate by Lineweaver-Burk double-reciprocal-plot and dixon plot analysis | Bioorg Med Chem 20: 2595-602 (2012) Article DOI: 10.1016/j.bmc.2012.02.044 BindingDB Entry DOI: 10.7270/Q22808MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50442402 (FLEMINGSIN) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qiqihar University Curated by ChEMBL | Assay Description Non-competitive inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometry based Lineweaver-Burk plot | Bioorg Med Chem Lett 26: 318-21 (2016) Article DOI: 10.1016/j.bmcl.2015.12.021 BindingDB Entry DOI: 10.7270/Q2C82C4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50278916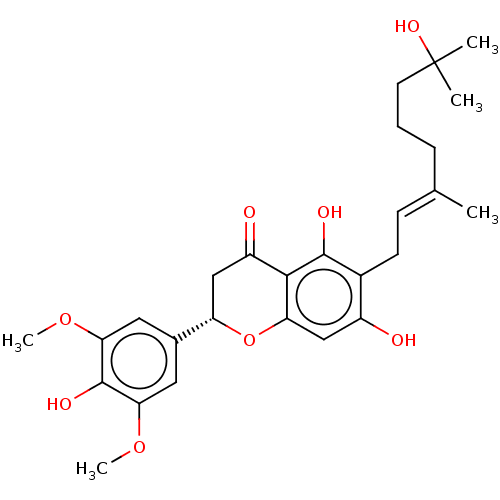 (CHEMBL4167477) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of human neutrophil elastase using varying levels of MeOSuc-AAPV-pNA as substrate measured after 30 mins by double-reciproc... | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50380198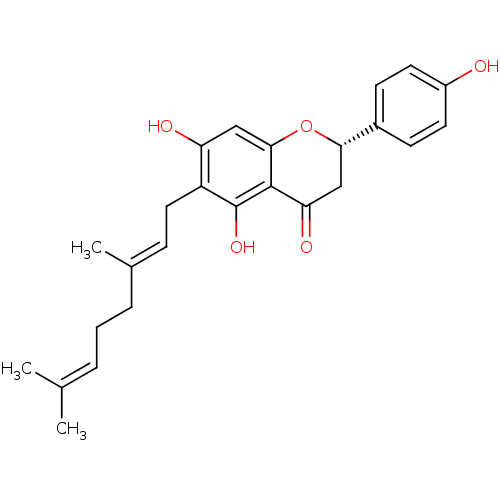 (BONANNIONE A) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 2.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Mixed type inhibition of equine BChE using butyrylthiocholine iodide as substrate by Lineweaver-Burk double-reciprocal-plot and dixon plot analysis | Bioorg Med Chem 20: 2595-602 (2012) Article DOI: 10.1016/j.bmc.2012.02.044 BindingDB Entry DOI: 10.7270/Q22808MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50380202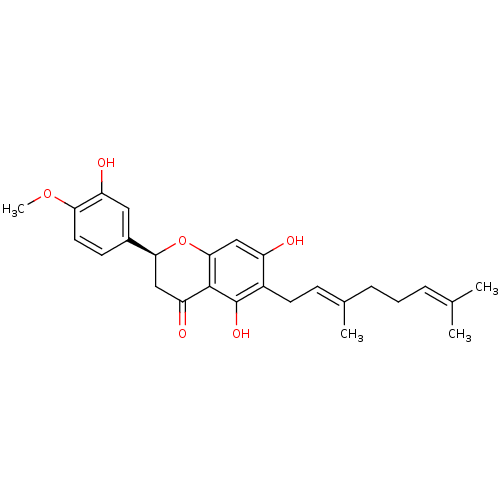 (CHEMBL2011404) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Mixed type inhibition of equine BChE using butyrylthiocholine iodide as substrate by Lineweaver-Burk double-reciprocal-plot and dixon plot analysis | Bioorg Med Chem 20: 2595-602 (2012) Article DOI: 10.1016/j.bmc.2012.02.044 BindingDB Entry DOI: 10.7270/Q22808MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50380203 (CHEMBL253152) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Mixed type inhibition of equine BChE using butyrylthiocholine iodide as substrate by Lineweaver-Burk double-reciprocal-plot and dixon plot analysis | Bioorg Med Chem 20: 2595-602 (2012) Article DOI: 10.1016/j.bmc.2012.02.044 BindingDB Entry DOI: 10.7270/Q22808MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50442397 (OSAJIN) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qiqihar University Curated by ChEMBL | Assay Description Non-competitive inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometry based Lineweaver-Burk plot | Bioorg Med Chem Lett 26: 318-21 (2016) Article DOI: 10.1016/j.bmcl.2015.12.021 BindingDB Entry DOI: 10.7270/Q2C82C4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 141 total ) | Next | Last >> |