

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50431782 (CHEMBL2347039) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Science Curated by ChEMBL | Assay Description Inhibition of human DPP4 using Gly-Pro-AMC as substrate treated with enzyme 10 mins prior to substrate addition measured after 10 mins | Bioorg Med Chem 21: 1749-55 (2013) Article DOI: 10.1016/j.bmc.2013.01.062 BindingDB Entry DOI: 10.7270/Q2R78GMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50431785 (CHEMBL2347036) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Science Curated by ChEMBL | Assay Description Inhibition of human DPP4 using Gly-Pro-AMC as substrate treated with enzyme 10 mins prior to substrate addition measured after 10 mins | Bioorg Med Chem 21: 1749-55 (2013) Article DOI: 10.1016/j.bmc.2013.01.062 BindingDB Entry DOI: 10.7270/Q2R78GMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50431784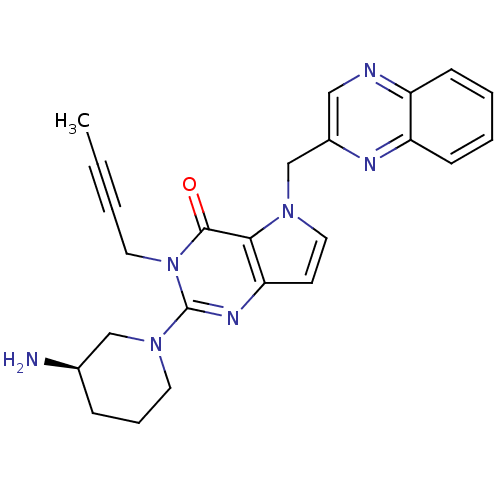 (CHEMBL2347037) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Science Curated by ChEMBL | Assay Description Inhibition of human DPP4 using Gly-Pro-AMC as substrate treated with enzyme 10 mins prior to substrate addition measured after 10 mins | Bioorg Med Chem 21: 1749-55 (2013) Article DOI: 10.1016/j.bmc.2013.01.062 BindingDB Entry DOI: 10.7270/Q2R78GMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50431791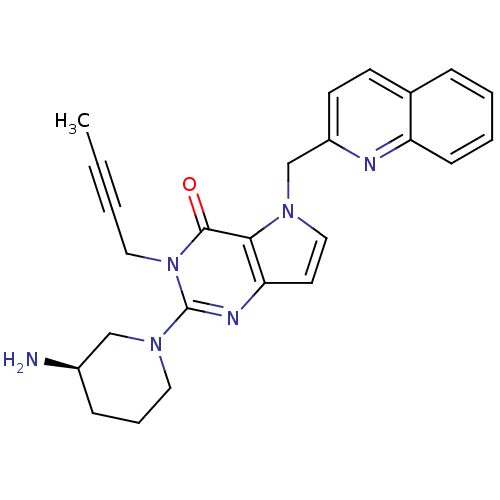 (CHEMBL2346926) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Science Curated by ChEMBL | Assay Description Inhibition of human DPP4 using Gly-Pro-AMC as substrate treated with enzyme 10 mins prior to substrate addition measured after 10 mins | Bioorg Med Chem 21: 1749-55 (2013) Article DOI: 10.1016/j.bmc.2013.01.062 BindingDB Entry DOI: 10.7270/Q2R78GMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50431786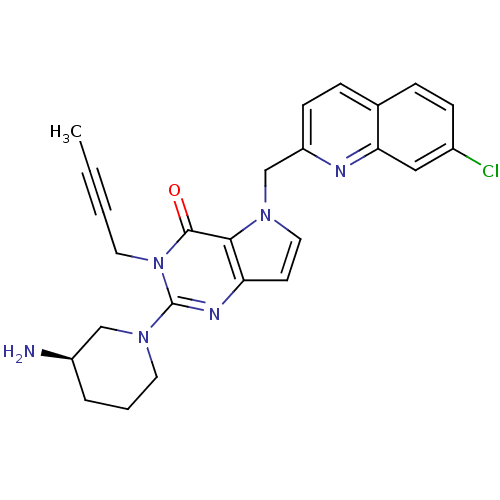 (CHEMBL2347035) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Science Curated by ChEMBL | Assay Description Inhibition of human DPP4 using Gly-Pro-AMC as substrate treated with enzyme 10 mins prior to substrate addition measured after 10 mins | Bioorg Med Chem 21: 1749-55 (2013) Article DOI: 10.1016/j.bmc.2013.01.062 BindingDB Entry DOI: 10.7270/Q2R78GMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50431788 (CHEMBL2346929) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Science Curated by ChEMBL | Assay Description Inhibition of human DPP4 using Gly-Pro-AMC as substrate treated with enzyme 10 mins prior to substrate addition measured after 10 mins | Bioorg Med Chem 21: 1749-55 (2013) Article DOI: 10.1016/j.bmc.2013.01.062 BindingDB Entry DOI: 10.7270/Q2R78GMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50431783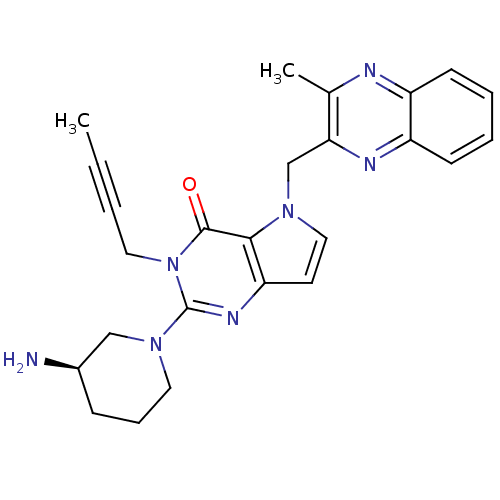 (CHEMBL2347038) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Science Curated by ChEMBL | Assay Description Inhibition of human DPP4 using Gly-Pro-AMC as substrate treated with enzyme 10 mins prior to substrate addition measured after 10 mins | Bioorg Med Chem 21: 1749-55 (2013) Article DOI: 10.1016/j.bmc.2013.01.062 BindingDB Entry DOI: 10.7270/Q2R78GMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM256459 (US10329256, Example 3 | US9487512, 3 | US9944601, ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (unknown origin) (catalytic domain 157 to 852 residues) expressed in Escherichia coli BL21 using H3K4me1 as substrate ... | Eur J Med Chem 175: 357-372 (2019) Article DOI: 10.1016/j.ejmech.2019.04.065 BindingDB Entry DOI: 10.7270/Q2X351W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50431793 (CHEMBL2346924) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Science Curated by ChEMBL | Assay Description Inhibition of human DPP4 using Gly-Pro-AMC as substrate treated with enzyme 10 mins prior to substrate addition measured after 10 mins | Bioorg Med Chem 21: 1749-55 (2013) Article DOI: 10.1016/j.bmc.2013.01.062 BindingDB Entry DOI: 10.7270/Q2R78GMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50431795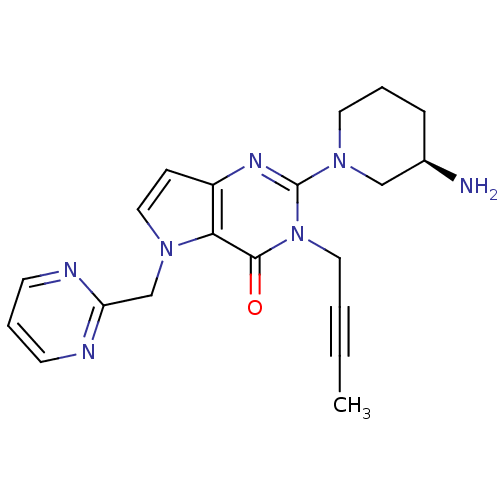 (CHEMBL2346922) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Science Curated by ChEMBL | Assay Description Inhibition of human DPP4 using Gly-Pro-AMC as substrate treated with enzyme 10 mins prior to substrate addition measured after 10 mins | Bioorg Med Chem 21: 1749-55 (2013) Article DOI: 10.1016/j.bmc.2013.01.062 BindingDB Entry DOI: 10.7270/Q2R78GMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50381853 (CHEMBL2023067) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Science Curated by ChEMBL | Assay Description Inhibition of human DPP4 using Gly-Pro-AMC as substrate treated with enzyme 10 mins prior to substrate addition measured after 10 mins | Bioorg Med Chem 21: 1749-55 (2013) Article DOI: 10.1016/j.bmc.2013.01.062 BindingDB Entry DOI: 10.7270/Q2R78GMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50431794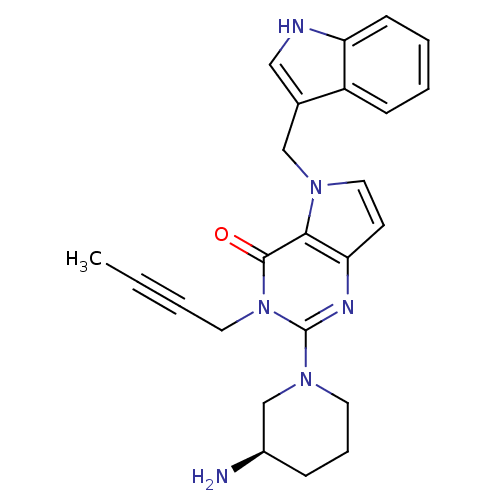 (CHEMBL2346923) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Science Curated by ChEMBL | Assay Description Inhibition of human DPP4 using Gly-Pro-AMC as substrate treated with enzyme 10 mins prior to substrate addition measured after 10 mins | Bioorg Med Chem 21: 1749-55 (2013) Article DOI: 10.1016/j.bmc.2013.01.062 BindingDB Entry DOI: 10.7270/Q2R78GMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50431792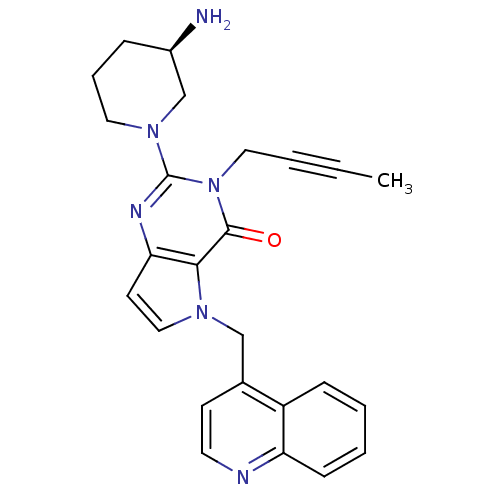 (CHEMBL2346925) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Science Curated by ChEMBL | Assay Description Inhibition of human DPP4 using Gly-Pro-AMC as substrate treated with enzyme 10 mins prior to substrate addition measured after 10 mins | Bioorg Med Chem 21: 1749-55 (2013) Article DOI: 10.1016/j.bmc.2013.01.062 BindingDB Entry DOI: 10.7270/Q2R78GMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50431787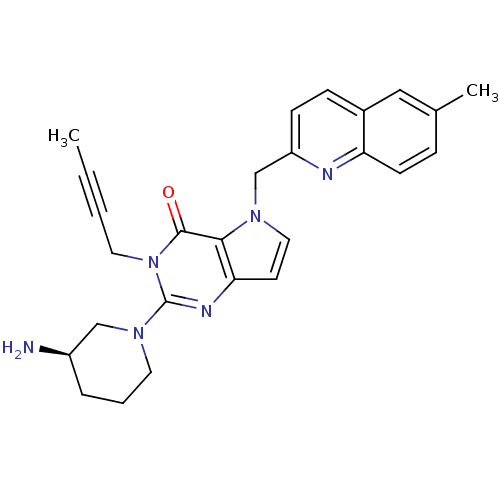 (CHEMBL2346930) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Science Curated by ChEMBL | Assay Description Inhibition of human DPP4 using Gly-Pro-AMC as substrate treated with enzyme 10 mins prior to substrate addition measured after 10 mins | Bioorg Med Chem 21: 1749-55 (2013) Article DOI: 10.1016/j.bmc.2013.01.062 BindingDB Entry DOI: 10.7270/Q2R78GMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50431789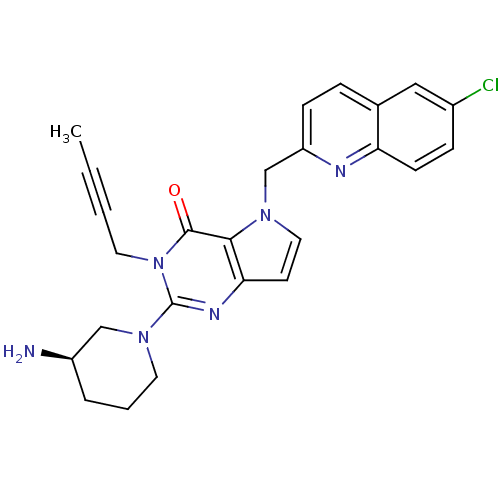 (CHEMBL2346928) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Science Curated by ChEMBL | Assay Description Inhibition of human DPP4 using Gly-Pro-AMC as substrate treated with enzyme 10 mins prior to substrate addition measured after 10 mins | Bioorg Med Chem 21: 1749-55 (2013) Article DOI: 10.1016/j.bmc.2013.01.062 BindingDB Entry DOI: 10.7270/Q2R78GMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50431790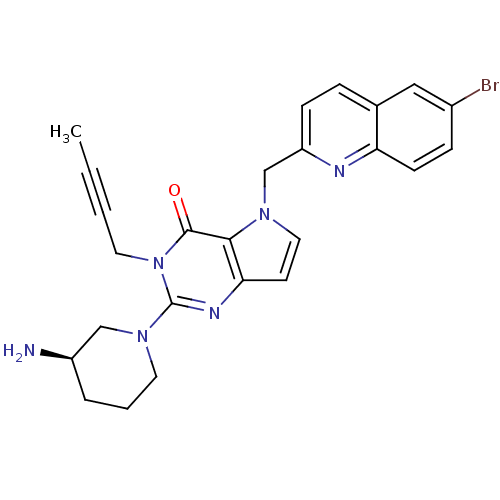 (CHEMBL2346927) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 139 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Science Curated by ChEMBL | Assay Description Inhibition of human DPP4 using Gly-Pro-AMC as substrate treated with enzyme 10 mins prior to substrate addition measured after 10 mins | Bioorg Med Chem 21: 1749-55 (2013) Article DOI: 10.1016/j.bmc.2013.01.062 BindingDB Entry DOI: 10.7270/Q2R78GMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50431781 (CHEMBL2346919) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 212 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Science Curated by ChEMBL | Assay Description Inhibition of human DPP4 using Gly-Pro-AMC as substrate treated with enzyme 10 mins prior to substrate addition measured after 10 mins | Bioorg Med Chem 21: 1749-55 (2013) Article DOI: 10.1016/j.bmc.2013.01.062 BindingDB Entry DOI: 10.7270/Q2R78GMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50431796 (CHEMBL2346921) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Science Curated by ChEMBL | Assay Description Inhibition of human DPP4 using Gly-Pro-AMC as substrate treated with enzyme 10 mins prior to substrate addition measured after 10 mins | Bioorg Med Chem 21: 1749-55 (2013) Article DOI: 10.1016/j.bmc.2013.01.062 BindingDB Entry DOI: 10.7270/Q2R78GMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50431797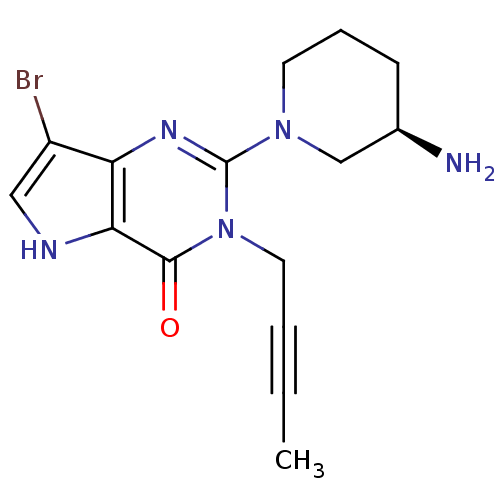 (CHEMBL2346920) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Science Curated by ChEMBL | Assay Description Inhibition of human DPP4 using Gly-Pro-AMC as substrate treated with enzyme 10 mins prior to substrate addition measured after 10 mins | Bioorg Med Chem 21: 1749-55 (2013) Article DOI: 10.1016/j.bmc.2013.01.062 BindingDB Entry DOI: 10.7270/Q2R78GMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50524592 (CHEMBL4550944) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (unknown origin) (catalytic domain 157 to 852 residues) expressed in Escherichia coli BL21 using H3K4me1 as substrate ... | Eur J Med Chem 175: 357-372 (2019) Article DOI: 10.1016/j.ejmech.2019.04.065 BindingDB Entry DOI: 10.7270/Q2X351W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50524624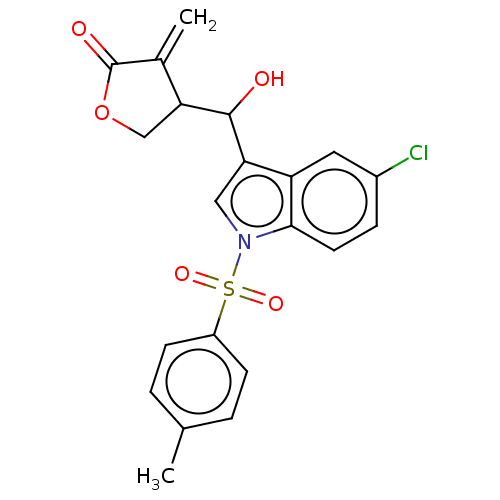 (CHEMBL4470161) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (unknown origin) (catalytic domain 157 to 852 residues) expressed in Escherichia coli BL21 using H3K4me1 as substrate ... | Eur J Med Chem 175: 357-372 (2019) Article DOI: 10.1016/j.ejmech.2019.04.065 BindingDB Entry DOI: 10.7270/Q2X351W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50524625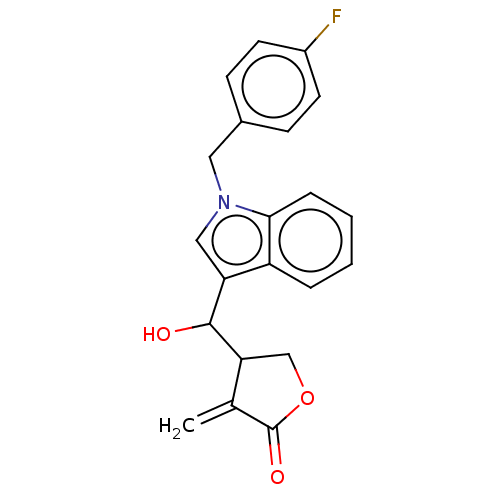 (CHEMBL4545973) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (unknown origin) (catalytic domain 157 to 852 residues) expressed in Escherichia coli BL21 using H3K4me1 as substrate ... | Eur J Med Chem 175: 357-372 (2019) Article DOI: 10.1016/j.ejmech.2019.04.065 BindingDB Entry DOI: 10.7270/Q2X351W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50524596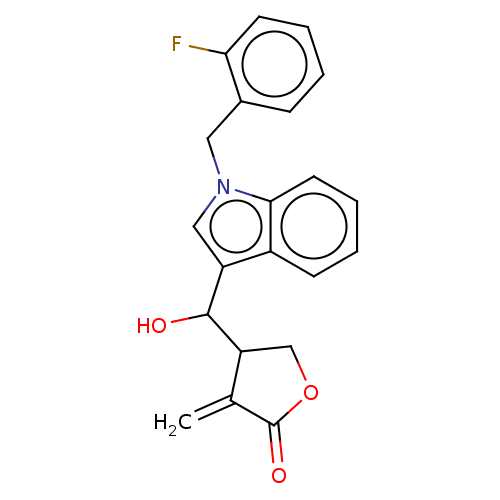 (CHEMBL4545461) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (unknown origin) (catalytic domain 157 to 852 residues) expressed in Escherichia coli BL21 using H3K4me1 as substrate ... | Eur J Med Chem 175: 357-372 (2019) Article DOI: 10.1016/j.ejmech.2019.04.065 BindingDB Entry DOI: 10.7270/Q2X351W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50524640 (CHEMBL4456313) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (unknown origin) (catalytic domain 157 to 852 residues) expressed in Escherichia coli BL21 using H3K4me1 as substrate ... | Eur J Med Chem 175: 357-372 (2019) Article DOI: 10.1016/j.ejmech.2019.04.065 BindingDB Entry DOI: 10.7270/Q2X351W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50524598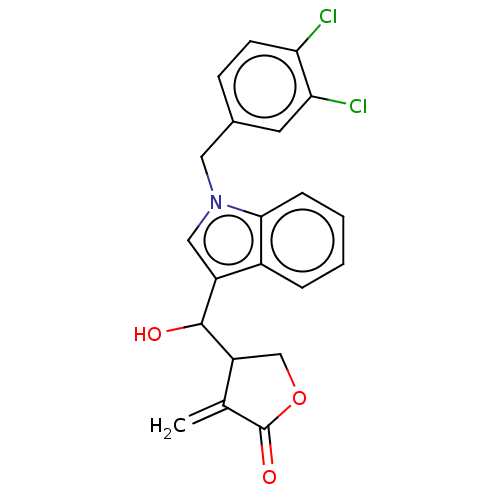 (CHEMBL4591471) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (unknown origin) (catalytic domain 157 to 852 residues) expressed in Escherichia coli BL21 using H3K4me1 as substrate ... | Eur J Med Chem 175: 357-372 (2019) Article DOI: 10.1016/j.ejmech.2019.04.065 BindingDB Entry DOI: 10.7270/Q2X351W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50524602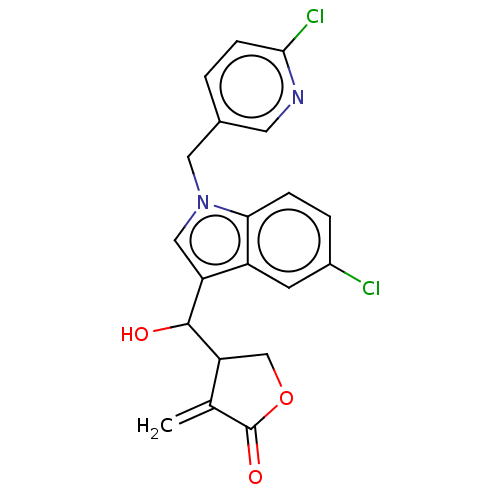 (CHEMBL4592421) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (unknown origin) (catalytic domain 157 to 852 residues) expressed in Escherichia coli BL21 using H3K4me1 as substrate ... | Eur J Med Chem 175: 357-372 (2019) Article DOI: 10.1016/j.ejmech.2019.04.065 BindingDB Entry DOI: 10.7270/Q2X351W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50524603 (CHEMBL4439541) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (unknown origin) (catalytic domain 157 to 852 residues) expressed in Escherichia coli BL21 using H3K4me1 as substrate ... | Eur J Med Chem 175: 357-372 (2019) Article DOI: 10.1016/j.ejmech.2019.04.065 BindingDB Entry DOI: 10.7270/Q2X351W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50524629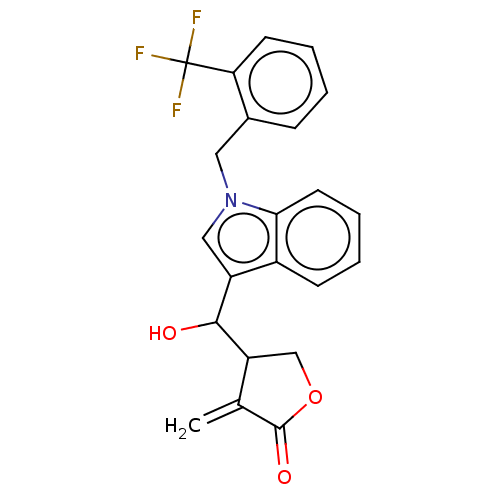 (CHEMBL4527359) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (unknown origin) (catalytic domain 157 to 852 residues) expressed in Escherichia coli BL21 using H3K4me1 as substrate ... | Eur J Med Chem 175: 357-372 (2019) Article DOI: 10.1016/j.ejmech.2019.04.065 BindingDB Entry DOI: 10.7270/Q2X351W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50524597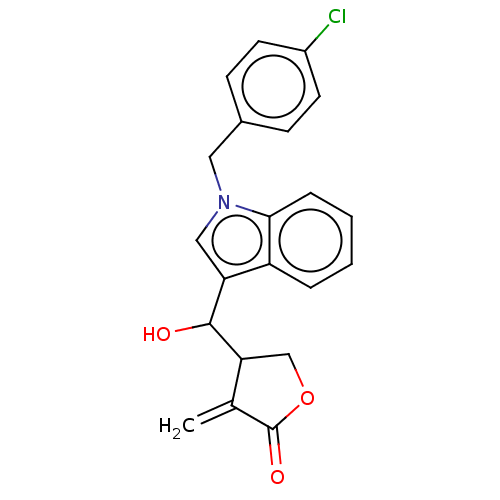 (CHEMBL4445502) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (unknown origin) (catalytic domain 157 to 852 residues) expressed in Escherichia coli BL21 using H3K4me1 as substrate ... | Eur J Med Chem 175: 357-372 (2019) Article DOI: 10.1016/j.ejmech.2019.04.065 BindingDB Entry DOI: 10.7270/Q2X351W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50524590 (CHEMBL4447670) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (unknown origin) (catalytic domain 157 to 852 residues) expressed in Escherichia coli BL21 using H3K4me1 as substrate ... | Eur J Med Chem 175: 357-372 (2019) Article DOI: 10.1016/j.ejmech.2019.04.065 BindingDB Entry DOI: 10.7270/Q2X351W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50524591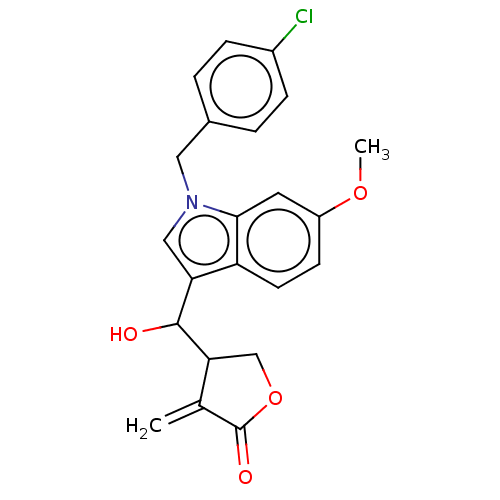 (CHEMBL4592269) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (unknown origin) (catalytic domain 157 to 852 residues) expressed in Escherichia coli BL21 using H3K4me1 as substrate ... | Eur J Med Chem 175: 357-372 (2019) Article DOI: 10.1016/j.ejmech.2019.04.065 BindingDB Entry DOI: 10.7270/Q2X351W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50524593 (CHEMBL4543148) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (unknown origin) (catalytic domain 157 to 852 residues) expressed in Escherichia coli BL21 using H3K4me1 as substrate ... | Eur J Med Chem 175: 357-372 (2019) Article DOI: 10.1016/j.ejmech.2019.04.065 BindingDB Entry DOI: 10.7270/Q2X351W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50524594 (CHEMBL4476237) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (unknown origin) (catalytic domain 157 to 852 residues) expressed in Escherichia coli BL21 using H3K4me1 as substrate ... | Eur J Med Chem 175: 357-372 (2019) Article DOI: 10.1016/j.ejmech.2019.04.065 BindingDB Entry DOI: 10.7270/Q2X351W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50524599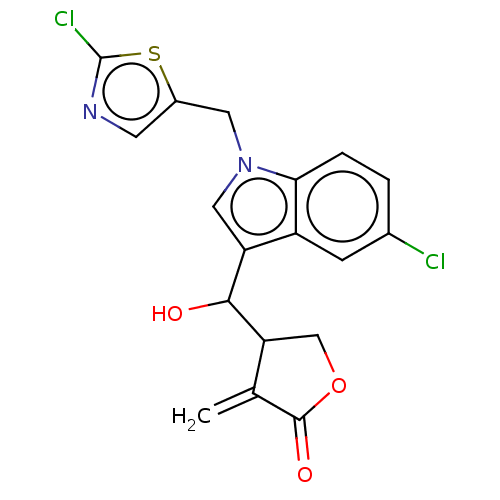 (CHEMBL4456857) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (unknown origin) (catalytic domain 157 to 852 residues) expressed in Escherichia coli BL21 using H3K4me1 as substrate ... | Eur J Med Chem 175: 357-372 (2019) Article DOI: 10.1016/j.ejmech.2019.04.065 BindingDB Entry DOI: 10.7270/Q2X351W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50524600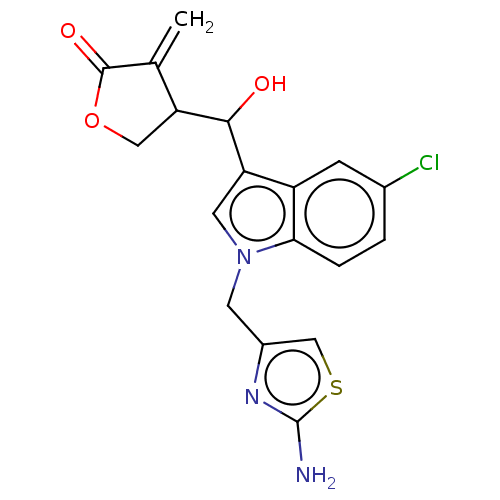 (CHEMBL4589600) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (unknown origin) (catalytic domain 157 to 852 residues) expressed in Escherichia coli BL21 using H3K4me1 as substrate ... | Eur J Med Chem 175: 357-372 (2019) Article DOI: 10.1016/j.ejmech.2019.04.065 BindingDB Entry DOI: 10.7270/Q2X351W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50524601 (CHEMBL4583255) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (unknown origin) (catalytic domain 157 to 852 residues) expressed in Escherichia coli BL21 using H3K4me1 as substrate ... | Eur J Med Chem 175: 357-372 (2019) Article DOI: 10.1016/j.ejmech.2019.04.065 BindingDB Entry DOI: 10.7270/Q2X351W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50524604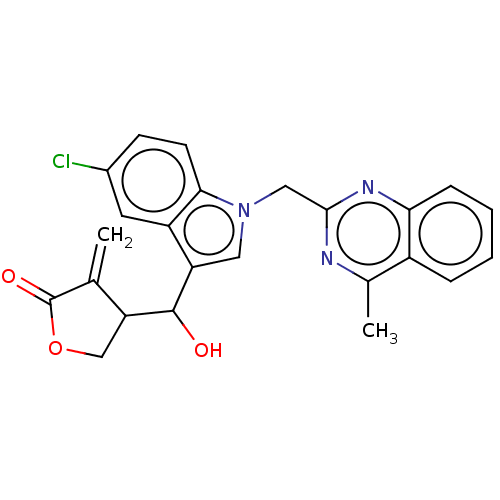 (CHEMBL4461712) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (unknown origin) (catalytic domain 157 to 852 residues) expressed in Escherichia coli BL21 using H3K4me1 as substrate ... | Eur J Med Chem 175: 357-372 (2019) Article DOI: 10.1016/j.ejmech.2019.04.065 BindingDB Entry DOI: 10.7270/Q2X351W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50524605 (CHEMBL4586915) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (unknown origin) (catalytic domain 157 to 852 residues) expressed in Escherichia coli BL21 using H3K4me1 as substrate ... | Eur J Med Chem 175: 357-372 (2019) Article DOI: 10.1016/j.ejmech.2019.04.065 BindingDB Entry DOI: 10.7270/Q2X351W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50524606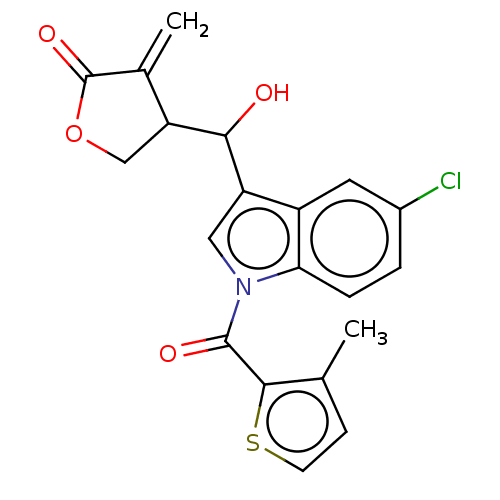 (CHEMBL4574897) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (unknown origin) (catalytic domain 157 to 852 residues) expressed in Escherichia coli BL21 using H3K4me1 as substrate ... | Eur J Med Chem 175: 357-372 (2019) Article DOI: 10.1016/j.ejmech.2019.04.065 BindingDB Entry DOI: 10.7270/Q2X351W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50524607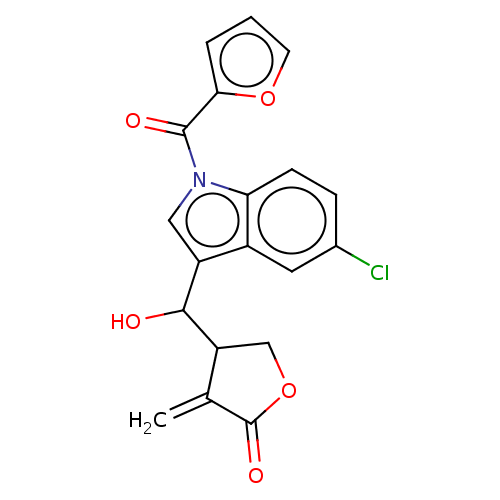 (CHEMBL4559325) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (unknown origin) (catalytic domain 157 to 852 residues) expressed in Escherichia coli BL21 using H3K4me1 as substrate ... | Eur J Med Chem 175: 357-372 (2019) Article DOI: 10.1016/j.ejmech.2019.04.065 BindingDB Entry DOI: 10.7270/Q2X351W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50524608 (CHEMBL4553752) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (unknown origin) (catalytic domain 157 to 852 residues) expressed in Escherichia coli BL21 using H3K4me1 as substrate ... | Eur J Med Chem 175: 357-372 (2019) Article DOI: 10.1016/j.ejmech.2019.04.065 BindingDB Entry DOI: 10.7270/Q2X351W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50524609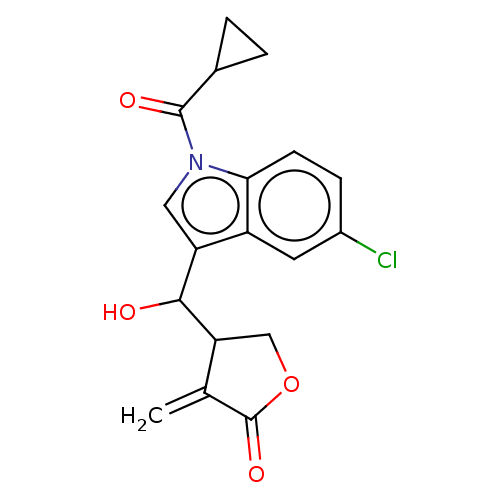 (CHEMBL4443586) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (unknown origin) (catalytic domain 157 to 852 residues) expressed in Escherichia coli BL21 using H3K4me1 as substrate ... | Eur J Med Chem 175: 357-372 (2019) Article DOI: 10.1016/j.ejmech.2019.04.065 BindingDB Entry DOI: 10.7270/Q2X351W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50524610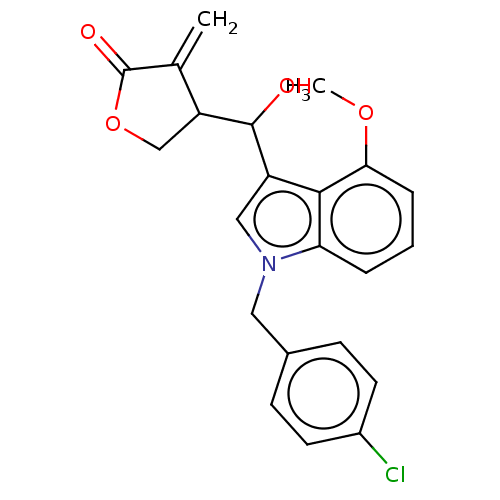 (CHEMBL4459346) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (unknown origin) (catalytic domain 157 to 852 residues) expressed in Escherichia coli BL21 using H3K4me1 as substrate ... | Eur J Med Chem 175: 357-372 (2019) Article DOI: 10.1016/j.ejmech.2019.04.065 BindingDB Entry DOI: 10.7270/Q2X351W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50524611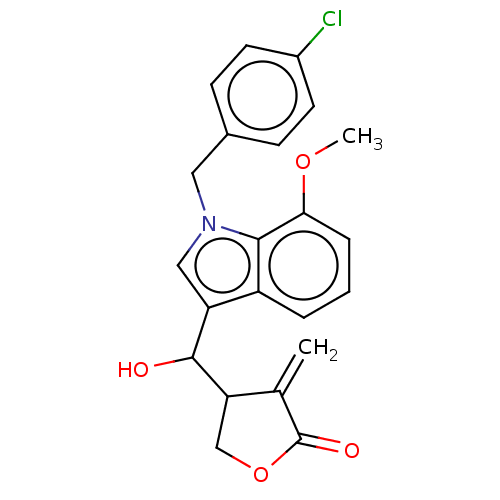 (CHEMBL4462953) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (unknown origin) (catalytic domain 157 to 852 residues) expressed in Escherichia coli BL21 using H3K4me1 as substrate ... | Eur J Med Chem 175: 357-372 (2019) Article DOI: 10.1016/j.ejmech.2019.04.065 BindingDB Entry DOI: 10.7270/Q2X351W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50524612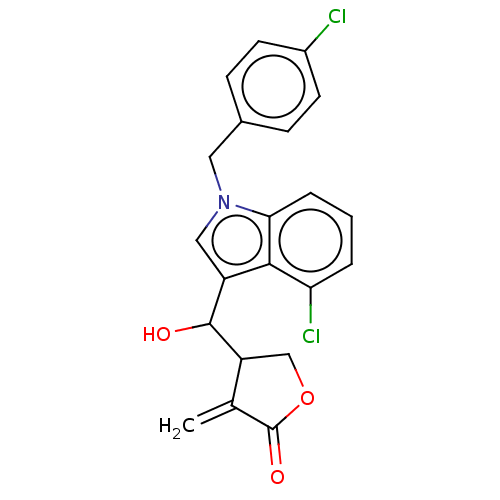 (CHEMBL4549040) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (unknown origin) (catalytic domain 157 to 852 residues) expressed in Escherichia coli BL21 using H3K4me1 as substrate ... | Eur J Med Chem 175: 357-372 (2019) Article DOI: 10.1016/j.ejmech.2019.04.065 BindingDB Entry DOI: 10.7270/Q2X351W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50524613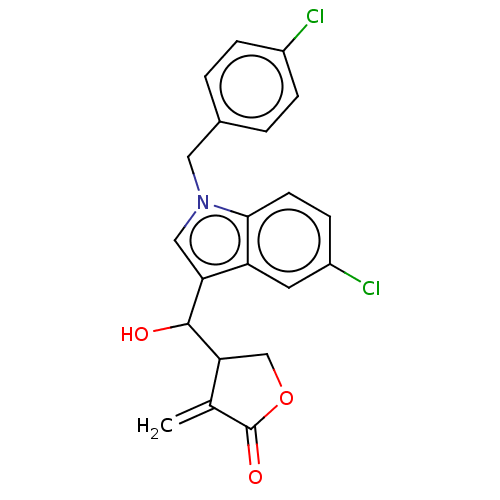 (CHEMBL4517041) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (unknown origin) (catalytic domain 157 to 852 residues) expressed in Escherichia coli BL21 using H3K4me1 as substrate ... | Eur J Med Chem 175: 357-372 (2019) Article DOI: 10.1016/j.ejmech.2019.04.065 BindingDB Entry DOI: 10.7270/Q2X351W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50524614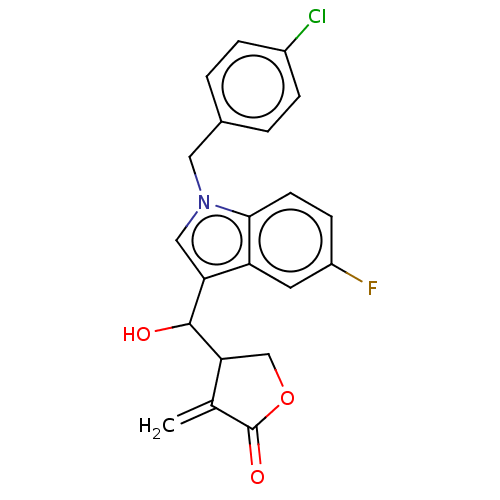 (CHEMBL4468238) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (unknown origin) (catalytic domain 157 to 852 residues) expressed in Escherichia coli BL21 using H3K4me1 as substrate ... | Eur J Med Chem 175: 357-372 (2019) Article DOI: 10.1016/j.ejmech.2019.04.065 BindingDB Entry DOI: 10.7270/Q2X351W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50524615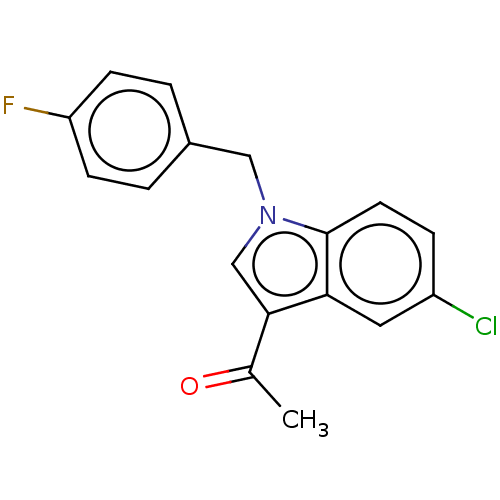 (CHEMBL4446079) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (unknown origin) (catalytic domain 157 to 852 residues) expressed in Escherichia coli BL21 using H3K4me1 as substrate ... | Eur J Med Chem 175: 357-372 (2019) Article DOI: 10.1016/j.ejmech.2019.04.065 BindingDB Entry DOI: 10.7270/Q2X351W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50524616 (CHEMBL4557004) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (unknown origin) (catalytic domain 157 to 852 residues) expressed in Escherichia coli BL21 using H3K4me1 as substrate ... | Eur J Med Chem 175: 357-372 (2019) Article DOI: 10.1016/j.ejmech.2019.04.065 BindingDB Entry DOI: 10.7270/Q2X351W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50524617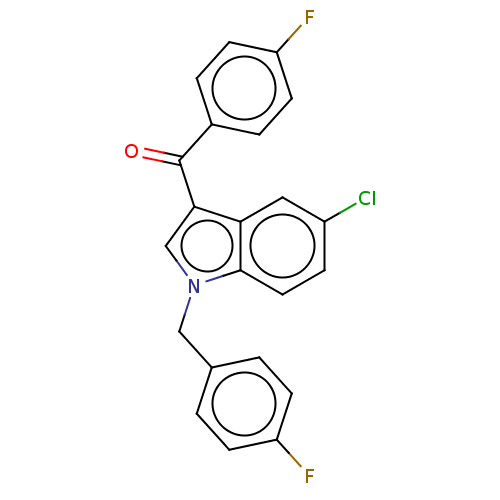 (CHEMBL4451864) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (unknown origin) (catalytic domain 157 to 852 residues) expressed in Escherichia coli BL21 using H3K4me1 as substrate ... | Eur J Med Chem 175: 357-372 (2019) Article DOI: 10.1016/j.ejmech.2019.04.065 BindingDB Entry DOI: 10.7270/Q2X351W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 73 total ) | Next | Last >> |