 Found 1442 hits with Last Name = 'zhang' and Initial = 'zy'
Found 1442 hits with Last Name = 'zhang' and Initial = 'zy' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
TYR_PHOSPHATASE_2 domain-containing protein
(Mycobacterium tuberculosis) | BDBM50544441
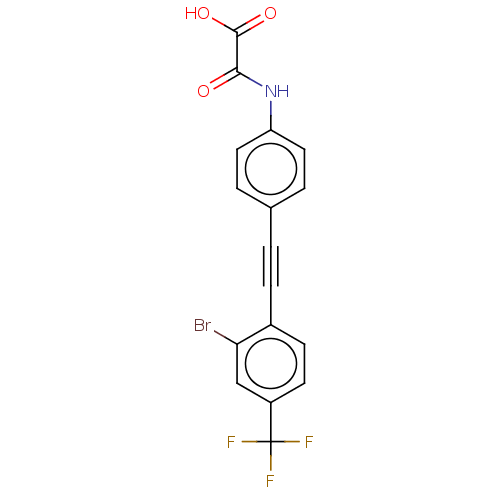
(CHEMBL4637373 | US11192850, Entry 4u)Show SMILES OC(=O)C(=O)Nc1ccc(cc1)C#Cc1ccc(cc1Br)C(F)(F)F Show InChI InChI=1S/C17H9BrF3NO3/c18-14-9-12(17(19,20)21)6-5-11(14)4-1-10-2-7-13(8-3-10)22-15(23)16(24)25/h2-3,5-9H,(H,22,23)(H,24,25) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Competitive inhibition of Mycobacterium tuberculosis PTPB expressed in Escherichia coli BL21 (DE3) using varying levels of p-nitrophenyl phosphate as... |
J Med Chem 63: 9212-9227 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00302
BindingDB Entry DOI: 10.7270/Q2QV3R3B |
More data for this
Ligand-Target Pair | |
Low molecular weight phosphotyrosine protein phosphatase
(Homo sapiens (Human)) | BDBM50607111
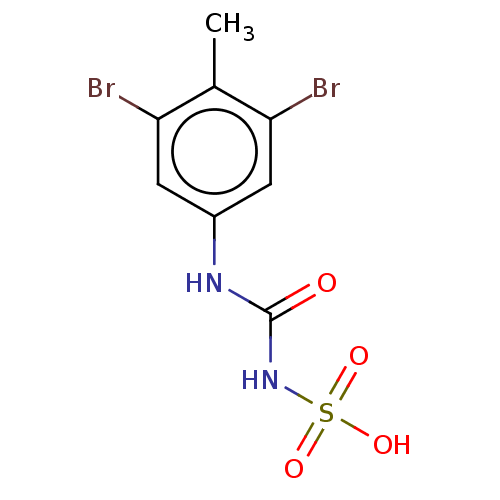
(CHEMBL5218807) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01143
BindingDB Entry DOI: 10.7270/Q2BK1HGF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50379185
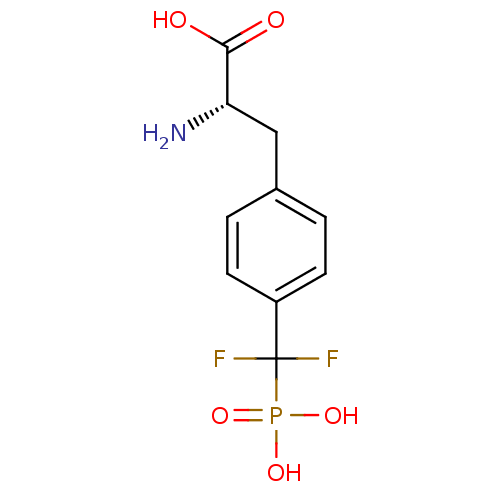
(CHEMBL1232859)Show SMILES N[C@@H](Cc1ccc(cc1)C(F)(F)P(O)(O)=O)C(O)=O |r| Show InChI InChI=1S/C10H12F2NO5P/c11-10(12,19(16,17)18)7-3-1-6(2-4-7)5-8(13)9(14)15/h1-4,8H,5,13H2,(H,14,15)(H2,16,17,18)/t8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B expressed in Escherichia coli BL21 (DE3) cells using p-nitrophenyl phosphate as substrate after 2 to 3 mins by spectrophotometric... |
Bioorg Med Chem 20: 1940-6 (2012)
Article DOI: 10.1016/j.bmc.2011.11.004
BindingDB Entry DOI: 10.7270/Q23J3DZQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50131550
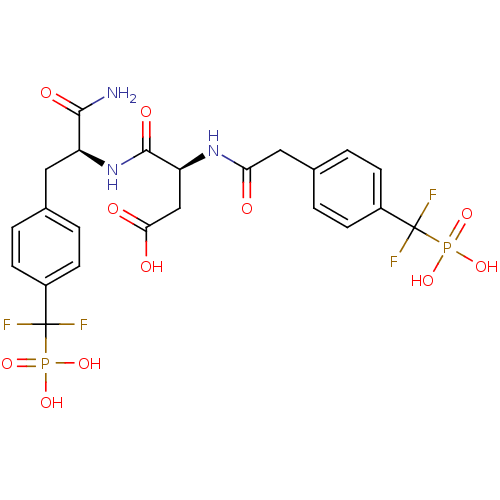
((3S)-3-{[(1S)-1-carbamoyl-2-{4-[difluoro(phosphono...)Show SMILES NC(=O)[C@H](Cc1ccc(cc1)C(F)(F)P(O)(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)Cc1ccc(cc1)C(F)(F)P(O)(O)=O |r| Show InChI InChI=1S/C23H25F4N3O11P2/c24-22(25,42(36,37)38)14-5-1-12(2-6-14)9-16(20(28)34)30-21(35)17(11-19(32)33)29-18(31)10-13-3-7-15(8-4-13)23(26,27)43(39,40)41/h1-8,16-17H,9-11H2,(H2,28,34)(H,29,31)(H,30,35)(H,32,33)(H2,36,37,38)(H2,39,40,41)/t16-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B expressed in human HepG2 cells |
J Med Chem 50: 856-64 (2007)
Article DOI: 10.1021/jm061146x
BindingDB Entry DOI: 10.7270/Q2B857SK |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
TYR_PHOSPHATASE_2 domain-containing protein
(Mycobacterium tuberculosis) | BDBM50544440
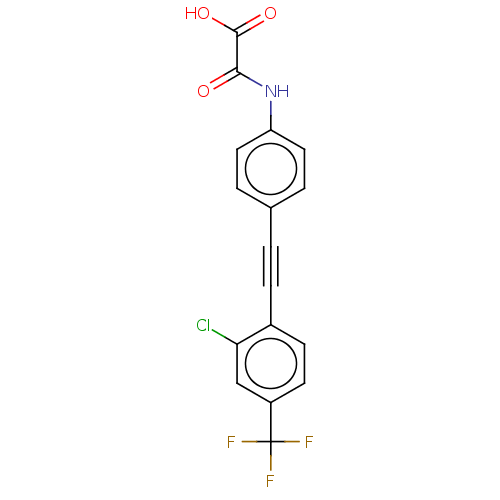
(CHEMBL4647367 | US11192850, Entry 4t)Show SMILES OC(=O)C(=O)Nc1ccc(cc1)C#Cc1ccc(cc1Cl)C(F)(F)F Show InChI InChI=1S/C17H9ClF3NO3/c18-14-9-12(17(19,20)21)6-5-11(14)4-1-10-2-7-13(8-3-10)22-15(23)16(24)25/h2-3,5-9H,(H,22,23)(H,24,25) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Competitive inhibition of Mycobacterium tuberculosis PTPB expressed in Escherichia coli BL21 (DE3) using varying levels of p-nitrophenyl phosphate as... |
J Med Chem 63: 9212-9227 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00302
BindingDB Entry DOI: 10.7270/Q2QV3R3B |
More data for this
Ligand-Target Pair | |
TYR_PHOSPHATASE_2 domain-containing protein
(Mycobacterium tuberculosis) | BDBM50544442
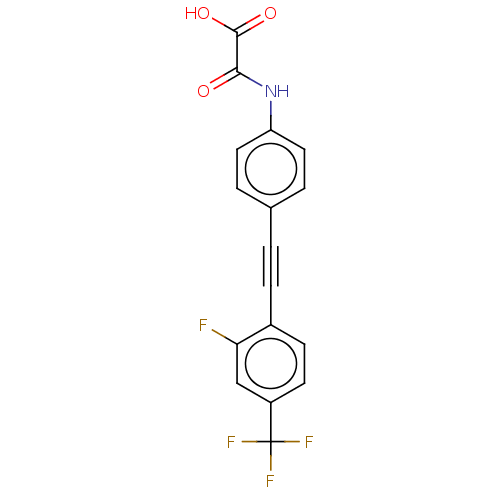
(CHEMBL4638011 | US11192850, Entry 4v)Show SMILES OC(=O)C(=O)Nc1ccc(cc1)C#Cc1ccc(cc1F)C(F)(F)F Show InChI InChI=1S/C17H9F4NO3/c18-14-9-12(17(19,20)21)6-5-11(14)4-1-10-2-7-13(8-3-10)22-15(23)16(24)25/h2-3,5-9H,(H,22,23)(H,24,25) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Competitive inhibition of Mycobacterium tuberculosis PTPB expressed in Escherichia coli BL21 (DE3) using varying levels of p-nitrophenyl phosphate as... |
J Med Chem 63: 9212-9227 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00302
BindingDB Entry DOI: 10.7270/Q2QV3R3B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50436357
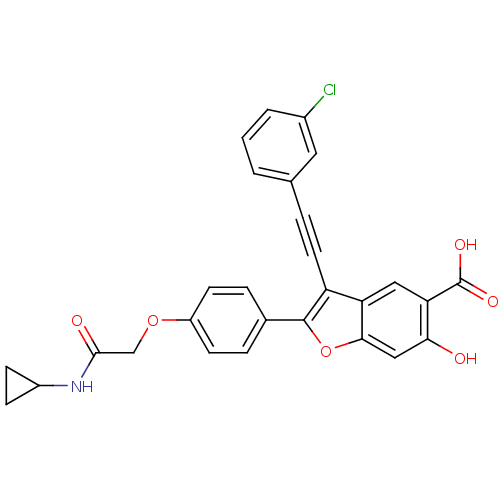
(CHEMBL2396719)Show SMILES OC(=O)c1cc2c(C#Cc3cccc(Cl)c3)c(oc2cc1O)-c1ccc(OCC(=O)NC2CC2)cc1 Show InChI InChI=1S/C28H20ClNO6/c29-18-3-1-2-16(12-18)4-11-21-22-13-23(28(33)34)24(31)14-25(22)36-27(21)17-5-9-20(10-6-17)35-15-26(32)30-19-7-8-19/h1-3,5-6,9-10,12-14,19,31H,7-8,15H2,(H,30,32)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Competitive inhibition of N-terminal 6xHis-tagged LYP catalytic domain (1 to 303) (unknown origin) expressed in Escherichia coli BL21(DE3) using pNPP... |
J Med Chem 56: 4990-5008 (2013)
Article DOI: 10.1021/jm400248c
BindingDB Entry DOI: 10.7270/Q2N017XW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50071330
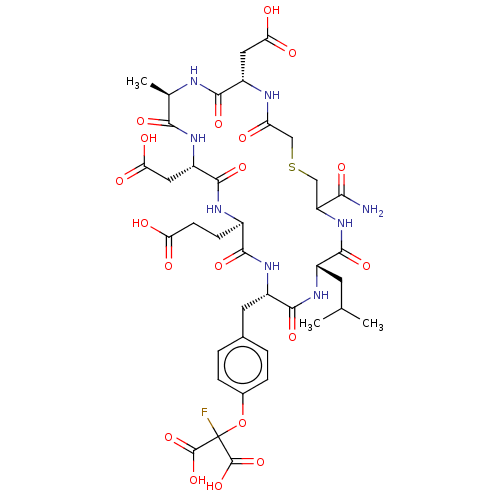
(CHEMBL2369450 | c(S-CH2-Ac-Asp-Ala-Asp-Glu-FOMT-Le...)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](Cc2ccc(OC(F)(C(O)=O)C(O)=O)cc2)NC(=O)[C@@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)CSCC(NC1=O)C(N)=O Show InChI InChI=1S/C39H51FN8O19S/c1-16(2)10-21-34(60)48-25(30(41)56)14-68-15-26(49)43-23(12-28(52)53)33(59)42-17(3)31(57)45-24(13-29(54)55)36(62)44-20(8-9-27(50)51)32(58)47-22(35(61)46-21)11-18-4-6-19(7-5-18)67-39(40,37(63)64)38(65)66/h4-7,16-17,20-25H,8-15H2,1-3H3,(H2,41,56)(H,42,59)(H,43,49)(H,44,62)(H,45,57)(H,46,61)(H,47,58)(H,48,60)(H,50,51)(H,52,53)(H,54,55)(H,63,64)(H,65,66)/t17-,20-,21+,22+,23+,24+,25?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for the binding affinity against protein-tyrosine phosphatase 1B (PTP1B) |
Bioorg Med Chem Lett 8: 2149-50 (1999)
BindingDB Entry DOI: 10.7270/Q27M0737 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50103243
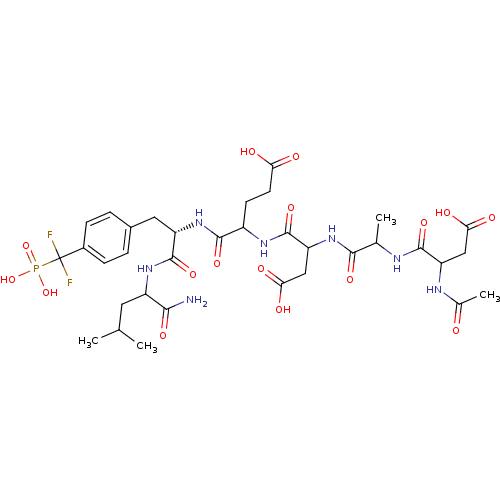
(4-{2-[2-(2-Acetylamino-3-carboxy-propionylamino)-p...)Show SMILES CC(C)CC(NC(=O)[C@H](Cc1ccc(cc1)C(F)(F)P(O)(O)=O)NC(=O)C(CCC(O)=O)NC(=O)C(CC(O)=O)NC(=O)C(C)NC(=O)C(CC(O)=O)NC(C)=O)C(N)=O Show InChI InChI=1S/C34H48F2N7O16P/c1-15(2)11-21(28(37)51)41-32(55)22(12-18-5-7-19(8-6-18)34(35,36)60(57,58)59)43-30(53)20(9-10-25(45)46)40-33(56)24(14-27(49)50)42-29(52)16(3)38-31(54)23(13-26(47)48)39-17(4)44/h5-8,15-16,20-24H,9-14H2,1-4H3,(H2,37,51)(H,38,54)(H,39,44)(H,40,56)(H,41,55)(H,42,52)(H,43,53)(H,45,46)(H,47,48)(H,49,50)(H2,57,58,59)/t16?,20?,21?,22-,23?,24?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Protein-tyrosinephosphatase 1B (PTP1B) |
J Med Chem 44: 2869-78 (2001)
BindingDB Entry DOI: 10.7270/Q237781C |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50112356
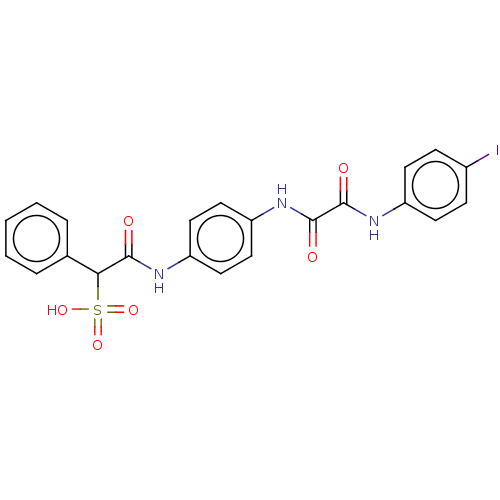
(CHEMBL3609373)Show SMILES OS(=O)(=O)C(C(=O)Nc1ccc(NC(=O)C(=O)Nc2ccc(I)cc2)cc1)c1ccccc1 Show InChI InChI=1S/C22H18IN3O6S/c23-15-6-8-16(9-7-15)25-21(28)22(29)26-18-12-10-17(11-13-18)24-20(27)19(33(30,31)32)14-4-2-1-3-5-14/h1-13,19H,(H,24,27)(H,25,28)(H,26,29)(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Competitive inhibition of phosphatase activity of SHP2 (unknown origin) using pNPP as a substrate Lineweaver-Burk plot analysis |
ACS Med Chem Lett 6: 782-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00118
BindingDB Entry DOI: 10.7270/Q2251M0S |
More data for this
Ligand-Target Pair | |
Low molecular weight phosphotyrosine protein phosphatase
(Homo sapiens (Human)) | BDBM50558461
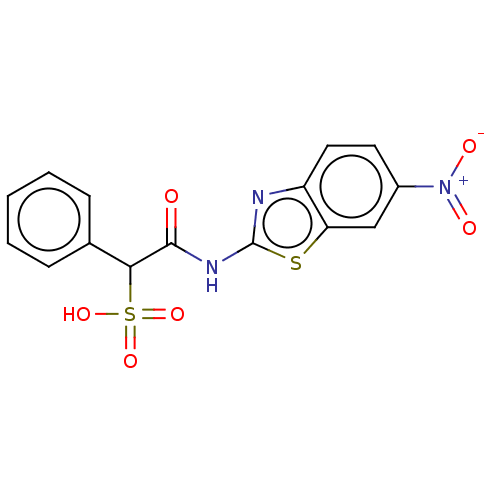
(CHEMBL4760367)Show SMILES OS(=O)(=O)C(C(=O)Nc1nc2ccc(cc2s1)[N+]([O-])=O)c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive inhibition of His-tagged LMW-PTP isoform A (unknown origin) expressed in Escherichia coli BL21 cells using para-nitrophenyl phosphate as ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b00993
BindingDB Entry DOI: 10.7270/Q2DN48Q4 |
More data for this
Ligand-Target Pair | |
Ubiquitin-like domain-containing CTD phosphatase 1
(Homo sapiens (Human)) | BDBM50087856
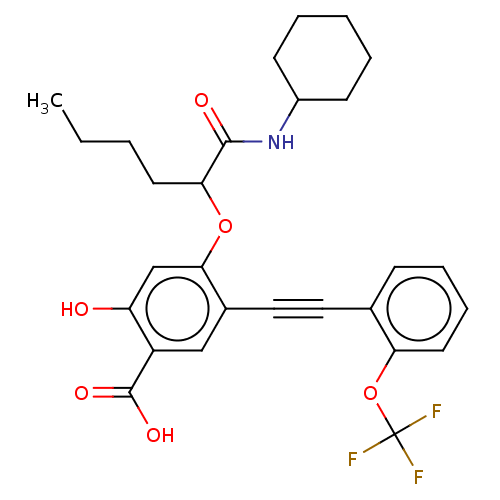
(CHEMBL3426913)Show SMILES CCCCC(Oc1cc(O)c(cc1C#Cc1ccccc1OC(F)(F)F)C(O)=O)C(=O)NC1CCCCC1 Show InChI InChI=1S/C28H30F3NO6/c1-2-3-12-24(26(34)32-20-10-5-4-6-11-20)37-25-17-22(33)21(27(35)36)16-19(25)15-14-18-9-7-8-13-23(18)38-28(29,30)31/h7-9,13,16-17,20,24,33H,2-6,10-12H2,1H3,(H,32,34)(H,35,36) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University
Curated by ChEMBL
| Assay Description
Competitive inhibition of His6-tagged UBLCP1 (unknown origin) expressed in Escherichia coli BL21 cells by Lineweaver-Burk plot analysis in presence o... |
Bioorg Med Chem 23: 2798-809 (2015)
Article DOI: 10.1016/j.bmc.2015.03.066
BindingDB Entry DOI: 10.7270/Q2VD7168 |
More data for this
Ligand-Target Pair | |
TYR_PHOSPHATASE_2 domain-containing protein
(Mycobacterium tuberculosis) | BDBM50343142
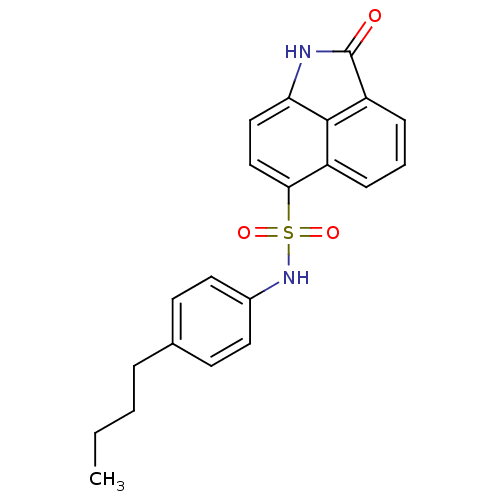
(CHEMBL1773168 | N-(4-butylphenyl)-2-oxo-1,2-dihydr...)Show SMILES CCCCc1ccc(NS(=O)(=O)c2ccc3NC(=O)c4cccc2c34)cc1 Show InChI InChI=1S/C21H20N2O3S/c1-2-3-5-14-8-10-15(11-9-14)23-27(25,26)19-13-12-18-20-16(19)6-4-7-17(20)21(24)22-18/h4,6-13,23H,2-3,5H2,1H3,(H,22,24) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of Mycobacterium tuberculosis N-terminal His6-tagged PTPB assessed as production of p-nitrophenol from pNPP substrate by L... |
ACS Med Chem Lett 1: 355-359 (2010)
Article DOI: 10.1021/ml1001135
BindingDB Entry DOI: 10.7270/Q26M37TG |
More data for this
Ligand-Target Pair | |
Low molecular weight phosphotyrosine protein phosphatase
(Homo sapiens (Human)) | BDBM50558482
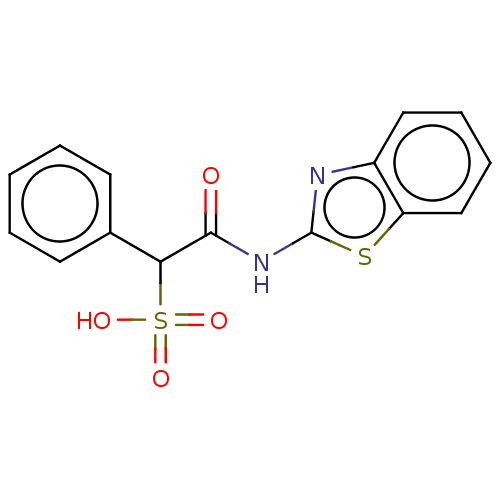
(CHEMBL4742123) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive inhibition of LMW-PTP isoform B (unknown origin) using para-nitrophenyl phosphate as substrate by Lineweaver-Burk plot analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b00993
BindingDB Entry DOI: 10.7270/Q2DN48Q4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50393461
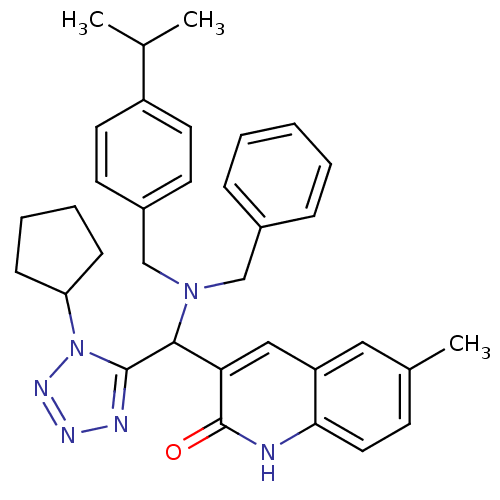
(CHEMBL2159938)Show SMILES CC(C)c1ccc(CN(Cc2ccccc2)C(c2nnnn2C2CCCC2)c2cc3cc(C)ccc3[nH]c2=O)cc1 Show InChI InChI=1S/C34H38N6O/c1-23(2)27-16-14-26(15-17-27)22-39(21-25-9-5-4-6-10-25)32(33-36-37-38-40(33)29-11-7-8-12-29)30-20-28-19-24(3)13-18-31(28)35-34(30)41/h4-6,9-10,13-20,23,29,32H,7-8,11-12,21-22H2,1-3H3,(H,35,41) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Inhibition of LYP expressed in Escherichia coli BL21 using increasing levels of ARLIED-NEpYTAREG substrate |
J Med Chem 54: 1640-54 (2011)
Article DOI: 10.1021/jm101202j
BindingDB Entry DOI: 10.7270/Q2RN38Z0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50335895
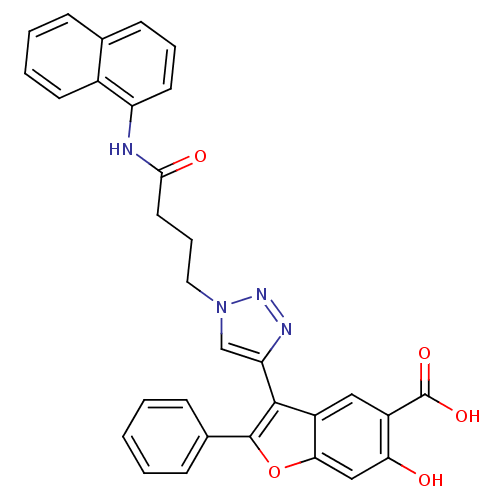
(6-hydroxy-3-(1-(4-(naphthalen-1-ylamino)-4-oxobuty...)Show SMILES OC(=O)c1cc2c(-c3cn(CCCC(=O)Nc4cccc5ccccc45)nn3)c(oc2cc1O)-c1ccccc1 Show InChI InChI=1S/C31H24N4O5/c36-26-17-27-23(16-22(26)31(38)39)29(30(40-27)20-9-2-1-3-10-20)25-18-35(34-33-25)15-7-14-28(37)32-24-13-6-11-19-8-4-5-12-21(19)24/h1-6,8-13,16-18,36H,7,14-15H2,(H,32,37)(H,38,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Competitive inhibition of N-terminal 6xHis-tagged LYP catalytic domain (1 to 303) (unknown origin) expressed in Escherichia coli BL21(DE3) using pNPP... |
J Med Chem 56: 4990-5008 (2013)
Article DOI: 10.1021/jm400248c
BindingDB Entry DOI: 10.7270/Q2N017XW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50393496
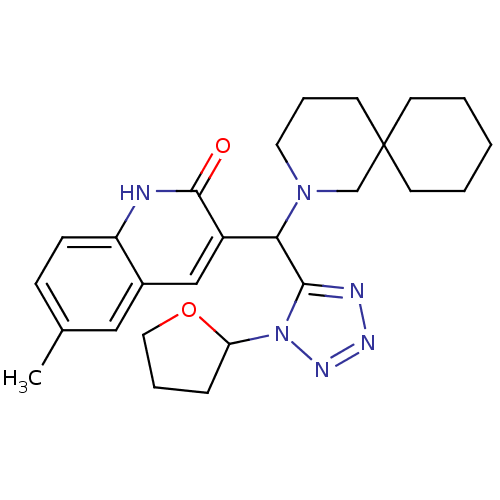
(CHEMBL2159935)Show SMILES Cc1ccc2[nH]c(=O)c(cc2c1)C(N1CCCC2(CCCCC2)C1)c1nnnn1C1CCCO1 Show InChI InChI=1S/C26H34N6O2/c1-18-8-9-21-19(15-18)16-20(25(33)27-21)23(24-28-29-30-32(24)22-7-5-14-34-22)31-13-6-12-26(17-31)10-3-2-4-11-26/h8-9,15-16,22-23H,2-7,10-14,17H2,1H3,(H,27,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Inhibition of LYP expressed in Escherichia coli BL21 using increasing levels of ARLIED-NEpYTAREG substrate |
J Med Chem 54: 1640-54 (2011)
Article DOI: 10.1021/jm101202j
BindingDB Entry DOI: 10.7270/Q2RN38Z0 |
More data for this
Ligand-Target Pair | |
TYR_PHOSPHATASE_2 domain-containing protein
(Mycobacterium tuberculosis) | BDBM50343147
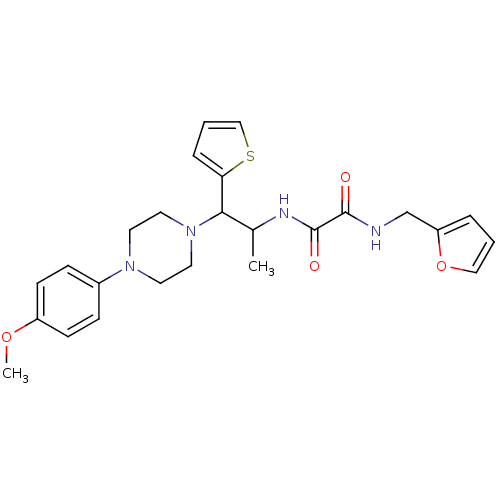
(CHEMBL1773180 | N1-(furan-2-ylmethyl)-N2-(1-(4-(4-...)Show SMILES COc1ccc(cc1)N1CCN(CC1)C(C(C)NC(=O)C(=O)NCc1ccco1)c1cccs1 Show InChI InChI=1S/C25H30N4O4S/c1-18(27-25(31)24(30)26-17-21-5-3-15-33-21)23(22-6-4-16-34-22)29-13-11-28(12-14-29)19-7-9-20(32-2)10-8-19/h3-10,15-16,18,23H,11-14,17H2,1-2H3,(H,26,30)(H,27,31) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Competitive inhibition of Mycobacterium tuberculosis N-terminal His6-tagged PTPB assessed as production of p-nitrophenol from pNPP substrate by Linew... |
ACS Med Chem Lett 1: 355-359 (2010)
Article DOI: 10.1021/ml1001135
BindingDB Entry DOI: 10.7270/Q26M37TG |
More data for this
Ligand-Target Pair | |
Low molecular weight phosphotyrosine protein phosphatase
(Homo sapiens (Human)) | BDBM50558482

(CHEMBL4742123) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive inhibition of His-tagged LMW-PTP isoform A (unknown origin) expressed in Escherichia coli BL21 cells using para-nitrophenyl phosphate as ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b00993
BindingDB Entry DOI: 10.7270/Q2DN48Q4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50103235

(5-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...)Show SMILES CC(C)CC(NC(=O)[C@H](Cc1ccc(OCC(O)=O)c(c1)C(O)=O)NC(=O)C(CCC(O)=O)NC(=O)C(CC(O)=O)NC(=O)C(C)NC(=O)C(CC(O)=O)NC(C)=O)C(N)=O Show InChI InChI=1S/C36H49N7O18/c1-15(2)9-21(30(37)53)41-34(57)22(11-18-5-7-25(61-14-29(51)52)19(10-18)36(59)60)43-32(55)20(6-8-26(45)46)40-35(58)24(13-28(49)50)42-31(54)16(3)38-33(56)23(12-27(47)48)39-17(4)44/h5,7,10,15-16,20-24H,6,8-9,11-14H2,1-4H3,(H2,37,53)(H,38,56)(H,39,44)(H,40,58)(H,41,57)(H,42,54)(H,43,55)(H,45,46)(H,47,48)(H,49,50)(H,51,52)(H,59,60)/t16?,20?,21?,22-,23?,24?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Protein-tyrosinephosphatase 1B (PTP1B) |
J Med Chem 44: 2869-78 (2001)
BindingDB Entry DOI: 10.7270/Q237781C |
More data for this
Ligand-Target Pair | |
TYR_PHOSPHATASE_2 domain-containing protein
(Mycobacterium tuberculosis) | BDBM50343148
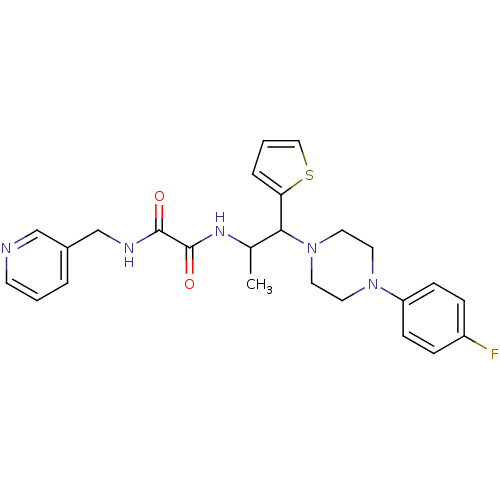
(CHEMBL1773181 | N1-(1-(4-(4-fluorophenyl)piperazin...)Show SMILES CC(NC(=O)C(=O)NCc1cccnc1)C(N1CCN(CC1)c1ccc(F)cc1)c1cccs1 Show InChI InChI=1S/C25H28FN5O2S/c1-18(29-25(33)24(32)28-17-19-4-2-10-27-16-19)23(22-5-3-15-34-22)31-13-11-30(12-14-31)21-8-6-20(26)7-9-21/h2-10,15-16,18,23H,11-14,17H2,1H3,(H,28,32)(H,29,33) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Competitive inhibition of Mycobacterium tuberculosis N-terminal His6-tagged PTPB assessed as production of p-nitrophenol from pNPP substrate by Linew... |
ACS Med Chem Lett 1: 355-359 (2010)
Article DOI: 10.1021/ml1001135
BindingDB Entry DOI: 10.7270/Q26M37TG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50348092
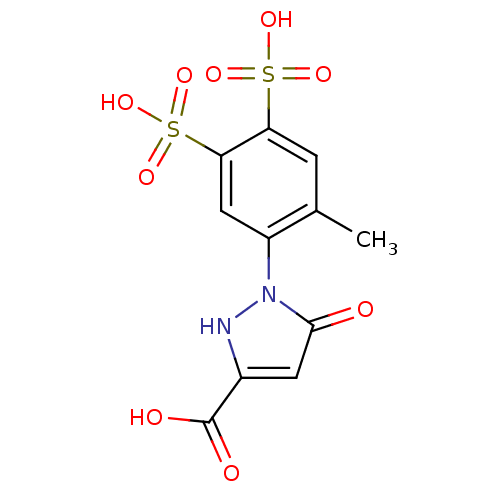
(CHEMBL1800273)Show SMILES Cc1cc(c(cc1-n1[nH]c(cc1=O)C(O)=O)S(O)(=O)=O)S(O)(=O)=O Show InChI InChI=1S/C11H10N2O9S2/c1-5-2-8(23(17,18)19)9(24(20,21)22)4-7(5)13-10(14)3-6(12-13)11(15)16/h2-4,12H,1H3,(H,15,16)(H,17,18,19)(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University
Curated by ChEMBL
| Assay Description
Competitive inhibition at SHP2 catalytic domain assessed as inhibition of pNPP to p-nitrophenol conversion by spectrophotometry |
Bioorg Med Chem Lett 21: 4238-42 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.078
BindingDB Entry DOI: 10.7270/Q2JQ11CJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50348092

(CHEMBL1800273)Show SMILES Cc1cc(c(cc1-n1[nH]c(cc1=O)C(O)=O)S(O)(=O)=O)S(O)(=O)=O Show InChI InChI=1S/C11H10N2O9S2/c1-5-2-8(23(17,18)19)9(24(20,21)22)4-7(5)13-10(14)3-6(12-13)11(15)16/h2-4,12H,1H3,(H,15,16)(H,17,18,19)(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University
Curated by ChEMBL
| Assay Description
Reversible inhibition at SHP2 catalytic domain assessed as inhibition of pNPP to p-nitrophenol conversion by spectrophotometry |
Bioorg Med Chem Lett 21: 4238-42 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.078
BindingDB Entry DOI: 10.7270/Q2JQ11CJ |
More data for this
Ligand-Target Pair | |
Carboxy-terminal domain RNA polymerase II polypeptide A small phosphatase 1
(Homo sapiens (Human)) | BDBM50585529

(CHEMBL5077686)Show SMILES FC(F)(F)Oc1ccc(cc1)S(=O)(=O)NCCCNC(=O)C1=Cc2ccccc2S1(=O)=O |t:22| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Covalent inhibition of wild type human SCP1 assessed as inhibition constant using Ac-EDLphosphoSPPSPPLPK-NH2 peptide as substrate preincubated for 0.... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01655
BindingDB Entry DOI: 10.7270/Q2251P2H |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50308158

(3-(1-(3-(Biphenyl-4-ylamino)-3-oxopropyl)-1H-1,2,3...)Show SMILES Cn1c(c(-c2cn(CCC(=O)Nc3ccc(cc3)-c3ccccc3)nn2)c2cc(C(O)=O)c(O)cc12)-c1ccccc1 Show InChI InChI=1S/C33H27N5O4/c1-37-28-19-29(39)26(33(41)42)18-25(28)31(32(37)23-10-6-3-7-11-23)27-20-38(36-35-27)17-16-30(40)34-24-14-12-22(13-15-24)21-8-4-2-5-9-21/h2-15,18-20,39H,16-17H2,1H3,(H,34,40)(H,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of SHP2 Src homology-2 domain expressed in Escherichia coli BL21 (DE3) assessed as inhibition of p-nitrophenyl phosphate hydrolysis by Lin... |
J Med Chem 53: 2482-93 (2010)
Article DOI: 10.1021/jm901645u
BindingDB Entry DOI: 10.7270/Q2639PVD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50432421
(CHEMBL2349152)Show SMILES COc1ccc(cc1)C(=O)CCc1ccc(cc1)C(=O)Nc1ccc(cc1)S(=O)(=O)Nc1nccs1 Show InChI InChI=1S/C26H23N3O5S2/c1-34-22-11-7-19(8-12-22)24(30)15-4-18-2-5-20(6-3-18)25(31)28-21-9-13-23(14-10-21)36(32,33)29-26-27-16-17-35-26/h2-3,5-14,16-17H,4,15H2,1H3,(H,27,29)(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of recombinant human PTP1B assessed as inhibition of hydrolysis of pNPP by Lineweaver-Burk plot |
Bioorg Med Chem Lett 23: 2313-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.073
BindingDB Entry DOI: 10.7270/Q2XK8GXS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50420258

(CEFSULODIN)Show SMILES NC(=O)c1cc[n+](CC2=C(N3[C@H](SC2)[C@H](NC(=O)C(c2ccccc2)S(O)(=O)=O)C3=O)C(O)=O)cc1 |t:8| Show InChI InChI=1S/C22H20N4O8S2/c23-18(27)13-6-8-25(9-7-13)10-14-11-35-21-15(20(29)26(21)16(14)22(30)31)24-19(28)17(36(32,33)34)12-4-2-1-3-5-12/h1-9,15,17,21H,10-11H2,(H4-,23,24,27,28,30,31,32,33,34)/p+1/t15-,17?,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Competitive inhibition of phosphatase activity of SHP2 (unknown origin) using pNPP as a substrate Lineweaver-Burk plot analysis |
ACS Med Chem Lett 6: 782-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00118
BindingDB Entry DOI: 10.7270/Q2251M0S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50393463
(CHEMBL2159940)Show SMILES Cc1ccc2[nH]c(=O)c(cc2c1)C(N(Cc1ccccc1)Cc1ccc(Cl)cc1)c1nnnn1C1CCCC1 Show InChI InChI=1S/C31H31ClN6O/c1-21-11-16-28-24(17-21)18-27(31(39)33-28)29(30-34-35-36-38(30)26-9-5-6-10-26)37(19-22-7-3-2-4-8-22)20-23-12-14-25(32)15-13-23/h2-4,7-8,11-18,26,29H,5-6,9-10,19-20H2,1H3,(H,33,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Inhibition of LYP expressed in Escherichia coli BL21 using increasing levels of ARLIED-NEpYTAREG substrate |
J Med Chem 54: 1640-54 (2011)
Article DOI: 10.1021/jm101202j
BindingDB Entry DOI: 10.7270/Q2RN38Z0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50393461

(CHEMBL2159938)Show SMILES CC(C)c1ccc(CN(Cc2ccccc2)C(c2nnnn2C2CCCC2)c2cc3cc(C)ccc3[nH]c2=O)cc1 Show InChI InChI=1S/C34H38N6O/c1-23(2)27-16-14-26(15-17-27)22-39(21-25-9-5-4-6-10-25)32(33-36-37-38-40(33)29-11-7-8-12-29)30-20-28-19-24(3)13-18-31(28)35-34(30)41/h4-6,9-10,13-20,23,29,32H,7-8,11-12,21-22H2,1-3H3,(H,35,41) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of LYP expressed in Escherichia coli BL21 using increasing levels of ARLIED-NEpYTAREG substrate |
J Med Chem 54: 1640-54 (2011)
Article DOI: 10.1021/jm101202j
BindingDB Entry DOI: 10.7270/Q2RN38Z0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50393462

(CHEMBL2159939)Show SMILES Cc1ccc2[nH]c(=O)c(cc2c1)C(N1CCN(CC1)C(c1ccccc1)c1ccccc1)c1nnnn1C1CCCC1 Show InChI InChI=1S/C34H37N7O/c1-24-16-17-30-27(22-24)23-29(34(42)35-30)32(33-36-37-38-41(33)28-14-8-9-15-28)40-20-18-39(19-21-40)31(25-10-4-2-5-11-25)26-12-6-3-7-13-26/h2-7,10-13,16-17,22-23,28,31-32H,8-9,14-15,18-21H2,1H3,(H,35,42) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Inhibition of LYP expressed in Escherichia coli BL21 using increasing levels of ARLIED-NEpYTAREG substrate |
J Med Chem 54: 1640-54 (2011)
Article DOI: 10.1021/jm101202j
BindingDB Entry DOI: 10.7270/Q2RN38Z0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50103240
(4-Carbamoyl-4-{[6-(difluoro-phosphono-methyl)-naph...)Show SMILES NC(=O)[C@@H](CCC(O)=O)NC(=O)c1ccc2cc(ccc2c1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C17H17F2N2O7P/c18-17(19,29(26,27)28)12-4-3-9-7-11(2-1-10(9)8-12)16(25)21-13(15(20)24)5-6-14(22)23/h1-4,7-8,13H,5-6H2,(H2,20,24)(H,21,25)(H,22,23)(H2,26,27,28)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Protein-tyrosinephosphatase 1B (PTP1B) |
J Med Chem 44: 2869-78 (2001)
BindingDB Entry DOI: 10.7270/Q237781C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM46264

(3-[2-azaspiro[5.5]undecan-2-yl-(1-cyclopentyl-1,2,...)Show SMILES Cc1ccc2[nH]c(=O)c(cc2c1)C(N1CCCC2(CCCCC2)C1)c1nnnn1C1CCCC1 Show InChI InChI=1S/C27H36N6O/c1-19-10-11-23-20(16-19)17-22(26(34)28-23)24(25-29-30-31-33(25)21-8-3-4-9-21)32-15-7-14-27(18-32)12-5-2-6-13-27/h10-11,16-17,21,24H,2-9,12-15,18H2,1H3,(H,28,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Inhibition of LYP expressed in Escherichia coli BL21 using increasing levels of ARLIED-NEpYTAREG substrate |
J Med Chem 54: 1640-54 (2011)
Article DOI: 10.1021/jm101202j
BindingDB Entry DOI: 10.7270/Q2RN38Z0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50050961
(2-{4-[(S)-2-((S)-2-{(S)-2-[(S)-2-((S)-2-Acetylamin...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccc(OC(C(O)=O)C(O)=O)cc1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(C)=O)C(N)=O Show InChI InChI=1S/C36H49N7O18/c1-15(2)11-21(29(37)51)41-33(55)22(12-18-5-7-19(8-6-18)61-28(35(57)58)36(59)60)43-31(53)20(9-10-25(45)46)40-34(56)24(14-27(49)50)42-30(52)16(3)38-32(54)23(13-26(47)48)39-17(4)44/h5-8,15-16,20-24,28H,9-14H2,1-4H3,(H2,37,51)(H,38,54)(H,39,44)(H,40,56)(H,41,55)(H,42,52)(H,43,53)(H,45,46)(H,47,48)(H,49,50)(H,57,58)(H,59,60)/t16-,20-,21-,22-,23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for the binding affinity against protein-tyrosine phosphatase 1B (PTP1B) |
Bioorg Med Chem Lett 8: 2149-50 (1999)
BindingDB Entry DOI: 10.7270/Q27M0737 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50132237

(CHEMBL320852 | [Difluoro-(6-methanesulfonylaminoca...)Show SMILES CS(=O)(=O)NC(=O)c1ccc2cc(ccc2c1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C13H12F2NO6PS/c1-24(21,22)16-12(17)10-3-2-9-7-11(5-4-8(9)6-10)13(14,15)23(18,19)20/h2-7H,1H3,(H,16,17)(H2,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIH
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards recombinant human Protein-tyrosine phosphatase 1B was determined |
Bioorg Med Chem Lett 13: 3005-7 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MV1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50393496

(CHEMBL2159935)Show SMILES Cc1ccc2[nH]c(=O)c(cc2c1)C(N1CCCC2(CCCCC2)C1)c1nnnn1C1CCCO1 Show InChI InChI=1S/C26H34N6O2/c1-18-8-9-21-19(15-18)16-20(25(33)27-21)23(24-28-29-30-32(24)22-7-5-14-34-22)31-13-6-12-26(17-31)10-3-2-4-11-26/h8-9,15-16,22-23H,2-7,10-14,17H2,1H3,(H,27,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of LYP expressed in Escherichia coli BL21 using increasing levels of ARLIED-NEpYTAREG substrate |
J Med Chem 54: 1640-54 (2011)
Article DOI: 10.1021/jm101202j
BindingDB Entry DOI: 10.7270/Q2RN38Z0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50393462

(CHEMBL2159939)Show SMILES Cc1ccc2[nH]c(=O)c(cc2c1)C(N1CCN(CC1)C(c1ccccc1)c1ccccc1)c1nnnn1C1CCCC1 Show InChI InChI=1S/C34H37N7O/c1-24-16-17-30-27(22-24)23-29(34(42)35-30)32(33-36-37-38-41(33)28-14-8-9-15-28)40-20-18-39(19-21-40)31(25-10-4-2-5-11-25)26-12-6-3-7-13-26/h2-7,10-13,16-17,22-23,28,31-32H,8-9,14-15,18-21H2,1H3,(H,35,42) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of LYP expressed in Escherichia coli BL21 using increasing levels of ARLIED-NEpYTAREG substrate |
J Med Chem 54: 1640-54 (2011)
Article DOI: 10.1021/jm101202j
BindingDB Entry DOI: 10.7270/Q2RN38Z0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50103227
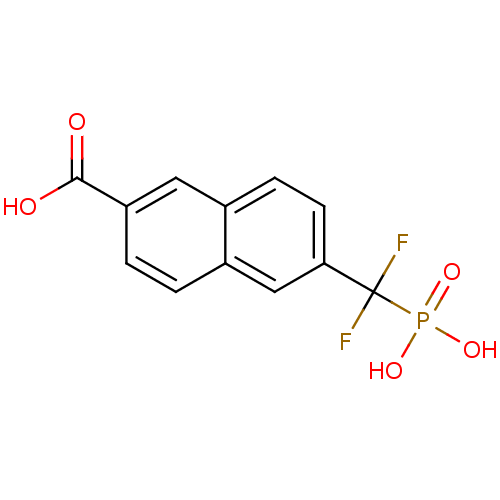
(6-(Difluoro-phosphono-methyl)-naphthalene-2-carbox...)Show InChI InChI=1S/C12H9F2O5P/c13-12(14,20(17,18)19)10-4-3-7-5-9(11(15)16)2-1-8(7)6-10/h1-6H,(H,15,16)(H2,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
PubMed
| 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIH
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards recombinant human Protein-tyrosine phosphatase 1B was determined |
Bioorg Med Chem Lett 13: 3005-7 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MV1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50103227

(6-(Difluoro-phosphono-methyl)-naphthalene-2-carbox...)Show InChI InChI=1S/C12H9F2O5P/c13-12(14,20(17,18)19)10-4-3-7-5-9(11(15)16)2-1-8(7)6-10/h1-6H,(H,15,16)(H2,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
PubMed
| 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Protein-tyrosinephosphatase 1B (PTP1B) |
J Med Chem 44: 2869-78 (2001)
BindingDB Entry DOI: 10.7270/Q237781C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50393463

(CHEMBL2159940)Show SMILES Cc1ccc2[nH]c(=O)c(cc2c1)C(N(Cc1ccccc1)Cc1ccc(Cl)cc1)c1nnnn1C1CCCC1 Show InChI InChI=1S/C31H31ClN6O/c1-21-11-16-28-24(17-21)18-27(31(39)33-28)29(30-34-35-36-38(30)26-9-5-6-10-26)37(19-22-7-3-2-4-8-22)20-23-12-14-25(32)15-13-23/h2-4,7-8,11-18,26,29H,5-6,9-10,19-20H2,1H3,(H,33,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of LYP expressed in Escherichia coli BL21 using increasing levels of ARLIED-NEpYTAREG substrate |
J Med Chem 54: 1640-54 (2011)
Article DOI: 10.1021/jm101202j
BindingDB Entry DOI: 10.7270/Q2RN38Z0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50131098

(2-(4-{2-((S)-1-(S)-Carbamoyl-3-methyl-butylcarbamo...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccc(OC(C(O)=O)C(O)=O)cc1)NC(=O)[C@H](CCC(O)=O)NC(=O)OCC1c2ccccc2-c2ccccc12)C(N)=O Show InChI InChI=1S/C38H42N4O12/c1-20(2)17-29(33(39)45)40-35(47)30(18-21-11-13-22(14-12-21)54-32(36(48)49)37(50)51)41-34(46)28(15-16-31(43)44)42-38(52)53-19-27-25-9-5-3-7-23(25)24-8-4-6-10-26(24)27/h3-14,20,27-30,32H,15-19H2,1-2H3,(H2,39,45)(H,40,47)(H,41,46)(H,42,52)(H,43,44)(H,48,49)(H,50,51)/t28-,29-,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibitory potency against human Protein-tyrosine phosphatase 1B expressed in E. coli BL21 (DE3) cells |
Bioorg Med Chem Lett 13: 2577-81 (2003)
BindingDB Entry DOI: 10.7270/Q2QC02VZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50335895

(6-hydroxy-3-(1-(4-(naphthalen-1-ylamino)-4-oxobuty...)Show SMILES OC(=O)c1cc2c(-c3cn(CCCC(=O)Nc4cccc5ccccc45)nn3)c(oc2cc1O)-c1ccccc1 Show InChI InChI=1S/C31H24N4O5/c36-26-17-27-23(16-22(26)31(38)39)29(30(40-27)20-9-2-1-3-10-20)25-18-35(34-33-25)15-7-14-28(37)32-24-13-6-11-19-8-4-5-12-21(19)24/h1-6,8-13,16-18,36H,7,14-15H2,(H,32,37)(H,38,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Inhibition of LYP expressed in Escherichia coli BL21 using increasing levels of ARLIED-NEpYTAREG substrate |
J Med Chem 54: 1640-54 (2011)
Article DOI: 10.1021/jm101202j
BindingDB Entry DOI: 10.7270/Q2RN38Z0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM46264

(3-[2-azaspiro[5.5]undecan-2-yl-(1-cyclopentyl-1,2,...)Show SMILES Cc1ccc2[nH]c(=O)c(cc2c1)C(N1CCCC2(CCCCC2)C1)c1nnnn1C1CCCC1 Show InChI InChI=1S/C27H36N6O/c1-19-10-11-23-20(16-19)17-22(26(34)28-23)24(25-29-30-31-33(25)21-8-3-4-9-21)32-15-7-14-27(18-32)12-5-2-6-13-27/h10-11,16-17,21,24H,2-9,12-15,18H2,1H3,(H,28,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of LYP expressed in Escherichia coli BL21 using increasing levels of ARLIED-NEpYTAREG substrate |
J Med Chem 54: 1640-54 (2011)
Article DOI: 10.1021/jm101202j
BindingDB Entry DOI: 10.7270/Q2RN38Z0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086645
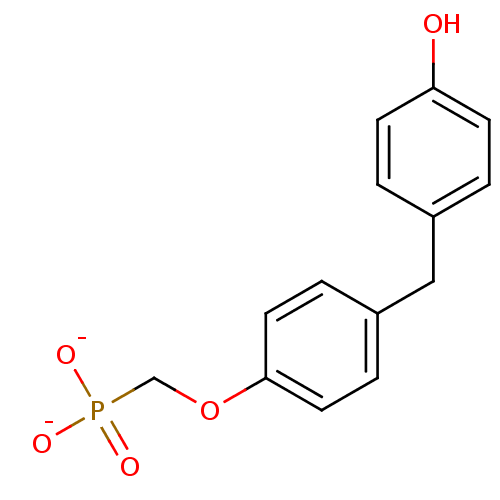
([4-(4-hydroxybenzyl)phenoxy]methylphosphonate)Show InChI InChI=1S/C14H15O5P/c15-13-5-1-11(2-6-13)9-12-3-7-14(8-4-12)19-10-20(16,17)18/h1-8,15H,9-10H2,(H2,16,17,18)/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 4.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
New York University
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition constant against Protein-tyrosine phosphatase (PTP1B ) |
Bioorg Med Chem Lett 10: 457-60 (2000)
BindingDB Entry DOI: 10.7270/Q2J966W3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50379182
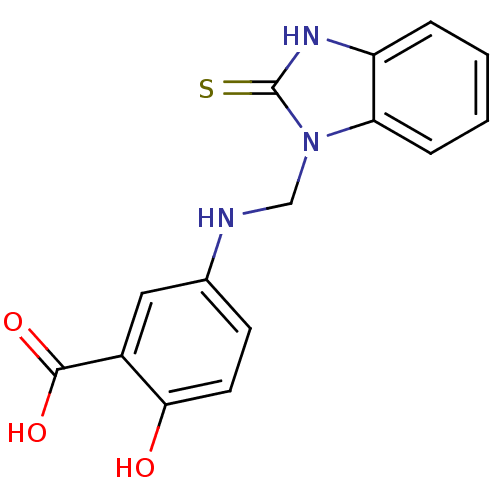
(CHEMBL289069)Show InChI InChI=1S/C15H13N3O3S/c19-13-6-5-9(7-10(13)14(20)21)16-8-18-12-4-2-1-3-11(12)17-15(18)22/h1-7,16,19H,8H2,(H,17,22)(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B expressed in Escherichia coli BL21 (DE3) cells using p-nitrophenyl phosphate as substrate after 2 to 3 mins by spectrophotometric... |
Bioorg Med Chem 20: 1940-6 (2012)
Article DOI: 10.1016/j.bmc.2011.11.004
BindingDB Entry DOI: 10.7270/Q23J3DZQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50075312

((Difluoro-naphthalen-2-yl-methyl)-phosphonic acid ...)Show InChI InChI=1S/C11H9F2O3P/c12-11(13,17(14,15)16)10-6-5-8-3-1-2-4-9(8)7-10/h1-7H,(H2,14,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.79E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIH
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards recombinant human Protein-tyrosine phosphatase 1B was determined |
Bioorg Med Chem Lett 13: 3005-7 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MV1 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50103238

(3-Carboxymethoxy-naphthalene-2,7-dicarboxylic acid...)Show InChI InChI=1S/C14H10O7/c15-12(16)6-21-11-5-7-1-2-8(13(17)18)3-9(7)4-10(11)14(19)20/h1-5H,6H2,(H,15,16)(H,17,18)(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Protein-tyrosinephosphatase 1B (PTP1B) |
J Med Chem 44: 2869-78 (2001)
BindingDB Entry DOI: 10.7270/Q237781C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086647
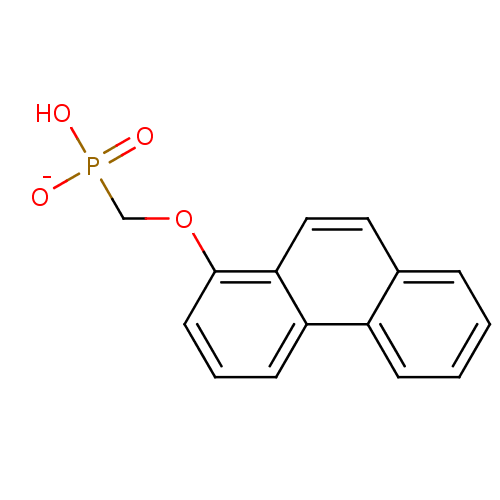
(hydrogen (1-phenanthryloxy)methylphosphonate)Show InChI InChI=1S/C15H13O4P/c16-20(17,18)10-19-15-7-3-6-13-12-5-2-1-4-11(12)8-9-14(13)15/h1-9H,10H2,(H2,16,17,18)/p-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 4.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
New York University
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition constant against Protein-tyrosine phosphatase (PTP1B ) |
Bioorg Med Chem Lett 10: 457-60 (2000)
BindingDB Entry DOI: 10.7270/Q2J966W3 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50086647

(hydrogen (1-phenanthryloxy)methylphosphonate)Show InChI InChI=1S/C15H13O4P/c16-20(17,18)10-19-15-7-3-6-13-12-5-2-1-4-11(12)8-9-14(13)15/h1-9H,10H2,(H2,16,17,18)/p-1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 5.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
New York University
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition constant against dual specificity phosphatase VHR |
Bioorg Med Chem Lett 10: 457-60 (2000)
BindingDB Entry DOI: 10.7270/Q2J966W3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50103234
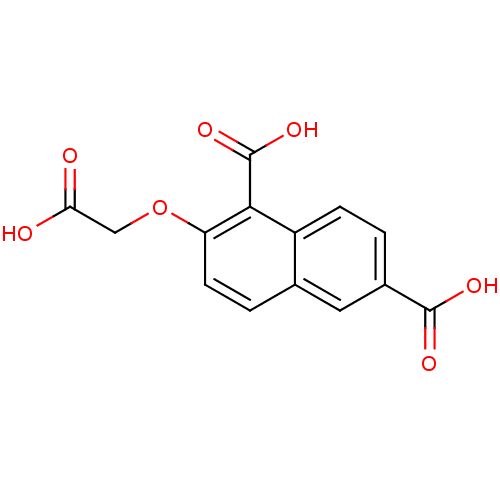
(2-Carboxymethoxy-naphthalene-1,6-dicarboxylic acid...)Show InChI InChI=1S/C14H10O7/c15-11(16)6-21-10-4-2-7-5-8(13(17)18)1-3-9(7)12(10)14(19)20/h1-5H,6H2,(H,15,16)(H,17,18)(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Protein-tyrosinephosphatase 1B (PTP1B) |
J Med Chem 44: 2869-78 (2001)
BindingDB Entry DOI: 10.7270/Q237781C |
More data for this
Ligand-Target Pair | |
Protein-tyrosine-phosphatase
(Yersinia pestis) | BDBM26193
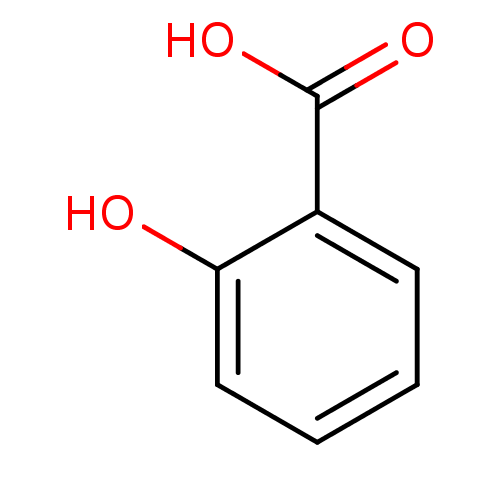
(2-Hydroxybenzoate, I | 2-hydroxybenzoic acid | CHE...)Show InChI InChI=1S/C7H6O3/c8-6-4-2-1-3-5(6)7(9)10/h1-4,8H,(H,9,10) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University
Curated by ChEMBL
| Assay Description
Inhibition of Yersinia pseudotuberculosis YopH |
Bioorg Med Chem 20: 1940-6 (2012)
Article DOI: 10.1016/j.bmc.2011.11.004
BindingDB Entry DOI: 10.7270/Q23J3DZQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data